Abstract
This protocol describes a method for efficient chemical synthesis of an analog of inositol-1,4,5-trisphosphate (IP3) hexakis acetoxymethyl ester having an ortho-nitroveratryl photochemical caging group on the 6-hydroxyl position. The six esters render the probe membrane permeant, such that it can be loaded into intact living cells in vitro or in vivo. Inside cells, the caged IP3 is inert until activated by two-photon excitation at 720 nm. The photoliberated signaling molecule can mobilize release of Ca2+ from intracellular stores on the endoplasmic reticulum. When co-loaded with the fluorescent Ca2+ indicator rhod-2, one laser can be used for stimulating and monitoring intracellular Ca2+ signaling with single-cell resolution. This protocol has chemistry and biology sections; the former describes the organic synthesis of the caged IP3, which requires 12 d, and the latter an application to a day-long study of astrocyte-regulated neuronal function in living brain slices acutely isolated from rats. As Ca2+ is the single most important intracellular second messenger and the IP3-Ca2+ signaling cascade is used by many cells to produce increases in Ca2+ concentration, this method should be widely applicable for the study of a variety of physiological processes in intact biological systems.
Introduction
Photochemical uncaging of signaling molecules has proven to be a useful technique for many areas of biology investigation1. A particularly powerful feature of caged compounds is that inert, intracellular messengers can be loaded into cells. Light, which passes through the plasma membrane, can then be used to activate a chosen process in the intracellular environment. The cytosol is relatively inaccessible to rapid solution changing; accordingly, uncaging can bypass any diffusional delays that would be involved in other methods of cell stimulation (e.g., microinjection of the signaling molecule). When uncaging is used in conjunction with imaging intracellular signaling, it becomes an even more impressive tool: light, with appropriate chemical probes, takes on a dual function as the activator and sensor of signaling cascades2. Since 1991, the most prominent pairing of sensor and activator chemical probes has been with fluorescent Ca2+ dyes and caged Ca2+ molecules3.
Using whole-cell dialysis, quantitative correlations of intracellular calcium concentrations [Ca2+]I and cell function have been made in several neuroendocrine cell types4–6. Even though this approach has proven to be extremely fruitful, there are a number of disadvantages to single-cell uncaging in this manner: (i) the necessity of patch clamping individual cells makes the approach quite laborious; (ii) typically only one cell can be studied at a time; and (iii) dialysis displaces vital intracellular components (e.g., G-actin and calmodulin from neurons). Loading cells with membrane-permeant probes is an important alternative approach to studying cell signaling using fluorescence microscopy7.
The extremely wide use of acetoxymethyl (AM) ester derivatives of the fluorescent Ca2+ indicators developed by Tsien and co-workers in the 1980s is the best example of such non-invasive loading and imaging; the 1985 fura-2/AM paper8 has been cited more than 17,000 times to date. In contrast to such fluorescent indicators, membrane-permeant caged compounds are comparatively recent, and thus have not been so widely used. It should be noted, however, that the first caged compound (cAMP9) was membrane permeant.
The development of two-photon microscopy allows facile imaging of [Ca2+]i and has proven to be especially useful to neuroscientists working in acute brain slice preparations or in vivo10. In another protocol, the synthesis of two-photon–sensitive caged neurotransmitters is described11. The reader is referred to that protocol for a more detailed discussion of two-photon microscopy. We have recently published studies with cell-permeant caged Ca2+12 and caged inositol-1,4,5-trisphosphate (IP3)13 probes in which these probes have been loaded into astrocytes in acutely isolated hippocampal brain slices. Two-photon uncaging in single cells was effected using the fluorescence recovery after photobleaching (FRAP) function in the microscope software. All commercial laser-scanning confocal microscopes have such a feature; hence, no custom programming is required for the uncaging experiments that we describe.
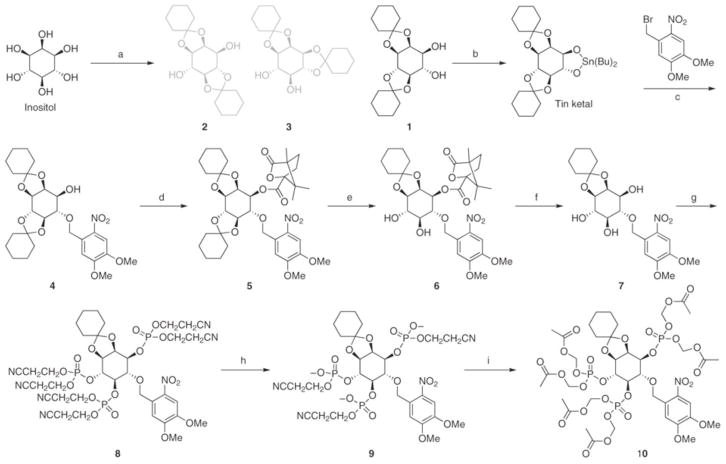
This protocol describes the synthesis of a membrane-permeant caged IP3 analog (Fig. 1) that undergoes facile two-photon uncaging at 720 nm using a mode-locked Ti:sapphire laser14, 15. We reveal that the key step in the high-yielding synthesis of membrane-permeant AM esters of caged inositol polyphosphate is the initial partial deprotection of the penultimate synthetic cyanoethylphosphate intermediate, followed by steps of reprotection with AM bromide and further cyanoethyl deprotections/AM bromide reactions, to create the final caged compound. This key step is performed on ~0.25 mmol but may be easily scaled up by two- to tenfold. The initial synthetic steps are performed in the range of 2–20 mmol.
Figure 1. Scheme for the synthesis of nitroveratryl-IP3/AM.
Reagents: (a) cyclohexanone; (b) dibutyltin oxide; (c) CSF; (d) camphanic chloride; (e) acetyl chloride; (f) KOH; (g) N,N-diisopropyl-bis(2-cyanoethyl)-phosphoramidite and then t-BuOOH; (h) Hünig’s base; and (i) AM bromide.
Application of cell-permeant caged IP3: experimental rationale and practical considerations
When the caged compound is co-loaded into cells with the fluorescent Ca2+ indicator rhod-2, a single laser may be used to image photo-evoked changes in [Ca2+]i at the same wavelength used for uncaging. As uncaging and imaging are effected in different power regimes (we use a tenfold difference, which equates to 100-fold in two-photon excitation), long-term imaging is essentially optically independent of uncaging at a single wavelength. This protocol outlines methods for such uncaging and imaging experiments in astrocytes from acutely isolated (i.e., immediately taken from a euthanized rat) brain slices. Given the ubiquity of the intracellular IP3-Ca2+ signaling cascade, the uncaging method we present is applicable to many cell types (e.g., smooth muscle, hepatocytes, neurons and so on). Such uncaging experiments allow one to address questions involving the importance of IP3, as photorelease separates the upstream signal that transduces IP3 release from the downstream effects of IP3. Second, questions on the timing of IP3 release can be addressed very precisely by uncaging. Third, as light may be focused on one cell in a large cluster or on one part of a cell, questions about the localization of IP3 signaling may be addressed with the uncaging technique.
The peak power of the focused two-photon laser beam is designed to be extremely high; therefore, the application of such lasers to uncaging needs to be done carefully, even more so than in normal uncaging experiments. Thus, for each cell type, microscope, laser and objective lens, the power threshold for laser damage must be quantified. In the case of two-photon uncaging inside cells, the same procedure for probe loading described in Steps 86–97 should be followed, except that the caged compound should be omitted from the loading solution. The power threshold for cell damage can then be determined by illuminating a calcium dye–loaded cell with a range of laser energies. Photodamage of astrocytes is readily apparent when the cell body becomes intensely saturated with fluorescent signal (i.e., it appears white on a pseudocolor scale), and this signal remains constant. We have found that the exact power level for photodamage is different for every system and must be determined for each type of experiment. As the efficiency of two-photon excitation is dependent on the fourth power of the numerical aperture (NA), the most important variable in any system is the objective lens. We have successfully used three types of objectives for two-photon uncaging (×40, 1.0 NA (this protocol); ×40, 0.8 NA11; ×20, 0.95 NA15). Once determined, we recommend using 30–50% of this power value for uncaging of IP3.
REAGENTS
Critical
HPLC-grade acetone is important for one synthetic step; otherwise no special care is required for any solvent used in any synthetic step. HPLC-grade acetonitrile, trifluoroacetic acid and water must be used for HPLC. Other reagents obtained from Sigma-Aldrich or Acros have been used with equal effectiveness.
Caution
Chemical safety considerations
Most of the reagents used in the protocol require protective goggles, gloves and lab coats. As several reagents are toxic or harmful, much (but not all) of the work must be carried out in a fume hood using round-bottomed flasks with ground glass joints.
Inositol (Sigma, cat. no. I5125)
Cyclohexanone (Aldrich, cat. no. W390909)
-
Para-toluenesulfonic acid
Caution: It is an irritant.
-
(1S)-(−)-camphanic chloride (Acros, cat. no. 20989)
Critical: This reagent is sensitive to atmospheric water, and thus should be purchased and used fresh.
Triethylamine
Dimethylaminopyridine
DMSO
-
Acetylchloride
Caution: It is a lachrymator.
Tetrazole (Fluka, cat. no. 17234)
Diisopropylphosphoramidous dichloride (Aldrich, cat. no. 307254)
2-Cynaoethanol
4,5-Dimethoxy-2-nitrobenzyl (ortho-nitroveratyl) bromide (Aldrich, cat. no. 392855)
Hünig’s base
Dibutyltin oxide
Aritificial cerebrospinal fluid (ACSF; see REAGENT SETUP)
Rhod-2 (Invitrogen, cat. no. R1244)
Bromomethyl acetate (AM bromide; Aldrich, cat. no. 303208)
Methylene chloride
-
Acetonitrile (HPLC grade)
Caution:It is flammable.
-
Methanol
Caution: It is flammable.
-
Chloroform
Caution: It is toxic.
-
Chloroform-d (CDCl3)
Caution: It is toxic.
-
Acetone
Caution: It is flammable.
-
Acetone-d6
Caution: It is flammable.
Tetramethylsilane (TMS)
-
Dimethyl formamide (DMF)
Caution: It is flammable.
-
Hexane
Caution: It is flammable.
-
Ethyl acetate
Caution: It is flammable.
-
Tert-BuOOH 30% (vol/vol) solution in water
Caution: It is an oxidizer.
Anisaldehyde
Acetic acid
Ethanol
H2SO4
KOH (1 N) solution
NaHCO3
NaH2PO4
K2HPO4
CaCl2
MgCl2
NaCl
KCl
Glucose
Sucrose
Nanopure water
Toluene
-
Silica gel 60 (230–400 mesh; Fisher, cat. no. S825)
Caution: This reagent is made up of fine particles; handle the dry powder in a fume hood.
Thin-layer chromatography (TLC) plates, silica gel 60 F254 (Whatman, cat. no. 4861-820)
Cyanoacrylate glue (e.g., Super Glue)
O2 (95%)/CO2 (5%) gas
-
Sprague-Dawley rats
Critical: Experiments must be conducted according to appropriate local and national animal care protocols.
EQUIPMENT
Round-bottomed flask
Fume hood
Rotary evaporator
Silica gel flash chromatography columns
Filter funnels
Reflux condensers
Azeotropic adaptor
Separatory funnels
Spatulas
Teflon-coated magnetic stirrer bars
Magnetic stirrer/hot plates
TLC-developing chambers
UV handheld lamp
Culture dish (12-well plate)
Two-photon microscope
Vibratome brain slicer
REAGENT SETUP
-
Anisaldehyde thin-layer chromatography (TLC) stain
Combine 18 mL anisaldehyde, 4 mL acetic acid, 350 ml ethanol and 10 mL concentrated H2SO4. Stored at 4°C, the stain is stable for several months.
-
ACSF (artificial cerebrospinal fluid)
Combine NaCl, 126 mM; KCl, 2.5 mM; NaHCO3, 26 mM; CaCl2, 1.5 mM; MgCl2, 1.5 mM; NaH2PO4, 1.25 mM; glucose, 10 mM; saturated with 95% O2/5% CO2. Prepare this solution fresh for each experiment.
-
Slicing solution
-
Combine NaCl, 87 mM; KCl, 2.5 mM; NaHCO3, 25 mM; CaCl2, 0.5 mM; MgCl2, 7 mM; NaH2PO4, 1.25 mM; glucose, 25 mM; sucrose, 75 mM; saturate with 95% O2/5% CO2.
Critical: Prepare this solution fresh for each experiment.
-
Steps 1 – 14: Synthesis of (±)-2,3-4,5-di-O-cyclohexilydene-myo-inositol. Timing: 48 h
-
1
In a 1-liter round-bottomed flask equipped with a stirrer bar, dissolve 36 g of inositol (36 g, 0.2 mol) and cyclohexanone (200 mL, 1.93 mol) in DMF (200 mL) and toluene (150 mL).
Critical step: All experiments must be conducted in a room with yellow-filtered lights to prevent photolysis of photosensitive compounds.
-
2
Thereafter, add 10 mL of a 10% solution (wt/wt) of para-toluenesulfonic acid in DMF.
-
3
Heat the reaction at reflux temperature for 22 h and collect the water side product (~11 mL) in an azeotropic adaptor.
-
4
Allow the reaction to cool to room temperature (RT, 20–25°C) and add triethylamine (50 mL).
-
5
TLC analysis (chloroform/acetone (2:1)) shows the three diol products (1, 2, 3) present in equal amounts (see Fig. 2).
-
1
Remove the solvents in vacuo (5 mmHg) on a rotary evaporator at 70–80 °C to produce a brown liquid. Diol 2 spontaneously precipitates and is removed by filtration, leaving a mixture of diols 1 and 3.
-
2
Fill a flash chromatography column with a bed of silica gel (dimensions 1.5 × 10 inches).
-
3
Equilibrate the column with running solvent (chloroform/acetone (1:10)) by shaking it. Empty the column of excess solvent by applying a low pressure (house compressed air is convenient). During this process, adjust the air pressure to give 5 cm min−1 linear flow of solvent.
Caution: Mixing solvent with silica is exothermic and causes the solvent to evaporate, so periodically relieve pressure by removing the stopcock.
-
4
Load 2 g of diols in an equal volume of running solvent onto the top of the column.
Critical step: This must be performed with care in order to form an even layer that does not disturb the silica gel bed.
-
5
Separate by silica gel chromatography, collecting 25-mL fractions.
-
6
Analyze each fraction by TLC to detect products.
-
7
Stain the TLC plate with anisaldehyde to visualize components by dipping the plate into a bath of stain and heating the plate on a hot plate until the spots develop.
-
8
Bulk all fractions with pure diol 1 in a 500-mL round-bottomed flask.
Critical step: Select only pure fractions, as purity at this stage is the basis of successful synthesis of the caged compound.
-
9
Remove the solvents by rotary evaporation at reduced pressure to yield diol 1 as a white solid.
Pause point: Compound 1 can be safely stored at RT for several years.
Figure 2. TLC plate for Step 5 of the procedure.
The relative migrations of diols 1–3 from the first step in the synthesis. Running solvent: chloroform/ acetone (2:1).
Steps 15 – 24: Synthesis of (±)-2,3-4,5-di-O-cyclohexilydene-6-O(ortho-nitroveratryl)-myo-inositol. Timing: 48 h
-
15
In a 125-mL round-bottomed flask equipped with a stirrer bar, dissolve diol 1 (1.7 g, 5.0 mmol) and dibutyltin oxide (1.25 g, 5.0 mmol) in benzene (30 mL). Toluene may be used in place of benzene.
-
16
Heat at reflux temperature with azeotropic distillation for 24 h.
-
17
Remove the benzene (toluene) in vacuo using rotary evaporation.
Pause point: The tin ketal can be safely stored at −20°C for several months.
-
18
Dissolve the tin ketal in DMF (25 mL) with ortho-nitroveratryl bromide (1.4 g, 5.1 mmol) and CSF (0.8 g, 0.52 mmol).
-
19
Stir at RT for 18 h.
-
20
Remove the solvent in vacuo on a rotary evaporator at 5 mmHg, at 70–80°C.
-
21
Dissolve the crude residue in water and extract with methylene chloride (3 washes of 25 mL each).
-
22
Dry the organic phase by adding sufficient solid MgSO4 until some of the drying agent appears to float as a fluffy solid, remove by filtration and concentrate in vacuo on a rotary evaporator at 30–40 °C.
-
23
Purify by silica gel chromatography as described above for compound 1, except use ethyl acetate/hexane (1:3) as the running solvent.
-
24
Isolate alcohol 4 (Rf = 0.26) in 59% yield.
Pause point: Compound 4 can be safely stored at −20°C for several months.
Steps 25 – 31: Synthesis of (±)-1-O-camphanyl-2,3-4,5-di-O-cyclohexilydene-6-O-(ortho-nitroveratryl)-myo-inositol. Timing: 18 h
-
25
In a 25-mL round-bottomed flask, prepare a solution of alcohol 4 (0.535 g, 1.0 mmol) and (1S)-(-)-camphanic chloride (0.25 g, 1.2 mmol) in methylene chloride (30 mL).
Critical step:Use fresh camphanic chloride.
Troubleshooting
-
26
Add triethylamine (0.5 ml) and catalytic dimethylaminopyridine (25 mg) to the reaction mixture (RM).
Troubleshooting
-
27
Allow the RM to stand at RT for 12 h.
Troubleshooting
-
28
Add water (25 mL) and separate the organic layer.
-
29
Dry over MgSO4, filter off MgSO4 and concentrate in vacuo on a rotary evaporator at 30–40°C.
-
30
Purify by silica gel chromatography as described above for compound 1, except use ethyl acetate/hexane (1:4) as the running solvent.
-
31
Isolate ester 5 (Rf = 0.35) in 89% yield.
Steps 32 – 44: Synthesis of (±)-2,3-O-cyclohexilydene-6-O-(ortho-nitroveratryl)-myo-inositol. Timing: 15 h
-
32
In a 25-mL round-bottomed flask, dissolve compound 5 (0.40 g, 0.56 mmol) in methylene chloride/methanol (9:1, 5 mL).
-
33
Cool with ice bath to 10°C.
-
34
Add acetylchloride (0.1 mL).
Troubleshooting
-
35
After 15 min, analyze the RM by TLC (ethyl acetate/hexane (1:1)) to confirm the disappearance of 5 (Rf = 0.83) and appearance of diol product 6 (Rf = 0.22).
Critical step: Allowing the reaction to stand too long also deprotects the 2,3 cyclohexylidene protecting group. In our hands, the use of trifluoroacetic acid for deprotection of ketal16 was unsuccessful.
Troubleshooting
-
33
Quench the reaction with triethylamine (1 ml) and wash the RM with water and separate the organic layer.
-
34
Dry over MgSO4, filter off MgSO4 and concentrate in vacuo on a rotary evaporator at 30–40°C.
-
35
Dissolve the crude diol 6 in methylene chloride/methanol (1:1, 5 mL).
-
36
Add KOH (0.25 ml 1 N) and stir for 12 h. TLC (100% ethyl acetate) shows the disappearance of diol 6 (Rf = 0.69) and appearance of triol product (Rf = 0.40).
-
37
Remove the methanol in vacuo using a rotary evaporator at 30–40°C.
-
38
Redissolve the RM in methylene chloride (25 mL), wash with water and separate the organic layer.
-
39
Dry over MgSO4, filter off MgSO4 and concentrate in vacuo on a rotary evaporator at 30–40°C.
-
40
Purify by silica gel chromatography as described above for compound 1, except use ethyl acetate.
-
41
Isolate triol 7 (Rf = 0.41) in 72% yield.
Pause point: Compound 7 can be safely stored at −20 °C for several months.
Timing: 48 h
-
45
In a round-bottomed flask equipped with a stirrer, cool a solution of diisopropylphosphoramidous dichloride (2.0 g, 9.9 mmol) and 2-cyanoethanol (1.45 g, 20 mmol) in methylene chloride (20 ml) to 10°C.
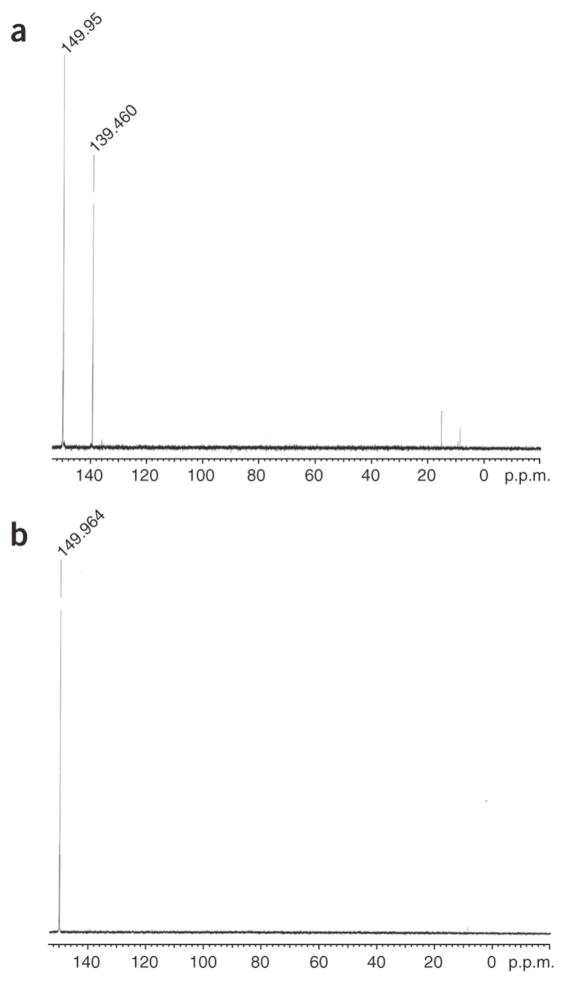
Critical step: The phosphitylation reagent must be freshly prepared. A single peak in 31P NMR at 149 p.p.m. (see Fig. 3) is indicative of a good reagent.
-
46
Add diisopropylethyl amine (2.8 g, 22 mmol).
-
46
Allow the RM to warm to RT and stir overnight (approximately 12–18 h).
-
47
Dilute the RM with methylene chloride (30 mL).
-
48
Wash the RM in a separatory funnel with saturated NaHCO3 (20 ml) and then with brine (20 mL).
-
49
Dry the organic phase over MgSO4, filter off the MgSO4 and separate the organic layer.
-
50
Concentrate in vacuo on a rotary evaporator at 30–40°C. The 31P NMR (CDCl3) will show several peaks (Fig. 3a).
-
51
Purify by silica gel chromatography, as described above for compound 1, except use ethyl acetate/hexane (1:4).
-
52
Isolate N,N-diisopropyl-bis(2-cyanoethyl)-phosphoramidite (Rf = 0.26) in 50% yield. The 31P NMR should now show a single peak at 150 p.p.m. (Fig. 3b).
Critical step: This reagent should be used immediately in the next step.
Troubleshooting
-
53
In a 25-ml round-bottomed flask equipped with a stirrer, mix a solution of triol 7 (0.112 g, 0.25 mmol) and phosphitylation reagent (0.42 g, 1.5 mmol) in methylene chloride (2 mL).
-
54
The RM at this juncture is clear and homogeneous.
-
55
Add tetrazole (0.1 g, 1.5 mmol) in acetonitrile (1 mL).
Critical step: The appearance of a milky white precipitate is indicative of the reaction proceeding successfully.
Troubleshooting
-
56
TLC analysis of the RM after 12–16 h should reveal one spot (Rf = 0.86, ethyl acetate).
Troubleshooting
-
57
Cool the RM to 5–10 °C with an ice bath.
-
58
Carefully add t-BuOOH (0.4 mL 30% solution in water) dropwise to the RM.
-
59
Allow the RM to stand at RT for 3 h.
Critical step: Oxidation to the desired phosphate product causes the RM to clarify.
-
60
Dilute with methylene chloride (20 mL), wash in a separatory funnel with saturated NaHCO3 (20 mL) and then with brine (20 mL).
-
61
Dry the organic phase over MgSO4, filter off the MgSO4 and then separate the organic layer.
-
62
Concentrate in vacuo on a rotary evaporator at 30–40°C.
-
63
Purify by silica gel chromatography as described for compound 1, except use ethyl acetate/methanol (95:5) as the running solvent.
-
64
Isolate trisphosphate 8 (Rf = 0.14 in ethyl acetate) in 56% yield.
Critical step: Three peaks in 31P NMR at −1.75, −2.14 and −2.23 p.p.m. are indicative of a pure product. We have found that phosphorus NMR is particularly useful in the diagnosis of phosphitylation reagents (Fig. 3) and reactions.
Pause point: Compound 8 can be safely stored at −20°C for several months.
Troubleshooting
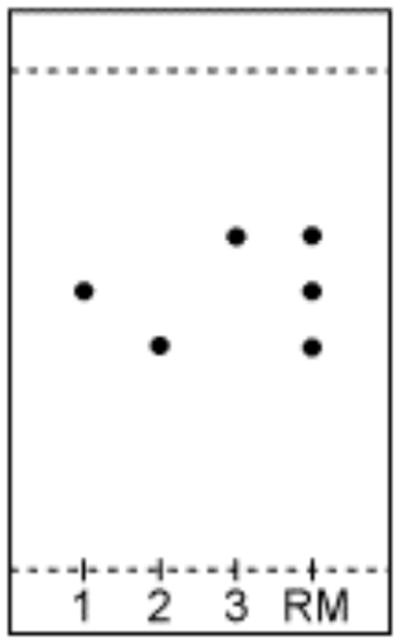
Figure 3. 31P NMR spectra.
(a,b) Panel a shows that of RM and b that of product (N,N-diisopropyl-bis(2-cyanoethyl)-phosphoramidite) for Step 52.
Steps 66 – 76: Synthesis of (±)-2,3-O-cyclohexilydene-6-O-(ortho-nitroveratryl)-myo-inositol-1,4,5-trisphosphate hexakis (acetoxymethyl)ester. Timing: 48 h
-
66
In a 25-mL round-bottomed flask equipped with a stirrer, mix a solution of hexakiscyanoethylphosphate 8 and ethyldiisopropyl amine (1 mL, Hunig’s base) in acetonitrile (2 mL).
-
67
After stirring for 24 h at RT, analyze by reverse-phase HPLC (elution at 1 ml min−1 on C-18 from 10% to 100% acetonitrile over 30 min).
-
68
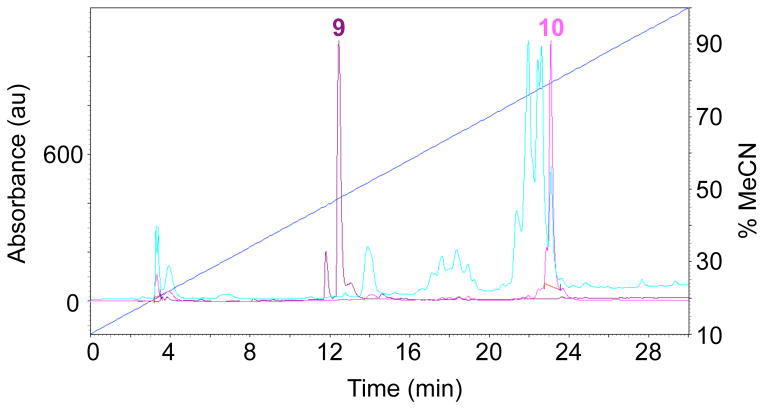
The RM should show one main peak with a retention time of 12.5 min (Fig. 4, purple trace).
-
69
This intermediate was shown by 1H NMR to be the triscyanoethylphosphate, compound 9.
-
70
Concentrate the RM to dryness and add dry acetonitrile (2 mL), Hunig’s base (0.3 mL) and AM bromide (0.2 mL).
-
71
HPLC after 5 h shows peaks between 18 and 23 min (Fig. 4, cyan trace).
-
72
The next day there will be essentially one peak in the HPLC (Fig. 4, pink trace).
Critical step: Enough reaction time must be allowed for only one peak at 12.5 min for the initial deprotection to appear, as the second cyanoethyl group at each reaction center is removed only after the first AM group is added to the phosphate. In our hands, use of ammonium hydroxide/methanol for deprotection of the phosphates16 has been unsuccessful.
Troubleshooting
-
67
Concentrate the RM to dryness by rotary evaporation at 30–40°C to yield a yellow/orange solid.
-
68
Purify by flash chromatography using a 10-ml Pasteur pipette as the column with 6 ml of silica gel (elute with ethyl acetate and then with 1% (vol/vol) methanol in ethyl acetate).
-
69
Collect small column fractions (~0.5 mL). Compound 10 has an Rf of 0.26.
-
70
Isolate the target-caged IP3 product (compound 10) in 35% yield.
Figure 4. HPLC of AM ester synthesis.
The purple trace shows the retention time of compound 9, the cyan trace the reaction of 9 with AM bromide after 5 h and the pink trace shows the composition of the final reaction mixture before silica gel chromatography. The target compound 10 is the major component of the RM.
Steps 77 – 97: Two-photon uncaging in astrocytes in living brain slices. Timing: 5 h
-
77
Critical step: All experiments on living tissue immediately isolated from rodents must be carried out according to appropriate institutional guidelines for the use and care of animals.
Prepare freshly oxygenated ACSF and slicing solutions (see REAGENT SETUP).
Critical step: The level of oxygenation and pH set by bubbling 95% O2/5% CO2 is crucial for the production of healthy acute brain slices. Continuously bubble all ACSF and slicing solutions.
-
78
Anesthetize a Sprague-Dawley rat (P15–19 for hippocampus and cortex; P15–21 for hypothalamus) with halothane.
-
79
Decapitate the rat.
-
80
Rapidly remove the brain.
Critical step: The speed of brain removal is essential to produce healthy acute brain slices.
-
81
Immerse the whole brain in ice-cold slicing solution.
-
82
Select the volume of brain to be sliced by rapidly dissecting the appropriate brain region.
-
83
Glue the brain portion to slicer using cyanoacrylate glue.
-
84
Prepare acute brain slices of 300- to 400-μm thickness in ice-cold slicing solution.
-
85
Allow the slices to recover in ACSF at 32.5°C for 1 h.
-
86
Dissolve 50 μg of caged IP3/AM (compound 1) and 50 μg of Ca2+ dye (rhod-2/AM ester) in 3-μL DMSO.
-
87
Sonicate for 5 min.
-
88
Add ACSF (0.5 mL) at RT to rhod-2/cage solution.
-
89
Provide fine bubbling of 95% O2/5% CO2 to the well (Fig. 5).
-
90
Transfer brain slices from a large recovery chamber to a small well chamber containing 2.5-mL ACSF at RT and then add the rhod-2/ cage solution (Fig. 5). Load for 1–1.5 h.
Critical step: The health of the brain slice is maintained by the gases. Care must be taken not to agitate the solution, as this will cause the brain slice to decay.
-
91
Transfer the slice to the microscope chamber.
-
92
Check for health of cells using infrared differential interference contrast (IR-DIC).
-
93
Image loaded cells by two-photon microscopy by imaging with low power (~4 mW after the objective) at 720 nm. Any water-dipping objective that passes two-photon wavelengths may be used.
Troubleshooting
-
94
Only astrocytes should be apparent, as shown by their distinctive shape (Fig. 6).
-
95
Select a cell visually for uncaging and define the area of uncaging by drawing a region of interest inside the cell using microscope software. All commercial microscopes have such subroutines available for FRAP experiments. This method uses the FRAP tool to liberate IP3 rather than locally bleach a chromophore.
-
96
Scan the region of interest at high power (~40–48 mW after the objective) using the FRAP software function using the same pixel dwell time used for imaging.
Troubleshooting
-
97
Return to full-frame Ca2+ imaging after photoactivation.
Troubleshooting
Figure 5. Loading astrocytes in acute brain slices with nitroveratyl-IP3/AM and the Ca2+ indicator rhod-2/AM.
Step 1: A single well in a multiwell plate is filled with 2.5 ml ACSF and bubbled with carbogen using a thin, flexible pipette-filling syringe tip. When the tip is placed at the bottom of the well, numerous fine bubbles are produced (shown in expanded image below). This is critical for providing adequate O2 and CO2 without physically disturbing the slices. Step 2: Carefully add brain slices from the large post-slicing recovery chamber to the well. Step 3: Add 0.5 mL of ACSF to the freshly sonicated rhod-2/AM–DMSO solution and then carefully add this to the well containing the slices. Step 4: Cover the well with a square piece of parafilm wax without disturbing the bubbler. A depression in the middle of the wax sheet will prevent the solution from gathering on the underside of the wax and instead allows the solution to fall back into the well. Incubate for 1–2 h.
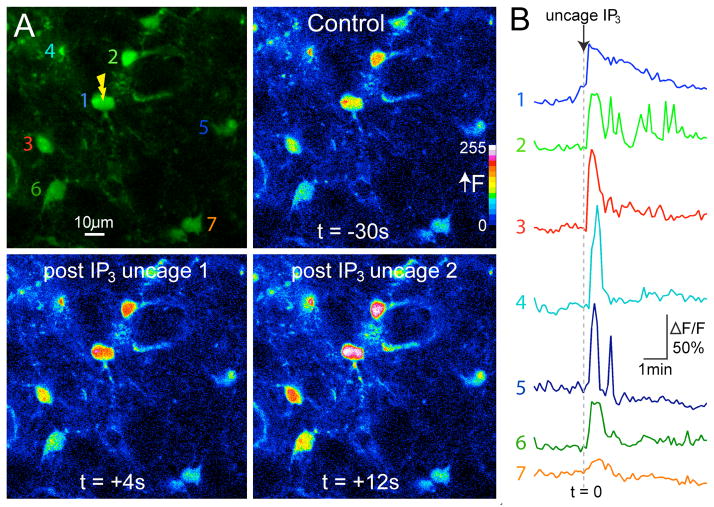
Figure 6. Two-photon imaging and IP3 uncaging (720 nm) in astrocytes in acute brain slices of the paraventricular nucleus of the hypothalamus.
(a) Loaded astrocytes showing resting rhod-2 fluorescence. Numbers indicate the somas of seven different astrocytes, which constitute the regions of interest for measuring increases in rhod-2 fluorescence as a consequence of elevations in free intracellular Ca2+. Pseudocolored images depict changes in free intracellular Ca2+ within astrocyte somas before and at two time points after IP3 uncaging in astrocyte 1. (b) Ca2+ signals in astrocyte somas in response to uncaging IP3 in astrocyte 1. Numbers correspond to the astrocytes in a.
Timing
Steps 1–14: 48 h
Steps 15–24: 48 h
Steps 25–31: 18 h
Steps 32–44: 15 h
Steps 45–65: 48 h
Steps 66–76: 48 h
Steps 77–97: 5 h
Anticipated results
Analytical data
NMR standards
TMS for 1H, CHCl3 or acetone for 13C and 70% phosphoric acid for 31P.
Compound 4
1H NMR δ (300 MHz, CDCl3) δ = 7.75 (s, 1H), 7.21 (s, 1H), 5.14 (dd, J = 7.4, 6.1, 2H), 4.48 (dd, J = 2.6, 1.3, 1H), 4.40 (t, J = 2.3, 1H), 4.1–4.2 (m, 2H), 4.08 (s, 3H), 3.94 (s, 3H), 3.87 (dd, J = 3.0, 0.9, 1H), 3.61 (dd, J = 3.9, 2.7, 1H), 2.6 (s, 1H) and 1.4–1.8 (m, 20H). MS ESI (mass spectrometry–electrospray ionization) m/z: 536 (M+H), 558 (M+Na).
Compound 5
1H NMR: δ (300 MHz, CDCl3) (NB: the diastereomers appear throughout this spectrum) 7.70 and 7.69 (s, 1H); 7.40 and 7.39 (s, 1H); 5.48–5.45 (m, 1H); 5.17 (Abq, J = 55.4, 15.0 Hz, 2H); 4.70–4.65 (m, 1H); 4.50 (dd, J = 8.0, 5.1 Hz); 4.10–3.99 (m, 3H); 3.99 (s, 3H); 3.97 (s, 3H); 3.80–3.72 (m, 1H); 2.53–2.42 (m, 1H); 2.08–1.83 (m, 2H) and 1.77–1.40 (m, 20H); six singlets for diastereomeric camphor methyl groups: 1.14; 1.07; 1.05; 1.04; 0.89 and 0.85. High-resolution mass spectrum (HRMS) C37H49NO13 requires 715.3204, 715.3238 (M+) found.
Compound 7
1H NMR: δ (300 MHz, CDCl3) 7.69 (s, 1H), 7.34 (s, 1H), 5.23 (s, 1H), 4.44 (dd, J = 5.9, 4.1 Hz, 1H), 4.08 (dd, J = 7.4, 5.8 Hz, 1H), 4.00 (s, 3H), 4.02–3.98 (m, 1H), 3.95, (s, 3H), 3.88–3.81 (m, 1H), 3.72 (t, J = 7.8 Hz, 1H), 3.52–3.45 (m, 1H), 2.76 (d, J = 2.6 Hz, 1H), 2.66 (d, J = 2.8 Hz, 1H), 2.51 (d, J = 6.3 Hz, 1H) and 1.74–1.42 (m, 10H). HRMS: C21H29NO10 455.1791, 455.1778 (M+) found.
Compound 8
1H NMR: δ (300 MHz, CDCl3) 7.69 (s, 1H), 7.30 (s, 1H), 5.16 (ABq, J = 42.1, 13.7 Hz, 2H), 4.91–4.86 (m, 1H), 4.83 (dd, J = 14.0, 8.4 Hz, 1H), 4.68 (dd, J = 8.7, 5.8 Hz, 1H), 4.66–4.62 (m, 1H), 4.42–4.21 (m, 14H), 4.04 (s, 3H), 3.96 (s, 3H), 2.86–2.80 (m, 8H), 2.79–2.66 (m, 4H) and 1.80–1.25 (m, 10H). 13C NMR: δ (70 MHz, acetone-d6) 156.56, 150.36, 141.28, 131.93, 119.90, 119.82, 119.66, 119.48, 119.41, 114.92, 112.92, 110.34, 82.72, 80.68, 78.56, 77.32, 76.15, 73.27, 65.74, 65.65, 65.58, 65.47, 65.40, 65.34, 58.59, 58.24, 39.33, 36.98, 27.29, 26.50, 26.24, 21.71, 21.66, 21.61, 21.47 and 21.36. 31P NMR: δ (121 MHz, CDCl3) −1.75, −2.14 and −2.23. HRMS: C39H50N7O19NaP3 requires 1,036.2272, 1,036.2309 (M+Na) found.
Compound 10
1H NMR: δ (300 MHz, CDCl3) 7.71 (s, 1H), 7.47 (s, 1H), 5.74–5.61 (m, 8H), 5.47–5.41 (m, 2H), 5.40–5.24 (m, 2H), .20 (s, 2H), 4.81 (td, J = 8.5, 3.7 Hz, 1H), 4.72 (dt, J = 8.8, 6.6, 1H), 4.64–4.54 (m, 2H), 4.31 (t, J = 6.2 Hz, 1H), 4.19 (t, J = 7.5 Hz, 1H), 4.05 (s, 3H), 3.95 (s, 3H), 2.15 (s, 3H), 2.14 (s, 3H), 2.13 (s, 3H), 2.11 (s, 3H), 2.03 (s, 3H), 2.01 (s, 3H), 1.82–1.41 (m, 10H). 31P NMR: δ (121 MHz, CDCl3) −3.67, −3.71 and −3.96. HRMS: C39H56NO31P3Na requires 1,150.1947, 1,150.1965 (M+Na) found.
Application of cell-permeant caged IP3
Our experience with the photolysis method described here is that, as long as dye-loaded cells can be clearly visualized with low power (TROUBLESHOOTING), cells respond extremely reliably to the uncaging protocol, using approximately ten times as much power required for imaging, with robust increases in intracellular calcium signals (Fig. 6). In a recent publication, we used the method described here to modulate the electrical activity of a patch-clamped neuron immediately adjacent to the photostimulated astrocyte in a living brain slice13. As the cell-permeant caged IP3 loaded multiple cells within the field of view of the microscope, we tested the spatial relationship between the photo-evoked calcium response and the change in neuronal electrical activity, and found that only those astrocytes intimately entwined with neurons could change their electrical properties. Such experiments are really only feasible with AM ester–loaded brain slices, as loading a succession of individual astrocytes around a single patch-clamped neuron with a second patch pipette is not very practical, even for the most skilled electrophysiologist. However, in certain experiments, whole-cell dialysis with calcium dye and caged probe is the method of choice, as this approach allows one to establish a quantitative relationship between the effects produced by uncaging and the biological readout. In contrast, AM ester loading with monowavelength calcium dyes allows only qualitative correlations to be defined.
Table 1.
Troubleshooting table.
| Step | Problem | Solution |
|---|---|---|
| 25–27 | Acylation gives low yield | Buy fresh reagent; should be a powder and not a gummy solid |
| 34,35 | Deprotection too slow | Distill acetyl chloride before use; follow up carefully by TLC |
| 34,35 | Deprotection too fast, very polar product on TLC (tetrahydroxyl) | Use less acetyl chloride, as 2,3-cyclohexyl group is somewhat labile |
| 53 | Phosphitylatlon reagent impure | Buy fresh dichloride and distill at 5 mmHg |
| 56 | No milky white precipitate | Make new phosphitylation reagent |
| 57 | Reaction shows multiple spots on TLC | Add more phosphitylation reagent |
| 65 | Multiple products | Add oxidizer (Step 59) very carefully, ensuring that temperature does not rise too high |
| 65 | Multiple peaks in 31P NMR spectrum | Re-purify by silica gel chromatography |
| 68 | Too many peaks in HPLC | Allow more time for first three cyanoethyl groups to deprotect |
| 72 | Product HPLC not pure | Add more AM bromide |
| 93 | Cells are poorly stained and show no calcium signal | Ensure that AM esters are dissolved in ACSF by using 20% pluronic/DMSO in place of DMSO and/or sonicate longer |
| 96 | Cells are stained with dye but show no calcium signal | Increase FRAP power or pixel dwell time by two- or threefold |
| 97 | Fluorescence signal does not eventually return to baseline after uncaging | Decrease FRAP power by two- or threefold |
Acknowledgments
This work was supported by a grant from US NIH (GM53395) to G.C.R.E.-D. B.A.M. is a Canada Research Chairperson, and the acute slice work was supported by an operating grant from the Canadian Institutes of Health Research. G.R.J.G. is supported by the Alberta Heritage Foundation for Medical Research, the Michael Smith Foundation for Health Research and the Natural Sciences and Engineering Council of Canada.
Footnotes
Contributions
S.K. prepared the caged compound. S.K. and G.C.R.E.-D. characterized the caged compound. G.C.R.E.-D. analyzed the NMR spectra. G.R.J.G. performed the cellular uncaging experiment. G.C.R.E.-D. wrote the paper with help from B.A.M. and G.R.J.G.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Ellis-Davies GCR. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miesenbock G, Kevrekidis IG. Optical imaging and control of genetically designated neurons in functioning circuits. Annu Rev Neurosci. 2005;28:533–563. doi: 10.1146/annurev.neuro.28.051804.101610. [DOI] [PubMed] [Google Scholar]
- 3.Ellis-Davies GCR. Neurobiology with caged calcium. Chem Rev. 2008;108:1603–1613. doi: 10.1021/cr078210i. [DOI] [PubMed] [Google Scholar]
- 4.Kasai H. Comparative biology of Ca2+-dependent exocytosis: implications of kinetic diversity for secretory function. Trends Neurosci. 1999;22:88–93. doi: 10.1016/s0166-2236(98)01293-4. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen JB. Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Pflugers Arch. 2004;448:347–362. doi: 10.1007/s00424-004-1247-8. [DOI] [PubMed] [Google Scholar]
- 6.Becherer U, Rettig J. Vesicle pools, docking, priming, and release. Cell Tissue Res. 2006;326:393–407. doi: 10.1007/s00441-006-0243-z. [DOI] [PubMed] [Google Scholar]
- 7.Tsien RY. Fluorescent probes of cell signaling. Annu Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- 8.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 9.Engels J, Schlaeger EJ. Synthesis, structure, and reactivity of adenosine cyclic 3′,5′-phosphate benzyl triesters. J Med Chem. 1977;20:907–911. doi: 10.1021/jm00217a008. [DOI] [PubMed] [Google Scholar]
- 10.Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Ellis-Davies GCR. A practical guide to the synthesis of dinitroindolinyl-caged neurotransmitters. Nat Protoc. 2011;6:314–326. doi: 10.1038/nprot.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GCR, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon GR, et al. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantevari S, et al. Synthesis and two-photon photolysis of 6-(ortho-nitroveratryl)-caged IP3 in living cells. Chembiochem. 2006;7:174–180. doi: 10.1002/cbic.200500345. [DOI] [PubMed] [Google Scholar]
- 15.Crowe SE, Kantevari S, Ellis-Davies GCR. Photochemically initiated intracellular calcium astrocytic waves in living mice using two-photon uncaging of IP3. ACS Chem Neurosci. 2010;1:575–585. doi: 10.1021/cn100052v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dakin K, Li WH. Cell membrane permeable esters of D-myo-inositol 1,4,5-trisphosphate. Cell Calcium. 2007;42:291–301. doi: 10.1016/j.ceca.2006.12.003. [DOI] [PubMed] [Google Scholar]