Abstract
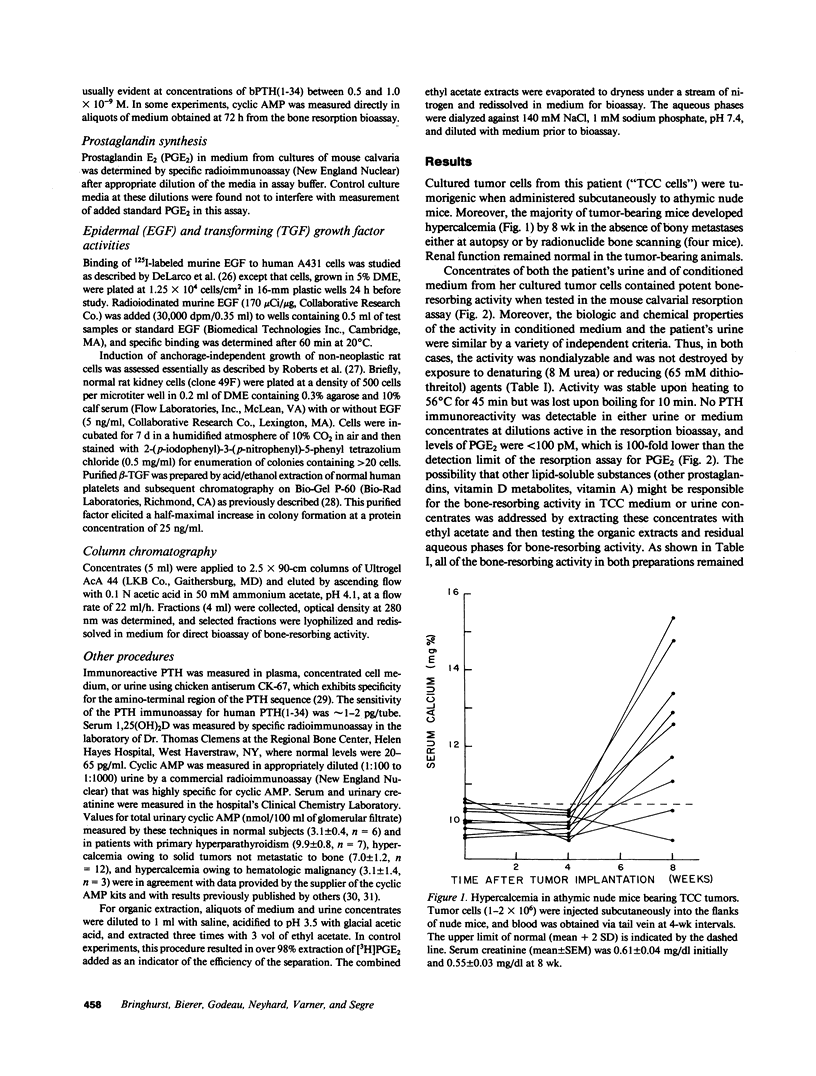
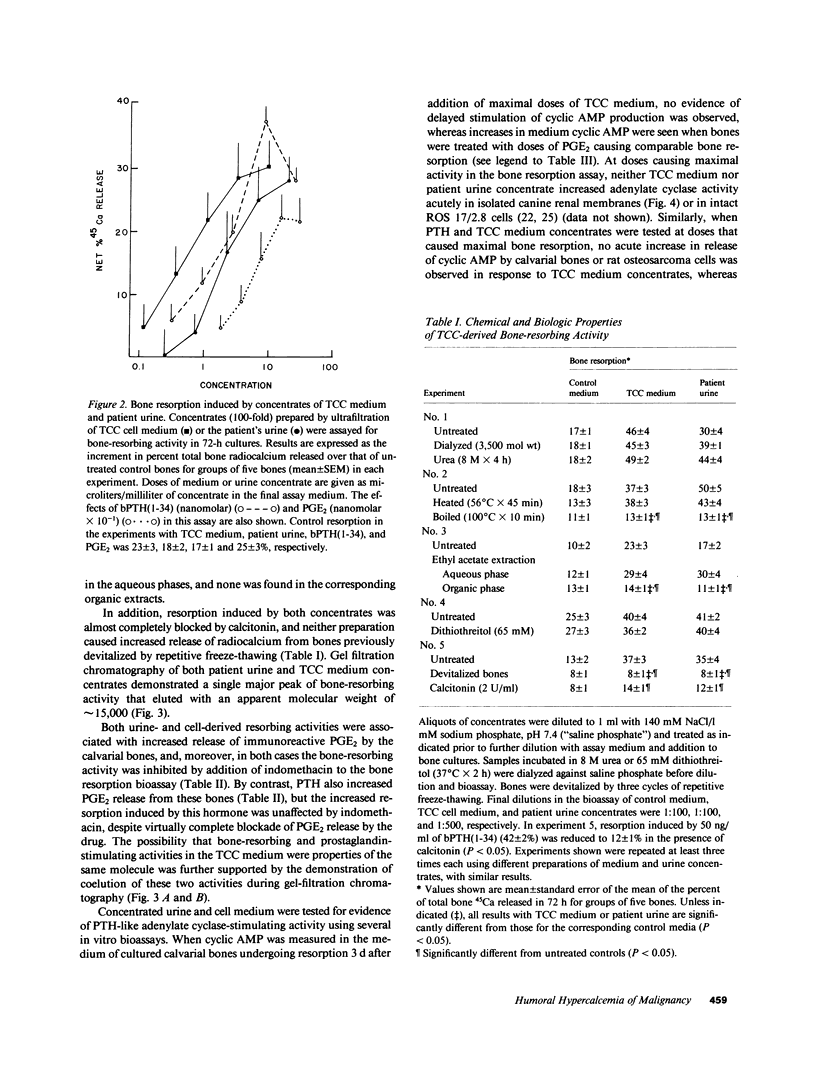
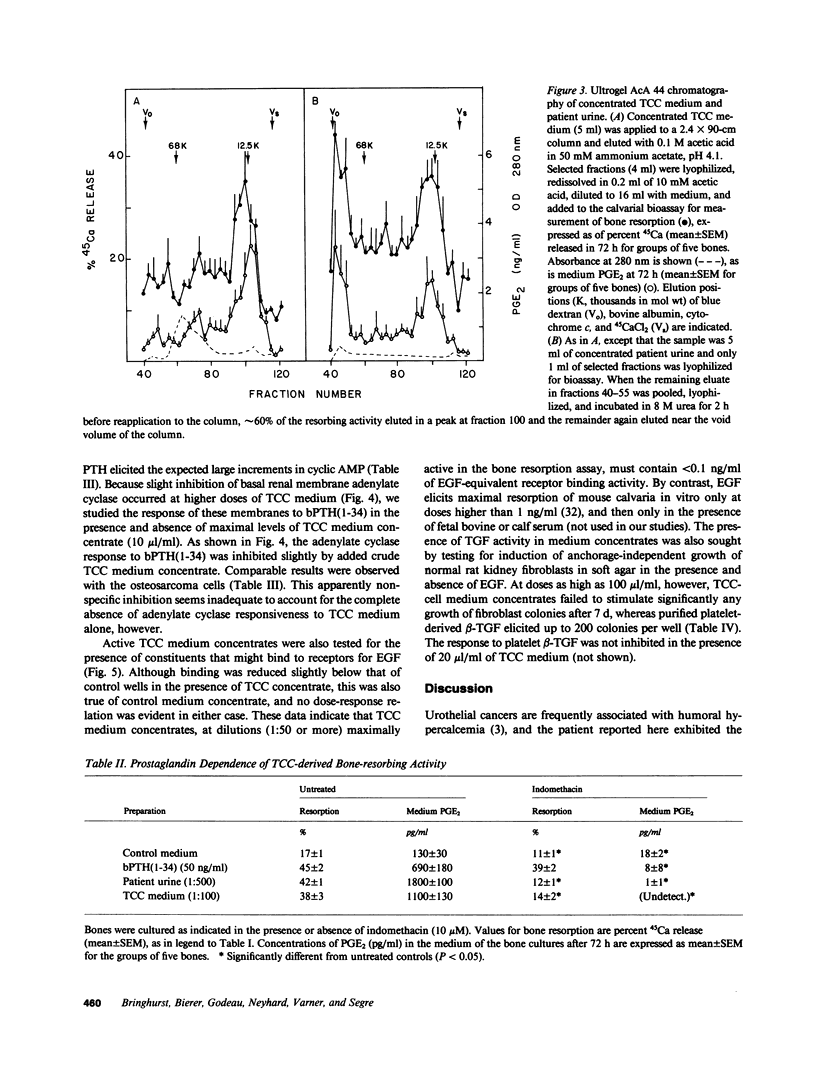
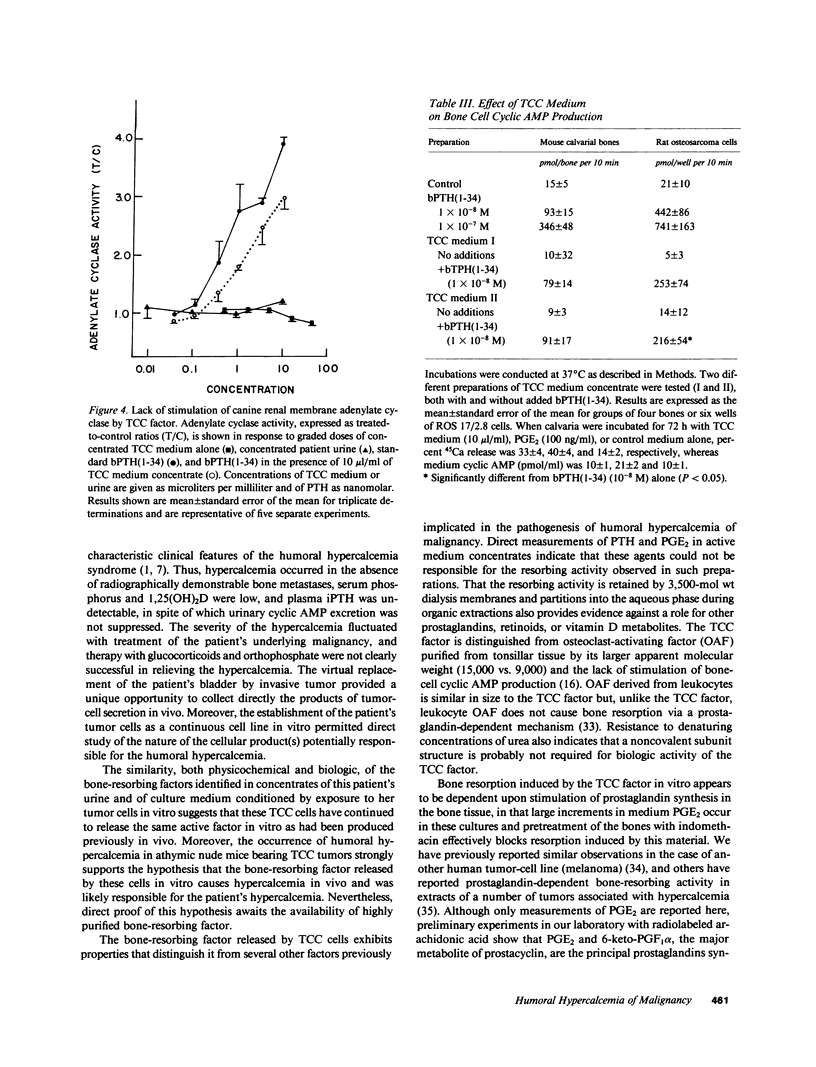
Secretion by tumor cells of circulating bone-resorbing factors may frequently underlie the hypercalcemia that occurs in patients with malignancy. Efforts to identify the responsible mediators have been hampered by a lack of available human tumor cell systems suitable for study of the pathogenesis of the humoral hypercalcemia syndrome. We have established a transitional-cell carcinoma (TCC) line in vitro from a patient with humoral hypercalcemia. These cells are tumorigenic and cause hypercalcemia in athymic nude mice. Culture medium conditioned by TCC cells contains potent bone-resorbing activity in vitro, the physical and biological properties of which are similar to those of bone-resorbing activity present in the original patient's urine. The bone-resorbing activity of the TCC factor is accompanied by increased prostaglandin release from bone and is blocked by indomethacin and calcitonin. The TCC-derived bone-resorbing activity coelutes with prostaglandin-stimulating activity during gel filtration with an approximate molecular weight of 15,000. This activity is nondialyzable, stable to concentrated urea and reducing agents, and destroyed by boiling. The TCC factor does not increase cyclic AMP production in bone or kidney bioassays and does not exhibit transforming growth factor activity. We conclude that a unique macromolecular factor released by TCC cells causes bone resorption by a mechanism dependent upon stimulation of bone cell cyclooxygenase, and that this factor is the probable cause of the hypercalcemia in vivo. The TCC cell line provides a new model for study of the human humoral hypercalcemia syndrome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Benson R. C., Jr, Riggs B. L., Pickard B. M., Arnaud C. D. Immunoreactive forms of circulating parathyroid hormone in primary and ectopic hyperparathyroidism. J Clin Invest. 1974 Jul;54(1):175–181. doi: 10.1172/JCI107739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. E., Harvey H. A., Lipton A., Demers L. A study of prostaglandin E2, parathormone, and response to indomethacin in patients with hypercalcemia of malignancy. Cancer. 1982 Feb 1;49(3):556–561. doi: 10.1002/1097-0142(19820201)49:3<556::aid-cncr2820490327>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bringhurst F. R., Segre G. V., Lampman G. W., Potts J. T., Jr Metabolism of parathyroid hormone by Kupffer cells: analysis by reverse-phase high-performance liquid chromatography. Biochemistry. 1982 Aug 31;21(18):4252–4258. doi: 10.1021/bi00261a011. [DOI] [PubMed] [Google Scholar]
- Broadus A. E. Nephrogenous cyclic AMP. Recent Prog Horm Res. 1981;37:667–701. doi: 10.1016/b978-0-12-571137-1.50019-0. [DOI] [PubMed] [Google Scholar]
- Coombes R. S., Ward M. K., Greenberg P. B., Hillyard C. J., Tulloch B. R., Morrison R., Joplin G. F. Calcium metabolism in cancer. Studies using calcium isotopes and immunoassays for parathyroid hormone and calcitonin. Cancer. 1976 Nov;38(5):2111–2120. doi: 10.1002/1097-0142(197611)38:5<2111::aid-cncr2820380539>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E., Mundy G. R. Relation of osteoclast activating factor production to extent of bone disease in multiple myeloma. Br J Haematol. 1981 Jan;47(1):21–30. doi: 10.1111/j.1365-2141.1981.tb02758.x. [DOI] [PubMed] [Google Scholar]
- Eilon G., Mundy G. R. Direct resorption of bone by human breast cancer cells in vitro. Nature. 1978 Dec 14;276(5689):726–728. doi: 10.1038/276726a0. [DOI] [PubMed] [Google Scholar]
- Fisken R. A., Heath D. A., Bold A. M. Hypercalcaemia--a hospital survey. Q J Med. 1980 Autumn;49(196):405–418. [PubMed] [Google Scholar]
- Greaves M., Ibbotson K. J., Atkins D., Martin T. J. Prostaglandins as mediators of bone resorption in renal and breast tumours. Clin Sci (Lond) 1980 Mar;58(3):201–210. doi: 10.1042/cs0580201. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., D'Souza S. M., Ng K. W., Osborne C. K., Niall M., Martin T. J., Mundy G. R. Tumor-derived growth factor increases bone resorption in a tumor associated with humoral hypercalcemia of malignancy. Science. 1983 Sep 23;221(4617):1292–1294. doi: 10.1126/science.6577602. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., D'Souza S. M., Smith D. D., Carpenter G., Mundy G. R. EGF receptor antiserum inhibits bone resorbing activity produced by a rat Leydig cell tumor associated with the humoral hypercalcemia of malignancy. Endocrinology. 1985 Jan;116(1):469–471. doi: 10.1210/endo-116-1-469. [DOI] [PubMed] [Google Scholar]
- Knill-Jones R. P., Buckle R. M., Parsons V., Calne R. Y., Williams R. Hypercalcemia and increased parathyroid-hormone activity in a primary hepatoma. Studies before and after hepatic transplantation. N Engl J Med. 1970 Mar 26;282(13):704–708. doi: 10.1056/NEJM197003262821302. [DOI] [PubMed] [Google Scholar]
- Kukreja S. C., Shemerdiak W. P., Lad T. E., Johnson P. A. Elevated nephrogenous cyclic AMP with normal serum parathyroid hormone levels in patients with lung cancer. J Clin Endocrinol Metab. 1980 Jul;51(1):167–169. doi: 10.1210/jcem-51-1-167. [DOI] [PubMed] [Google Scholar]
- Kukreja S. C., Shemerdiak W. P., York P. A., Lad T. E., Abramson E. C., Thomas P. A., Mir J. Presence of prostaglandin E in lung tumors from normocalcemic patients. Am J Med. 1982 May;72(5):737–742. doi: 10.1016/0002-9343(82)90538-1. [DOI] [PubMed] [Google Scholar]
- Luben R. A. An assay for osteoclast-activating factor (OAF) in biological fluids: detection of OAF in the serum of myeloma patients. Cell Immunol. 1980 Jan;49(1):74–80. doi: 10.1016/0008-8749(80)90057-x. [DOI] [PubMed] [Google Scholar]
- MYERS W. P. Hypercalcemia in neoplastic disease. Arch Surg. 1960 Feb;80:308–318. doi: 10.1001/archsurg.1960.01290190128024. [DOI] [PubMed] [Google Scholar]
- Minkin C., Fredericks R. S., Pokress S., Rude R. K., Sharp C. F., Jr, Tong M., Singer F. R. Bone resorption and humoral hypercalcemia of malignancy: stimulation of bone resorption in vitro by tumor extracts is inhibited by prostaglandin synthesis inhibitors. J Clin Endocrinol Metab. 1981 Nov;53(5):941–947. doi: 10.1210/jcem-53-5-941. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Luben R. A., Raisz L. G., Oppenheim J. J., Buell D. N. Bone-resorbing activity in supernatants from lymphoid cell lines. N Engl J Med. 1974 Apr 18;290(16):867–871. doi: 10.1056/NEJM197404182901601. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Martin T. J. The hypercalcemia of malignancy: pathogenesis and management. Metabolism. 1982 Dec;31(12):1247–1277. doi: 10.1016/0026-0495(82)90012-9. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Rick M. E., Turcotte R., Kowalski M. A. Pathogenesis of hypercalcemia in lymphosarcoma cell leukemia. Role of an osteoclast activating factor-like substance and a mechanism of action for glucocorticoid therapy. Am J Med. 1978 Oct;65(4):600–606. doi: 10.1016/0002-9343(78)90847-1. [DOI] [PubMed] [Google Scholar]
- Nissenson R. A., Abbott S. R., Teitelbaum A. P., Clark O. H., Arnaud C. D. Endogenous biologically active human parathyroid hormone: measurement by a guanyl nucleotide-amplified renal adenylate cyclase assay. J Clin Endocrinol Metab. 1981 May;52(5):840–846. doi: 10.1210/jcem-52-5-840. [DOI] [PubMed] [Google Scholar]
- Nolan R. D., Partridge N. C., Godfrey H. M., Martin T. J. Cyclo-oxygenase products of arachidonic acid metabolism in rat osteoblasts in culture. Calcif Tissue Int. 1983 May;35(3):294–297. doi: 10.1007/BF02405049. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Lamb L. C., Newton D. L., Sporn M. B., De Larco J. E., Todaro G. J. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Fischer M. K., Egan J. J., Epstein P. M., Rodan G. A. The effect of dexamethasone on parathyroid hormone stimulation of adenylate cyclase in ROS 17/2.8 cells. Endocrinology. 1984 Sep;115(3):951–958. doi: 10.1210/endo-115-3-951. [DOI] [PubMed] [Google Scholar]
- Rodan S. B., Insogna K. L., Vignery A. M., Stewart A. F., Broadus A. E., D'Souza S. M., Bertolini D. R., Mundy G. R., Rodan G. A. Factors associated with humoral hypercalcemia of malignancy stimulate adenylate cyclase in osteoblastic cells. J Clin Invest. 1983 Oct;72(4):1511–1515. doi: 10.1172/JCI111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude R. K., Sharp C. F., Jr, Fredericks R. S., Oldham S. B., Elbaum N., Link J., Irwin L., Singer F. R. Urinary and nephrogenous adenosine 3',5'-monophosphate in the hypercalcemia of malignancy. J Clin Endocrinol Metab. 1981 Apr;52(4):765–771. doi: 10.1210/jcem-52-4-765. [DOI] [PubMed] [Google Scholar]
- Seyberth H. W., Segre G. V., Morgan J. L., Sweetman B. J., Potts J. T., Jr, Oates J. A. Prostaglandins as mediators of hypercalcemia associated with certain types of cancer. N Engl J Med. 1975 Dec 18;293(25):1278–1283. doi: 10.1056/NEJM197512182932502. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Horst R., Deftos L. J., Cadman E. C., Lang R., Broadus A. E. Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and nonhumoral groups. N Engl J Med. 1980 Dec 11;303(24):1377–1383. doi: 10.1056/NEJM198012113032401. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Insogna K. L., Goltzman D., Broadus A. E. Identification of adenylate cyclase-stimulating activity and cytochemical glucose-6-phosphate dehydrogenase-stimulating activity in extracts of tumors from patients with humoral hypercalcemia of malignancy. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1454–1458. doi: 10.1073/pnas.80.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. F., Vignery A., Silverglate A., Ravin N. D., LiVolsi V., Broadus A. E., Baron R. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endocrinol Metab. 1982 Aug;55(2):219–227. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- Strewler G. J., Williams R. D., Nissenson R. A. Human renal carcinoma cells produce hypercalcemia in the nude mouse and a novel protein recognized by parathyroid hormone receptors. J Clin Invest. 1983 Mar;71(3):769–774. doi: 10.1172/JCI110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Ivey J. L., Delclos B., Levine L. Stimulation of prostaglandin production in bone by phorbol diesters and melittin. Prostaglandins. 1978 Aug;16(2):221–232. doi: 10.1016/0090-6980(78)90023-0. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Levine L. Epidermal growth factor stimulates prostaglandin production and bone resorption in cultured mouse calvaria. Biochem Biophys Res Commun. 1978 Dec 14;85(3):966–975. doi: 10.1016/0006-291x(78)90638-1. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T., Mundy G. R. Monocytes regulate osteoclast-activating factor production by releasing prostaglandins. J Exp Med. 1979 Aug 1;150(2):338–350. doi: 10.1084/jem.150.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]


