Abstract
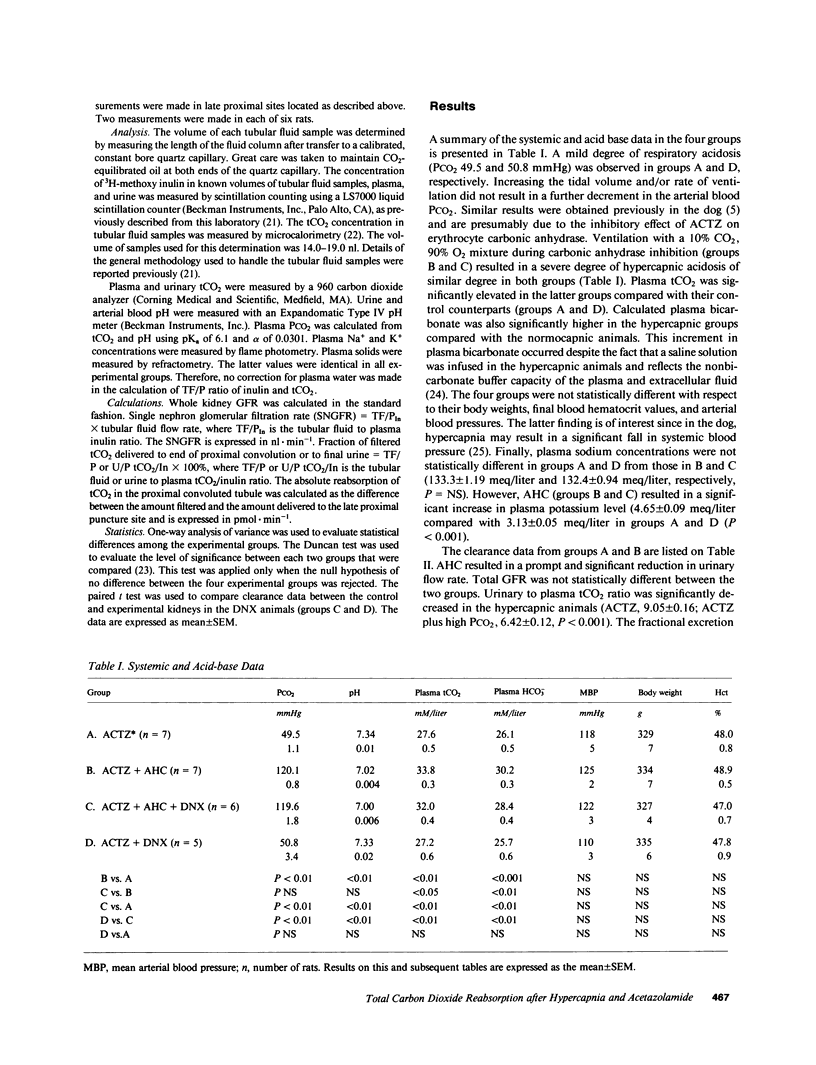
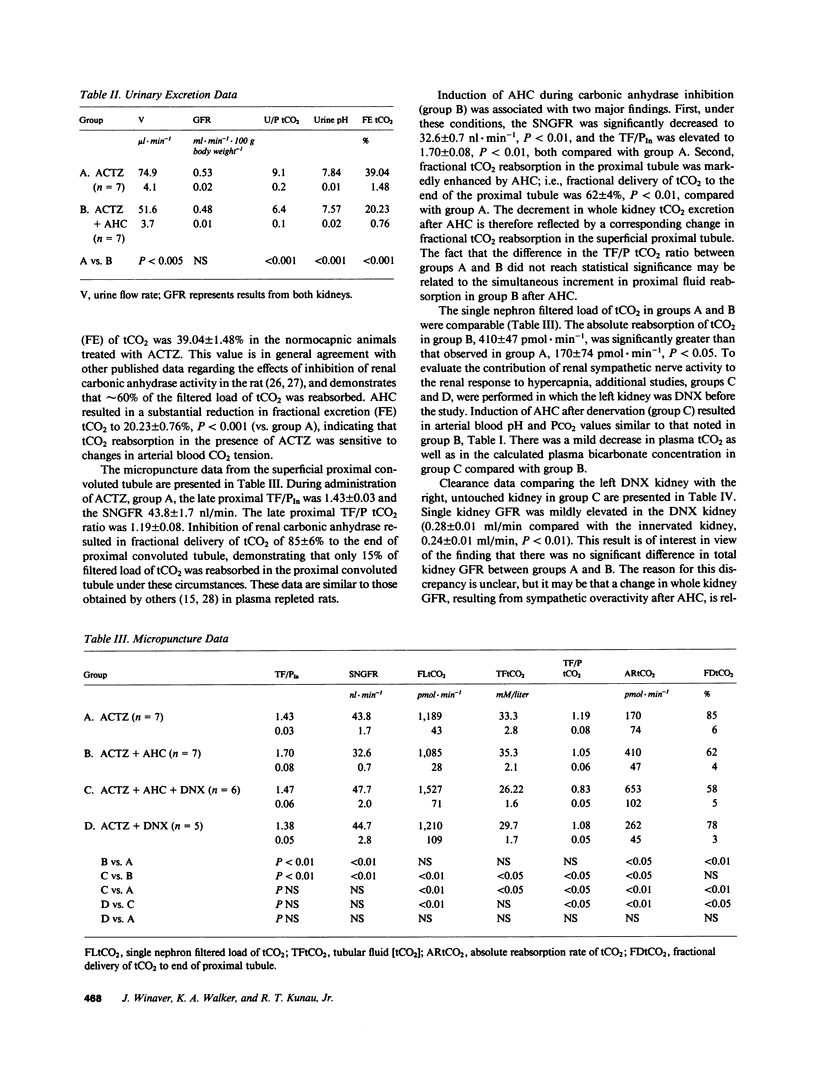
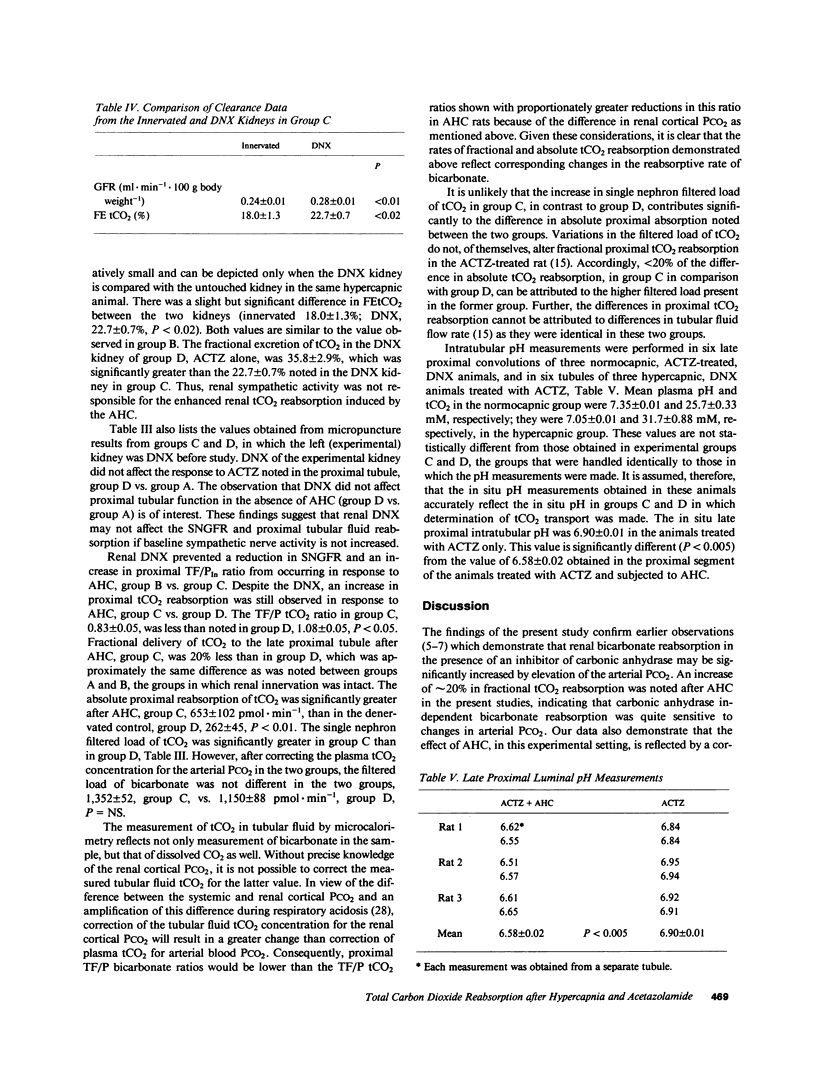
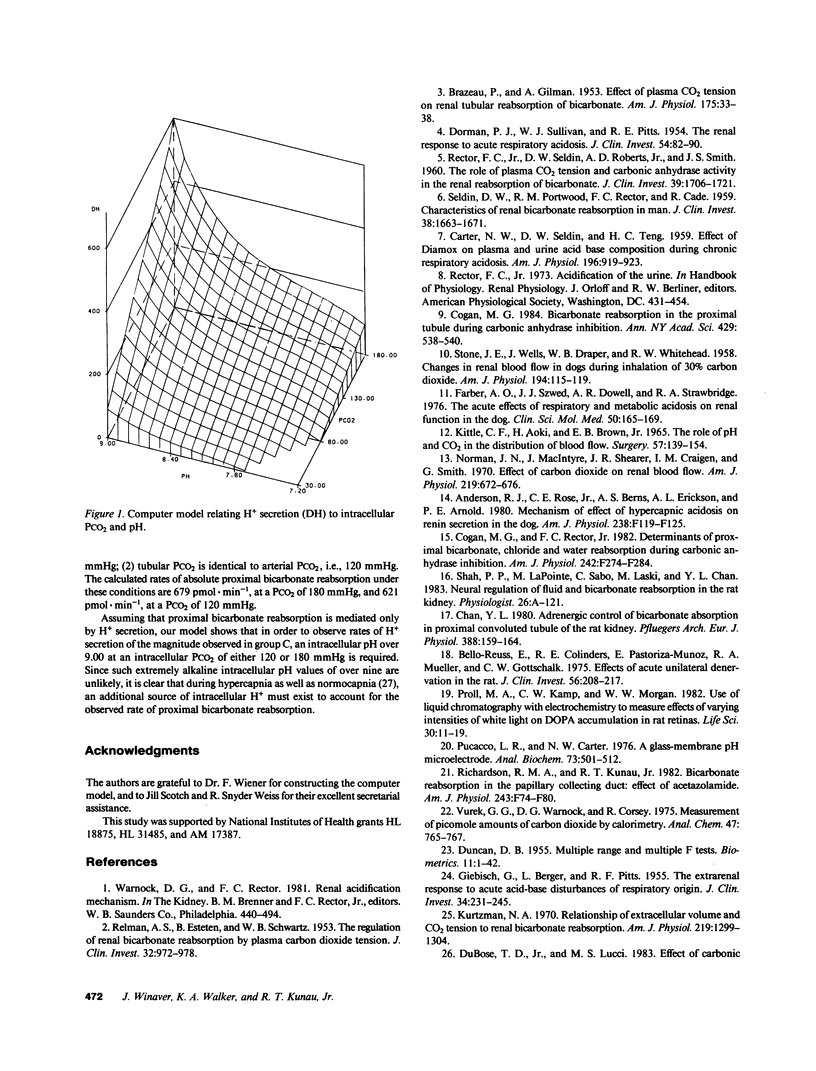
The present study evaluates the effect of acute hypercapnia on renal total CO2 (tCO2) reabsorption after inhibition of renal carbonic anhydrase. Simultaneous renal clearance studies and free-flow micropuncture studies of the superficial proximal tubule were performed on plasma-repleted Sprague-Dawley rats treated with acetazolamide, 50 mg/kg body weight. Acute hypercapnia (arterial PCO2, 120 mmHg; blood pH, 7.02) was induced by ventilation with a 10% CO2-90% O2 gas mixture. Control rats (PCO2, 49.5 mmHg, pH 7.34) were ventilated with room air. The renal fractional excretion of tCO2 was approximately 20% lower in the hypercapnic group compared with the rats given acetazolamide alone. Acute hypercapnia reduced the fractional delivery of tCO2 to the late proximal tubule by a comparable amount. The absolute proximal reabsorption of tCO2 was increased by hypercapnia to 410 +/- 47 vs. 170 +/- 74 pmol X min-1, P less than 0.05. The single nephron glomerular filtration rate was 32.6 +/- 0.7 nl X min-1 in the hypercapnic group and 43.8 +/- 1.7 nl X min-1 in the rats given acetazolamide only, P less than 0.01. Acute hypercapnia enhances renal sympathetic nerve activity. To eliminate this effect, additional experiments were performed in which the experimental kidney was denervated before study. Denervation prevented the change in the single nephron filtration rate during acute hypercapnia, but absolute and fractional proximal tCO2 reabsorption remained elevated in comparison to denervated controls. The concentration of H2CO3 in the late proximal tubule, calculated from the measured luminal pH and bicarbonate concentration and the estimated cortical PCO2, was higher in the hypercapnic group, which was a finding compatible with H2CO3 cycling from lumen into proximal tubular cell, which provided a source of hydrogen ions for secretion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J., Cogan M. G., Rector F. C., Jr Effect of luminal bicarbonate concentration on proximal acidification in the rat. Am J Physiol. 1982 Jul;243(1):F53–F59. doi: 10.1152/ajprenal.1982.243.1.F53. [DOI] [PubMed] [Google Scholar]
- Alpern R. J., Cogan M. G., Rector F. C., Jr Flow dependence of proximal tubular bicarbonate absorption. Am J Physiol. 1983 Oct;245(4):F478–F484. doi: 10.1152/ajprenal.1983.245.4.F478. [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Rose C. E., Jr, Berns A. S., Erickson A. L., Arnold P. E. Mechanism of effect of hypercapnic acidosis on renin secretion in the dog. Am J Physiol. 1980 Feb;238(2):F119–F125. doi: 10.1152/ajprenal.1980.238.2.F119. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- BRAZEAU P., GILMAN A. Effect of plasma CO2 tension on renal tubular reabsorption of bicarbonate. Am J Physiol. 1953 Oct;175(1):33–38. doi: 10.1152/ajplegacy.1953.175.1.33. [DOI] [PubMed] [Google Scholar]
- Bello-Reuss E., Colindres R. E., Pastoriza-Muñoz E., Mueller R. A., Gottschalk C. W. Effects of acute unilateral renal denervation in the rat. J Clin Invest. 1975 Jul;56(1):208–217. doi: 10.1172/JCI108069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTER N. W., SELDIN D. W., TENG H. C. Effect of diamox on plasma and urine acid-base composition during chronic respiratory acidosis. Am J Physiol. 1959 Apr;196(4):919–923. doi: 10.1152/ajplegacy.1959.196.4.919. [DOI] [PubMed] [Google Scholar]
- Chan Y. L. Adrenergic control of bicarbonate absorption in the proximal convoluted tubule of the rat kidney. Pflugers Arch. 1980 Nov;388(2):159–164. doi: 10.1007/BF00584122. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Malnic G., Giebisch G. Passive driving forces of proximal tubular fluid and bicarbonate transport: gradient dependence of H+ secretion. Am J Physiol. 1983 Nov;245(5 Pt 1):F622–F633. doi: 10.1152/ajprenal.1983.245.5.F622. [DOI] [PubMed] [Google Scholar]
- Cogan M. G. Bicarbonate reabsorption in the proximal tubule during carbonic anhydrase inhibition. Ann N Y Acad Sci. 1984;429:538–540. doi: 10.1111/j.1749-6632.1984.tb12383.x. [DOI] [PubMed] [Google Scholar]
- Cogan M. G. Effects of acute alterations in PCO2 on proximal HCO-3, Cl-, and H2O reabsorption. Am J Physiol. 1984 Jan;246(1 Pt 2):F21–F26. doi: 10.1152/ajprenal.1984.246.1.F21. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Liu F. Y. Metabolic alkalosis in the rat. Evidence that reduced glomerular filtration rather than enhanced tubular bicarbonate reabsorption is responsible for maintaining the alkalotic state. J Clin Invest. 1983 May;71(5):1141–1160. doi: 10.1172/JCI110864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan M. G., Maddox D. A., Warnock D. G., Lin E. T., Rector F. C., Jr Effect of acetazolamide on bicarbonate reabsorption in the proximal tubule of the rat. Am J Physiol. 1979 Dec;237(6):F447–F454. doi: 10.1152/ajprenal.1979.237.6.F447. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Rector F. C., Jr Determinants of proximal bicarbonate, chloride, and water reabsorption during carbonic anhydrase inhibition. Am J Physiol. 1982 Mar;242(3):F274–F284. doi: 10.1152/ajprenal.1982.242.3.F274. [DOI] [PubMed] [Google Scholar]
- DORMAN P. J., SULLIVAN W. J., PITTS R. F. The renal response to acute respiratory acidosis. J Clin Invest. 1954 Jan;33(1):82–90. doi: 10.1172/JCI102874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Lucci M. S. Effect of carbonic anhydrase inhibition on superficial and deep nephron bicarbonate reabsorption in the rat. J Clin Invest. 1983 Jan;71(1):55–65. doi: 10.1172/JCI110751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Seldin D. W., Carter N. W. Direct determination of PCO2 in the rat renal cortex. J Clin Invest. 1978 Aug;62(2):338–348. doi: 10.1172/JCI109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber M. O., Szwed J. J., Dowell A. R., Strawbridge R. A. The acute effects of respiratory and metabolic acidosis on renal function in the dog. Clin Sci Mol Med. 1976 Mar;50(3):165–169. doi: 10.1042/cs0500165. [DOI] [PubMed] [Google Scholar]
- GIEBISCH G., BERGER L., PITTS R. F. The extrarenal response to acute acid-base disturbances of respiratory origin. J Clin Invest. 1955 Feb;34(2):231–245. doi: 10.1172/JCI103076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITTLE C. F., AOKI H., BROWN E. B., Jr THE ROLE OF PH AND CO2 IN THE DISTRIBUTION OF BLOOD FLOW. Surgery. 1965 Jan;57:139–154. [PubMed] [Google Scholar]
- Kleinman J. G., Brown W. W., Ware R. A., Schwartz J. H. Cell pH and acid transport in renal cortical tissue. Am J Physiol. 1980 Nov;239(5):F440–F444. doi: 10.1152/ajprenal.1980.239.5.F440. [DOI] [PubMed] [Google Scholar]
- Kurtzman N. A. Relationship of extracellular volume and CO2 tension to renal bicarbonate reabsorption. Am J Physiol. 1970 Nov;219(5):1299–1304. doi: 10.1152/ajplegacy.1970.219.5.1299. [DOI] [PubMed] [Google Scholar]
- Levine D. Z. Effect of acute hypercapnia on proximal tubular water and bicarbonate reabsorption. Am J Physiol. 1971 Oct;221(4):1164–1170. doi: 10.1152/ajplegacy.1971.221.4.1164. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbon dioxide equilibria in the kidney: the problems of elevated carbon dioxide tension, delayed dehydration, and disequilibrium pH. Kidney Int. 1978 Nov;14(5):395–405. doi: 10.1038/ki.1978.144. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Chemistry of the renal reabsorption of bicarbonate. Can J Physiol Pharmacol. 1974 Dec;52(6):1041–1050. doi: 10.1139/y74-138. [DOI] [PubMed] [Google Scholar]
- Mello Aires M., Malnic G. Peritubular pH and PCO'2 in renal tubular acidification. Am J Physiol. 1975 Jun;228(6):1766–1774. doi: 10.1152/ajplegacy.1975.228.6.1766. [DOI] [PubMed] [Google Scholar]
- Norman J. N., MacIntyre J., Shearer J. R., Craigen I. M., Smith G. Effect of carbon dioxide on renal blood flow. Am J Physiol. 1970 Sep;219(3):672–676. doi: 10.1152/ajplegacy.1970.219.3.672. [DOI] [PubMed] [Google Scholar]
- Proll M. A., Kamp C. W., Morgan W. W. Use of liquid chromatography with electrochemistry to measure effects of varying intensities of white light on DOPA accumulation in rat retinas. Life Sci. 1982 Jan 4;30(1):11–19. doi: 10.1016/0024-3205(82)90630-0. [DOI] [PubMed] [Google Scholar]
- Pucacco L. R., Carter N. W. A glass-membrane pH microelectrode. Anal Biochem. 1976 Jun;73(2):501–512. doi: 10.1016/0003-2697(76)90200-1. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., ROBERTS A. D., Jr, SMITH J. S. The role of plasma CO2 tension and carbonic anhydrase activity in the renal reabsorption of bicarbonate. J Clin Invest. 1960 Nov;39:1706–1721. doi: 10.1172/JCI104193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RELMAN A. S., ETSTEN B., SCHWARTZ W. B. The regulation of renal bicarbonate reabsorption by plasma carbon dioxide tension. J Clin Invest. 1953 Oct;32(10):972–978. doi: 10.1172/JCI102823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R. M., Kunau R. T., Jr Bicarbonate reabsorption in the papillary collecting duct: effect of acetazolamide. Am J Physiol. 1982 Jul;243(1):F74–F80. doi: 10.1152/ajprenal.1982.243.1.F74. [DOI] [PubMed] [Google Scholar]
- SELDIN D. W., PORTWOOD R. M., RECTOR F. C., Jr, CADE R. Characteristics of renal bicarbonate reabsorption in man. J Clin Invest. 1959 Oct;38:1663–1671. doi: 10.1172/JCI103944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE J. E., WELLS J., DRAPER W. B., WHITEHEAD R. W. Changes in renal blood flow in dogs during the inhalation of 30% carbon dioxide. Am J Physiol. 1958 Jul;194(1):115–119. doi: 10.1152/ajplegacy.1958.194.1.115. [DOI] [PubMed] [Google Scholar]
- Struyvenberg A., Morrison R. B., Relman A. S. Acid-base behavior of separated canine renal tubule cells. Am J Physiol. 1968 May;214(5):1155–1162. doi: 10.1152/ajplegacy.1968.214.5.1155. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]



