Abstract
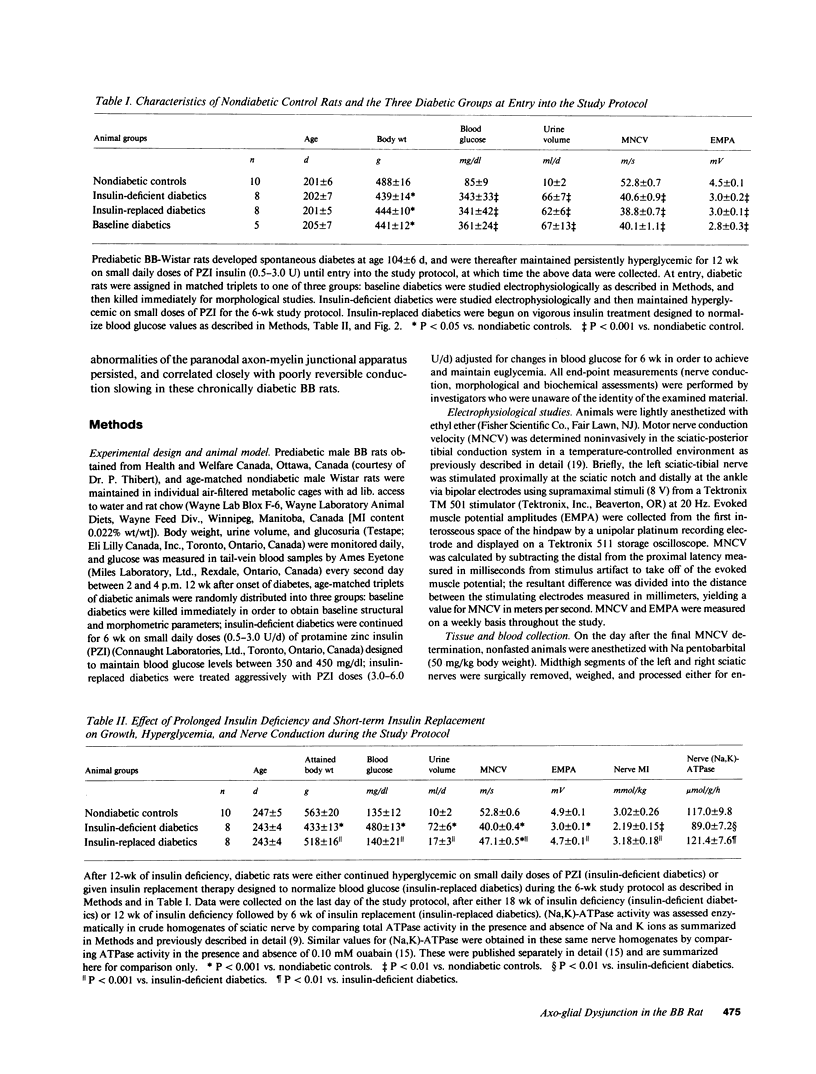
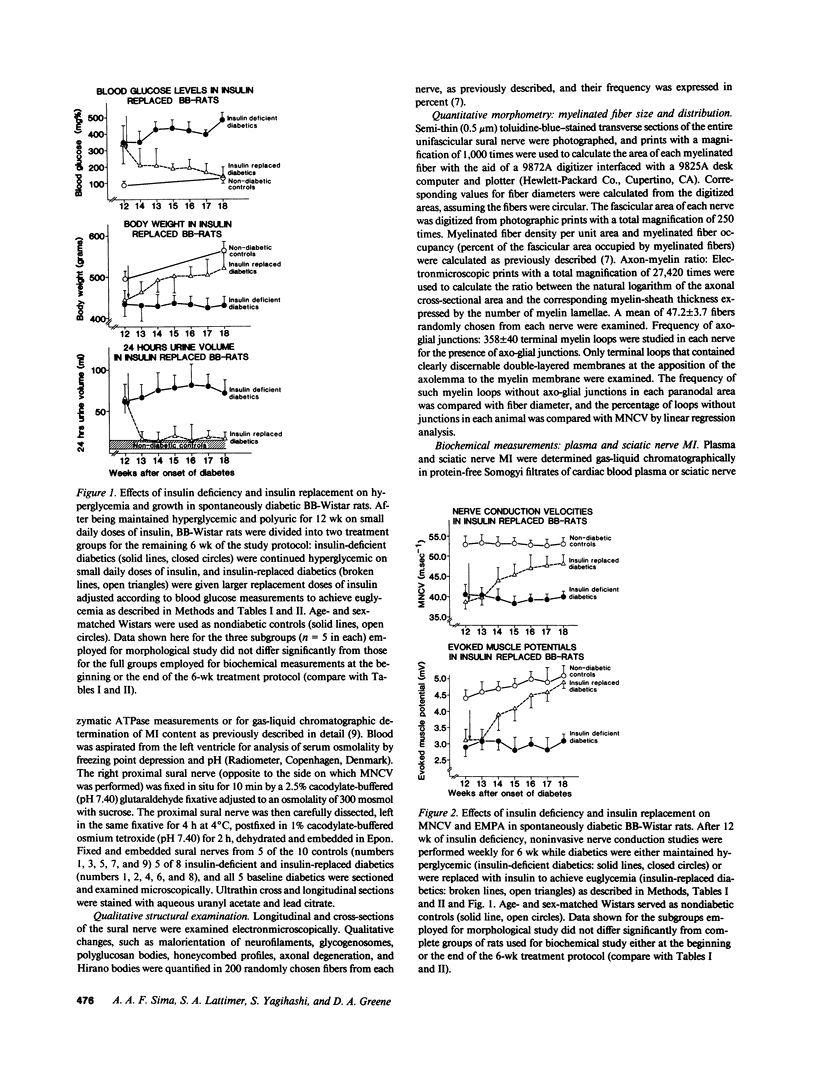
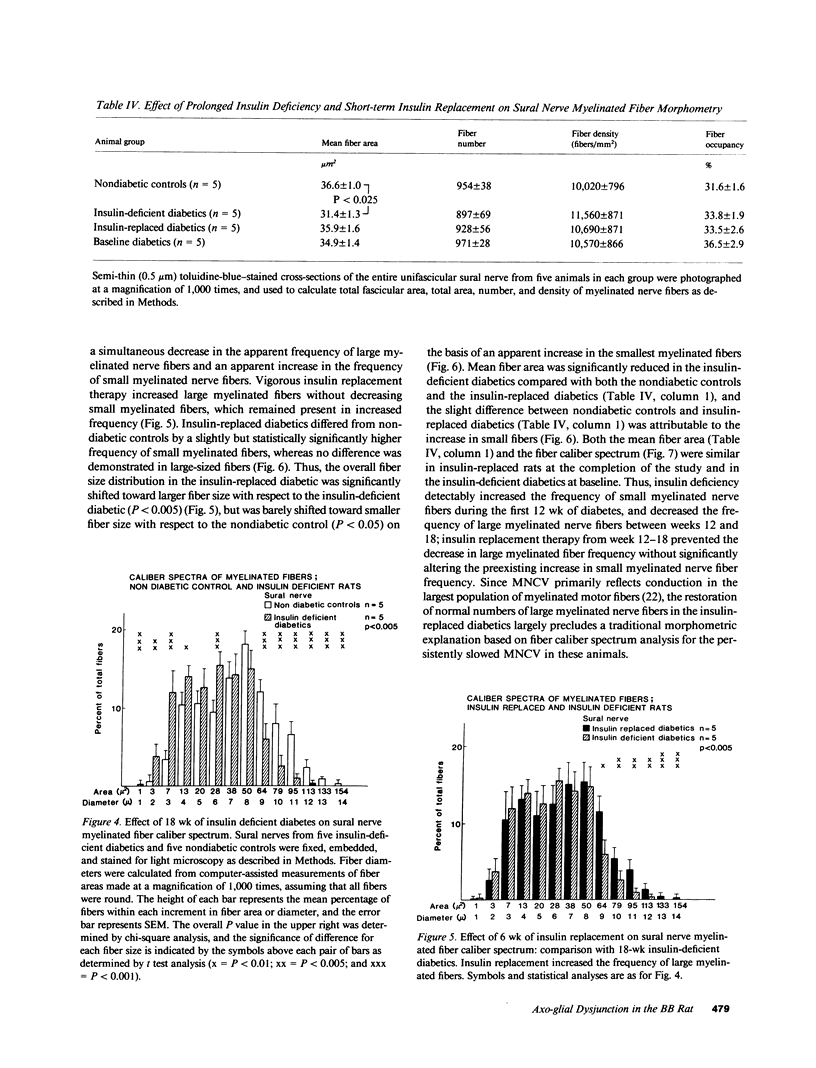
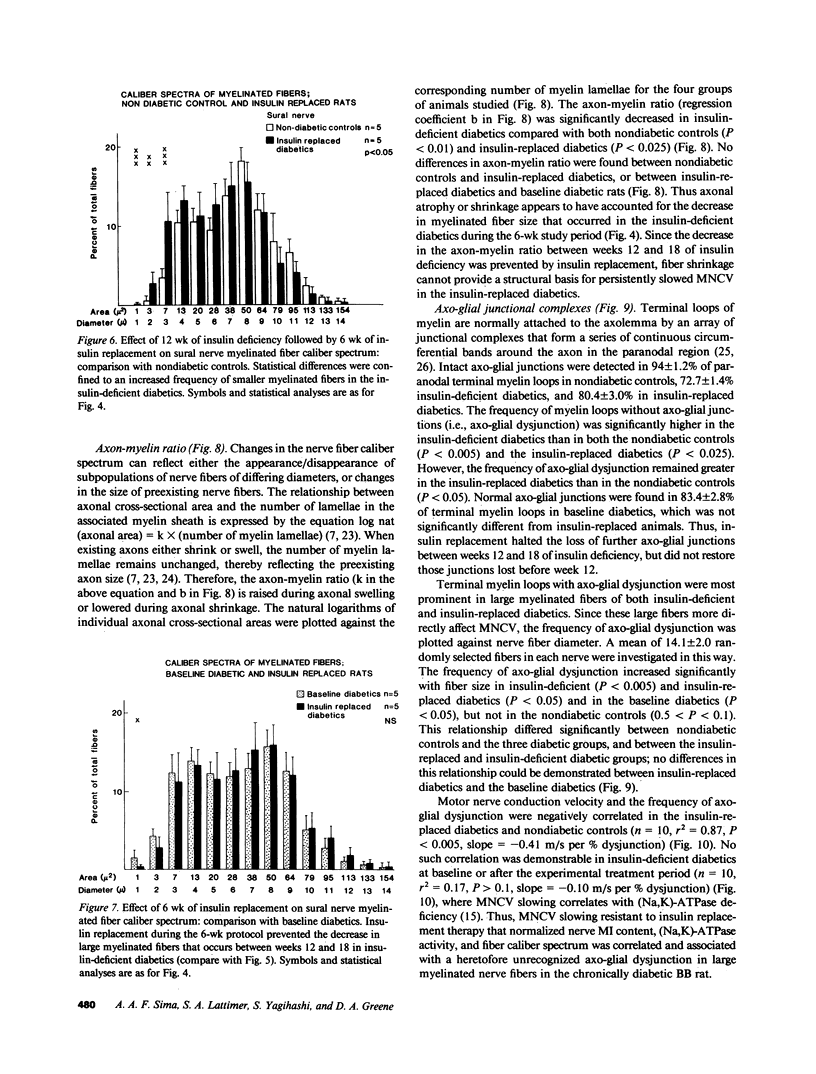
Biochemical abnormalities in peripheral nerve are thought to precede and condition the development of diabetic neuropathy, but metabolic intervention in chronic diabetic neuropathy produces only limited acute clinical response. The residual, metabolically unresponsive neurological deficits have never been rigorously defined in terms of either persistent metabolic derangements or irreversible structural defects because human nerve tissue is rarely accessible for anatomical and biochemical study and experimentally diabetic animals do not develop the structural hallmarks of human diabetic neuropathy. Detailed neuroanatomical-functional-biochemical correlation was therefore undertaken in long-term spontaneously diabetic BB-Wistar rats that functionally and structurally model human diabetic neuropathy. Vigorous insulin replacement in chronically diabetic BB rats essentially normalized both the sural nerve fiber caliber spectrum and the decreased sciatic nerve myo-inositol and (Na,K)-ATPase levels generally associated with conduction slowing in diabetic animals; yet, nerve conduction was only partially restored toward normal. Morphometric analysis revealed a striking disappearance of paranodal axo-glial junctional complexes that was not corrected by insulin replacement. Loss of these strategic junctional complexes, which are thought to limit lateral migration of axolemmal Na channels away from nodes of Ranvier, correlates with and can account for the diminished nodal Na permeability and resultant nodal conduction delay characteristic of chronic diabetic neuropathy in this animal model.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behse F., Buchthal F., Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977 Nov;40(11):1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Neuropathy-functional abnormalities in the BB rat. Metabolism. 1983 Jul;32(7 Suppl 1):112–117. doi: 10.1016/s0026-0495(83)80023-7. [DOI] [PubMed] [Google Scholar]
- Brismar T., Sima A. A. Changes in nodal function in nerve fibres of the spontaneously diabetic BB-Wistar rat: potential clamp analysis. Acta Physiol Scand. 1981 Dec;113(4):499–506. doi: 10.1111/j.1748-1716.1981.tb06928.x. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr Diabetic neuropathy--new concepts of its etiology. Diabetes. 1979 Jun;28(6):604–611. doi: 10.2337/diab.28.6.604. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Lambert E. H., Windebank A. J., Lais A. A., Sparks M. F., Karnes J., Sherman W. R., Hallcher L. M., Low P. A., Service F. J. Acute hyperosmolar hyperglycemia causes axonal shrinkage and reduced nerve conduction velocity. Exp Neurol. 1981 Mar;71(3):507–514. doi: 10.1016/0014-4886(81)90028-5. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Low P. A., Sparks M. F., Hexum L. A., Karnes J. L. Effect of serum hyperosmolality on morphometry of healthy human sural nerve. J Neuropathol Exp Neurol. 1980 May;39(3):285–295. doi: 10.1097/00005072-198005000-00005. [DOI] [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N. Sorbitol, inositol and nerve conduction in diabetes. Life Sci. 1983 Apr 25;32(17):1943–1947. doi: 10.1016/0024-3205(83)90045-0. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Brown M. J., Braunstein S. N., Schwartz S. S., Asbury A. K., Winegrad A. I. Comparison of clinical couse and sequential electrophysiological tests in diabetics with symptomatic polyneuropathy and its implications for clinical trials. Diabetes. 1981 Feb;30(2):139–147. doi: 10.2337/diab.30.2.139. [DOI] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Action of sorbinil in diabetic peripheral nerve. Relationship of polyol (sorbitol) pathway inhibition to a myo-inositol-mediated defect in sodium-potassium ATPase activity. Diabetes. 1984 Aug;33(8):712–716. doi: 10.2337/diab.33.8.712. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983 Sep;72(3):1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982 Nov;70(5):1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A. Metabolic abnormalities in diabetic peripheral nerve: relation to impaired function. Metabolism. 1983 Jul;32(7 Suppl 1):118–123. doi: 10.1016/s0026-0495(83)80024-9. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Yagihashi S., Lattimer S. A., Sima A. A. Nerve Na+-K+-ATPase, conduction, and myo-inositol in the insulin-deficient BB rat. Am J Physiol. 1984 Oct;247(4 Pt 1):E534–E539. doi: 10.1152/ajpendo.1984.247.4.E534. [DOI] [PubMed] [Google Scholar]
- Gregersen G. Variations in motor conduction velocity produced by acute changes of the metabolic state in diabetic patients. Diabetologia. 1968 Nov;4(5):273–277. doi: 10.1007/BF01309900. [DOI] [PubMed] [Google Scholar]
- Griffin J. W., Price D. L. Demyelination in experimental beta, beta'-iminodipropionitrile and hexacarbon neuropathies. Evidence for an axonal influence. Lab Invest. 1981 Aug;45(2):130–141. [PubMed] [Google Scholar]
- Mayer J. H., Tomlinson D. R. Prevention of defects of axonal transport and nerve conduction velocity by oral administration of myo-inositol or an aldose reductase inhibitor in streptozotocin-diabetic rats. Diabetologia. 1983 Nov;25(5):433–438. doi: 10.1007/BF00282524. [DOI] [PubMed] [Google Scholar]
- Mendell J. R., Sahenk Z., Warmolts J. R., Marshall J. K., Thibert P. The spontaneously diabetic BB Wistar rat. Morphologic and physiologic studies of peripheral nerve. J Neurol Sci. 1981 Oct;52(1):103–115. doi: 10.1016/0022-510x(81)90139-8. [DOI] [PubMed] [Google Scholar]
- Norido F., Canella R., Zanoni R., Gorio A. Development of diabetic neuropathy in the C57BL/Ks (db/db) mouse and its treatment with gangliosides. Exp Neurol. 1984 Feb;83(2):221–232. doi: 10.1016/S0014-4886(84)90094-3. [DOI] [PubMed] [Google Scholar]
- Ochs S. Trophic functions of the neuron. 3. Mechanisms of neurotrophic interactions. Systems of material transport in nerve fibers (axoplasmic transport) related to nerve function and trophic control. Ann N Y Acad Sci. 1974 Mar 22;228(0):202–223. doi: 10.1111/j.1749-6632.1974.tb20511.x. [DOI] [PubMed] [Google Scholar]
- Pietri A., Ehle A. L., Raskin P. Changes in nerve conduction velocity after six weeks of glucoregulation with portable insulin infusion pumps. Diabetes. 1980 Aug;29(8):668–671. doi: 10.2337/diab.29.8.668. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Glial membrane specializations in extraparanodal regions. J Neurocytol. 1978 Dec;7(6):709–719. doi: 10.1007/BF01205146. [DOI] [PubMed] [Google Scholar]
- Saida K., Saida T., Kayama H., Nishitani H. Rapid alterations of the axon membrane in antibody-mediated demyelination. Ann Neurol. 1984 Jun;15(6):581–589. doi: 10.1002/ana.410150611. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Bouchier M., Christensen H. Axonal atrophy in sensory nerves of the diabetic BB-Wistar rat: a possible early correlate of human diabetic neuropathy. Ann Neurol. 1983 Mar;13(3):264–272. doi: 10.1002/ana.410130307. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Brismar T. Reversible diabetic nerve dysfunction: structural correlates to electrophysiological abnormalities. Ann Neurol. 1985 Jul;18(1):21–29. doi: 10.1002/ana.410180105. [DOI] [PubMed] [Google Scholar]
- Sima A. A. Can the BB-rat help to unravel diabetic neuropathy? Neuropathol Appl Neurobiol. 1985 Jul-Aug;11(4):253–264. doi: 10.1111/j.1365-2990.1985.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Hay K. Functional aspects and pathogenetic considerations of the neuropathy in the spontaneously diabetic BB-Wistar rat. Neuropathol Appl Neurobiol. 1981 Sep-Oct;7(5):341–350. doi: 10.1111/j.1365-2990.1981.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Lorusso A. C., Thibert P. Distal symmetric polyneuropathy in the spontaneously diabetic BB-wistar rat. An ultrastructural and teased fiber study. Acta Neuropathol. 1982;58(1):39–47. doi: 10.1007/BF00692696. [DOI] [PubMed] [Google Scholar]
- Sima A. A. The development and structural characterization of the neuropathies in the spontaneously diabetic BB Wistar rat. Metabolism. 1983 Jul;32(7 Suppl 1):106–111. doi: 10.1016/s0026-0495(83)80022-5. [DOI] [PubMed] [Google Scholar]
- Tuck R. R., Schmelzer J. D., Low P. A. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984 Sep;107(Pt 3):935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- Ward J. D., Barnes C. G., Fisher D. J., Jessop J. D., Baker R. W. Improvement in nerve conduction following treatment in newly diagnosed diabetics. Lancet. 1971 Feb 27;1(7696):428–430. doi: 10.1016/s0140-6736(71)92415-9. [DOI] [PubMed] [Google Scholar]
- Williams S. K., Howarth N. L., Devenny J. J., Bitensky M. W. Structural and functional consequences of increased tubulin glycosylation in diabetes mellitus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6546–6550. doi: 10.1073/pnas.79.21.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad A. I., Simmons D. A., Martin D. B. Has one diabetic complication been explained? N Engl J Med. 1983 Jan 20;308(3):152–154. doi: 10.1056/NEJM198301203080309. [DOI] [PubMed] [Google Scholar]
- Yoda A., Yoda S. A new simple preparation method for NaK-ATPase-rich membrane fragments. Anal Biochem. 1981 Jan 1;110(1):82–88. doi: 10.1016/0003-2697(81)90115-9. [DOI] [PubMed] [Google Scholar]






