Abstract
A predictive model of the fate of coral reef fish larvae in a reef system is proposed that combines the oceanographic processes of advection and turbulent diffusion with the biological process of horizontal swimming controlled by olfactory and auditory cues within the timescales of larval development. In the model, auditory cues resulted in swimming towards the reefs when within hearing distance of the reef, whereas olfactory cues resulted in the larvae swimming towards the natal reef in open waters by swimming against the concentration gradients in the smell plume emanating from the natal reef. The model suggested that the self-seeding rate may be quite large, at least 20% for the larvae of rapidly developing reef fish species, which contrasted with a self-seeding rate less than 2% for non-swimming coral larvae. The predicted self-recruitment rate of reefs was sensitive to a number of parameters, such as the time at which the fish larvae reach post-flexion, the pelagic larval duration of the larvae, the horizontal turbulent diffusion coefficient in reefal waters and the horizontal swimming behaviour of the fish larvae in response to auditory and olfactory cues, for which better field data are needed. Thus, the model suggested that high self-seeding rates for reef fish are possible, even in areas where the ‘sticky water’ effect is minimal and in the absence of long-term trapping in oceanic fronts and/or large-scale oceanic eddies or filaments that are often argued to facilitate the return of the larvae after long periods of drifting at sea.
Keywords: currents, larval dispersion, horizontal swimming, auditory cues, olfactory cues, connectivity
1. Introduction
Some hard corals (Scleractinia) spawn at discrete times and places, and have a dispersive larval stage [1–3]. The coral eggs transform into larvae that have negligible horizontal swimming ability when compared with crustacean and fish larvae [4]. Thus, they drift passively with the water currents until they settle on a suitable substrate that they presumably find using chemical and tactile cues [5,6]. The fastest-developing coral larvae may be able to settle 2–5 days after spawning, and these may settle on their natal reefs as a result of trapping in convergence zones, island wakes or the sticky water effect [7–10]. The remaining larvae may drift long distances (typically km to tens of km) downstream with the mainstream currents while oceanic turbulence facilitates dispersal [11–13]. However, phase eddies near isolated reefs and sticky water effects in a reef matrix of reefs also restrict physical dispersal [14,15], and thus help keep the larvae in open waters but in the vicinity of the natal reefs. The probability of a coral larva recruiting to ‘sink’ reefs downstream of the natal (or ‘source’) reef is controlled by the dispersal kernel and is typically less than a few per cent [16–22]. The behaviour of the larvae alters patterns of recruitment. Indeed, some coral larvae change their depth in the water column in response to environmental cues to help find a suitable habitat where they may settle, whereas soft corals and other brooder corals have been shown to be competent quite rapidly and to settle immediately in the vicinity (less than 10 m) of the parent colonies [23–25]. By contrast, a high percentage (possibly up to 30%) of invertebrate and fish larvae can return to the reef from which they were spawned [26,27].
For coral larvae with a short (less than 7 days) pelagic larval duration (PLD), larval retention in open waters near the natal reefs is, however, possible, because phase eddies abound in areas where reefs are isolated [14] and because of the sticky water effect in areas where reefs are present at high density [9,15]. By contrast, reef fish larvae are not retained or trapped in eddies behind their natal reefs for the long periods (typically more than 10 days) necessary before they are competent to settle [28,29], because this period is too long for trapping in a reef wake [30]. However, when they are competent to settle the fish larvae may accumulate near reefs, and possibly in reef wakes, while they search for a suitable place to settle [31–33].
Most reef fishes have a pelagic larval life-history stage that starts with the release and fertilization of planktonic eggs or the hatching of demersal eggs into free-swimming larvae [34]. The eggs, yolk-sac and pre-flexion larvae are pelagic and believed to suffer high mortality and to be largely free drifting until post-flexion. Despite short duration phase-eddy retention [14], it is generally predicted that dispersal of larvae will be to reefs well away from their natal reefs [13,22]. Nevertheless, a surprisingly large number of reef fish do recruit to their natal reefs [27,35,36] and such findings may be explained by earlier studies of oceanography processes such as trapping of the larvae in long-lasting oceanic fronts [37–39], transport at sea and later return to the natal reefs by oceanic gyres [40,41] or vertical gradients in oceanic currents comprising offshore surface wind-driven currents and onshore transport through deep ocean currents [12]. These studies have generally focused on isolated reefs with little or no shelf. However, the oceanographic processes of the Great Barrier Reef (GBR) do not facilitate such larvae retention processes; for instance, long-lasting oceanic fronts do not exist in the GBR shelf and oceanic eddies do not penetrate much the GBR matrix and those are exceptionally rare events of short duration owing to high bottom friction over shallow waters [42,43].
Whether owing to self-seeding of fish larvae to their natal reefs, or to connectivity of fish larvae between reefs, field observations reveal the presence of patches of high recruitment of larval reef fishes at specific areas of a reef for some fish families, and modelling has indicated that these patches can be generated by settlement-ready reef fish larvae that swim directionally in oceanic waters towards the sound of the reef [44,45].
Most reef larval fish species have a horizontal swimming ability, which varies greatly by taxa and developmental form. They can therefore potentially affect their location through directional swimming especially for the last half of their planktonic lives [29,46–48]. Post-flexion reef fish larvae can swim directionally towards reefs, because they can hear reefs [49–54], smell reefs [50,55–57] and even use sun-compass orientation [58]. Auditory cues are probably restricted spatially to less than 2 km from reefs, although the largest fish larvae may hear the reef for much longer distances [59]. Despite the view that reef fish larvae may use a hierarchy of different cues and related senses, it is unclear how efficiently the larvae locate settlement sites and the spatial scales of detection [4,50,58,60,61].
Tuna in oceanic waters and bonefish in coastal waters can aggregate by swimming directionally in relation to environmental characteristics (e.g. temperature or food) [62,63]. Reef fish larvae competent to settle aggregate primarily at the upstream and downstream separation points of coral reefs by swimming directionally towards reef sound [44]. While these studies are about fish aggregating and not navigating, they provide a conceptual framework to model reef fish larvae dispersing in a reef matrix but at the same time using smell and sound cues to orient their swimming. Even the smallest coral fish larvae at which olfaction can be assessed can detect dissolved materials [64]. The intensity of the odour of their natal reef will generally decrease with distance downstream in a somewhat stochastic manner owing to turbulent diffusion in the ocean; nevertheless, the fish larvae may be able to rely on this cue [65], even at very low concentrations, to choose a swimming direction by searching for an odour gradient in the turbulent odour landscape using taxis, kinesis, rheotaxis or eddy chemotaxis [62,63,65,66].
We demonstrate that the most logical way for a fish to return to its natal reef is by swimming using olfactory cues for orientation when out of hearing range of the reefs and by swimming straight to reefs using auditory cues when they are within hearing distance. Additional cues may also be available for the larvae to orient themselves, such as the sun compass [58]. A fish larva far from a reef is immersed in the water and does not see the substrate in order to determine the direction of the currents. The simplest method to find this direction is gradient response via restricted-area search using olfactory cues, based on the fact that diffusion causes the olfactory cues from its natal reef to decrease with increasing distance downstream from the natal reef. The fish cannot overcome the maximum tidal currents [29]; however, they do not need to, because these tidal currents simply advect them back and forth with minimal net displacement; what is important is whether they can overcome the net currents. By swimming against the concentration gradient of the smell plume emanating from their natal reef, the fish, in practice, swim against the net currents—which are commonly much smaller than the tidal currents—and this reinforces the gradient response and informs the fish that it is going the right way. Olfactory cues thus can generate directional swimming in open waters even many kilometres away from the reefs as long as the chemical cues can be smelled. Indeed, filaments of lagoonal water can be observed kilometres from reefs and suggest a navigational opportunity for larvae [46,67].
We compare self-recruitment rates by coral larvae and coral reef fish larvae on the GBR shelf and we also estimate the dependence of the coral reef fish larvae on their pre-flexion timescales and PLD, their ability to detect sound of olfactory cues, and their swimming cruise speed. We determine that on the GBR shelf the reef fish larvae can self-recruit in high numbers (more than 20% and possibly as high as 30%) by using horizontal directional swimming towards auditory and olfactory clues within the larvae development timescales. Additionally, we demonstrate that this can happen even in the presence of strong tidal currents. We also suggest that the capture of the remaining larvae in sink reefs immediately downstream of the natal reef is also high (up to 10%) as a result of directional swimming following auditory cues. Finally, we show that without horizontal swimming (e.g. for corals) and in areas where reefs are widely scattered, so that the ‘sticky water’ effect is negligible, the rate of larval recruitment to natal reefs is small (less than 2%).
2. Methods
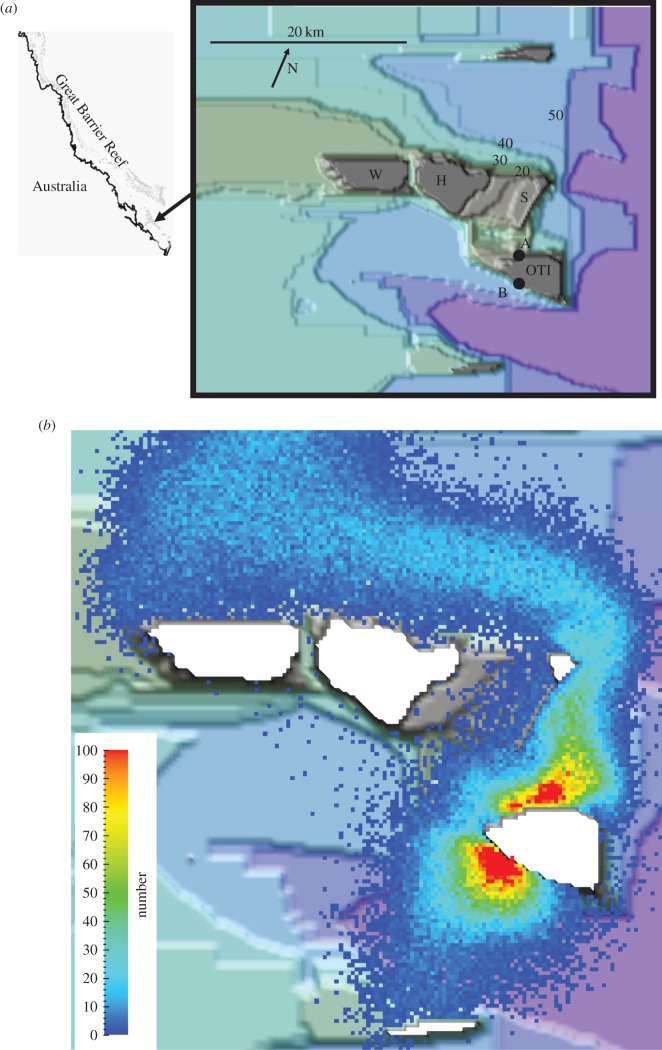
The study area was the Capricorn Bunker Group in the southern GBR (figure 1a) as described in [55]. The model domain and the open boundary conditions were the same as in [55]; however, our grid size was smaller (200 m as opposed to 300 m in the original study). The new bathymetry data were provided by R. Beaman at JCU eReef. The hydrodynamic model was re-run. The time step was 10 s. The waters are shallow and vertically well-mixed, justifying the use of the depth-averaged two-dimensional model [68]; this model removed the numerical error in calculating diffusion over a sloping seafloor [68] that in many other depth-averaged models numerically transports larvae towards shallow waters.
Figure 1.
(a) A general location map and a bathymetric map (depth in metres) of the hydrodynamic modelling area. The reefs are Wistari (W), Sykes (S), Heron (H) and One Tree Island (OTI). A and B are the model spawning sites at OTI. (b) The predicted tidally averaged smell plume from OTI. The scale bar is in number of smell tracers per cell (200 × 200 m).
Larvae were modelled as vertically well-mixed waterborne particles within the water column; vertical mixing occurs in a few hours after spawning for non-buoyant particles in reefal waters [30]. A total of 100 000 particles were released at two points (labelled A and B in figure 1a) on both the east and west sides of One Tree Island (OTI) to simulate spawning. The advection–dispersion model was run separately for each scenario; over each time step, each larva was advected by a velocity calculated as the vectorial sum of the water velocity plus the swimming velocity of the larva and then allowed to disperse in a Markov diffusion process following [68] and parametrized by a horizontal turbulent diffusion coefficient Kx. The time step for the advection–dispersion model was 30 min. Various scenarios listed in table 1 were studied using realistic values of the oceanographic processes and biological variables determining the fate of these larvae.
Table 1.
Predicted self-seeding rate for OTI and downstream capture rates at reefs W, H and S at time Tmax + 4 days. The model was run for various scenarios determined by the following physical and biological parameters, namely (i) the subgrid-scale turbulent horizontal diffusion coefficient Kx, (ii) the maximum swimming cruise velocity Umax of the fish larvae, (iii) the pre-flexion time Tpre-flexion of the larvae, (iv) the time Tmax at which the larvae start swimming at maximum swimming cruise velocity, (v) the width W of the band around reefs in which larvae can hear the reefs, and (vi) the ability to detect a odour gradient where the concentration is at least 0.1% (odour = 0.1), 1% (odour = 1) and 10% (odour = 10) that at 400 m from the source. The model accounted only for the larvae that survive. Non-swimming larvae were assumed to start to recruit on day 5 after spawning. Swimming larvae were assumed to be able to recruit when T > Tpre-flexion. The larvae were assumed to stop swimming on reaching the pelagic larval duration PLD (PLD = Tmax + 4 days).
| scenario number | Kx (m2 s−1) | Umax (m s−1) | Tpre-flexion (day) | Tmax (day) | PLD (day) | W (m) | odour | self-seeding rate (%) | downstream capture rate (%) | directional swimming |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 5.9 | 0.04 | N | |||||
| 2 | 5 | 0 | 1.2 | 0.5 | N | |||||
| 3 | 10 | 0 | 0.6 | 1.3 | N | |||||
| 4 | 5 | 0.15 | 7 | 10 | 14 | 2000 | 0.1 | 19.3 | 7.6 | Y (sound+smell) |
| 4a | 5 | 0.15 | 7 | 10 | 14 | 0 | 0.1 | 4.2 | 1.2 | Y (smell only) |
| 4b | 5 | 0.15 | 7 | 10 | 14 | 2000 | no | 7.9 | 4.3 | Y (sound only) |
| 5 | 1 | 0.15 | 7 | 10 | 14 | 2000 | 0.1 | 28.8 | 0.44 | Y (sound + smell) |
| 6 | 3 | 0.15 | 7 | 10 | 14 | 2000 | 0.1 | 27.3 | 4.5 | Y (sound + smell) |
| 7 | 7 | 0.15 | 7 | 10 | 14 | 2000 | 0.1 | 14 | 8.5 | Y (sound + smell) |
| 8 | 10 | 0.15 | 7 | 10 | 14 | 2000 | 0.1 | 9.3 | 9.1 | Y (sound + smell) |
| 9 | 5 | 0.05 | 7 | 10 | 14 | 2000 | 0.1 | 10.7 | 5.3 | Y (sound + smell) |
| 10 | 5 | 0.1 | 7 | 10 | 14 | 2000 | 0.1 | 15.9 | 8.1 | Y (sound + smell) |
| 11 | 5 | 0.2 | 7 | 10 | 14 | 2000 | 0.1 | 19.8 | 7.9 | Y (sound + smell) |
| 12 | 5 | 0.25 | 7 | 10 | 14 | 2000 | 0.1 | 20 | 8.2 | Y (sound + smell) |
| 13 | 5 | 0.15 | 7 | 10 | 14 | 1000 | 0.1 | 14.8 | 4.8 | Y (sound + smell) |
| 14 | 5 | 0.15 | 13 | 17 | 21 | 2000 | 0.1 | 10.8 | 5.9 | Y (sound+smell) |
| 15 | 5 | 0.15 | 15 | 18 | 22 | 2000 | 0.1 | 3 | 3 | Y (sound + smell) |
| 16 | 5 | 0.15 | 24 | 27 | 31 | 2000 | 0.1 | 0.4 | 0.5 | Y (sound + smell) |
| 17 | 5 | 0.15 | 7 | 10 | 14 | 2000 | 1 | 12 | 5 | Y (sound + smell) |
| 18 | 5 | 0.15 | 7 | 10 | 14 | 2000 | 10 | 8.1 | 4.5 | Y (sound + smell) |
The exact value of Kx is unknown; for a grid size of 200 m, Kx ∼ 0.5 m2 s−1 in open waters away from the salient topography of reefs [69], whereas its value is approximately 1.8 m2 s−1 in reef-free coastal GBR waters [15]. The value of Kx could be even larger in reefal waters in view of near-reef shear effects that initially produce linear features that are then readily dispersed by turbulence [70,71]; indeed, Kx ∼ 7 m2 s−1 in reefal waters near Bowden Reef [22]. We adopted Kx ∼ 5 m2 s−1 as the most likely value [15,42,68]. Thus, we tested in scenarios 4–8 (table 1) the dependence of the fate larvae on the value of Kx varying between its minimum likely value of 1 m2 s−1 and its maximum likely value of 10 m2 s−1.
The smell plume that provided olfactory cues to the fish larvae was calculated by continuously releasing at sites A and B passive particles representing the reef chemical cues. It is shown in figure 1b. The data of the concentration in the smell plume at 200 m resolution were used to calculate the direction of the maximum concentration gradient for each cell. Because odour is diffused in the ocean, the ability of fish larvae to follow a gradient of odour is expected to vary with the strength of the signal [66,72]. The sensitivity of this process was evaluated by three scenarios whereby the larvae could detect the odour gradient everywhere in the smell plume, including in the far-field (scenarios 4–16), in the intermediate-field (scenario 17) and only in the near-field (scenario 18). The far-field, the intermediate-field and the far-field of the smell plume were defined as the areas where the concentration value was, respectively, at least 0.1%, 1% and 10% that at 400 m from the source.
The fish larvae were assumed [29] to have a swimming speed Uswim which was zero at pre-flexion, and which increased linearly in time starting at post-flexion until the larvae reached the maximum swimming cruise speed Umax (also called ‘sustained swimming ability’ [73] and ‘routine speed’ [74]) after which time Uswim remained constant until 4 days later when they reached their PLD. At that time, the larvae were assumed to be exhausted and to stop swimming (figure 2). This was expressed mathematically as
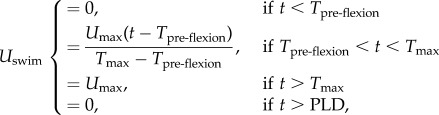 |
2.1 |
where t is the time, Tpre-flexion is the time at which the fish larva starts swimming, Tmax is the time at which the post-flexion larva reaches maximum cruise speed, and PLD = Tmax + 4 days. The values of Umax, Tpre-flexion and Tmax are species-dependent [29].
Figure 2.
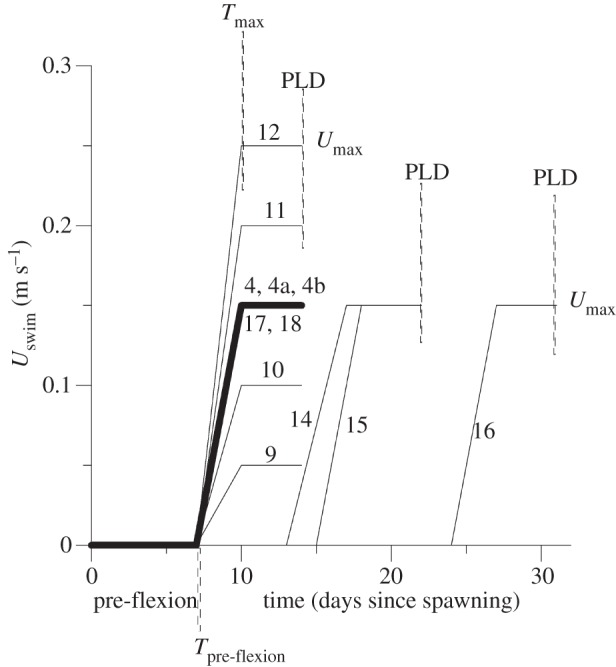
Plot of the fish larva horizontal swimming velocity Uswim as function of time after spawning for the various swimming scenarios 4, 9–16 listed in table 1. Following equation (2.1), Umax is the maximum swimming cruise speed, Tpre-flexion is the pre-flexion time, Tmax is the time at which the post-flexion larvae reach maximum cruise speed and PLD (=Tmax + 4 days) is the pelagic larval duration. Scenario 13 was the same as scenario 4 except that the hearing distance W was reduced from 2000 to 1000 m. Scenarios 5–8 were the same as scenario 4 except for different values of the subgrid-scale horizontal turbulent diffusion coefficient Kx.
When the larvae swam directionally, the velocity at which the larvae moved was the vectorial sum of the water velocity plus their swimming velocity. This was calculated for each larva at each time step. When the larvae were within the hearing distance W from a point along the perimeter of a reef, the swimming velocity was oriented directly towards that point, because one would expect that the larvae swam directly towards the reef sound. When the larvae were outside of hearing range of a reef, the swimming direction was oriented against the maximum concentration gradient at that point. Thus, directional swimming by using auditory cues was limited to a band of width W (expressed in m) around the reefs, whereas directional swimming using olfactory cues occurred everywhere in the smell plume except where the larvae could hear the reef and then they swam directly to the reef.
The larval oceanography model thus had several independent parameters, namely the oceanographic parameter Kx and the biological parameters Umax, Tpre-flexion, Tmax, W and the ability of the larvae to detect the odour gradient. The influence of these parameters was quantified by running the model for a number of scenarios for which one parameter was changed at a time. The values of these parameters for the various scenarios are listed in table 1. Scenarios 1–3 assumed no directional swimming by the larvae, and the subgrid-scale horizontal diffusion Kx was set in a range between 1 and 10 m2 s−1. The other scenarios 4–16 were for swimming larvae. Scenario 4 was the standard model run for larvae swimming to sound and smell cues; in this run Kx = 5 m2 s−1, the larval fish swam at a cruise speed Umax = 0.15 m s−1, the time of pre-flexion Tpre-flexion was 7 days, the time Tmax was 10 days, the fish larvae heard the reefs at a distance W = 2000 m; the PLD was 14 days (figure 2); this scenario applies to fast-developing larvae. Scenarios 5–8 were the same as scenario 4 except that the horizontal diffusion coefficient Kx was varied in the range of likely values. Scenarios 9–16 tested the sensitivity of the various parameters Kx, Umax, Tpre-flexion, Tmax and W compared with the results of scenario 4. Scenario 4a was the same as scenario 4 except that the fish larvae swam to smell cues only and not to sound cues. Scenario 4b was the same as scenario 4 except that the fish larvae swam to sound cues only and not to smell cues. Scenarios 9–12 were the same as scenario 4 except that the swimming cruise speed Umax varied in a range of the likely values 0.05–0.25 m s−1. Scenario 13 was the same as scenario 4 except that the distance W that the larvae heard a reef was reduced from 2000 to 1000 m. Scenarios 13–16 were the same as scenario 4 except that the pre-flexion time Tpre-flexion and the time Tmax were increased by steps to maximum values of 24 and 27 days, respectively.
These scenarios were realistic; indeed, scenario 2 was that expected for fast-developing coral larvae, scenarios 4–13 were that expected for fast-developing reef fish larvae with a short PLD, and scenarios 14–16 were that expected for slower developing reef fish larvae with a long PLD (e.g. 15–27 days for Pomacentrus coelestis; [29,33]).
Because we focused on the recruitment of larvae that settled, the model considered only the larvae that survived during the PLD; thus, the calculations did not include the larvae that died at sea from predation, starvation, disease and unknown causes [74]. If the daily mortality rate m of larvae is age-independent, then the number of surviving larvae decreases exponentially with time t as {exp(−mt)} and thus, considering the surviving larvae, mortality does not affect the dispersal pattern (i.e. the shape of the dispersing larval plume). However, if m is age-dependent, then mortality will affect the dispersal pattern.
We calculated the self-seeding rate of OTI as the number of larvae spawned at OTI and that recruited back to OTI up to time equal to the PLD. It was expressed in percentage of the total number of larvae released. We also calculated the number of larvae that recruited to the three reefs Wistari (W), Sykes (S) and Heron (H) located north of OTI. This was called the downstream capture rate, and it was also expressed in percentage of the total number of larvae released initially at OTI.
3. Results
3.1. Smell plume
The smell plume for scenario 4 extended far from the reef (figure 1b). This smell plume varied slightly with the tides in the far-field and more so in the near-field; however, the larvae were always in the smell plume of their natal reef.
From an initial high concentration near the release point of the natal reef water, the plume dispersed northwards with the mean currents and eastwards and westwards with the tidal currents. The plume meandered around the downstream reefs during its northward trajectory. This smell plume was somewhat thinner than that predicted by [55] because of the sensitivity of the model to the times of spawning with respect to the tides, the location of the spawning sites, the smaller mesh size and the adopted value of Kx.
3.2. Larval plumes
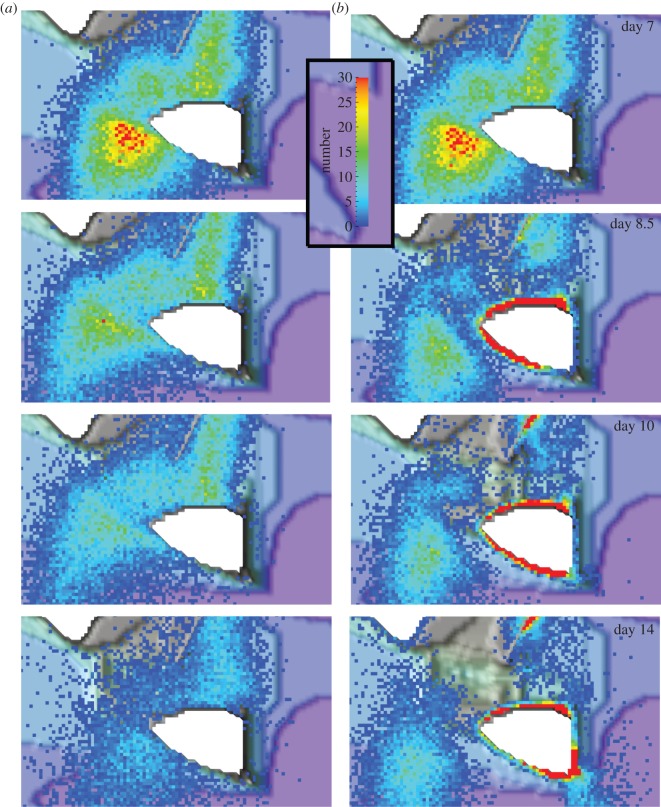
On day 7, the plume (figure 3) had already spread far downstream and showed two maxima around OTI, which were due to the two spawning events (i.e. the particle release points) at sites A and B (figure 1). On that day, the larval plume was the same for scenarios 2 (passive larvae) and 4 (swimming larvae), because at that time, the reef fish larvae had not yet begun to swim. On subsequent days, the non-swimming larvae (scenario 2) increasingly dispersed over time. By contrast, the swimming larvae s accumulated in high concentration near the natal reef and the downstream reefs (figure 3).
Figure 3.
The predicted synoptic distribution of (a) the larval plume around OTI for scenario 2 (non-swimming larvae) and (b) for scenario 4 (swimming larvae) on days 7, 8.5, 10 and 14 after spawning at sites A and B (shown in figure 1). The scale bar is in number of larvae per cell (200 × 200 m). Larval mortality was not included in this model, i.e. the model only follows living larvae.
3.3. Recruitment rates for passive larvae
For passive larvae (e.g. coral larvae that were competent to recruit on day 5), the model predicted (see scenarios 1–3 in table 1) that the self-seeding rate decreased from 5.9% to 0.6% with increasing values of Kx (with a self-seeding rate of 1.2% for scenario 3). The downstream capture rate increased from 0.04% to 1.3% with increasing values of Kx (i.e. with increasing oceanic turbulence).
3.4. Recruitment rates for swimming larvae
The self-seeding rate also decreased with increasing values of Kx for swimming larvae and the downstream capture rate also decreased with increasing values of Kx (scenarios 4, 5–8 in table 1 and figure 4).
Figure 4.
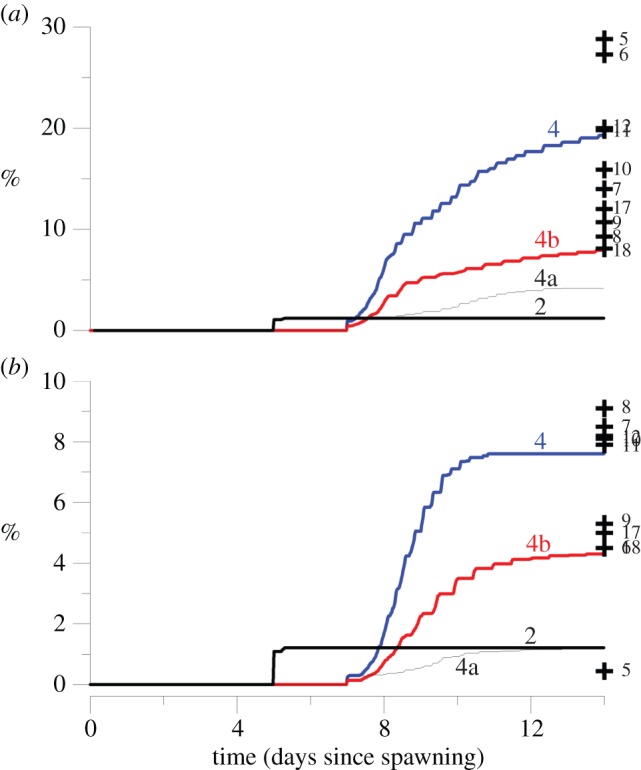
Lines: time-series plot of (a) the self-seeding rate and (b) the downstream capture rate of the coral fish larvae, after spawning at OTI for scenarios 2 (passive larvae), 4 (larvae swimming to sound and smell cues), 4a (larvae swimming to smell only) and 4b (larvae swimming to sound only). The plus symbols show the final recruitment rates for the other scenarios.
The self-seeding rate and the downstream capture rates increased with increasing values of the maximum swimming cruise speed Umax between 0.05 and 0.15 m s−1, and these recruitment rates varied little for Umax > 0.15 m s−1 (scenarios 4, 9–12 in table 1 and figure 4).
The self-seeding and the downstream capture rates decreased by about 30% when the hearing distance W was halved (compare scenarios 4 and 13 in table 1).
The self-seeding and the downstream capture rates decreased markedly with increasing values of the PLD (compare scenario 4 with scenarios 14–15 in table 1 and figure 4). For instance, they were, respectively, 19.3% and 7.6% for scenario 4 (PLD = 14 days) and 0.4% and 0.5% for scenario 16 (PLD = 31 days; all other physical and biological parameters remaining unchanged).
The results were also dependent on the time that the larvae were weak swimmers, i.e. the pre-flexion time of the larvae, Tpre-flexion. When Tpre-flexion was increased for 7 days (scenario 4) to 13, 15 and 24 days (scenarios 14–16), the self-seeding rate decreased from 19.3% to 10.8%, 3% and 0.4% respectively, whereas the downstream capture rate also decreased from 7.6% to, respectively, 5.9%, 3% and 0.5% (table 1).
Swimming larvae using only smell only to choose their swimming direction self-recruited much less (4.2%) than those using both smell and sound (19.3%; compare scenarios 4 and 4a; figure 4 and table 1). Similarly, swimming larvae using only sound only to choose their swimming direction self-recruited much less (7.9%) than those using both smell and sound (19.3%).
The ability of swimming larvae to detect an odour gradient at small values of the odour concentration increased the self-seeding rate (19.3%, 12% and 8.1% when the larvae can detect a gradient when the concentration was 0.1%, 1% and 10%, respectively that at 400 m from the source; compare scenarios 4, 17 and 18 in table 1).
4. Discussion
4.1. Influence of the oceanography
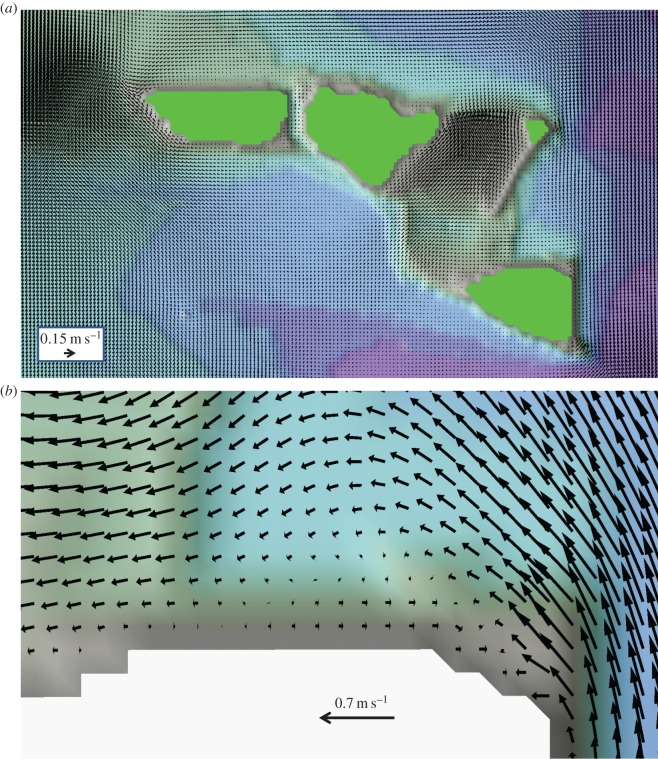
The reefs around OTI provided an obstacle generating a small ‘sticky water’ effect, forcing the net water circulation to be deflected around the reef matrix [9]. The net currents around OTI were smaller by a factor of two than those to the east and west of the reef matrix (figure 5a). Swift tidal currents prevailed around OTI and generated phase eddies at tidal frequency (figure 5b). Although phase eddies decreased the advection of larvae away from their natal reefs [14], these eddies did not trap larvae for long durations (e.g. figure 3 for passive larvae). The location and size of the smell plume (figure 1b) and non-swimming larval plume (figure 3) were determined by this water circulation. Fish larvae were transported at scales of a few kilometres to a few tens of kilometres in the first few weeks of their life. However, in other areas of the GBR where reefs are closely located, the ‘sticky water’ effect would be much larger and result in a much higher self-seeding rate [75]. Predictions of the influence of the oceanography on self-seeding rates are thus site-specific.
Figure 5.
(a) Synoptic distribution of the tidally averaged mean currents for a prevailing northward longshore flow in the far-field. (b) Examples of phase eddies shed by the reefs by the swift, reversing tidal currents, to the north of OTI.
The intensity of oceanic turbulence in inter-reefal waters remains poorly known [9,15,75]. Oceanic turbulence was parametrized here by the subgrid-scale horizontal eddy diffusion coefficient Kx, and values of Kx from 1 to 10 m2 s−1 were chosen to represent the expected range of values. More intense turbulence (larger values of Kx) diffused larvae faster away from the natal reef and decreased the self-seeding rate; more intense turbulence diffused more larvae to downstream reefs from the larval plume and thus generally increased the downstream capture rate.
The recruitment rate of passive larvae (e.g. coral) at OTI was less than 2% for larvae that were competent to recruit on day 5. This is consistent with the predictions by other models of very small coral larvae recruitment rates [9,76]. The prediction from the latter model is that at OTI 63% of the larvae are flushed out in 1.5 days, i.e. on day 5 only 3.5% of the larvae would remain. If the coral larvae started to recruit on day 10, then the self-seeding rate would be even smaller (less than 0.1%; not shown).
4.2. Influence of the animal behaviour
Very high fish larvae concentration resulted near OTI and downstream reefs, and these larvae can be assumed to maintain position until they recruited. Indeed, there is strong field evidence for a near-reef accumulation phase prior to settlement [3,32].
Swimming larvae using both smell and sound recruited more both to their natal reefs and the downstream reefs than non-swimming larvae. Their level of self-seeding asymptoted towards 20% (scenario 4), a value which is much larger than that (less than 2%) of the passive coral larvae (scenario 2). Thus, we clearly demonstrated that the swimming larvae have a much greater chance to recruit to their natal reef than larvae that do not swim. The self-seeding rates were sensitive to the ability of the larvae to orient to sound and smell cues. For fast-developing fish larvae, the self-seeding rates were 19.3% for a sound detection limit of 2000 m (scenario 4) and 14.8% for a sound detection limit of 1000 m (scenario 13; table 1). These self-seeding rates were sensitive to the odour concentration value; for fast-developing larvae, these rates decreased from 19.3% to 8.1% if the larvae were able to detect the odour gradient in the far-field (scenario 4) or only in the near-field (scenario 18). Further, swimming larvae using either smell or sound to choose their swimming direction recruited much less than those using both smell and sound (compare scenarios 4, 4a and 4b in figure 4 and table 1). This emphasizes the importance of multiple senses [4,58].
4.3. The importance of physics–biology links
This study revealed the competing effects on the fate of larvae of (i) dispersal away from natal reefs, which was facilitated by the water circulation and hindered by the oceanic dispersion, and (ii) the horizontal directional swimming that facilitated retention. The first process was physical, the second process was biological.
For swimming larvae, the bulk of the self-seeding occurred progressively and continuously over a few days as the larvae reached maximum swimming cruise speed. By contrast, the bulk of the downstream capture rate (i.e. the connectivity between reefs) was much more a hit-or-miss process, because the larval plume needed to pass in the vicinity of downstream reefs; they can recruit to a non-natal reef when they come within hearing distance of that reef and they have to recruit quickly before they are advected away by the water currents. However, they have more than one chance to hear that reef, and thus to recruit, owing to the reversing tidal currents transporting back and forth and thus occasionally at times them within hearing distance of that reef. Therefore, the downstream recruitment occurred as a series of spike event (figure 4).
Our study thus demonstrated that even when the sticky water effect is small and, therefore, the potential for expatriation from reefs is high, there can still be a high level of self-recruitment (at least 20%) of coral reef fish. In addition, the predicted early arrival of many reef fish larvae close to natal reefs early in their typical PLD demonstrates the potential for near shore accumulation prior to settlement [77] and suggests that such accumulation may be more common than previously thought.
Figure 6 summarizes the linked physical–biological processes that enable recruitment of coral fish larvae. The larvae disperse around the natal reef to form a larval plume that spreads downstream while mixing with surrounding waters. Most larvae are exported downstream of the natal reef in that plume. The dispersal from the natal reef decreases if the near-reef turbulent horizontal diffusion is small and/or if the sticky water effect prevails; tidal phase eddies can decrease this dispersal over a few days only. These processes enable some larvae to remain within the vicinity of their natal reef. When reaching swimming competence at post-flexion the larvae slow down their advection by horizontal swimming following olfactory cues; the tidal currents are strong and sweep this plume back and forth around the reef, enabling some larvae to come within hearing distance of the reef; they can then swim to the reef using auditory cues. This is an evolving process and this recruitment occurs over many days. With that strategy the self-seeding rate could have values of 20%, and possibly more, for fast-developing reef fish larvae. For slow developing reef larvae the self-seeding rate may have much smaller values (0.4% for the most pessimistic scenario) though this would depend on the duration of the pre-flexion phase and the swimming ability and sensory capabilities at post-flexion. For instance some larvae such as Acanthuridae and Chaetodontidae have long PLDs (i.e. 39–84 days; [78]), but are very large at settlement and have impressive swimming capabilities. For larval recruitment on downstream reefs, high oceanic diffusion and strong reversing tidal currents in reefal waters are needed bring the larvae to within the hearing distance of the reef, which enables the larvae to swim directionally to the reef following auditory cues. The nature of oceanographic filaments [79], social interactions among larvae that may modify directional swimming [80], the need to forage that modifies directional swimming [61] and the use of other sensors (e.g. sun-compass orientation; [58]) may also further influence the recruitment rates.
Figure 6.
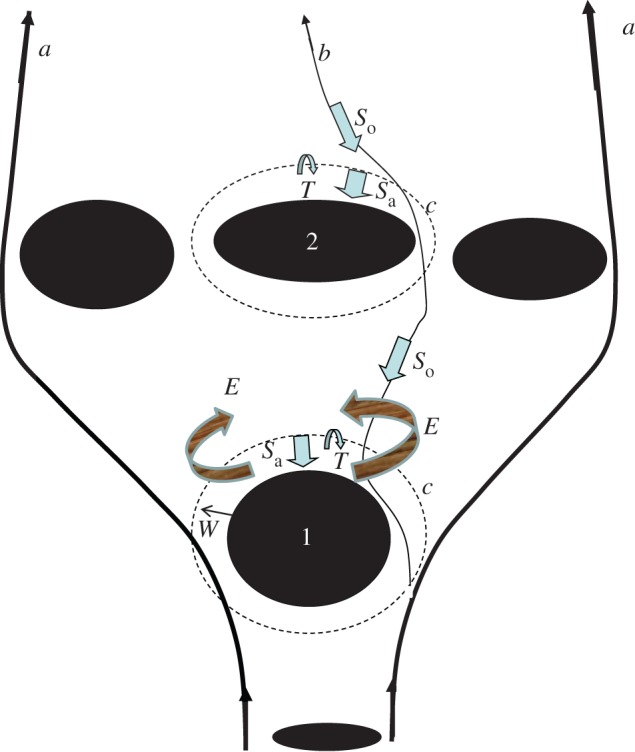
A diagram explaining the key physical and biological processes controlling the fate of reef fish larvae around OTI. 1, natal reef; 2, sink reef; a, streamline of the strong net currents around the reef matrix; b, streamline of the smaller net currents within the reef matrix and the smell plume; E, transient phase eddies; T, turbulent diffusion; So , directional swimming from olfactory cues; Sa, directional swimming from auditory cues; c (dotted line), limit where the larvae can hear the reef; W, width of the band around the reefs where the larvae can hear the reef. (Online version in colour.)
In this study, it was assumed that the larvae swam towards the whole reef when they were at a distance W of the reef. In the field, W probably varies spatially as a function of the habitats on the reef, because various taxa of settling fishes prefer different habitats [81,82]. Fine tuning of our model could accommodate this process, provided that data on the location of such habitats for individual reefs were available. This would generate patchiness and schooling of the recruited larvae [44,61,62].
This study suggests that fast-developing reef fish larvae (Tmax ≤ 14 days; [29,78]) can successfully recruit in large numbers even in areas with a small reef density and a small ‘sticky water’ effect [9], even in the absence of long-duration oceanic fronts/eddies/filaments that control recruitment rates in open waters [79]. For slow developing fish larvae (Tmax > 20 days; [39,78]), the net current commonly reverses direction with that of the wind at periods of typically 20–30 days in summer [42]; in such cases, some of these slow developing fish larvae may be advected backward to their natal reefs by these reversing wind-driven net currents, hence recruitment would be enhanced.
Based on the sensitivity analysis in this study, several key processes are particularly important in determining the self-seeding rates and downstream capture rates of reefs and these should be studied in the field in the future, among them (i) how far downstream can reef fish larvae smell their natal reef, (ii) how far away from reefs do larvae of different sizes hear the reef, (iii) how efficiently do reef fish larvae use olfactory and auditory cues to orient their horizontal swimming, (iv) the exact value of the horizontal turbulent diffusivity Kx in reefal waters, (v) while there is no evidence that in the shallow waters of the GBR shelf reef fish larvae select different depths [83], an observation that may due to the a high level of vertical mixing [42], do reef fish larvae in the GBR shelf select different depths to maximize their self-recruitment as they do in deep oceanic waters [12,18], and (vi) to what level do fish larvae use other senses such as sun compass for directional swimming [58]. Until such physical and biological data become available, models of reef larval fish oceanography should probably not be made much more complex.
In conclusion, the mobility of larvae appears to make great differences in terms of levels of self-recruitment. The study reveals the importance of multiple senses in enabling directional swimming. Our estimates of self-recruitment for fishes (at least 20%) are much higher than researchers had expected. Tagging studies elsewhere [26,27] also reported a high value (up to 30%), once again much larger than expected, for the percentage of newly recruited fish from a given location that came from that same location. Our study suggests that some kind of behaviour is probably necessary to explain these large values of coral reef fish self-recruitment levels.
These findings have to be held in the context that OTI is relatively well flushed compared with many reefs in a reef matrix of much greater ‘sticky water’ effect. Thus, the self-seeding rate of fish in a reef matrix may be larger than that at OTI. Further, our study suggests that many larvae arrive close to natal reefs in the early stages of their PLD at post-flexion, in agreement with observations of near shore accumulation prior to settlement. We also demonstrated that, for larvae that have weak swimming and orientation abilities, short PLDs and/or downstream trapping effects such as phase eddies and sticky waters are needed to ensure self-seeding in areas where flushing is swift. Accurate, models of dispersal and self-recruitment clearly require information on the oceanography and the sensory and swimming abilities and habitat preference of larvae, which will vary by taxa.
References
- 1.Babcock R, Mundy C, Keesing J, Oliver J. 1992. Predictable and unpredictable spawning events: in situ behavioural data from free-spawning coral reef invertebrates. Invert. Reprod. Dev. 22, 213–228. ( 10.1080/07924259.1992.9672274) [DOI] [Google Scholar]
- 2.Baird AH, Guest JR, Willis BL. 2009. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 40, 551–571. ( 10.1146/annurev.ecolsys.110308.120220) [DOI] [Google Scholar]
- 3.Van Woesik R. 2010. Calm before the spawn: global coral spawning patterns are explained by regional wind fields. Proc. R. Soc. B 277, 715–722. ( 10.1098/rspb.2009.1524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kingsford MJ, Leis J, Shanks A, Lindeman K, Morgan S, Pineda J. 2002. Sensory environments, larval abilities and local self-recruitment. Bull. Mar. Sci. Suppl. 70, 309–340. [Google Scholar]
- 5.Koehl MAR, Hadfield MG. 2004. Soluble settlement cue in slowly moving water within coral reefs induces larval adhesion to surfaces. J. Mar. Syst. 49, 75–88. ( 10.1016/j.jmarsys.2003.06.003) [DOI] [Google Scholar]
- 6.Price N. 2010. Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163, 747–758. ( 10.1007/s00442-010-1578-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sammarco PW, Andrews JC. 1988. Localised dispersal and recruitment in Great Barrier Reef corals: the Helix experiment. Science 239, 1422–1424. ( 10.1126/science.239.4846.1422) [DOI] [PubMed] [Google Scholar]
- 8.Sammarco PW, Andrews JC. 1989. The Helix experiment: differential localized dispersal and recruitment patterns in Great Barrier Reef corals. Limnol. Oceanogr. 34, 896–912. ( 10.4319/lo.1989.34.5.0896) [DOI] [PubMed] [Google Scholar]
- 9.Andutta F, Kingsford M, Wolanski E. 2012. ‘Sticky water’ enables the retention of larvae in a reef mosaic. Estuarine, Coastal Shelf Sci. 101, 54–63. ( 10.1016/j.ecss.2012.02.013) [DOI] [Google Scholar]
- 10.Golbuu Y, Wolanski E, Idechong JW, Victor S, Isechal AL, Oldiais NW, Idip D, Jr, Richmond RH, van Woesik R. 2012. Predicting coral recruitment in Palau's complex reef archipelago. PLoS ONE 7, e50998 ( 10.1371/journal.pone.0050998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis BL, Oliver JK. 1990. Direct tracking of coral larvae: implications for the dispersal of planktonic larvae in topographically complex environments. Ophelia 32, 145–162. ( 10.1080/00785236.1990.10422029) [DOI] [Google Scholar]
- 12.Cowen RK, Lwiza KMM, Sponaugle S, Limouzy-Paris CB, Olson DB. 2000. Connectivity of marine populations: open or closed? Science 287, 857–859. ( 10.1126/science.287.5454.857) [DOI] [PubMed] [Google Scholar]
- 13.Van Oppen MJH, Lutz A, De'ath G, Peplow L, Kinnimonth S. 2008. Genetic traces of recent long-distance dispersal in a predominantly self-recruiting coral. PLoS ONE 3, e3401 ( 10.1371/journal.pone.0003401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess SC, Kingsford MJ, Black KP. 2007. Influence of tidal eddies and wind on the distribution of presettlement fishes around One Tree Island, Great Barrier Reef. Mar. Ecol. Prog. Ser. 341, 233–242. ( 10.3354/meps341233) [DOI] [Google Scholar]
- 15.Andutta F, Ridd PV, Wolanski E. 2011. Dynamics of hypersaline coastal waters in the Great Barrier Reef. Estuarine, Coastal Shelf Sci. 94, 299–305. ( 10.1016/j.ecss.2011.06.009) [DOI] [Google Scholar]
- 16.Graham EM, Baird AH, Connolly SR. 2008. Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27, 529–539. ( 10.1007/s00338-008-0361-z) [DOI] [Google Scholar]
- 17.Siegel DA, Kinlan BP, Gaylord B, Gaines SD. 2003. Lagrangian descriptions of marine larval dispersal. Mar. Ecol. Prog. Ser. 260, 83–96. ( 10.3354/meps260083) [DOI] [Google Scholar]
- 18.Cowen RK, Gawarkiewicz G, Pineda J, Thorrold SR, Werner FE. 2007. Population connectivity in marine systems. An overview. Oceanography 20, 14–21. ( 10.5670/oceanog.2007.26) [DOI] [Google Scholar]
- 19.Gawarkiewicz G, Monismith S, Largier J. 2007. Observing larval transport processes affecting population connectivity. Progress and challenges. Oceanography 20, 40–53. ( 10.5670/oceanog.2007.28) [DOI] [Google Scholar]
- 20.Buston PM, Jones GP, Planes S, Thorrold SR. 2012. Probability of successful dispersal declines fivefold over one kilometer in a coral reef fish. Proc. R. Soc. B 279, 1883–1888. ( 10.1098/rspb.2011.2041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hrycik JM, Chasse J, Ruddick BR, Taggart CT. 2013. Dispersal kernel estimation: a comparison of empirical and modelled particle dispersion in a coastal marine system. Estuarine, Coastal Shelf Sci. 133, 11–22. ( 10.1016/j.ecss.2013.06.023) [DOI] [Google Scholar]
- 22.Wolanski E, Burrage B, King B. 1989. Trapping and diffusion of coral eggs near Bowden Reef, Great Barrier Reef following mass coral spawning. Continental Shelf Res. 9, 479–496. ( 10.1016/0278-4343(89)90011-3) [DOI] [Google Scholar]
- 23.Stake JL, Sammarco PW. 2003. Effects of pressure on swimming behavior in planula larvae of the coral Porites astreoides (Cnidaria, Scleractinia). J. Exp. Mar. Biol. Ecol. 288, 181–201. ( 10.1016/S0022-0981(03)00018-2) [DOI] [Google Scholar]
- 24.Gutiérrez-Rodríguez C, Lasker HR. 2005. Reproductive biology, development, and planula behavior in the Caribbean gorgonian Pseudopterogorgia elisabethae. Invert. Biol. 123, 54–67. ( 10.1111/j.1744-7410.2004.tb00141.x) [DOI] [Google Scholar]
- 25.Vermeij MJA, Marhaver KL, Huijbers CM, Nagelkerken I, Simpson SD. 2010. Coral larvae move toward reef sounds. PLoS ONE 5, e10660 ( 10.1371/journal.pone.0010660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones GP, Almany GR, Russ GR, Sale PF, Steneck RS, van Oppen MJH, Willis BL. 2009. Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28, 307–325. ( 10.1007/s00338-009-0469-9) [DOI] [Google Scholar]
- 27.Harrison HB, et al. 2012. Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr. Biol. 22, 1023–1028. ( 10.1016/j.cub.2012.04.008) [DOI] [PubMed] [Google Scholar]
- 28.Williams DMcB, English S. 1992. Distribution of fish larvae around a coral reef: direction detection of a meso-scale, multi-specific patch? Continental Shelf Res. 12, 923–937. ( 10.1016/0278-4343(92)90052-L) [DOI] [Google Scholar]
- 29.Fisher R. 2005. Swimming speeds of larval coral reef fishes: impacts on self-recruitment and dispersal. Mar. Ecol. Prog. Ser. 285, 223–232. ( 10.3354/meps285223) [DOI] [Google Scholar]
- 30.Hamner WH, Largier JL. 2011. Oceanography of the planktonic stages of aggregation spawning reef fishes. In Reef fish spawning aggregations: biology, research and management (eds de Mitcheson YS, Colin PL.), pp. 159–190. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 31.Doherty PJ, Kingsford MJ, Booth D, Carleton J. 1996. Habitat selection before settlement by Pomacentrus coelestis. Mar. Freshw. Res. 47, 391–399. ( 10.1071/MF9960391) [DOI] [Google Scholar]
- 32.Patterson HM, Kingsford MJ, McCullough MT. 2005. Resolution of the early life history of a reef fish using otolith chemistry. Coral Reefs 24, 222–229. ( 10.1007/s00338-004-0469-8) [DOI] [Google Scholar]
- 33.Kingsford M, Smith FJA, Flood MJ. 2011. Growth and pelagic larval duration of presettlement and newly settled neon damselfish, Pomacentrus coelestis, at multiple spatial scales. Coral Reefs 30, 203–214. ( 10.1007/s00338-010-0692-4) [DOI] [Google Scholar]
- 34.de Mitcheson YS, Colin PL. 2011. Reef fish spawning aggregations: biology, research and management, 621 p Dordrecht, The Netherlands: Springer. [Google Scholar]
- 35.Jones GP, Milicich MJ, Emslie MJ, Lunow C. 1999. Self-recruitment in a coral reef fish population. Nature 402, 802–804. ( 10.1038/45538) [DOI] [Google Scholar]
- 36.Jones GP, Planes S, Thorrold SR. 2005. Coral reef fish larvae settle close to home. Curr. Biol. 15, 1314–1318. ( 10.1016/j.cub.2005.06.061) [DOI] [PubMed] [Google Scholar]
- 37.Curtis DL, McGaw IJ. 2008. A year in the life of a Dungeness crab: methodology for determining microhabitat conditions experienced by large decapod crustaceans in estuaries. J. Zool. 274, 375–385. ( 10.1111/j.1469-7998.2007.00397.x) [DOI] [Google Scholar]
- 38.Eggleston DB, et al. 1998. Estuarine fronts as conduits for larval transport: hydrodynamics and spatial distribution of Dungeness crab postlarvae. Mar. Ecol. Prog. Ser. 164, 73–82. ( 10.3354/meps164073) [DOI] [Google Scholar]
- 39.Marancik KE, Richardson DR, Lyczkowski-Shultz J, Cowen RK, Konieczna M. 2012. Spatial and temporal distribution of grouper larvae (Serranidae: Epinephelinae: Epinephelini) in the Gulf of Mexico and Straits of Florida. Fish Bull. 110, 1–20. [Google Scholar]
- 40.Lobel PS, Robinson AR. 1986. Transport and entrapment of fish larvae by ocean mesoscale eddies and currents in Hawaiian waters. Deep Sea Res. A 33, 483–500. ( 10.1016/0198-0149(86)90127-5) [DOI] [Google Scholar]
- 41.Lee TN, Rooth C, Williams E, McGowan M, Szmant AF, Clarke ME. 1992. Influence of Florida Current, gyres and wind-driven circulation on transport of larvae and recruitment in the Florida Keys coral reefs. Continental Shelf Res. 12, 971–1002. ( 10.1016/0278-4343(92)90055-O) [DOI] [Google Scholar]
- 42.Wolanski E. 1994. Physical oceanographic processes of the Great Barrier Reef, 194 p. Boca Raton, FL: CRC Press. [Google Scholar]
- 43.Weeks SJ, Bakun A, Steinberg CR, Brinkman R, Hoegh-Guldberg O. 2010. The Capricorn Eddy: a prominent driver of the ecology and future of the southern Great Barrier Reef. Coral Reefs 29, 975–985. ( 10.1007/s00338-010-0644-z) [DOI] [Google Scholar]
- 44.Wolanski E, Doherty P, Carleton J. 1997. Directional swimming of fish larvae determines connectivity of fish populations on the Great Barrier Reef. Naturwissenschaften 84, 6, 262–268. ( 10.1007/s001140050394) [DOI] [Google Scholar]
- 45.Carleton JH, Brinkman R, Doherty PJ. 2001. The effect of water flow around coral reefs on the distribution of pre-settlement fish (Great Barrier Reef, Australia). In Oceanographic processes of coral reefs. Physical and biological links in the Great Barrier Reef (ed. Wolanski E.), pp. 209–230. Boca Raton, FL: CRC Press. [Google Scholar]
- 46.Atema J, Kingsford MJ, Gerlach G. 2002. Larval reef fish could use odour for detection, retention and orientation to reefs. Mar. Ecol. Prog. Ser. 241, 151–160. ( 10.3354/meps241151) [DOI] [Google Scholar]
- 47.Fisher R, Bellwood DR. 2003. Undisturbed swimming behaviour and nocturnal activity of coral reef fish larvae. Mar. Ecol. Prog. Ser. 263, 177–188. ( 10.3354/meps263177) [DOI] [Google Scholar]
- 48.Fisher R, Hogan JD. 2007. Morphological predictors of swimming speed: a case study of pre-settlement juvenile coral reef fishes. J. Exp. Biol. 210, 2436–2443. ( 10.1242/jeb.004275) [DOI] [PubMed] [Google Scholar]
- 49.Leis JM, Carson-Ewart NM. 1997. In situ swimming speeds of the late pelagic larvae of some Indo-Pacific coral-reef fishes. Mar. Ecol. Prog. Ser. 159, 165–174. ( 10.3354/meps159165) [DOI] [Google Scholar]
- 50.Wright KJ, Higgs DM, Belanger AJ, Leis JM. 2005. Auditory and olfactory abilities of pre-settlement larvae and post-settlement juveniles of a coral reef damselfish (Pisces: Pomacentridae). Mar. Biol. 147, 1425–1434. ( 10.1007/s00227-005-0028-z) [DOI] [Google Scholar]
- 51.Wright KJ, Higgs DM, Leis JM. 2011. Ontogenetic and interspecific variation in hearing ability in marine fish larvae. Mar. Ecol. Prog. Ser. 424, 1–13. ( 10.3354/meps09004) [DOI] [Google Scholar]
- 52.Mann DA, Casper BM, Boyle KS, Tricas TC. 2007. On the attraction of larval fishes to reef sounds. Mar. Ecol. Prog. Ser. 338, 307–310. ( 10.3354/meps338307) [DOI] [Google Scholar]
- 53.Leis JM, Sweatman HPA, Reader SE. 1996. What the pelagic fishes of coral reef fishes are doing out in blue water: daytime field observations of larval behavioural capabilities. Mar. Freshw. Res. 47, 101–411. ( 10.1071/MF9960401) [DOI] [Google Scholar]
- 54.Leis JM, Hay AC, Lockett MM, Chen J-P, Fang L-S. 2007. Ontogeny of swimming speed in larvae of pelagic-spawning, tropical, marine fishes. Mar. Ecol. Prog. Ser. 349, 255–267. ( 10.3354/meps07107) [DOI] [Google Scholar]
- 55.Gerlach G, Atema J, Kingsford M, Black KP, Miller-Sims V. 2007. Smelling home can prevent dispersal of reef fish larvae. Proc. Natl Acad. Sci. USA 104, 858–863. ( 10.1073/pnas.0606777104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dixson DL, Jones GP, Munday PL, Planes S, Pratchett MS, Srinivasan M, Syms C, Thorrold SR. 2008. Coral reef fish smell leaves to find island homes. Proc. R. Soc. B 275, 2831–2839. ( 10.1098/rspb.2008.0876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paris CB, Atema J, Irisson J-O, Kingsford M, Gerlach G, Guigand CM. 2013. Reef odor: a wake up call for navigation in reef fish larvae. PLoS ONE 8, e72808 ( 10.1371/journal.pone.0072808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mouritsen H, Atema J, Kingsford MJ, Gerlach G. 2013. Sun compass orientation helps coral reef fish larvae return to their natal reef. PLoS ONE 8, e66039 ( 10.1371/journal.pone.0066039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radford CA, Tindle CT, Montgomery JC, Jeffs AG. 2011. Modelling a reef as an extended sound source increases the predicted range at which reef noise may be heard by fish larvae. Mar. Ecol. Prog. Ser. 438, 157–174. ( 10.3354/meps09312) [DOI] [Google Scholar]
- 60.Armsworth PR. 2000. Modelling the swimming response of late stage larval reef fish to different stimuli. Mar. Ecol. Prog. Ser. 195, 231–247. ( 10.3354/meps195231) [DOI] [Google Scholar]
- 61.Simpson SD, Piercy JJB, King J, Codling EA. 2013. Modelling larval dispersal and behaviour of coral reef fishes. Ecol. Complex. 16, 68–76. ( 10.1016/j.ecocom.2013.08.001) [DOI] [Google Scholar]
- 62.Humston R, Ault JS, Lutcavage M, Olson DB. 2000. Schooling and migration of large pelagic fishes relative to environmental cues. Fish. Oceanogr. 9, 136–146. ( 10.1046/j.1365-2419.2000.00132.x) [DOI] [Google Scholar]
- 63.Humston R, Olson DB, Ault JS. 2004. Behavioural assumptions in models of fish movement and their influence on population dynamics. Trans. Am. Fish. Soc. 133, 1304–1328. ( 10.1577/T03-040.1) [DOI] [Google Scholar]
- 64.Leis JM. 2007. Behaviour as input for modelling dispersal of fish larvae: behaviour, biogeography, hydrodynamics, ontogeny, physiology and phylogeny meet hydrography. Mar. Ecol. Prog. Ser. 347, 185–193. ( 10.3354/meps06977) [DOI] [Google Scholar]
- 65.Staaterman E, Paris CB, Helgers J. 2012. Orientation behavior in fish larvae: a missing piece to Hjort's critical period hypothesis. J. Theor. Biol. 304, 188–196. ( 10.1016/j.jtbi.2012.03.016) [DOI] [PubMed] [Google Scholar]
- 66.Gardiner JM, Atema J. 2007. Sharks need the lateral line to locate odor sources: rheotaxis and eddy chemotaxis. J. Exp. Biol. 210, 1925–1934. ( 10.1242/jeb.000075) [DOI] [PubMed] [Google Scholar]
- 67.Booth DJ, Kingsford MJ, Doherty PJ, Berretta GA. 2000. Recruitment of damselfishes in One Tree Island lagoon: persistent interannual spatial patterns. Mar. Ecol. Prog. Ser. 202, 212–230. ( 10.3354/meps202219) [DOI] [Google Scholar]
- 68.Spagnol S, Wolanski E, Deleersnijder E, Brinkman R, McAllister F, Cushman-Roisin F, Hanert E. 2002. An error frequently made in the evaluation of advective transport in two-dimensional Lagrangian models of advection–diffusion in coral reef waters. Mar. Ecol. Prog. Ser. 235, 299–302. ( 10.3354/meps235299) [DOI] [Google Scholar]
- 69.Okubo A. 1974. Some speculations on oceanic diffusion diagrams. Rapport des Procès-Verbaux du Conseil International de l'Exploration de la Mer 167, 77–85. [Google Scholar]
- 70.Wolanski E, Hammer WM. 1988. Topographically controlled fronts in the ocean and their biological influence. Science 241, 177–181. ( 10.1126/science.241.4862.177) [DOI] [PubMed] [Google Scholar]
- 71.Kingsford MJ, Wolanski E, Choat JH. 1991. Influence of tidally induced fronts and Langmuir circulations on the distribution and movements of presettlement fish around a coral reef. Mar. Biol. 109, 167–180. ( 10.1007/BF01320244) [DOI] [Google Scholar]
- 72.Moore P, Crimaldi J. 2004. Odor landscapes and animal behavior: tracking odor plumes in different physical worlds. J. Mar. Syst. 49, 55–64. ( 10.1016/j.jmarsys.2003.05.005) [DOI] [Google Scholar]
- 73.Stobutzki I, Bellwood DR. 1997. Sustained swimming abilities of the late pelagic stages of coral reef fishes. Mar. Ecol. Prog. Ser. 149, 35–41. ( 10.3354/meps149035) [DOI] [Google Scholar]
- 74.Pec kMA, Huebert KB, Llopis KL. 2012. Intrinsic and extrinsic factors driving match–mismatch dynamics during the early life history of marine fishes. Adv. Ecol. Res. 47, 177–302. [Google Scholar]
- 75.Andutta F, Ridd PV, Wolanski E. 2013. The age and the flushing time of the Great Barrier Reef waters. Continental Shelf Res. 53, 11–19. ( 10.1016/j.csr.2012.11.016) [DOI] [Google Scholar]
- 76.Connolly SR, Baird A. 2010. Estimating dispersal potential for marine larvae: dynamic models applied to scleractinian corals. Ecology 91, 3572–3583. ( 10.1890/10-0143.1) [DOI] [PubMed] [Google Scholar]
- 77.Kingsford MJ, Choat JH. 1989. Horizontal distribution patterns of presettlement reef fish: are they influenced by the proximity of reefs? Mar. Biol. 100, 285–297. ( 10.1007/BF00428124) [DOI] [Google Scholar]
- 78.Brothers EB, McWilliams DMcB, Sale PF. 1983. Length of larval life in twelve families of fishes at ‘One Tree Lagoon’, Great Barrier Reef, Australia. Mar. Biol. 76, 319–324. ( 10.1007/BF00393035) [DOI] [Google Scholar]
- 79.Harrison CS, Siegel DA, Mitarai S. 2013. Filamentation and eddy-eddy interactions in marine larval accumulation and transport. Mar. Ecol. Prog. Ser. 472, 27–44. ( 10.3354/meps10061) [DOI] [Google Scholar]
- 80.Bode NWF, Franks DW, Wood AJ, Piercy JJB, Croft DP, Codling EA. 2012. Distinguishing social from non-social navigation in moving animal groups. Am. Nat. 179, 621–632. ( 10.1086/665005) [DOI] [PubMed] [Google Scholar]
- 81.Sale PF, Douglas WA, Doherty PJ. 1984. Choice of microhabitats by coral-reef fishes at settlement. Coral Reefs 3, 91–99. ( 10.1007/BF00263759) [DOI] [Google Scholar]
- 82.Wen C, Pratchett MS, Almany GR, Jones GP. 2013. Patterns of recruitment and microhabitat associations for three predatory coral reef fishes on the southern Great Barrier Reef, Australia. Coral Reefs 32, 389–398. ( 10.1007/s00338-012-0985-x) [DOI] [Google Scholar]
- 83.Fisher R, Bellwood DR. 2002. A light trap design for stratum-specific sampling of reef fish larvae. J. Exp. Mar. Biol. Ecol. 269, 27–37. ( 10.1016/S0022-0981(01)00384-7) [DOI] [Google Scholar]