Abstract
Araneomorph spiders have evolved different silks with dissimilar material properties, serving different purposes. The two-compound pyriform secretion is used to glue silk threads to substrates or to other threads. It is applied in distinct patterns, called attachment discs. Although ubiquitously found in spider silk applications and hypothesized to be strong and versatile at low material consumption, the performance of attachment discs on different substrates remains unknown. Here, we analyse the detachment forces and fracture mechanics of the attachment discs spun by five different species on three different substrates, by pulling on the upstream part of the attached thread. Results show that although the adhesion of the pyriform glue is heavily affected by the substrate, even on Teflon it is frequently strong enough to hold the spider's weight. As plant surfaces are often difficult to wet, they are hypothesized to be the major driving force for evolution of the pyriform secretion.
Keywords: silk, adhesion, tensile test, compound material, hierarchical structure, Araneae
1. Introduction
Natural silks are biofibres that fascinate through their outstanding material properties and versatility. Araneomorph spiders have evolved different kinds of silks with altered material properties serving different purposes—they are equipped with a ‘silken toolkit’ [1–4]. The pyriform gland secretion is used to glue silk threads to substrates [2,5,6] or with each other [7,8]. It is applied in a distinct pattern, called attachment discs, and was formerly hypothesized to be a two-compound material including spidroins (silk proteins) as the first phase and an amorphous hydrocarbon-rich cement-like substance as the second phase [9,10]. Attachment discs are fundamental for locomotion, prey capture and reproduction: they secure climbing spiders in the case of a fall or enable them to reach distant places by descending [2,11], provide robust anchorage for webs [5,12,13], retreats and egg sacs [14], stabilize jumping [15] and on-water movements [16] and facilitate navigation [7] and brood care [8]. Through an adapted spinning behaviour, they can even be used to produce special traps such as trapdoors, stumbling threads [17] and catapulting lines [13]. As there is such a broad and frequent use of attachment discs in a spider's life, they must provide a strong attachment capability on various surfaces, with a broad bandwidth of surface chemistry and topography (universality) at the minimum amount of the secretion applied (economy). This also makes them very interesting for potential biomimetic applications. Initial attempts have been made to use this principle for technical applications, including electro spun artificial attachment discs [18] and ‘dragline attachment’ in robots [19]. Nonetheless, the pyriform secretion and its use in the generation of attachment discs are remarkably scarcely studied.
The morphology of attachment discs spun by different spiders including representatives of Araneidae, Linyphiidae, Filistatidae, Salticidae and Pholcidae was studied by Schütt [5]. She found differences in the architecture depending on phylogeny and hunting style of the spider. Basically, the structure includes a bilateral symmetric network of pyriform threads firmly attached to the ground and bundled in a central part, where it forms an envelope around the dragline, which is composed of paired (or more) major ampullate silk threads. The close location of the major ampullate spigot to the numerous pyriform spigots on the anterior lateral spinnerets provides an easy and fast way to spin such rather complex structure [20]. Ultrastructural analyses of the pyriform glands showed that two distinct types of secretory cells are involved, which produce at least two different substances and which are extruded by the same spigot [9,21]. It was proposed that the pyriform spidroins (silk proteins) produced by these glands self-assemble into fibres in a liquid environment [10]. This could be shown for biotechnologically produced pyriform spidroins [22]. Evaporation of a solvent, like water, may lead to quick solidifying of the matrix, cementing the threads to the substrate and to each other.
The first biomechanical study on attachment discs was recently published by Sahni et al. [13]. They reported that the attachment discs adhere very strongly to smooth surfaces like glass. However, during their measurements, they did not directly pull on the dragline, but on thin underlying nylon threads, which does not reflect the natural way the structure performs. They explained the function of the attachment disc by a geometrical staple-pin model with parallel peeling membranes [13]. Pugno et al. [12] proposed an alternative model, which is based on the simultaneous peeling of multiple membranes. Their theoretical study, however, is not based on experimental data. Despite this, nothing is known about the function of attachment discs, especially how strong they adhere to natural substrates, for which these secretion products have been presumably optimized. It is likely that the discs behave very differently on different substrates, because the strength of a glue-based attachment highly depends on surface properties, such as surface free energy and microtopography [23]. Nevertheless, in models on spider web biomechanics [24–31] and dragline security during falling-spider events [11,32–34], thread anchoring has always been presumed to be robust enough to keep the spider body weight and therefore neglected for biomechanical models. Furthermore, spider species of different ecology and size may have different demands on thread anchorage or their discs may have different phylogenetic constraints. In order to understand the functional morphology of the disc, and to evaluate, whether its structure is universal or shows specializations, it is important to structurally and experimentally compare discs of different species.
The aim of this study is thus to compare the disc attachment performance on (i) different substrates and (ii) between different spider species with different ecology. We used three different substrates: (1) glass, which is hydrophilic and provides a high surface free energy (this control surface was also used in previous experiments [13]), (2) Teflon, which has a very low free surface energy and is thus highly anti-adhesive, and (3) upper surface of leaves of sycamore maple (Acer pseudoplatanus) as a wide spread generic plant surface. We used the large orb web spiders Argiope trifasciata (Araneae) and Nephila senegalensis (Nephilidae) and the cobweb weaver Parasteatoda tepidariorum (Theridiidae), which was used in the previous study by Sahni et al. [13], the large wandering spider Cupiennius salei (Ctenidae) and the flower-associated ambush predator Thomisus onustus (Thomisidae). We performed tensile tests, in which we measured forces resisting pulling onto the dragline. To evaluate which part of the disc structure is in direct contact to the substrate and estimate where the forces are exactly applied and energy dissipated within the structure, we studied attachment discs using transmission (TLM) and coaxial light microscopy (CLM) and scanning electron microscopy (SEM), and analysed structure deformation and fracture behaviour in pull-off tests combined with video recording.
2. Material and methods
2.1. Animals and harvesting of attachment discs
Adult females of the species A. trifasciata Forsskål 1775 (Araneidae), N. senegalensis Walckenaer 1842 (Nephilidae) and C. salei Keyserling 1877 (Ctenidae) were obtained from laboratory stocks, P. tepidariorum C. L. Koch 1841 (Theridiidae) was collected in the greenhouses of the botanical garden of Kiel University and T. onustus Walckenaer 1805 (Thomisidae) was collected during a field trip to Sardinia (Italy) in May 2013. Spiders were kept at 23–26°C and sprinkled daily with water. The ground substrate (turf-sand) was constantly kept humid. Spiders were weekly fed. Argiope trifasciata and N. senegalensis were kept in terraria (40 × 40 × 40 cm) equipped with wooden frames and fed with flies (Musca domestica) and juvenile grasshoppers (Locusta migratoria). Cupiennius salei were kept in cylindrical glasses (diameter: 10 cm, height: 25 cm) and fed with house crickets (Acheta domestica). Parasteatoda tepidariorum and T. onustus were kept in plastic tubes (diameter: 5 cm, height: 10 cm) and fed with flies (Drosophila melanogaster and M. domestica) and cricket larvae (A. domestica). Spiders were weighted on an AG 204 Delta Range scale (Mettler Toledo GmbH, Greifensee, Switzerland) one day after feeding.
Attachment discs were collected directly before experiments. For this purpose, three different substrates were used: glass (microscopy glass slide), the upper surface of sycamore (A. pseudoplatanus) leaves and Teflon (PTFE) foil. Tree leafs and Teflon foils were cut to the size of 76 × 26 mm and mounted on microscopy glass slides. To harvest attachment discs, spiders were stimulated to walk and climb on the prepared substrate, which was held by tweezers. Spiders usually spun attachment discs instantly, when brought in an upside-down position. In the large C. salei, the substrate was carefully put under the opisthosoma of the resting spider and the existing dragline was cut with a fine pair of scissors, which usually triggered the spider to renew the attachment. After the spider has spun an attachment disc and a sufficient length of the dragline, the dragline was grasped with a pair of tweezers and cut 3 cm above the attachment disc with a fine pair of scissors.
2.2. Microscopy
The morphology of attachment discs spun on glass was investigated with the means of a stereo microscope (Leica M205 A, Leica Microsystems GmbH, Wetzlar, Germany) equipped with a camera (Leica DFC420), using a reflected and a coaxial light source. Using coaxial light, the area of direct contact was made visible as a dark contrast. Contact area was measured from the obtained still images with ImageJ 1.47v (Wayne Rasband, National Institutes of Health, USA).
For SEM, small pieces of the substrates including attachment discs were cut out and mounted on stubs in the fresh condition. Teflon and glass samples were sputter coated with 10 nm Au-Pd and viewed at 3.0 kV in a Hitachi S 4800 scanning electron microscope (Hitachi Ltd, Tokio, Japan). Leaf samples were immediately shock frozen in liquid nitrogen using Gatan ALTO-2500 cryo system (Gatan Inc., Abingdon, UK). Samples were then sputter coated in a frozen state at −140°C with 10 nm Au-Pd and viewed at 3.0 kV and −120°C in the Hitachi S 4800 scanning electron microscope.
2.3. Tensile tests
The upstream dragline (the one spun last and leading to the animal) of freshly harvested attachment discs was glued to the cantilever of a load cell force transducer (FORT-10 with 10 g force range, World Precision Instruments Inc., Sarasota, FL, USA) with a molten droplet of beeswax at a length of about 20 mm. The force transducer could be moved vertically by a micromanipulator (DC3001R with controller MS314, World Precision Instruments Inc.) at a speed of 0.2 mm s−1. The signal of the force transducer was amplified and processed by a Biopac MP-100 acquisition system (Biopac Systems Ltd, Goleta, CA, USA). Force curves were recorded using the AcqKnowledge 3.7.0 software (Biopac Systems Ltd). Tension tests were simultaneously filmed using a Firefly pro GT 800 camera (Firefly Global, Belmont, CA, USA).
Pull-off forces were taken as the highest forces occurring during attachment disc pulling. Attachment strength was calculated as the quotient of pull-off force and the mean contact area of attachment discs by the species measured previously. Safety factor was calculated as the quotient of pull-off force and the weight of the spider. Data were statistically analysed using Kruskal–Wallis rank sum test with α = 0.05 and Mann–Whitney U-test with FDR alpha adjusting for pairwise comparison in SigmaStat 12.5 (Systat Software GmbH, Erkrath, Germany). We compared the attachment strength on the three different substrates for each species separately, with pooled individual data. To exclude an effect of individuals on the analysis, we previously performed a two-way ANOVA (F1 = individual, F2 = substrate) on the pull-off force data of each species in R (R Core Development team, http://www.r-project.org/; electronic supplementary material, S2). We found that the substrate type has a much more significant effect on the detachment force than the individual in the large species A. trifasciata, N. senegalensis and C. salei. We conclude that the bias by individuals is neglectable and maintain the procedure of pooling data from individuals for the Kruskal–Wallis tests. However, in both T. onustus and P. tepidariorum, the differences between pull-off forces from glass and leaf substrates are not significant in single individuals. This result was taken into account in the following analysis. Species were compared with pooled substrate data.
3. Results
3.1. Architecture of attachment discs
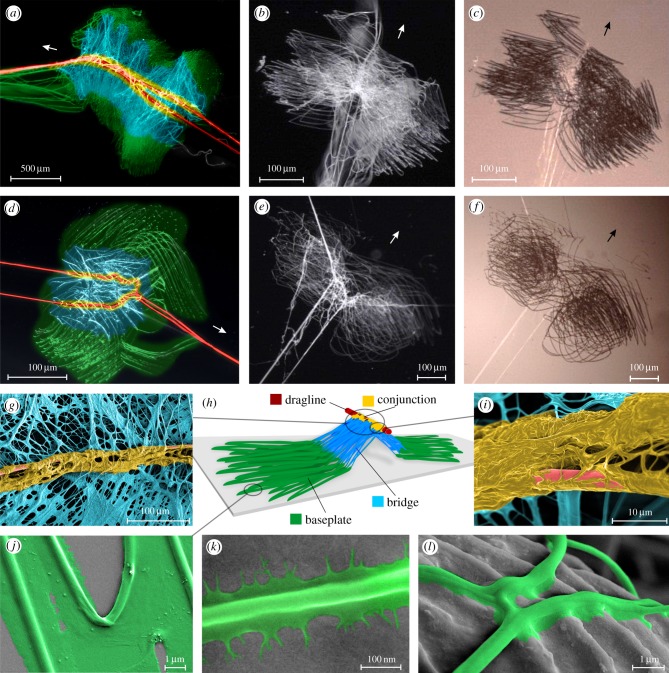
Attachment discs of the analysed spider species basically consist of a network of pyriform glue-coated fibres, which cement the dragline in the central part of the disc and are attached to the substrate in the lateral parts of the disc (figure 1; electronic supplementary material, S1a,f). The dragline cementation is present as thick bundles of pyriform fibres and glue forming an envelope around the double stranded dragline (figure 1g,i; electronic supplementary material, S1a). We term this part the conjunction. It is not in direct contact with the substrate, which can easily recognized by comparing reflection and coaxial light micrographs (figure 1b,c,e,f). Substrate cementation consists of numerous parallel and crossing loops of pyriform glue-coated fibres (electronic supplementary material, S1b) that are denser in the central part of the disc and more separated in the lateral parts (electronic supplementary material, S1f). We term this part of substrate anchorage the baseplate.
Figure 1.
Morphology of spider attachment discs. Panels (a–f) show light microscopy images of attachment discs spun by different spider species on glass slides (arrows indicate the direction of spinning, upstream): (a) A. trifasciata, (b,c) P. tepidariorum and (d–f) T. onustus. In panels (a,b,d,e), reflected light was used. In panels (c,f), same specimens were used as in panels (b,e), respectively, are imaged using coaxial light, which displays the contact area appearing dark. Panels (a,d) are coloured to depict different functional regions within the attachment disc, as schematically illustrated in panel (h). In the central part, the pyriform secretions form an envelope around the dragline (red), which we term the conjunction (yellow). A network of pyriform glue-coated fibres spreads to both lateral parts towards the substrate, which we call the bridge (blue). The direct substrate anchorage is called the baseplate (green). The coloured SEM micrographs shown in panels (g,i–l) reveal fine structural details of the silken disc structure. Panels (g,i) show details of the central part, with the dragline, the conjunction and the bridge. Panels (j–l) show single pyriform glue-coated fibres of the baseplate, whereas in panel (j) those are attached to glass, in panel (k) to Teflon and in panel (l) to the upper surface of an A. pseudoplatanus leaf.
The glue of pyriform glands wets the three tested substrates differently (figure 1j–l; electronic supplementary material, S1c,d). On glass it spreads widely (figure 1j), whereas Teflon has a repellent effect (figure 1k). On the surface of the sycamore, leaf wetting is hampered by cuticular micro ripples (figure 1l; electronic supplementary material, S1d) and (more obvious on the underside of the leaf) crystalline waxes (electronic supplementary material, S1c). Between the conjunction and the baseplate, a meshwork of pyriform glue-coated fibres is spread, which branches towards the substrate (figure 1g; electronic supplementary material, S1a,f). We call this part the bridge.
Comparison of attachment discs of different species shows that the disc morphology is relatively similar. However, in T. onustus the loops of the baseplate are more round, resulting in steeper angles of pyriform fibre crossings and a more radial shape of the attachment disc (figure 1d–f, compare with a–c). The upstream part (last spun, in the direction to the animal) often exhibits a larger width than the downstream part (most apparent in T. onustus and C. salei). The contact area of the attachment disc scales with species weight, ranging from 40 µm² in P. tepidariorum to 1400 µm² in C. salei and N. senegalensis (table 1).
Table 1.
Body mass (m) and contact area (A) of attachment discs of different spider species used in experiments. Mean values are given ± s.d. N, number of spiders; n, number of measurements.
| species | m (g) | A (×1000 µm²) |
|---|---|---|
| A. trifasciata (N = 6) | 0.59 ± 0.15 | 0.98 ± 0.13 (n = 10) |
| N. kenianensis (N = 3) | 0.75 ± 0.11 | 1.35 ± 0.29 (n = 11) |
| C. salei (N = 4) | 2.48 ± 0.82 | 1.41 ± 0.33 (n = 20) |
| T. onustus (N = 2) | 0.09 ± 0.02 | 0.09 ± 0.01 (n = 10) |
| P. tepidariorum (N = 4) | 0.03 ± 0.01 | 0.04 ± 0.01 (n = 11) |
3.2. Fracture mechanics
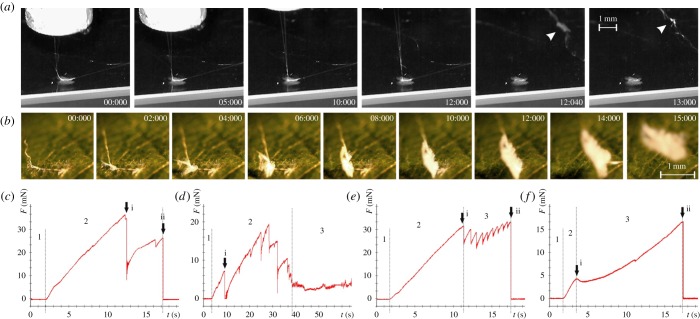
During tensile tests, four different failure modes were observed: (i) dragline-mode, (ii) conjunction-mode, (iii) bridge-mode, and (iv) baseplate-mode. The dragline-mode is due to dragline breakage above or directly at the attachment disc. Force–time curves in those cases often show two peaks, as the dragline usually consists of a double strand (figure 2c). The conjunction failure occurred at the interface between the dragline and pyriform glue. In these cases, rupture could not usually be seen in the video, but was followed by a sliding of the dragline through the conjunction envelope, which caused considerable friction forces (figure 2d). When the bridge failed, the conjunction was totally removed from the attachment disc (figure 2a; electronic supplementary material, S1e). According to the meshwork character of the bridge, its rupture often caused multiple force peaks (figure 2e). When the failure occurred at the interface between the pyriform glue and the substrate (baseplate-mode), a total peel-off (delamination) of the attachment disc from the substrate was observed (figure 2b). In those cases, the time–force curve usually did not show force peaks between the crack initiation event and completed peel-off, just a smooth slope (figure 2f).
Figure 2.
Results of tensile tests of spider attachment discs pulled at the upstream dragline. Panels (a,b) show two video sequences recorded during individual tensile tests showing different failure modes. In panel (a), the tested attachment disc was spun on a smooth glass slide and failed at the bridge. Arrowheads point to the conjunction and bridge remnants on the dragline, broken off the attachment disc. In panel (b), the tested attachment disc was spun on the upper surface of an A. pseudoplatanus leaf and failure occurred at the baseplate–substrate interface, leading to a total peel-off of the disc structure from the substrate. Panels (c–f) show exemplary time–force curves recorded in the tensile tests, with different parts of the attachment disc failing. Arrows indicate the first crack (i) and final rupture (ii). In panel (c), dragline fails. After thread is tightened (1), it is strained (2), leading to a linear increase in force, until the material breaks. Two force peaks are typical, as the dragline is constituted of a double strand. If the conjunction fails (d), several rupture events are followed by sliding friction (3), caused by the dragline sliding through the envelope. Bridge failure (e) caused one or multiple similar force peaks (3), depending on the length of the attachment disc. The baseplate failure (total detachment) event included time–force curves with multiple peaks similar to those in (e), but with a higher variation in peak size, or a flattening of the slope after initial crack induction, similar to that in panel (f). (Online version in colour.)
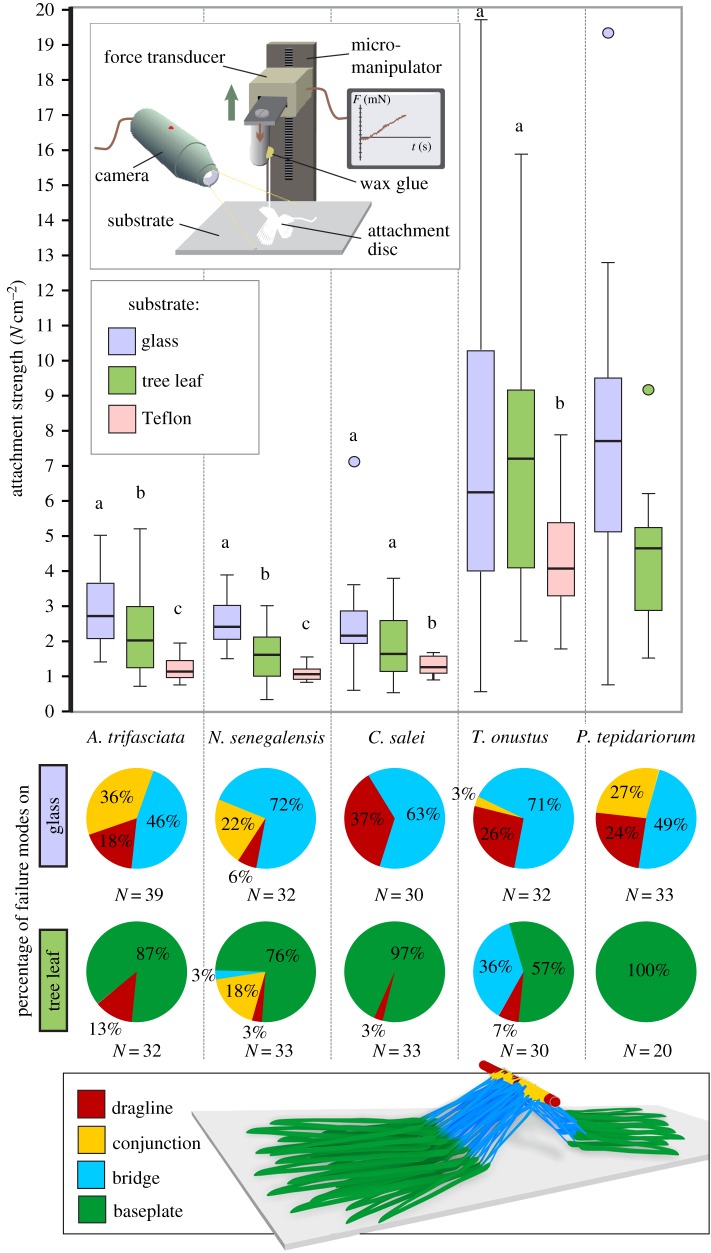
In which part the attachment disc breaks, heavily depends on its attachment strength to the substrate (figure 3). On glass, where the highest pull-off forces were recorded, the bridge failed in the majority of cases, from about half in A. trifasciata to three-fourths in N. senegalensis and C. salei. The second most frequent failure occurred at the conjunction in A. trifasciata, N. senegalensis and P. tepidariorum, as well as at the dragline in C. salei and T. onustus. On Teflon, where significantly lower pull-off forces were recorded than on glass, there was always a total delamination of the attachment disc from the underlying substrate. On the sycamore leaf, the majority of attachment discs also showed total delamination, however, in N. senegalensis, the conjunction failed in one-fifth of the cases, and in T. onustus, the bridge failed in over one-third of the cases (figure 3).
Figure 3.
Attachment strength and failure analysis. The attachment strength was calculated as the quotient of the maximal force during the tensile test and the mean contact area of attachment discs typically spun by the corresponding species. Boxplots illustrate the median value, interquartile range and the extreme values, outliers are indicated by circles. Letters above indicate significant differences between the substrates (within species). Inset shows set-up of the tensile tests. Pie diagrams below illustrate the percentage of failure modes on glass (upper row) and the upper surface of an A. pseudoplatanus leaf (lower row). Which part failed first was analysed from combined evaluations of video recordings and time–force curves. Failure modes on Teflon are not displayed, because there was only a total peel-off detachment of discs in all cases.
3.3. Attachment strength and safety factor
Pull-off forces differed significantly between substrates in all spider species studied, except P. tepidariorum (Kruskal–Wallis rank sum test with p < 0.05, d.f. = 2) (figure 3 and table 2). Pull-off forces of attachment discs spun on glass reached mean values of 36 mN in N. senegalensis, 35 mN in C. salei, 28 mN in A. trifascata, 7 mN in T. onustus and 3 mN in P. tepidariorum. Pull-off forces on the sycamore leaf were significantly lower (about one-third), except in T. onustus. Lowest forces were obtained on Teflon (always significantly different to both other substrates), with mean values about 5 mN in A. trifasciata and N. senegalensis, 8 mN in C. salei and 3 mN in T. onustus, which is only one-sixth to one-seventh of the force measured on glass in the first three species, and one-half in T. onustus (attachment discs of P. tepidariorum were not tested on Teflon). Pull-off forces scale with body mass with the power law of two-thirds, if individuals of all species are taken into account (figure 4). This indicates that the forces are dependent on the contact area of the attachment disc. Thus, the attachment strength was calculated (pull-off force divided by average contact area measured for each species), to compare the performance of attachments discs spun by different species. The attachment strength did not differ significantly between A. trifasciata, N. senegalensis and C. salei, but was about three times higher in the smaller T. onustus and P. tepidariorum, with mean values ranging from 3.4 to 7.8 N cm−2 depending on the substrate (figure 3).
Table 2.
Pull-off force (F), attachment strength (τ) and safety factor (sf) of attachment discs measured in tensile tests. Mean values are given ± s.d. n, number of measurements.
| species | substrate | F (mN) | τ (N cm−²) | sf | n |
|---|---|---|---|---|---|
| A. trifasciata | glass | 28.3 ± 9.1 | 2.8 ± 0.9 | 4.3 ± 1.5 | 54 |
| tree leaf | 23.2 ± 10.8 | 2.2 ± 1.1 | 3.3 ± 1.5 | 32 | |
| Teflon | 5.0 ± 3.1 | 0.4 ± 0.3 | 0.8 ± 0.5 | 31 | |
| N. senegalensis | glass | 35.7 ± 8.9 | 2.5 ± 0.7 | 4.6 ± 1.2 | 32 |
| tree leaf | 22.3 ± 9.5 | 1.7 ± 0.7 | 2.9 ± 1.2 | 33 | |
| Teflon | 5.2 ± 2.9 | 0.3 ± 0.2 | 0.7 ± 0.4 | 21 | |
| C. salei | glass | 35.1 ± 16.5 | 2.2 ± 1.2 | 1.4 ± 0.6 | 30 |
| tree leaf | 26.0 ± 11.8 | 1.7 ± 0.8 | 1.0 ± 0.4 | 33 | |
| Teflon | 8.0 ± 3.6 | 0.5 ± 0.3 | 0.4 ± 0.2 | 15 | |
| T. onustus | glass | 6.7 ± 3.9 | 6.3 ± 4.3 | 7.5 ± 3.4 | 32 |
| tree leaf | 6.5 ± 3.2 | 7.3 ± 3.5 | 7.1 ± 2.8 | 30 | |
| Teflon | 3.3 ± 1.4 | 3.4 ± 1.6 | 3.6 ± 1.6 | 20 | |
| P. tepidariorum | glass | 2.9 ± 1.4 | 7.8 ± 3.6 | 13.0 ± 4.5 | 33 |
| tree leaf | 1.8 ± 0.8 | 4.7 ± 2.0 | 8.4 ± 7.6 | 20 |
Figure 4.
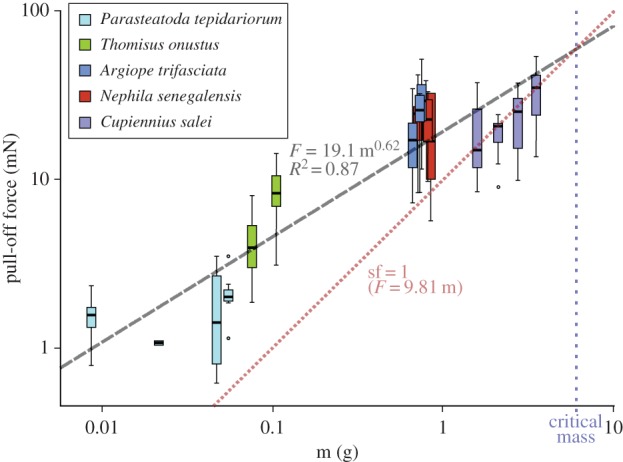
Scaling effect of body mass on the attachment disc pull-off force. The pull-off force of attachment discs spun on A. pseudoplatanus leaf surface scales with body mass by the power law of 2/3. This indicates that forces are dependent on contact area. As the surface area increases by a power lower than the power of the volume, the safety factor (detachment force divided by the weight force) decreases with increasing spider size. The critical mass indicates the limit of spider mass, at which the mean safety factor falls below 1, thus getting insufficient to hold the animal. Indeed, araneomorph spiders rarely exceed this size. Boxplots are defined as in figure 3.
Safety factor (pull-off forces divided the weight of the spider individual) did not differ between the arboreal web-builder N. senegalensis and the low vegetation dwelling web-builder A. trifasciata (4 on glass, 3 on tree leaf and 0.8 on Teflon). In the large arboreal hunting spider C. salei, the safety factor of the attachment disc is significantly lower (about 1 on glass and tree leaf and 0.4 on Teflon). In small species, T. onustus and P. tepidariorum, the safety factor is significantly higher than in the other species. On glass, P. tepidariorum reached a higher safety factor (sf = 13) than T. onustus (sf = 8). On the tree leaf, both species did not differ, with means about 8 in P. tepidariorum and 7 in T. onustus. On Teflon, T. onustus had a reduction of the safety factor only about one-half, if compared with glass. The safety factor decreases with increasing body mass of the spider, if individuals of all species are taken into account (figure 4).
4. Discussion
Attachment discs represent an important part of the relationship of araneomorph spiders to their environment. Our data show that the discs provide strong attachment to different substrates due to their specific hierarchical structure. Sahni et al. [13] recently described two different architectures of attachment discs, spun by the cobweb spider P. tepidariorum for different purposes. The usual ‘staple-pin’ architecture is used in the so-called scaffolding disc, which is for firm cementing of draglines to substrates. This one is comparable to the dragline anchors of other spiders [5,13]. The other type with the dendritic architecture is used to attach so-called gumfoot threads. These ‘gumfoot discs’ are only loosely attached to the substrate and can be pulled off by much lower forces. They are connected to preloaded threads and easily detach, if walking prey touches the thread, causing the prey to catapult into the web. In contrast to the experimental data provided by Sahni et al. [13], the multiple peeling theory by Pugno et al. [12] predicts that such dendritic architecture must be the optimal one for strong attachment at low material cost. The latter paper is based on the so-called theory of multiple peeling suggesting that a simultaneous detachment of opposing adhesive tapes leads to much higher adhesion than the sum of detachment forces needed to peel-off each tape separately [35], a model that is highly applicable to biological locomotory attachment systems, such as gecko feet or arthropod adhesive foot pads [36]. The previous models of the ‘staple-pin’ architecture [12,13] presume that initially the dragline is directly cemented to the substrate by means of the pyriform threads. However, our results show that the central part of the discs, where the dragline is attached, is rarely in contact with the substrate. This might be fundamental for the observed strong performance of the attachment disc: the separation of the dragline attachment and substrate attachment provides high flexibility of the disc structure and should support the force distribution within.
Further, we found that the architecture of an attachment disc follows a hierarchy from the dragline glued with dense bundles of pyriform fibres (conjunction) which spread and get separated towards the substrate, where they are separately cemented, distributed over a large area. This architecture might have several advantages: (i) A large contact area can be generated at low material cost, (ii) forces may be distributed over a high number of branches and therefore the stress generated in each single structure is kept low, (iii) propagating cracks may be arrested because of the inhomogeneous material distribution, and (iv) peeling angles are kept low, which means that higher forces are necessary to detach the cementation [12,35].
Which part of the attachment disc fails under load highly depends on the adhesion energy between the cement and the substrate. Our results on glass show that the dragline is usually stronger than the pyriform silk network, in contrast to the observation by Sahni et al. [13]. This makes sense because a failure within the disc is usually not accompanied by a total failure of spider attachment. For example, in the case of the conjunction failure, the dragline was further continuously pulled through the pyriform envelope, generating high friction. A bridge failure occurred stepwise in most cases. Both events significantly delay the total structural failure. Previous studies calculated the safety factor of draglines in spider fall scenarios, always assuming that failure occurs at the level of the dragline [11,32–34]. This also holds for various models of web biomechanics, in which thread anchors were presumed as stable [24–31]. Although these studies show that a high amount of energy can be absorbed by thread deformation, significant forces may reach the substrate anchors, especially in short threads. Our findings show that thread anchors can fail before the thread fails and should thus be considered in further estimations of the mechanical stability of spider webs. Additionally, non-uniform crack propagation due to non-continuous distribution of the pyriform glue leads to the further energy dissipation during detachment.
Our comparison of spider species with different ecology and phylogenetic background revealed that the high attachment strength is consistent with the relatively conserved structure of the discs. This indicates that web building and wandering spiders, as well as dwellers of the lower and the higher vegetation, might have similar demands on thread anchoring. However, in the crab spider T. onustus, the attachment strength is surprisingly less affected by the substrate.
Scaling effects on the attachment disc strength have very important implications for biology of species studied. Comparing large and small species indicates that safety factor of the disc attachment decreases with the spider's weight, thus making both mechanical anchorage and its function as a protection against falling from the height less efficient. This with spider size increasing loss of safety in climbing situations was previously hypothesized for the dragline [11] and leads to one of potential explanations of the extreme sexual size dimorphism in some spiders [37]. Males are more active in locomotion as they need to wander around, in order to find potential mating partners. In spiders that live on vegetation, males are often much smaller than females, which may be extreme in some orb web spiders and crab spiders, and which was previously interpreted by facilitation of the thread-based locomotion [37]. Interestingly, the high sexual size dimorphism is absent in large wandering spiders (i.e. Ctenidae, Sparassidae), of which the arboreal representatives possess highly efficient adhesive foot pads [36,38–40]. This is also the case for the large wandering spider C. salei, whose attachment discs exhibit only an insufficient safety factor. Future studies, testing the change in attachment disc performance during ontogeneny, may reveal scaling effects in the safety factor of dragline anchors.
For the spider, the most uncontrollable part of the attachment disc performance is the adhesive strength between the pyriform glue and substrate. Our results show that cementation fails on surfaces with a low free surface energy like Teflon. This also counts, to some extent, for plant surfaces. The cuticle of plant leaves, stems and flowers is often covered with cuticular folds and crystalline waxes that create roughness on a micro- and nanoscale. Waxes may easily detach, if glued, which is reported to be an adaptation against herbivorous insects [41–43]. Spiders, although not being plant consumers, are faced with the same problem of adhesion reduction on these surfaces. Thus, they might have had the need to participate in the arms race of plants and herbivores, as they have to forage at the same sites. Whereas the basic architecture of the attachment disc seems to be relatively conserved (i.e. compare discs spun by Haplogynes and Entelegynes in [5]), there might have been strong selective pressure on the wetting and adhesion properties of the pyriform glue. Convergently, insects that glue their eggs onto such waxy surfaces use proteinaceous cement [44], which might be functionally analogous to the pyriform glue. Crab spiders (Thomisidae), whose tested representative T. onustus showed no significant reduction of the attachment strength on the sycamore leaf surface and even on Teflon demonstrated remarkably high values, are often associated with herbal plants, where they capture prey items larger than themselves [45]. In contrast to many free hunting spiders, thomisids lack the hairy adhesive foot pads [39], thus, they may largely rely on their exceptionally strong dragline attachment.
As the surface geometry and properties of natural surfaces may vary in a very wide range, the stability of web attachment points may be influenced by substrates. Comparing the arboreal N. senegalensis with A. trifasciata that occupies lower vegetation, no difference in attachment disc performance could be found, which speaks against adaptation of the pyriform glue to particular microhabitat. This, in turn, means that the evolution of the pyriform glue might have been driven towards high universality. This seems to be plausible, as surfaces may be highly inhomogeneous even within a particular microhabitat. On the other hand, there is certain sensibility of disc attachment to the substrate type. Possible ethological counter adaptations, like adjusting the number and/or size of anchors depending on the substrate or choosing a proper attachment site, remain to be studied in the future.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Prof. Dr Jutta Schneider (University of Hamburg, Germany) and Dr Clemens Schaber (University of Kiel, Germany) for providing experimental animals. Heike Scholz (Botanical Garden Kiel) and her colleagues helped collecting experimental animals in the greenhouses. Phillipp Hofmann and Mike Schindler (University of Applied Sciences, Bocholt, Germany) assisted in handling the living spiders during the experiments. Matthias Foellmer (Adelphi University, NY, USA) and an anonymous reviewer are acknowledged for their constructive criticism of the manuscript.
Funding statement
This work was supported by the German Science Foundation to S.G. (DFG, no. GO 995/10-1 and Project no. C-10 within SFB 677) and by the German National Merit Foundation (Studienstiftung des deutschen Volkes) to J.O.W.
References
- 1.Blackledge TA, Hayashi CY. 2006. Silken toolkits: biomechanics of silk fibers spun by the orb web spider Argiope argentata (Fabricius 1775). J. Exp. Biol. 209, 2452–2461. ( 10.1242/Jeb.02275) [DOI] [PubMed] [Google Scholar]
- 2.Apstein C. 1889. Bau und Funktion der Spinndrüsen der Araneida. Arch. Naturg. 55, 29–74. [Google Scholar]
- 3.Peakall DB. 1964. Composition function and glandular origin of silk fibroins of spider Araneus diadematus Cl. J. Exp. Zool. 156, 345 ( 10.1002/jez.1401560310) [DOI] [PubMed] [Google Scholar]
- 4.Vollrath F. 1992. Spider webs and silks. Sci. Am. 266, 70–76. ( 10.1038/scientificamerican0392-70) [DOI] [Google Scholar]
- 5.Schütt K. 1996. Wie Spinnen ihre Netze befestigen. Mikrokosmos 85, 274–278. [Google Scholar]
- 6.Saffre F, Mailleux AC, Deneubourg JL. 1999. Dragline attachment pattern in the neotropical social spider Anelosimus eximius (Araneae: Theridiidae). J. Insect Behav. 12, 277–282. ( 10.1023/A:1020927102896) [DOI] [Google Scholar]
- 7.Gorb SN, Landolfa MA, Barth FG. 1998. Dragline-associated behaviour of the orb web spider Nephila clavipes (Araneoidea, Tetragnathidae). J. Zool. 244, 323–330. ( 10.1111/j.1469-7998.1998.tb00036.x) [DOI] [Google Scholar]
- 8.Townley MA, Tillinghast EK. 2003. On the use of ampullate gland silks by wolf spiders (Araneae, Lycosidae) for attaching the egg sac to the spinnerets and a proposal for defining nubbins and tartipores. J. Arachnol. 31, 209–245. ( 10.1636/0161-8202(2003)031[0209:Otuoag]2.0.Co;2) [DOI] [Google Scholar]
- 9.Kovoor J, Zylberberg L. 1980. Fine-structural aspects of silk secretion in a spider (Araneus diadematus). 1. Elaboration in the pyriform glands. Tissue Cell 12, 547–556. ( 10.1016/0040-8166(80)90044-0) [DOI] [PubMed] [Google Scholar]
- 10.Blasingame E, et al. 2009. Pyriform spidroin 1, a novel member of the silk gene family that anchors dragline silk fibers in attachment discs of the black widow spider, Latrodectus hesperus. J. Biol. Chem. 284, 29 097–29 108. ( 10.1074/jbc.M109.021378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortlepp C, Gosline JM. 2008. The scaling of safety factor in spider draglines. J. Exp. Biol. 211, 2832–2840. ( 10.1242/jeb.014191) [DOI] [PubMed] [Google Scholar]
- 12.Pugno NM, Cranford SW, Buehler MJ. 2013. Synergetic material and structure optimization yields robust spider web anchorages. Small 9, 2747–2756. ( 10.1002/smll.201201343) [DOI] [PubMed] [Google Scholar]
- 13.Sahni V, Harris J, Blackledge TA, Dhinojwala A. 2012. Cobweb-weaving spiders produce different attachment discs for locomotion and prey capture. Nat. Commun. 3, 1106 ( 10.1038/Ncomms2099) [DOI] [PubMed] [Google Scholar]
- 14.Hajer J, Rehakova D. 2003. Spinning activity of the spider Trogloneta granulum (Araneae, Mysmenidae): web, cocoon, cocoon handling behaviour, draglines and attachment discs. Zoology 106, 223–231. ( 10.1078/0944-2006-00117) [DOI] [PubMed] [Google Scholar]
- 15.Chen YK, Liao C-P, Tsai F-Y, Chi K-J. 2013. More than a safety line: jump-stabilizing silk of salticids. J. R. Soc. Interface 10, 20130572 ( 10.1098/rsif.2013.0572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorb SN, Barth FG. 1994. Locomotor behavior during prey-capture of a fishing spider, Dolomedes plantarius (Araneae, Araneidae): galloping and stopping. J. Arachnol. 22, 89–93. [Google Scholar]
- 17.Stern H, Kullmann E. 1975. Leben am seidenen Faden: die rätselvolle Welt der Spinnen. Gütersloh, Germany: C. Bertelsmann. [Google Scholar]
- 18.Jain D, Sahni V, Dhinojwala A. 2014. Synthetic adhesive attachment discs inspired by spider's pyriform silk architecture. J. Polym. Sci. B Polym. Phys. 52, 553–560. ( 10.1002/polb.23453) [DOI] [Google Scholar]
- 19.Wang L, Culha U, Iida F. 2014. A dragline-forming mobile robot inspired by spiders. Bioinspir. Biomim. 9, 016006 ( 10.1088/1748-3182/9/1/016006) [DOI] [PubMed] [Google Scholar]
- 20.Eberhard WG. 2010. Possible functional significance of spigot placement on the spinnerets of spiders. J. Arachnol. 38, 407–414. ( 10.1636/B09-97.1) [DOI] [Google Scholar]
- 21.Kovoor J, Zylberberg L. 1982. Fine-structural aspects of silk secretion in a spider. 2. Conduction in the pyriform glands. Tissue Cell 14, 519–530. ( 10.1016/0040-8166(82)90044-1) [DOI] [PubMed] [Google Scholar]
- 22.Geurts P, et al. 2010. Synthetic spider silk fibers spun from pyriform spidroin 2, a glue silk protein discovered in orb-weaving spider attachment discs. Biomacromolecules 11, 3495–3503. ( 10.1021/Bm101002w) [DOI] [PubMed] [Google Scholar]
- 23.Kinloch AJ. 1987. Adhesion and adhesives: science and technology. London, UK: Chapman and Hall. [Google Scholar]
- 24.Tarakanova A, Buehler MJ. 2012. The role of capture spiral silk properties in the diversification of orb webs. J. R. Soc. Interface 9, 3240–3248. ( 10.1098/rsif.2012.0473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmer AMT, Blackledge TA, Madin JS, Herberstein ME. 2011. High-performance spider webs: integrating biomechanics, ecology and behaviour. J. R. Soc. Interface 8, 457–471. ( 10.1098/rsif.2010.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko FK, Jovicic J. 2004. Modeling of mechanical properties and structural design of spider web. Biomacromolecules 5, 780–785. ( 10.1021/Bm0345099) [DOI] [PubMed] [Google Scholar]
- 27.Lin LH, Edmonds DT, Vollrath F. 1995. Structural-engineering of an orb-spiders web. Nature 373, 146–148. ( 10.1038/373146a0) [DOI] [Google Scholar]
- 28.Cranford SW, Tarakanova A, Pugno NM, Buehler MJ. 2012. Nonlinear material behaviour of spider silk yields robust webs. Nature 482, U72–U91. ( 10.1038/Nature10739) [DOI] [PubMed] [Google Scholar]
- 29.Sensenig AT, Agnarsson I, Blackledge TA. 2011. Adult spiders use tougher silk: ontogenetic changes in web architecture and silk biomechanics in the orb-weaver spider. J. Zool. 285, 28–38. ( 10.1111/j.1469-7998.2011.00809.x) [DOI] [Google Scholar]
- 30.Sensenig AT, Lorentz KA, Kelly SP, Blackledge TA. 2012. Spider orb webs rely on radial threads to absorb prey kinetic energy. J. R. Soc. Interface 9, 1880–1891. ( 10.1098/rsif.2011.0851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoyanagi Y, Okumura K. 2010. Simple model for the mechanics of spider webs. Phys. Rev. Lett. 104, 038102 ( 10.1103/Physrevlett.104.038102) [DOI] [PubMed] [Google Scholar]
- 32.Brandwood A. 1985. Mechanical-properties and factors of safety of spider drag-lines. J. Exp. Biol. 116, 141–151. [Google Scholar]
- 33.Osaki S. 1996. Spider silk as mechanical lifeline. Nature 384, 419 ( 10.1038/384419a0) [DOI] [Google Scholar]
- 34.Osaki S. 2003. Safety coefficient of the mechanical lifeline of spiders. Polym. J. 35, 261–265. ( 10.1295/polymj.35.261) [DOI] [Google Scholar]
- 35.Pugno NM. 2011. The theory of multiple peeling. Int. J. Fracture 171, 185–193. ( 10.1007/s10704-011-9638-2) [DOI] [Google Scholar]
- 36.Wohlfart E, Wolff JO, Arzt E, Gorb SN. 2014. The whole is more than the sum of all its parts: collective effect of spider attachment organs. J. Exp. Biol. 217, 222–224. ( 10.1242/Jeb.093468) [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Girones MA, Corcobado G, Moya-Larano J. 2010. Silk elasticity as a potential constraint on spider body size. J. Theor. Biol. 266, 430–435. ( 10.1016/j.jtbi.2010.06.031) [DOI] [PubMed] [Google Scholar]
- 38.Homann H. 1957. Haften Spinnen an Einer Wasserhaut. Naturwissenschaften 44, 318–319. ( 10.1007/BF00630926) [DOI] [Google Scholar]
- 39.Wolff JO, Nentwig W, Gorb SN. 2013. The great silk alternative: multiple co-evolution of web loss and sticky hairs in spiders. PLoS ONE 8, e62682 ( 10.1371/journal.pone.0062682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapinski W, Tschapka M. 2013. Habitat use in an assemblage of Central American wandering spiders. J. Arachnol. 41, 151–159. ( 10.1636/P11-88.1) [DOI] [Google Scholar]
- 41.Eigenbrode SD. 2001. Attachment by predatory insects to waxy plant surfaces: mechanisms and ecological implications. Am. Zool. 41, 1435–1436. [Google Scholar]
- 42.Eigenbrode SD. 2004. The effects of plant epicuticular waxy blooms on attachment and effectiveness of predatory insects. Arthropod. Struct. Dev. 33, 91–102. ( 10.1016/j.asd.2003.11.004) [DOI] [PubMed] [Google Scholar]
- 43.Whitney HM, Federle W. 2013. Biomechanics of plant–insect interactions. Curr. Opin. Plant Biol. 16, 105–111. ( 10.1016/j.pbi.2012.11.008) [DOI] [PubMed] [Google Scholar]
- 44.Voigt D, Gorb S. 2010. Egg attachment of the asparagus beetle Crioceris asparagi to the crystalline waxy surface of Asparagus officinalis. Proc. R. Soc. B 277, 895–903. ( 10.1098/rspb.2009.1706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nentwig W, Wissel C. 1986. A comparison of prey lengths among spiders. Oecologia 68, 595–600. ( 10.1007/Bf00378777) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.