Abstract
Animals navigate using a variety of sensory cues, but how each is weighted during different phases of movement (e.g. dispersal, foraging, homing) is controversial. Here, we examine the geomagnetic and olfactory imprinting hypotheses of natal homing with datasets that recorded variation in the migratory routes of sockeye (Oncorhynchus nerka) and pink (Oncorhynchus gorbuscha) salmon returning from the Pacific Ocean to the Fraser River, British Columbia. Drift of the magnetic field (i.e. geomagnetic imprinting) uniquely accounted for 23.2% and 44.0% of the variation in migration routes for sockeye and pink salmon, respectively. Ocean circulation (i.e. olfactory imprinting) predicted 6.1% and 0.1% of the variation in sockeye and pink migration routes, respectively. Sea surface temperature (a variable influencing salmon distribution but not navigation, directly) accounted for 13.0% of the variation in sockeye migration but was unrelated to pink migration. These findings suggest that geomagnetic navigation plays an important role in long-distance homing in salmon and that consideration of navigation mechanisms can aid in the management of migratory fishes by better predicting movement patterns. Finally, given the diversity of animals that use the Earth's magnetic field for navigation, geomagnetic drift may provide a unifying explanation for spatio-temporal variation in the movement patterns of many species.
Keywords: movement ecology, magnetic navigation, geomagnetic secular variation, migration, homing, salmon
1. Introduction
Predicting the movement patterns of animals is central to successfully managing species [1,2]. The integration of physiological information at the organismal-level and large-scale environmental data into models of animal movement is a powerful way to predict spatio-temporal variation in species distributions [3,4]. Largely lacking in these models, however, are mechanisms of spatial orientation and navigation [5–8]. Although efforts to depict animal movement as a random process might appear to circumvent the need for information on navigation [9–11], other work suggests that ignoring navigation mechanisms produces misleading predictions of animal movement and its role in ecological and evolutionary processes [12–15]. Knowledge of navigation mechanisms may be especially important in species that travel long-distances, as slight differences in orientation that result from different navigation strategies can lead to highly divergent movement paths as the area of travel increases [13,16].
However, the sensory basis of animal navigation remains subject to considerable speculation and debate as results often appear contradictory from one study to the next [17]. For instance, a number of laboratory-based experiments clearly demonstrate that diverse animals are capable of using the Earth's magnetic field for navigation, as a map to assess their location and as a compass to maintain a heading [18–31]. By contrast, field-based experiments that deprive animals of magnetic cues often show minor or no effects compared to controls [32–38]. Do animals in the laboratory use magnetic orientation because other (and preferred) environmental cues are not present [39]? Do animals in the field successfully navigate without magnetic information because they possess back-up (and seldom-used) orientation systems for use during the occasional magnetic storm or rare encounter with a crustal magnetic anomaly [12]? Both laboratory and field-based manipulations have limitations and it is challenging to know the importance of magnetic cues to an animal navigating under normal (i.e. entirely unmanipulated) conditions. Here, we make use of a unique dataset in which movement patterns in the homing migration of two species of Pacific salmon were recorded over a period spanning six decades; originally collected for fisheries management, we use these data to examine hypotheses of magnetic and olfactory navigation.
Anadromous Pacific salmon enter the ocean as juveniles where they remain for several months to years, often travelling thousands of kilometres to forage. Upon reaching maturity they return with remarkable precision to the vicinity of their natal site to reproduce [40]. There is growing consensus that the navigational challenge faced by homing salmon is solved in two parts: a large-scale strategy to travel from the open ocean to the river mouth followed by a fine-scale strategy to reach the natal site once in freshwater [40–44]. Experimental evidence strongly suggests that the freshwater phase of the migration is based on olfactory imprinting, whereas the mechanism responsible for the oceanic migration is unknown [40,45].
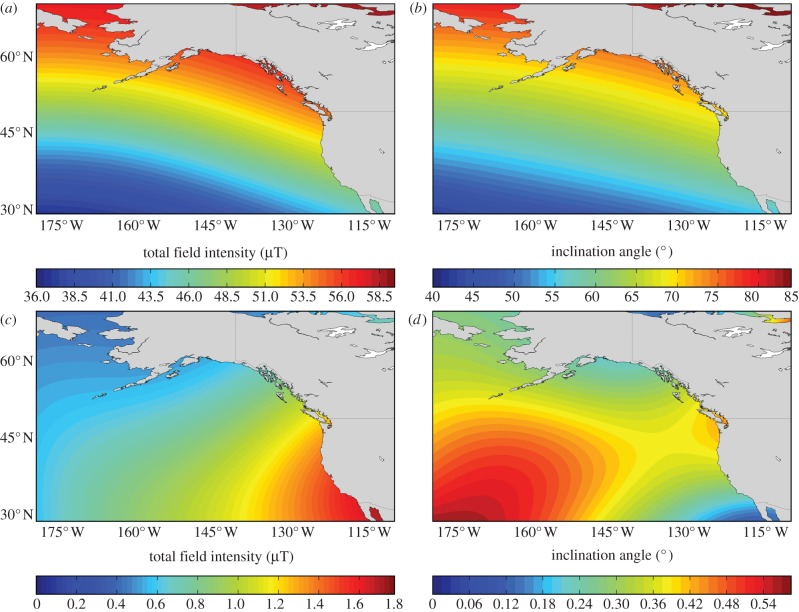
In a recent paper, we presented evidence that the ocean-phase of the homing migration is accomplished by geomagnetic imprinting [46]. Geomagnetic imprinting hypothesizes that marine animals remember the magnetic field values (e.g. total field intensity and/or inclination angle) at the onset of their oceanic migration and, upon reaching maturity, follow the large-scale magnetic gradients (figure 1a,b) to relocate this same magnetic value [42,47]. Simple models of geomagnetic imprinting suggest that this navigation mechanism would consistently return Pacific salmon to the vicinity of the mouth of their home river [42,46,48]; however, gradual drift (secular variation) of the magnetic field (figure 1c,d) might cause long-term spatio-temporal variation in the migratory routes used. In the earlier paper, we tested this prediction using a dataset cataloging the migration routes of sockeye salmon (Oncorhynchus nerka) returning from the North Pacific to spawn in the Fraser River, British Columbia [49,50]. Homing fish are confronted by Vancouver Island, an approximately 400 km long obstacle that must be circumnavigated to the north via Queen Charlotte Strait (QC) or to the south via Juan de Fuca Strait (JdF) (figure 2a). The proportion of sockeye salmon migrating through the northern route (i.e. the ‘diversion rate’) has been monitored since the 1950s to manage the shared fisheries resource between Canada and the USA (salmon travelling through the northern route are only accessible to Canadian fisheries, whereas those taking the southern route are exploited by both Canadian and US fisheries) [49,50]. Consistent with the geomagnetic imprinting hypothesis, the proportion of sockeye salmon (Oncorhynchus nerka) using a northern or southern route to return to the Fraser River was correlated with geomagnetic drift relative to the likely imprinting site [46].
Figure 1.
Large-scale gradients of (a) total field intensity and (b) inclination angle (shown here as estimates based on the International Geomagnetic Reference Field (IGRF-11) for 2014) allow animals, such as salmon, to use magnetic information as a proxy for geographical position. A complication for animals using such a system is that fields marking particular areas drift over time, as shown by (c) the annual standard deviation of mean total field intensity from 1900 to 2014 and (d) the annual standard deviation of mean inclination angle from 1900 to 2014. The rate of field drift over ecological timescales is sufficiently great that it is likely an important factor to consider when studying animals that rely on geomagnetic cues for navigation.
Figure 2.
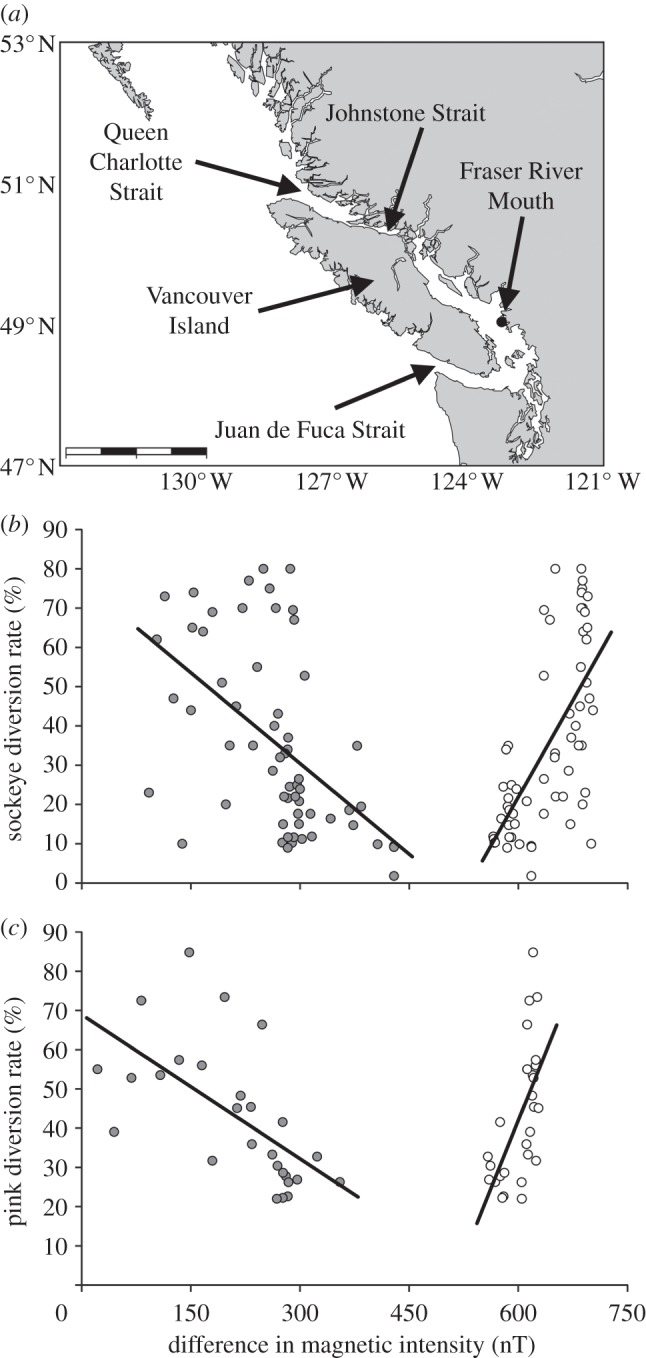
(a) Salmon returning to the Fraser River must travel around Vancouver Island to the north via the QC and Johnstone Strait or to the south through JdF. The proportion of Fraser River pink and sockeye salmon that migrate through the northerly route is the ‘diversion rate.’ Scale bar is 225 km. (b) Grey circles plot the relationship between the sockeye salmon diversion rate and the difference in magnetic intensity between the mouth of the Fraser River and QC. White circles plot the relationship between the sockeye salmon diversion rate and the difference in magnetic intensity between the mouth of the Fraser River and JdF. Trend lines in each graph are estimated by linear regression. (c) The same conventions are used here as in (b), but for pink salmon diversion rate.
In this paper, our goal was to test the robustness of our earlier findings by performing similar analyses using estimates of sockeye diversion rate that were updated to reflect the more accurate method adopted by the Pacific Salmon Commission in the summer of 2013. We test the generality of the geomagnetic imprinting hypothesis by applying the same modelling approach developed for sockeye salmon to a previously unpublished dataset on Fraser River pink salmon. Moreover, we extend this work by examining the primary oceanographic and fluvial factors that could influence the proportion of salmon returning to the Fraser River by either a northern or southern route, with particular attention paid to environmental correlates likely associated with the olfactory imprinting hypothesis [45]. With these analyses, we examine whether olfactory imprinting is sufficient to predict variation in the oceanic migratory routes of salmon, exclusive of a ‘large-scale’ navigation strategy. Finally, to assess whether our approach could be used for fisheries management we compare the predictive ability of the models that we developed based on navigation mechanisms in salmon to those currently used by the Pacific Salmon Commission.
2. Material and methods
2.1. Diversion rate
Data on the diversion rates are obtained by separately calculating the proportion of total Fraser River sockeye and pink salmon that enter QC and migrate through Johnstone Strait compared to JdF (figure 2a)
| 2.1 |
The number of sockeye and pink salmon (N) migrating through both Straits is estimated based on catch per unit effort (CPUE) data obtained from test fishing vessels in these two areas. The catchability (q) is estimated based on historic CPUE data from the test fishery in both areas and the total abundance in the river
| 2.2 |
and
| 2.3 |
For Fraser River sockeye salmon, four different management groups exist that differ in terms of the timing of the returning salmon stocks, i.e. Early Stuart, Early Summer run, Summer run and Late run stocks. To evaluate the abundance of the earlier timed Early Stuart and Early Summer run sockeye stocks, gillnet test fishing vessels are used. Later in the season, purse seine test fishing vessels are used to evaluate the abundance of Summer and Late Run sockeye as well as Fraser River pink salmon. Gillnet efficiency is the same in Johnstone Strait and JdF [51]
| 2.4 |
Purse seine test vessels differ in efficiency of catching sockeye and pink salmon. Given possible variability in the CPUE data and error estimating total salmon abundance in the river, it is difficult to determine the catchability of both areas using equation (2.3). We therefore related the catchability of the salmon in both Johnstone Strait and JdF to the area swept by the fishing net. JdF is wider than Johnstone Strait and, based on the migration areas for sockeye salmon and the size of the nets used in each area, the ratio relating the catchability of Johnstone Strait to JdF was set at 1/2.2, meaning that it is 2.2 times harder to catch a sockeye salmon in the JdF compared with Johnstone Strait
| 2.5 |
Pink salmon are more widely distributed across the channel compared to sockeye resulting in a ratio of 3 instead of 2.2
| 2.6 |
In the case that the ratios relating QC and JdF catchability are incorrect, this would have an impact on the magnitude of the diversion rate. The annual differences between diversion rates however, which are the focus of our study, are unlikely to be affected. Moreover, diversion rates were computed ‘blind’ with respect to the hypotheses tested here. Diversion rates have been calculated for Fraser River sockeye salmon from 1953 to 2012 (n = 60 years). The estimates for sockeye diversion rate we use are updated to reflect the more accurate method recently adopted by the Pacific Salmon Commission (summer 2013); the differences between these and previous estimates [46] range between −5% and +8% for a given year and include four additional years of data. Fraser River pink salmon diversion rates have been calculated from 1959 to 2011, for the odd years when spawning occurs (n = 27 years). Pink salmon occur as obligate odd—or even—year spawning populations [40].
2.2. Geomagnetic imprinting
Recent laboratory experiments demonstrate that juvenile Pacific salmon use both magnetic field intensity and inclination angle to assess location [30]. We hypothesize that juvenile salmon imprint on the magnetic field when they make the transition from freshwater to seawater and use the specific values of intensity and inclination angle as return markers during the homing migration [42,46]. If true, then fish leaving the Fraser River would imprint on the magnetic values at the river mouth and thus on the eastern side of Vancouver Island. When the fish return, their migration route, either through the northern or southern inlet, might reflect how closely the field at each entryway resembles the field that fish experienced when they left the Fraser River as juveniles [46,48]. As the difference between the magnetic fields at QC and the Fraser River decreases, a greater number of fish should enter by that route (diversion rate increases). Similarly, as the difference between the magnetic fields at JdF and the Fraser River decreases, a greater proportion of fish might enter the southern route (diversion rate decreases).
We used the International Geomagnetic Reference Field model (IGRF-11) [52] to determine magnetic field strength (total field intensity) and inclination angle at the mouth of the Fraser River (49.1° N, 123.25° W), the seaward entry to QC (51.0° N, 128.0° W) and the seaward entry to JdF (48.45° N, 124.6° W) (figure 2a). For sockeye salmon, we assumed a 2 year lag between fish leaving the river as juveniles and returning to spawn [40]. Thus, to predict the sockeye diversion rate in 1953 we took the 1951 magnetic value at the Fraser River (e.g. intensity = 57312.8 nT) and subtracted it from the 1953 magnetic values at QC (e.g. intensity = 56883.8 nT, a difference of 429.0 nT) and the 1953 magnetic values at JdF (e.g. intensity = 56695.2 nT, a difference of 617.6 nT). The process was repeated for both intensity and inclination angle for each year with diversion rate estimates available. Magnetic values were taken at 1 January of each year. The IGRF model is updated every 5 years and linearly interpolates field changes between the updates [52]; thus choosing a specific day or range within a year would imply finer measurement precision than is warranted. For pink salmon, we assumed a 1 year lag between fish leaving the river as juveniles and returning to spawn [40]. In this case, to predict pink diversion rate in 1959 we took the 1958 value at the Fraser River (e.g. intensity = 57115.8 nT) and subtracted it from the 1959 magnetic values at QC (e.g. intensity = 56761.1 nT, a difference of 354.7 nT) and the 1959 magnetic values at JdF (e.g. intensity = 56547.3 nT, a difference of 568.5 nT). The process was repeated for both intensity and inclination angle for each year with diversion rate estimates available in the same manner as described for sockeye.
2.3. Olfactory imprinting
Classic experiments demonstrate that juvenile salmon can imprint upon odours during their downstream migration and use those same odours to relocate their natal river during their spawning migration [45]. The chemicals salmon use to discriminate rivers are unknown, and thus their persistence in the environment and distances over which they might be detected are unknown as well [43]. However, if olfactory imprinting were the primary way that salmon navigate from the ocean to the river then we would predict that spatio-temporal variation in the homing migration would be related to variation in the transport of fluvial odours in the vicinity of Vancouver Island [12,45]. This variation in odour transport is likely correlated with several readily available metrics associated with river and ocean water velocity.
Groot & Quinn [53] suggested that increased water discharge from the Fraser River might enhance the odour signal at the more distant QC, thus increasing the proportion of salmon that enter through this route. Following their methods, we used mean flow volume between April and June at Hope, British Columbia (approx. 165 km from the mouth of the Fraser River) as a measure of the amount of odour entering the ocean during the homing phase of sockeye and pink salmon [53]. Data were provided by Water Survey of Canada (http://www.wsc.ec.gc.ca/staflo/index_e.cfm). In addition to flow volume, another possible indication as to whether odour from the Fraser River extends into the Pacific is water velocity at the mouth of each strait. When water currents are directed offshore (westward), odours from the Fraser River are more likely to extend into the open ocean and serve as a long-distance homing cue. Based on the olfactory imprinting hypothesis, we predict that when water velocity is more westward at QC (and, likewise, when water velocity is more eastward at JdF) the diversion rate might be expected to increase.
For estimates of water velocity, we used the output from two ocean circulation models, Simple Ocean Data Assimilation (SODA) and the Global Hybrid Coordinate Ocean Model (Global HYCOM). SODA assimilates hydrographic profile data, ocean station data, moored temperature and salinity time series, surface temperature and salinity observations of various types, and night-time infrared satellite sea surface temperature (SST) data into an ocean general circulation model to generate estimates that match actual oceanic conditions (i.e. observations) [54]. SODA output is provided as monthly averages at 0.5° grid resolution from January 1871 to 2010 (http://apdrc.soest.hawaii.edu/datadoc/soda_2.2.4.php). Similarly, Global HYCOM assimilates satellite altimetry data, SST and in situ measurements from a global array of expendable bathythermographs, Argos floats and moored buoys to produce realistic ‘hindcast’ output [55]. Global HYCOM output is provided at much finer resolution, daily snapshots at 0.08° grid spacing, but is only available from 2003 to present (http://hycom.org). Therefore, we used SODA output for the years 1953–2002 and higher resolution Global HYCOM output once it became available, for the years 2003–2012.
Eastward ocean current velocity (u-velocity) was estimated for QC by taking the mean value across the surface layer of an area encompassing 50.75–51.25° N and 129.25–128.25° W. For JdF, the mean values were computed across an area encompassing 48.25–48.75° N and 125.75–124.75° W. We used the surface layer because it is most constrained by observations within the models, and thus is likely to provide the best approximation of ocean conditions [54,55]. The spawning migration of sockeye and pink salmon occurs during the late spring and through the summer, with sockeye beginning approximately in May and pink beginning in June [40,50]. We computed means of eastward ocean velocity between May and June for sockeye and June and July for pink, the period when most fish are moving through these waters towards the Fraser River [50,53,56].
2.4. Factors influencing salmon distribution offshore
In addition to variation in navigation cues, the variation in the distribution of salmon at sea is also likely to influence the diversion rate [40,46,49–51,53,56]. Previous work in sockeye salmon indicate that the diversion rate is positively correlated with SST in April, the month before salmon begin to arrive at Vancouver Island [46,50,53]. It is thought that warmer temperatures cause fish to move poleward and thus increase the proportion of the population that migrate through the northern route. SST exceeding the preferred upper limit for sockeye and pink salmon would likely increase the diversion rate whether or not geomagnetic or olfactory imprinting was used for navigation. However, temperature effects might also act synergistically with navigational factors (e.g. years with warmer SST that coincide with a smaller difference in magnetic field values between QC and the Fraser River might have a particularly high diversion rate) [46]. Thus, a positive correlation between the diversion rate and temperature does not necessarily exclude either of the navigational hypotheses.
Another factor that could influence salmon distribution at sea is ocean current velocity, by directly pushing salmon a particular direction [56,57]. For instance, if salmon approach Vancouver Island and are advected northward by ocean currents, a higher diversion rate might result [56]. This possibility is likely if salmon do not use magnetic cues to assess their location to stay on a particular course during their migration, but rather choose a general ‘landward’ compass direction and seek an olfactory signal associated with their home river [58]. Thus, a positive correlation between the diversion rate and northward current velocity is compatible with the olfactory imprinting hypothesis, but would be inconsistent with the geomagnetic imprinting hypothesis (at least as we have formulated it).
For estimates of SST and northward current velocity (v-velocity), we used SODA [54] and HYCOM [55] output as described above. In this case, however, both values were averaged over an area extending offshore of Vancouver Island (latitudinal range of 47.25–52.25° N and a longitudinal range of 134.25–123.75° W). This area was chosen to be large enough to encompass waters around both QC and JdF s, but restricted enough so that we could detect an effect of SST and northward current velocity as they acted on the portion of the oceanic migration where fish are, presumably, pinpointing the natal river. As in earlier studies, mean SST over this area was computed for the month before salmon begin to arrive at Vancouver Island (April for sockeye, May for pink) [46,53]. Northward ocean velocity means were computed for the months May through June for sockeye and June through July for pink, the period when fish are moving through these waters towards the Fraser River [56].
2.5. Analyses
Spearman's correlation test, a non-parametric regression, identified the relationship between individual variables and diversion rate. Variables with Spearman R-values of greater than 0.3 or less than or equal to 0.3 were selected for further analysis. Multiple linear regressions and variance partitioning analyses assessed the relative importance of the variables associated with geomagnetic imprinting, olfactory imprinting and offshore distribution [46]. We then compared the results of the regression models based on navigation mechanisms to a regression using the current predictive metrics for the diversion rate. April SST data from Kains Island Lighthouse (50.27° N, 128.02° W), provided by Fisheries and Oceans Canada (http://www.pac.dfo-mpo.gc.ca/science/oceans/data-donnees/lighthouses-phares/index-eng.htm) are used to predict diversion rates for sockeye fisheries management [50]. For pink salmon, the diversion rate is predicted using the previous run's diversion rate (e.g. the 1959 diversion rate is used to predict the diversion rate in 1961, the 1961 diversion rate is used to predict the diversion rate in 1963, etc.) [50].
3. Results
3.1. Spearman correlations
Consistent with the geomagnetic imprinting hypothesis, as the difference between the magnetic intensity at QC and the mouth of the Fraser River decreased, diversion rates for both sockeye and pink salmon increased. Likewise, as the difference between the magnetic intensity at JdF and the mouth of the Fraser River decreased, diversion rates for both species decreased (table 1). The difference between the inclination angle at the Fraser River and QC was also well correlated with both sockeye and pink diversion rates. However, this was not the case for the comparisons of inclination angle at JdF; no relationship was observed with diversion rate in sockeye or pink salmon (table 1).
Table 1.
Results of Spearman's correlation tests relating diversion rate of sockeye salmon (1953–2012) and pink salmon (1959–2011) to geomagnetic, fluvial and oceanic variables that might influence variation in migratory route. See Material and methods for details.
| variable (location) | sockeye Spearman's r (p-value) | pink Spearman's r (p-value) |
|---|---|---|
| Δ magnetic intensity (QC) | −0.580 (<0.0000012) | −0.747 (<0.00000752) |
| Δ magnetic intensity (JdF) | 0.636 (<0.000000047) | 0.653 (<0.000225) |
| Δ magnetic inclination (QC) | −0.578 (<0.0000013) | −0.718 (0.000025) |
| Δ magnetic inclination (JdF) | −0.179 (0.172) | −0.198 (0.322) |
| Fraser River Discharge | −0.098 (0.4558) | 0.247 (0.214) |
| eastward ocean velocity (QC) | −0.395 (0.0018) | −0.308 (0.118) |
| eastward ocean velocity (JdF) | −0.173 (0.186) | −0.056 (0.781) |
| northward ocean velocity (offshore) | 0076 (0.563) | −0.085 (0.672) |
| SST (offshore) | 0.492 (0.000064) | 0.179 (0.372) |
The only variable associated with the olfactory imprinting hypothesis that was correlated with diversion rate was eastward ocean velocity at QC (decreased eastward flow increased the diversion rate for sockeye, but not pink salmon). Other variables, Fraser River flow volume and eastward current velocity at JdF were unrelated to diversion rates in either sockeye or pink salmon. Of the variables tested that could influence salmon distribution prior to reaching Vancouver Island, the sole correlation was a positive relationship between ocean temperature and sockeye diversion rate (but not pink diversion rate). No relationship was observed between northward ocean velocity and diversion rate for either sockeye or pink salmon (table 1).
3.2. Variance partitioning
To explore the relative importance of the different navigational hypotheses for predicting the diversion rate, the metrics that had Spearman R-values of greater than 0.3 or less than −0.3 (table 1) were grouped according to whether they were associated with geomagnetic imprinting (the difference in intensity and the difference in inclination angle between QC and the Fraser River, and the difference in intensity between JdF and the Fraser River), olfactory imprinting (eastward current velocity at QC) or the offshore distribution of salmon (SST, for sockeye only). Multiple linear regression indicated that the full model accounted for 62.8% of the variation in sockeye diversion rate. Variance partitioning uniquely ascribed 23.2% of the variation in diversion rate to geomagnetic imprinting, 13.0% to offshore SST and 6.1% to olfactory imprinting. Positive interactive effects were observed for geomagnetic imprinting and offshore SST as well as geomagnetic and olfactory imprinting (table 2). For pink salmon, multiple linear regression indicated that 47.0% of the variation in diversion rate could be accounted for with the full model. Variance partitioning analyses uniquely ascribed 44.0% of the variation in diversion rate to geomagnetic imprinting and 0.1% to olfactory imprinting. A small positive interactive effect was observed between geomagnetic and olfactory imprinting (table 2); offshore SST was not examined as no correlation was observed in the initial analyses (table 1).
Table 2.
Results of variance partitioning analyses of multiple regression analyses with variables identified as possible predictors using Spearman's correlation (r > 0.3). Correlates are based on whether they are associated with geomagnetic imprinting (the difference in total field intensity between the mouth of the Fraser River and QC, the difference in total field intensity between the mouth of the Fraser River and JdF, and the difference in inclination angle between the mouth of the Fraser River and QC), olfactory imprinting (eastward ocean velocity at QC) and offshore distribution (offshore SST). Numbers indicate the percentage of variance in diversion rate uniquely explained by a given set of factors.
| predictors | variance in sockeye diversion rate explained | variance in pink diversion rate explained |
|---|---|---|
| full model | 62.8 | 47.0 |
| geomagnetic imprinting | 23.2 | 44.0 |
| olfactory imprinting | 6.1 | 0.1 |
| temperature | 13.0 | — |
| geomagnetic imprinting × temperature | 10.7 | — |
| olfactory imprinting × temperature | −1.2 | — |
| geomagnetic imprinting × olfactory imprinting | 13.0 | 2.8 |
| geomagnetic imprinting × olfactory imprinting × temperature | −2.0 | — |
3.3. Comparison of navigation-based models to fisheries managers' predictions
April SST at Kains Island Lighthouse (the metric used by sockeye fisheries managers) could account for up to 41.1% of the variation in diversion rate (p < 0.000001, n = 60). Linear regression indicates that the previous run's diversion rate could account for up to 17.1% of the variation in pink diversion rate (p = 0.036, n = 26). In comparison, geomagnetic imprinting accounted for up to 45.0% of the variation in sockeye diversion rate (p < 0.000001, n = 60) and 46.8% of the variation in pink diversion rate (p = 0.0019, n = 27). The olfactory imprinting hypothesis accounted for as much as 15.9% of the variation in sockeye diversion rate (p = 0.0025, n = 60) and 2.9% of the variation in pink diversion rate (p = 0.404, n = 27). The full models, as described in the section above, account for 62.8% of the variation in sockeye diversion rate (p < 0.000000001, n = 60) and 47.0% of the variation in pink diversion rate (p = 0.013, n = 27). Including the metric used by fisheries managers to predict sockeye diversion rate to the full model adds little predictive ability (+5.4%), as the Kain's Island measure of SST is highly correlated with the offshore measure of SST (Pearson's R = 0.85, p < 0.00000001). For pink salmon, including the metric used by fisheries managers to the full model decreases its predictive ability (−1.2%) because using the previous run's diversion rate necessarily excludes the first year of data.
4. Discussion
Our analyses indicate that geomagnetic imprinting provides a unifying explanation for spatio-temporal variation in the homing migration of Fraser River sockeye and pink salmon. This is not to say that olfaction is an unimportant component of salmon homing, however, at the scale of our analysis (several hundred kilometres) geomagnetic cues clearly predominate. Likewise, variation in migration route was much more sensitive to geomagnetic drift than changes in ocean temperature (table 2). As global climate continues to change and as humans further modify habitats the need to predict long-term trends in animal movements and distribution is growing [1,2,5]. Our work suggests that relying exclusively on projected changes in temperature will be insufficient to predict changes in migration patterns for many species. Although navigation in salmon (and other animals that migrate long-distances in the ocean) is far from being adequately described [8], it appears that even basic information relating to the navigation mechanisms (in this case geomagnetic imprinting) can provide a simple and intuitive way to predict their oceanic movement patterns [15,42].
Pink and sockeye salmon from the Fraser River differ in many ways: pink spawn only in odd numbered years, have a shorter freshwater residence period as juveniles, remain at sea for only 1 year rather than 2, spawn closer to the river mouth [40], and have better aerobic performance across a wider and warmer range of temperatures than sockeye [59]. The greater thermal tolerance of pink salmon may explain why their diversion rate is less influenced by SST than sockeye's (tables 1 and 2). During warm years, sockeye might be constrained to cooler, more northern latitudes (and thus more fish return by the northern route) [46] whereas pink salmon are not. Likewise, olfactory imprinting provided some predictive ability for sockeye but not pink salmon (table 2). Why sockeye appear to be more sensitive to water flow variables is less clear, but could be related to differences in the freshwater migration. Because most sockeye populations spawn further inland than do pink salmon [40], sockeye might have evolved to be more sensitive to odours when homing. Comparative experiments are needed to assess this possibility. Despite these differences, geomagnetic drift predicts a similar percentage of the variation in diversion rate for both species (multiple regressions of magnetic variables give R2 values of 0.468 for pink and 0.450 for sockeye salmon). This implies that the precision with which magnetic fields are detected, the geomagnetic navigation strategy used, and its role in shaping oceanic movements are similar in these species (figure 2).
Navigation to a specific location requires at least two coordinates, though they need not be orthogonal [60,61]. Experiments in juvenile Chinook salmon [30], steelhead trout [31], sea turtles [19–21,25,28,29] and lobsters [24] suggest that these animals use the combination of magnetic intensity and inclination angle as an indicator of their geographical position. (Newts [22,23] and birds [18,27,60] probably do as well, though evidence in these animals is less conclusive [62,63].) For pink and sockeye salmon, the gradients of magnetic intensity and inclination angle across the North Pacific could provide the information necessary to complete the oceanic phase of the homing migration (figures 1a,b and 3). For more than a century, the mouth of the Fraser River has been unambiguously marked by specific pairings of intensity and inclination angle values (figure 3). Thus, with these magnetic gradients and some basic spatial information (e.g. that they are west of the home river) salmon could approximate the direction towards and, to a lesser extent, distance from the Fraser River over much of their oceanic range.
Figure 3.
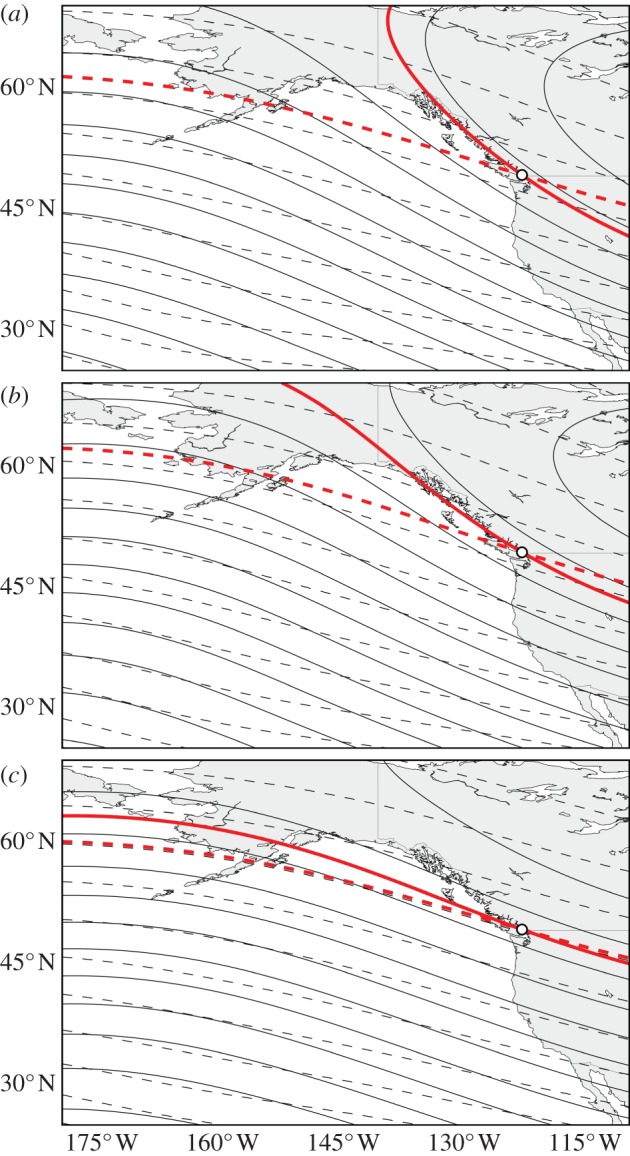
Contour maps of magnetic intensity (solid lines, 2 μT contours) and inclination angle (dashed lines, 4° contours) across the Northeast Pacific based on the IGRF-11. If salmon imprint upon the value of intensity and inclination angle (shown in red) as they depart the Fraser River (white circle) as juveniles, they could return to the vicinity of their natal river by relocating the particular pairing of intensity and inclination angle, as these values unambiguously demarcate the mouth of the Fraser River. Though the gradients of intensity and inclination angle have shifted over time (a) 1900, (b) 1955, (c) 2010, they consistently provide sockeye and pink salmon an indication of their location at sea relative to their home river. The non-parallel gradients of these two magnetic features are compatible with a number of different navigation strategies that have been proposed, ranging from single-coordinate latitude detection [42,48] to more sophisticated [60,61] or unconventional [42,47,62] bicoordinate navigation strategies.
Precisely how salmon use the magnetic field for homing cannot be determined from our correlations, but several observations are worth mentioning. Over the study period, magnetic intensity at the Fraser River decreased by 4.3%, whereas inclination angle decreased by 1.6%. A decrease in these field elements corresponds with northward drift, consistent with the general trend of increased diversion rates for both sockeye and pink salmon. Additionally, the basin-scale gradient of magnetic intensity has shifted much more in the past century than has inclination (figure 3). Assuming that fish weight both field elements equally for determining position [30], a reasonable expectation is that the precision with which salmon navigate to the Fraser River is primarily influenced by the least stable coordinate (magnetic intensity). Consistent with this possibility, correlations between diversion rate and drift of intensity were observed at both QC and JdF, whereas correlations with drift of inclination angle were only observed at QC.
Furthermore, it is worth noting that pink and sockeye salmon appear to have a preference for the southern route, through JdF, even though QC has been consistently more magnetically similar to the Fraser River mouth (figure 2) and ocean current velocity was consistently more westward at QC than at JdF for sockeye (T-test, p < 1.04 × 10−10 n = 60) and pink salmon (T-test, p = 0.0009, n = 27). We speculate that Fraser River salmon may have a preference to travel through the JdF due to the ease of migration after reaching Vancouver Island. Fish travelling through the northern route must swim approximately 400 km through a complex array of islands, whereas those travelling through the southern route swim closer to 200 km and primarily through open water. If predation risk or energy expenditure is lower when migrating through JdF, natural selection may have favoured homing salmon that: (i) biased their compass orientation slightly southward, (ii) preferred a slightly weaker value of intensity than what they imprinted upon at the mouth of the Fraser River or (iii) preferred a slightly less steep value of inclination angle than was imprinted upon (figure 3). Any of these three possibilities would provide a simple way to increase the relative proportion of salmon moving through the southern route. How salmon could accomplish a southward bias using only olfactory cues is less apparent.
That geomagnetic models can help answer long-standing questions related to fisheries management highlights the value of a broad, interdisciplinary and comparative perspective in the applied sciences. Information on navigation mechanisms coupled with these models greatly improves our ability to predict salmon migration routes around Vancouver Island compared to the current approach of relying on the previous run's diversion rate (for pink salmon) and SST (for sockeye salmon) [50]. Accurate predictions of the proportion of salmon migrating around the northern or southern end of Vancouver Island allows for better planning by Canadian and US fisheries and is of great interest for fisheries management. Fish migrating through QC are not accessible to US fisherman and high diversion rates mean that US fishermen will need to fish harder early in the season in order to reach their share of the total allowable catch. Under-predicting the diversion rate could result in unrealistic catch expectations for US fishermen. Presumably, with more complete information on how pink and sockeye salmon perceive the geomagnetic field, use the field to guide their return migration, and weight geomagnetic information relative to other sensory input, a better depiction of the migration could be obtained (and greater variance in diversion rate could be explained). Acquiring such information, through direct experimentation in the laboratory and in the field, should be prioritized [30,31,64]. More generally, we encourage a renewed focus on navigation mechanisms as an elegant approach to generate insight into the ecological, evolutionary and economic implications of animal movement [1,65–67].
Finally, accumulating evidence indicates that diverse animals derive positional (‘map’) information from the Earth's magnetic field to navigate over a wide range of spatial scales [18–31]. As such, our work suggests that drift of the magnetic field could play an important role in the spatio-temporal variation in movement patterns for many species [67–70]. The rate of field drift can vary substantially across different regions of the Earth and through time [42,47,62]. A detailed consideration of this global environmental feature relative to animal navigation might reveal a surprising degree of similarity in the movements of geographically disparate and phylogenetically divergent populations.
Supplementary Material
Acknowledgements
Steve Latham of the Pacific Salmon Commission and two anonymous reviewers provided constructive feedback on the paper.
Data accessibility
Data used in this publication have been made available in the electronic supplementary material.
Funding statement
Financial support was provided by Oregon Sea Grant, Oregon State University and the Oregon Department of Fish and Wildlife.
References
- 1.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowlin MS, et al. 2010. Grand challenges in migration biology. Integr. Comp. Biol. 50, 261–279. ( 10.1093/icb/icq013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrovskii S, Petrovskaya N. 2012. Computational ecology as an emerging science. Interface Focus 2, 241–254. ( 10.1098/rsfs.2011.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putman NF, Mansfield KM, He R, Shaver DJ, Verley P. 2013. Predicting the distribution of oceanic-stage Kemp's Ridley sea turtles. Biol. Lett. 9, 20130345 ( 10.1098/rsbl.2013.0345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa DP, Breed G, Robinson PW. 2012. New insights in pelagic migrations: implications for ecology and conservation. Annu. Rev. Ecol. Syst. 43, 73–96. ( 10.1146/annurev-ecolsys-102710-145045) [DOI] [Google Scholar]
- 6.Holyoak M, Casagrandi R, Nathan R, Revilla E, Spiegel O. 2008. Trends and missing parts in the study of movement ecology. Proc. Natl Acad. Sci. USA 105, 19 060–19 065. ( 10.1073/pnas.0800483105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyer D, Walsh PD. 2010. Modelling the mobility of living organisms in heterogeneous landscapes: does memory improve foraging success? Phil. Trans. R. Soc. A 368, 5645–5659. ( 10.1098/rsta.2010.0275) [DOI] [PubMed] [Google Scholar]
- 8.Byron CJ, Burke BJ. In press. Salmon ocean migration models suggest a variety of population-specific strategies. Rev. Fish Biol. Fish. ( 10.1007/s11160-014-9343-0) [DOI] [Google Scholar]
- 9.Bartumeus F, Da Luz MGE, Viswanathan GM, Catalan J. 2005. Animal search strategies: a quantitative random-walk analysis. Ecology 86, 3078–3087. ( 10.1890/04-1806) [DOI] [Google Scholar]
- 10.Sims DW, et al. 2008. Scaling laws of marine predator search behaviour. Nature 451, 1098–1102. ( 10.1038/nature06518) [DOI] [PubMed] [Google Scholar]
- 11.Humphries NE, et al. 2010. Environmental context explains Levy and Brownian movement patterns of marine predators. Nature 465, 1066–1069. ( 10.1038/nature09116) [DOI] [PubMed] [Google Scholar]
- 12.Lohmann KJ, Lohmann CMF, Endres CS. 2008. The sensory ecology of ocean navigation. J. Exp. Biol. 211, 1719–1728. ( 10.1242/jeb.015792) [DOI] [PubMed] [Google Scholar]
- 13.Putman NF, Verley P, Shay TJ, Lohmann KJ. 2012. Simulating transoceanic migrations of young loggerhead sea turtles: merging magnetic navigation behavior with an ocean circulation model. J. Exp. Biol. 215, 1863–1870. ( 10.1242/jeb.067587) [DOI] [PubMed] [Google Scholar]
- 14.Staaterman E, Paris CB, Helgers J. 2012. Orientation behavior in fish larvae: a missing piece to Hjort's critical period hypothesis. J. Theor. Biol. 304, 186–196. ( 10.1016/j.jtbi.2012.03.016) [DOI] [PubMed] [Google Scholar]
- 15.Burke BJ, Anderson JJ, Baptista AM. 2014. Evidence for multiple navigational sensory capabilities of Chinook salmon. Aqu. Biol. 20, 77–90. ( 10.3354/ab00541) [DOI] [Google Scholar]
- 16.Alerstam T, Gudmundsson GA, Green M, Hedenstrom A. 2001. Migration along orthodromic sun compass routes by Arctic birds. Science 291, 300–303. ( 10.1126/science.291.5502.300) [DOI] [PubMed] [Google Scholar]
- 17.Alerstam T. 2006. Conflicting evidence about long-distance animal navigation. Science 313, 791–794. ( 10.1126/science.1129048) [DOI] [PubMed] [Google Scholar]
- 18.Beck W, Wiltschko W. 1988. Magnetic factors control the migratory direction of pied flycatchers, Fidecula hypoleuca. Proc. Int. Ornithol. Congr. 19, 1955–1962. [Google Scholar]
- 19.Lohmann KJ, Lohmann CMF. 1996. Detection of magnetic field intensity by sea turtles. Nature 380, 59–61. ( 10.1038/380059a0) [DOI] [Google Scholar]
- 20.Lohmann KJ, Lohmann CMF. 1994. Detection of magnetic inclination angle by sea turtles: a possible mechanism for determining latitude. J. Exp. Biol. 194, 23–32. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann KJ, Cain SD, Dodge SA, Lohmann CMF. 2001. Regional magnetic fields as navigational markers for sea turtles. Science 294, 364–366. ( 10.1126/science.1064557) [DOI] [PubMed] [Google Scholar]
- 22.Fischer JH, Freake MJ, Borland SC, Phillips JB. 2001. Evidence for the use of magnetic map information by an amphibian. Anim. Behav. 62, 1–10. ( 10.1006/anbe.2000.1722) [DOI] [Google Scholar]
- 23.Phillips JB, Freake MJ, Fischer JH, Borland SC. 2002. Behavioral titration of a magnetic map coordinate. J. Comp. Physiol. 188, 157–160. ( 10.1007/s00359-002-0286-x) [DOI] [PubMed] [Google Scholar]
- 24.Boles LC, Lohmann KJ. 2003. True navigation and magnetic maps in spiny lobsters. Nature 421, 60–63. ( 10.1038/nature01226) [DOI] [PubMed] [Google Scholar]
- 25.Lohmann KJ, Lohmann CMF, Ehrhart LM, Bagley DA, Swing T. 2004. Geomagnetic map used in sea-turtle navigation. Nature 428, 909–910. ( 10.1038/428909a) [DOI] [PubMed] [Google Scholar]
- 26.Stapput K, Thalau P, Wiltschko R, Wiltschko W. 2008. Orientation of birds in total darkness. Curr. Biol. 18, 602–606. ( 10.1016/j.cub.2008.03.046) [DOI] [PubMed] [Google Scholar]
- 27.Henshaw I, Fransson T, Jakobsson S, Kullberg C. 2010. Geomagnetic field affects spring migratory direction in a long distance migrant. Behav. Ecol. Sociobiol. 64, 1317–1323. ( 10.1007/s00265-010-0946-8) [DOI] [Google Scholar]
- 28.Putman NF, Endres CS, Lohmann CMF, Lohmann KJ. 2011. Longitude perception and bicoordinate magnetic maps in sea turtles. Curr. Biol. 21, 463–466. ( 10.1016/j.cub.2011.01.057) [DOI] [PubMed] [Google Scholar]
- 29.Lohmann KJ, Putman NF, Lohmann CMF. 2012. The magnetic map of hatchling loggerhead sea turtles. Curr. Opin. Neurobiol. 22, 336–342. ( 10.1016/j.conb.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 30.Putman NF, Scanlan MM, Billman EJ, O'Neil JP, Couture RB, Quinn TP, Lohmann KJ, Noakes DLG. 2014. Inherited magnetic map guides ocean navigation in juvenile Pacific salmon. Curr. Biol. 24, 446–450. ( 10.1016/j.cub.2014.01.017) [DOI] [PubMed] [Google Scholar]
- 31.Putman NF, Meinke AM, Noakes DLG. 2014. Rearing in a distorted magnetic field disrupts the ‘map sense’ of juvenile steelhead trout. Biol. Lett. 10, 20140169 ( 10.1098/rsbl.2014.0169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano A, Ogura M, Sato A, Sakaki Y, Shimizu Y, Baba N, Nagasawa K. 1997. Effect of modified magnetic field on the ocean migration of maturing chum salmon, Oncorhynchus keta. Mar. Biol. 129, 523–530. ( 10.1007/s002270050193) [DOI] [Google Scholar]
- 33.Papi F, Luschi P, Akesson S, Capogrossi S, Hays GC. 2000. Open-sea migration of magnetically disturbed sea turtles. J. Exp. Biol. 203, 3435–3443. [DOI] [PubMed] [Google Scholar]
- 34.Benhamou S, Bonadonna F, Jouventin P. 2003. Successful homing of magnet-carrying white-chinned petrels released in the open sea. Anim. Behav. 65, 729–734. ( 10.1006/anbe.2003.2092) [DOI] [PubMed] [Google Scholar]
- 35.Bonadonna F, Chamaille-Jammes S, Pinaud D, Weimerskirch H. 2003. Magnetic cues: are they important in black-browed albatross Diomedea melanophris orientation? Ibis 145, 152–155. ( 10.1046/j.1474-919X.2003.00117.x) [DOI] [Google Scholar]
- 36.Bonadonna F, Bajzak C, Benhamou S, Igloi K, Jouventin P, Lipp HP, Dell'Omo G. 2005. Orientation in the wandering albatross: interfering with magnetic perception does not affect orientation performance. Proc. R. Soc. B 272, 489–495. ( 10.1098/rspb.2004.2984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benhamou S, Sudre J, Bourjea J, Ciccione S, De Santis A, Luschi P. 2011. The role of geomagnetic cues in green turtle open sea navigation. PLoS ONE 6, e26672 ( 10.1371/journal.pone.0026672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagliardo A, Bried J, Lambardi P, Luschi P, Wikelski M, Bonadonna F. 2013. Olfactory oceanic navigation in an Atlantic seabird. J. Exp. Biol. 216, 2798–2805. ( 10.1242/jeb.085738) [DOI] [PubMed] [Google Scholar]
- 39.Akesson S, Hedenstrom A. 2007. How migrants get there: migratory performance and orientation. BioScience 57, 123–133. ( 10.1641/B570207) [DOI] [Google Scholar]
- 40.Quinn TP. 2005. The behavior and ecology of pacific salmon and trout. Seattle, WA: University of Washington Press. [Google Scholar]
- 41.Quinn TP. 1982. A model for salmon navigation on the high seas. In Salmon and Trout Migratory Behavior Symposium (eds Brannon EL, Salo EO.), pp. 229–237. Seattle: School of Fisheries, University of Washington. [Google Scholar]
- 42.Lohmann KJ, Putman NF, Lohmann CMF. 2008. Geomagnetic imprinting: a unifying hypothesis of natal homing in salmon and sea turtles. Proc. Natl Acad. Sci. USA 105, 19 096–19 101. ( 10.1073/pnas.0801859105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueda H. 2014. Homing ability and migration success in Pacific salmon: mechanistic insights from biotelemetry, endocrinology, and neurophysiology. Mar. Ecol. Progr. Ser. 496, 219–232. ( 10.3354/meps10636) [DOI] [Google Scholar]
- 44.Keefer ML, Caudill CC. 2014. Homing and straying by anadromous salmonids: a review of mechanisms and rates. Rev. Fish Biol. Fish. 24, 333–368. ( 10.1007/s11160-013-9334-6) [DOI] [Google Scholar]
- 45.Scholz AT, Horrall RM, Cooper JC, Hasler AD. 1976. Imprinting to chemical cues: the basis for home stream selection in salmon. Science 192, 1247–1249. ( 10.1126/science.1273590) [DOI] [PubMed] [Google Scholar]
- 46.Putman NF, Lohmann KJ, Putman EM, Klimley AP, Quinn TP, Noakes DLG. 2013. Evidence for geomagnetic imprinting as a homing mechanism in Pacific salmon. Curr. Biol. 23, 312–316. ( 10.1016/j.cub.2012.12.041) [DOI] [PubMed] [Google Scholar]
- 47.Putman NF, Lohmann KJ. 2008. Compatibility of magnetic imprinting and secular variation. Curr. Biol. 18, R596–R597. ( 10.1016/j.cub.2008.05.008) [DOI] [PubMed] [Google Scholar]
- 48.Bracis C, Anderson JJ. 2012. An investigation of the geomagnetic imprinting hypothesis for salmon. Fish. Oceanogr. 21, 170–181. ( 10.1111/j.1365-2419.2012.00617.x) [DOI] [Google Scholar]
- 49.International Pacific Salmon Fisheries Commission. 1986. Annual Report 1985. New Westminster, Canada: International Pacific Salmon Fisheries Commission.
- 50.Pacific Salmon Commission. 2012. Report of the Fraser River Panel to the Pacific Salmon Commission on the 2008 Fraser River Sockeye Salmon Fishing Season. Vancouver, Canada: Pacific Salmon Commission.
- 51.Cave JD, Gazey WJ. 1993. A preseason simulation model for fisheries on Fraser River sockeye salmon (Onchorhynchus nerka). Can. J. Fish. Aquat. Sci. 51, 1535–1549. ( 10.1139/f94-153) [DOI] [Google Scholar]
- 52.Finlay CC, et al. 2010. International Geomagnetic Reference Field: the eleventh generation. Geophys. J. Int. 183, 1216–1230. ( 10.1111/j.1365-246X.2010.04804.x) [DOI] [Google Scholar]
- 53.Groot C, Quinn TP. 1987. Homing migration of sockeye salmon, Oncorhynchus nerka, to the Fraser River. Fishery Bull. 85, 455–469. [Google Scholar]
- 54.Carton JA, Giese BS. 2008. A reanalysis of ocean climate using simple ocean data assimilation (SODA). Mon. Weather Rev. 136, 2999–3017. ( 10.1175/2007MWR1978.1) [DOI] [Google Scholar]
- 55.Chassignet EP, Hurlburt HE, Smedstad OM, Halliwell GR, Hogan PJ, Wallcraft AJ, Baraille R, Bleck R. 2007. The HYCOM (Hybrid Coordinate Ocean Model) data assimilative system. J. Mar. Syst. 65, 60–83. ( 10.1016/j.jmarsys.2005.09.016) [DOI] [Google Scholar]
- 56.Thomson KA, Ingraham WJ, Healey MC, Leblond PH, Groot C, Healey CG. 1992. The influence of ocean currents on latitude of landfall and migration speed of sockeye salmon returning to the Fraser River. Fish. Oceanogr. 1, 163–179. ( 10.1111/j.1365-2419.1992.tb00035.x) [DOI] [Google Scholar]
- 57.Gaspar P, Georges J-Y, Fossette S, Lenoble A, Ferraroli S, Maho YL. 2006. Marine animal behaviour: neglecting ocean currents can lead us up the wrong track. Proc. R. Soc. B 273, 2697–2702. ( 10.1098/rspb.2006.3623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lohmann KJ, Luschi P, Hays GC. 2008. Goal navigation and island-finding in sea turtles. J. Exp. Mar. Biol. Ecol. 356, 83–95. ( 10.1016/j.jembe.2007.12.017) [DOI] [Google Scholar]
- 59.Clark TD, Jeffries KM, Hinch SG, Farrell AP. 2011. Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. J. Exp. Biol. 214, 3074–3081. ( 10.1242/jeb.060517) [DOI] [PubMed] [Google Scholar]
- 60.Gould JL. 1982. The map sense of pigeons. Nature 296, 205–211. ( 10.1038/296205a0) [DOI] [Google Scholar]
- 61.Benhamou S. 2003. Bicoordinate navigation based on non-orthogonal gradient fields. J. Theor. Biol. 225, 235–239. ( 10.1016/S0022-5193(03)00242-X) [DOI] [PubMed] [Google Scholar]
- 62.Lohmann KJ, Lohmann CMF, Putman NF. 2007. Magnetic maps in animals: nature's GPS. J. Exp. Biol. 210, 3697–3705. ( 10.1242/jeb.001313) [DOI] [PubMed] [Google Scholar]
- 63.Thorup K, Holland RA. 2009. The bird GPS—long-range navigation in migrants. J. Exp. Biol. 212, 3597–3604. ( 10.1242/jeb.021238) [DOI] [PubMed] [Google Scholar]
- 64.Staaterman E, Paris CB. 2014. Modelling larval fish navigation: the way forward. ICES J. Mar. Sci. 71, 918–924. ( 10.1093/icesjms/fst103) [DOI] [Google Scholar]
- 65.Neave F. 1964. Ocean migrations of Pacific salmon. J. Fish. Res. Board Can. 21, 1227–2144. ( 10.1139/f64-104) [DOI] [Google Scholar]
- 66.Royce WF, Smith LS, Hartt AC. 1968. Models of oceanic migrations of Pacific salmon and comments on guidance mechanisms. Fish. Bull. 66, 441–462. [Google Scholar]
- 67.Gould JL. 2014. Animal navigation: map for all seasons. Curr. Biol. 24, R153–R155. ( 10.1016/j.cub.2014.01.030) [DOI] [PubMed] [Google Scholar]
- 68.Feldheim KA, Gruber SH, DiBattista JD, Babcock EA, Kessel ST, Hendry AP, Pikitch EK, Ashley MV, Chapman DD. 2014. Two decades of genetic profiling yields first evidence of natal philopatry and long-term fidelity to parturition sites in sharks. Mol. Ecol. 23, 110–117. ( 10.1111/mec.12583) [DOI] [PubMed] [Google Scholar]
- 69.Block BA, et al. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. ( 10.1038/nature10082) [DOI] [PubMed] [Google Scholar]
- 70.Lohmann KJ, Lohmann CMF, Brothers JR, Putman NF. 2013. Natal homing and imprinting in sea turtles. In Biology of sea turtles, vol. 3 (eds Wyneken J, Lohmann KJ, Musick JA.), pp. 59–73. Boca Raton, FL: CRC Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this publication have been made available in the electronic supplementary material.