Abstract
Collembola, also known as springtails, are soil-dwelling arthropods that typically respire through the cuticle. To avoid suffocating in wet conditions, Collembola have evolved a complex, hierarchically nanostructured, cuticle surface that repels water with remarkable efficiency. In order to gain a more profound understanding of the cuticle characteristics, the chemical composition and architecture of the cuticle of Tetrodontophora bielanensis was studied. A stepwise removal of the different cuticle layers enabled controlled access to each layer that could be analysed separately by chemical spectrometry methods and electron microscopy. We found a cuticle composition that consisted of three characteristic layers, namely, a chitin-rich lamellar base structure overlaid by protein-rich nanostructures, and a lipid-rich envelope. The specific functions, composition and biological characteristics of each cuticle layer are discussed with respect to adaptations of Collembola to their soil habitat. It was found that the non-wetting characteristics base on a rather typical arthropod cuticle surface chemistry which confirms the decisive role of the cuticle topography.
Keywords: Collembola, cuticle, lipid, protein, chitin
1. Introduction
Liquid-repellent, non-fouling and self-cleaning characteristics of natural surfaces receive particular attention in biomimicry research. Unravelling the chemical and morphological origin of the surface properties is crucial to reproduce and translate these characteristics into engineered surfaces [1,2]. The non-wetting and self-cleaning properties of plant surfaces were intensively studied [3–5] over the last 20 years. Plant surfaces are typically decorated by wax crystals, which are rather fragile but regenerate after mechanical destruction [6,7]. Mimicking the needle-like or platelet-shaped crystal structures into artificial surface coatings was widely studied [8–10]; however, the inherent fragility of such micro- and nanostructures limits their durability [11]. Recent studies on the functional morphology of the cuticles of Collembola (springtails) revealed surfaces with higher stability against wear and friction, and outstanding resistance against wetting, even with low-surface-tension liquids [12,13]. While structural features of Collembola cuticles were investigated in detail [14], the impact of the molecular composition on the cuticle characteristics required further elucidation.
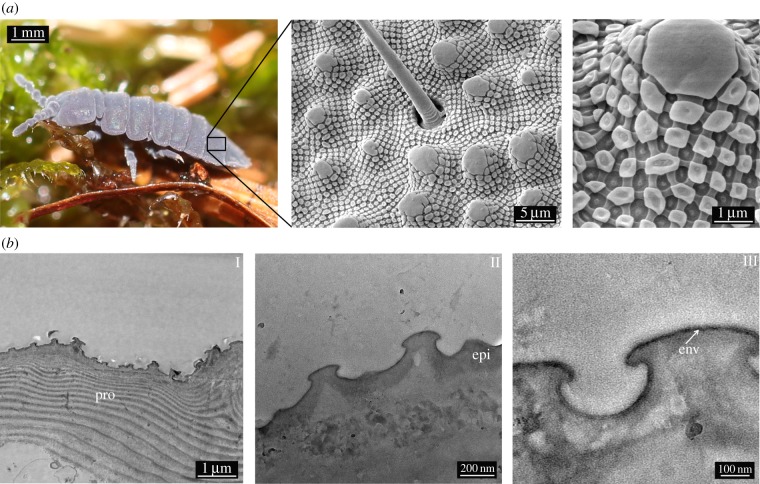
Representing more than 8000 species [15], Collembola are the most abundant and widespread arthropods and an important part of the community of soil-dwelling animals [16]. To enable cutaneous respiration [17,18], the cuticle surface needs to be kept clean and dry in the presence of soil microorganisms and water that is often contaminated by surfactants originating from decaying organic matter [19]. Consequently, the collembolan cuticle is adapted to the soil habitat by a robust and repellent surface consisting of nanoscopic, comb-like structures (figure 1a) [14,20]. Polymer replication methods were applied to demonstrate that the particular structures enable a robust non-wetting state, even with low-surface-tension liquids, and, thus, protect the animals against suffocation, even when immersed [21,22].
Figure 1.
(a) SEM studies of the cuticular morphology of T. bielanensis. SEM images showing papillose microstructures (secondary granules), covered by a rhombic comb-like mesh exhibiting nanoscopic tubercles (primary granules). (b) TEM sections of the cuticle, revealing the layered structure to consist of (I) the lamellar procuticle (pro), covered by (II) the epicuticle (epi) and (III) a thin envelope (env) as the topmost layer. (Online version in colour.)
While the unique surface morphology of collembolan cuticle was recently intensively studied, its chemical composition was not yet comprehensively analysed. A thorough analysis of both structure and composition of the cuticle is, however, needed for reconstituting the non-wetting and non-fouling surface characteristics in the development of bioinspired materials. Earlier studies found the collembolan cuticle to exhibit a layered structure commonly observed for arthropods [23]. Based upon electron micrographs, the authors classified several layers (from inside to outside): an endocuticle, an exocuticle and an epicuticle. The epicuticle was further divided into an internal, a cuticulin, a wax and a cement layer [24–26]. The waxy-like surface coating was suggested to support hydrophobicity under wet conditions and to prevent desiccation under arid conditions [27,28].
As a detailed chemical analysis of each cuticle layer was not yet available, we chose to investigate the cuticle of Tetrodontophora bielanensis with respect to the composition of each layer. Morphological characteristics were analysed using electron microscopy, and chemical analyses were performed by time of flight secondary ion mass spectrometry (TOF-SIMS), thin layer chromatography (TLC) and gas chromatography mass spectrometry (GC-MS). Additionally, hydrolysed cuticle samples were analysed by high-performance liquid chromatography (HPLC) and electrospray ionization mass spectrometry (ESI-MS). The combined results of these analyses allowed for establishing a detailed compositional model of the Collembola cuticle.
2. Results
The hierarchical structure of the cuticle of T. bielanensis is displayed in figure 1a. The scanning electron microscopy (SEM) images show papillose microstructures (designated as secondary granules) covered by a rhombic, comb-like pattern featuring nanoscopic tubercles (designated as primary granules) at the points of intersection. These structures completely cover the cuticle of T. bielanensis and were previously shown to prevent wetting and enable skin respiration under humid conditions [11,12]. Transmission electron microscopy (TEM) studies revealed a layer composition of the cuticle cross-section (figure 1b). Referring to recent studies of arthropod cuticles [28,29], three characteristic layers can be distinguished: the inner procuticle (combination of exo- and endocuticle) forms the basis of the exoskeleton and exhibits a characteristic lamellar structure (I). The 5–20 µm thick procuticle is overlaid by the non-lamellar 100 nm thick epicuticle layer, which includes nanoscopic surface features (II). The surface structures are covered by a thin 10 nm thick envelope (III). This outermost layer is stained by osmium tetroxide indicating a high unsaturated lipid content [30].
A stepwise removal of the cuticle layers was performed for a selective analysis of their chemical composition. As cuticle analyses on complete animals is challenging, moulted cuticles were analysed in comparison. During the moulting process, the entire cuticle is dorsally ruptured and shed, whereas the new cuticle is already present underneath (figure 2).
Figure 2.
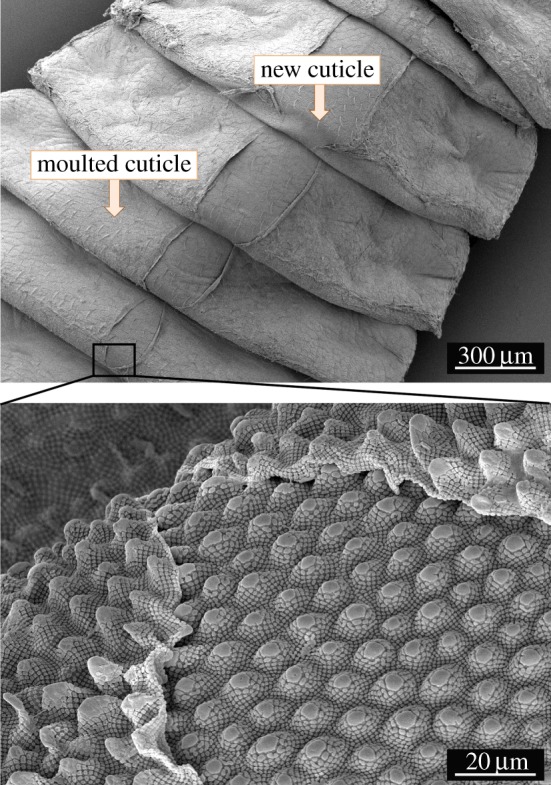
An individual of T. bielanensis during the moulting process. The images show the dorsal rupture and shedding of the old cuticle with its entire morphology, while the newly formed cuticle is present underneath. (Online version in colour.)
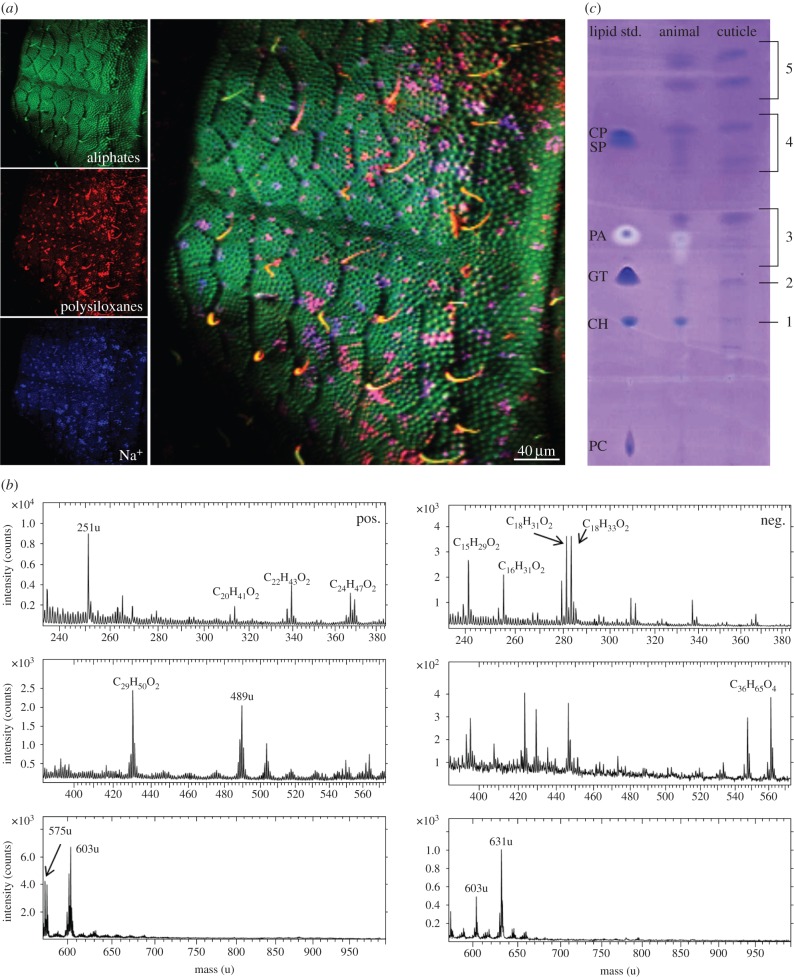
Information about the composition of the topmost cuticle layer of T. bielanensis was obtained by TOF-SIMS measurements, which analyses a few molecular monolayers. Surface mapping revealed a chemically homogeneous distribution of aliphatic hydrocarbons covering the entire surface (figure 3a). Contaminations (sodium ions and polysiloxanes) were assumed to be caused by the sample storage. The spectral data of the measurements (figure 3b) showed lipids, such as fatty acids (m/z = 255; 279; 281; 283) and higher molecular weight aliphatic compounds (m/z = 489; 561; 575; 603; 631). Hexane/dichloromethane extracts of the examined animal sample were applied to glass surfaces and also analysed by TOF-SIMS. The resulting data (electronic supplementary material, figure S1) were similar to those of the untreated cuticle surface, demonstrating the relevance of the extracted sample. As TOF-SIMS only allows for the detection of fragments, TLC was also performed to obtain further insights into the cuticle lipid composition using a standard lipid mixture of phospholipids, steroids, triglycerides, fatty acids, fatty esters and steryl esters as a reference. With the exception of phospholipids, all lipid classes were detected in the extracts of the moulted cuticles and the complete animals (figure 3c). In addition, terpenes, which are not included into the standard lipid mixture, were detected (electronic supplementary material, figure S2). The intensity of the TLC spots revealed that fatty acids, esters and terpenes dominated the extracts of both sources. The extract of the complete animals contained a higher amount of steroids, fatty acids and esters. This might be due to lower amounts of lipids retained in the moulted cuticle extract.
Figure 3.
Analysis of the topmost lipid layer by TOF-SIMS and TLC. (a) TOF-SIMS imaging of the cuticle surface shows a homogeneous distribution of an aliphatic (lipid) layer. (b) TOF-SIMS data indicating aliphatic hydrocarbon layers covering the collembolan cuticle (left, positive secondary ion spectra; right, negative secondary ion spectra). (c) TLC of moulted cuticle (cuticle) and complete animal (animal) hexane/dichloromethane extracts. Standard lipid mixture containing phospholipids (phosphatidylcholine; PC), steroids (cholesterol; CH), triglycerides (glyceryltrioleate; GT), fatty acids (palmitic acid; PA), fatty esters (stearyl palmitate; SP) and steryl esters (cholesteryl palmitate; CP). TLC of both extracts revealed steroids (1), triglycerides (2), fatty acids (3), esters (4) and terpenes (5).
GC-MS analysis was performed to identify the lipids as detected by TLC (electronic supplementary material, figures S3–S9). For the moulted cuticle extract, steroids, fatty acids and one terpene were detected. The detected steroids were identified as cholesterol and desmosterol, the fatty acids as palmitic and stearic acid and the terpene as lycopaen (table 1). In case of the complete animal extract, steroids, fatty acids, esters and terpenes could be detected, which was in accordance with the TLC measurements. The steroids again were identified as cholesterol and desmosterol, in addition the fatty acids palmitic acid, stearic acid, oleic acid and linoleic acid. The esters were identified as linolenyl myristate and linoleyl palmitate, which are common wax esters. The terpenes were in line with the results from [29] identified as lycopan, lycopaen and lycopadien (table 1). The extract of moulted cuticles contained fewer substances, which might be due to lower amounts of lipids retained.
Table 1.
Lipid components of the moulted cuticle and complete animal hexane/dichloromethane extract as detected by TLC and GC-MS.
 |
Extraction of the outermost lipid layer of freshly shed cuticles left the epicuticle and the procuticle behind for further analysis. The epicuticle was subsequently dissolved in a 2.5 M KOH solution for hydrolysis of proteins (figure 4). Figure 4b depicts the destruction of the comb-like structure after 0.5 h incubation time. After 24 h, the protein-rich epicuticle was completely dissolved. In order to verify the proteinaceous nature of the nanoscopic surface features, the solution was further analysed by HPLC. Table 2 summarizes the amino acid composition and the particular concentrations of the moulted cuticle extract. Glycine represents more than 50% of the detected amino acids. Furthermore, the sample showed a high amount of tyrosine and serine residues. For comparison, the amino acid compositions of structural proteins, such as fibroin (Bombyx mori), collagen (Periplaneta americana) and resilin (Schistocerca gregaria) [30–32] are included in table 2. A common feature of structural proteins, glycine was the dominating amino acid in all proteins. Therefore, the epicuticular structures of the collembolan cuticle are concluded to also consist of structural proteins. Additionally, decellularized (TritonX treated) animals were analysed by amino acid analysis. The results clearly differed (the glycine amount was reduced to 35%, the amounts of alanine and serine increased) determining the decellularization of animals by TritonX to be unsuitable in identifying cuticle proteins.
Figure 4.
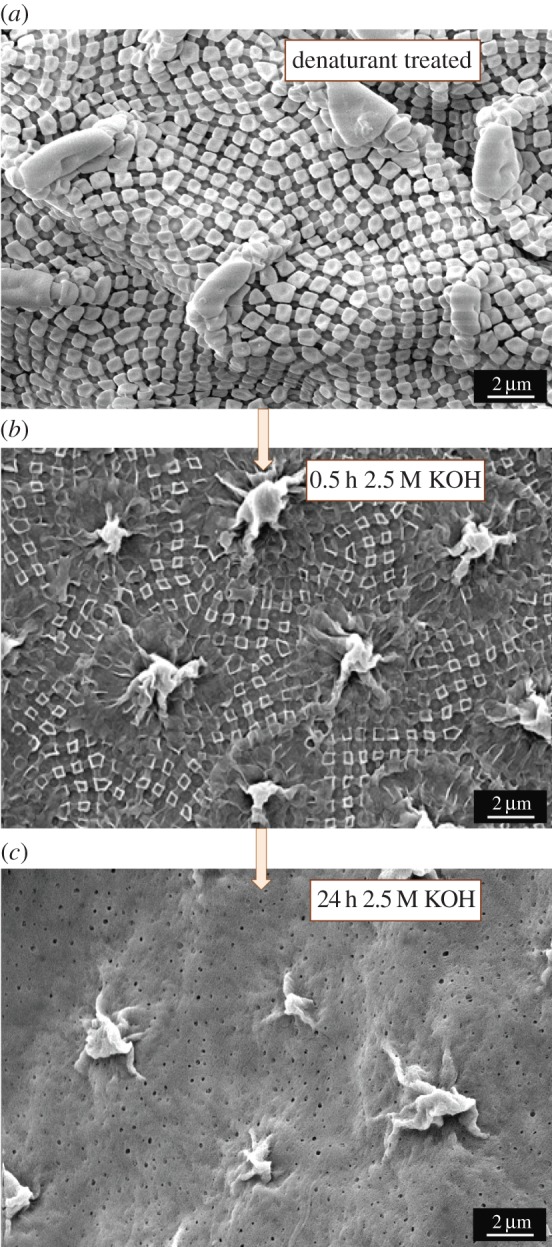
Separation and analysis of the procuticle and epicuticle. (a) Cuticle treated with 8 M urea, 6 M guanidine hydrochloride, 20% SDS and guanidinium thiocyanate for one week at 60°C without changes in the epicuticular structures. (b) Cuticle samples treated with 2.5 M KOH solution for 0.5 h for dissolving the cuticle structures. The KOH solution was used for HPLC analysis. (c) Further treatment of the cuticle with 2.5 M KOH led to complete dissolution of the epicuticular proteins uncovering the chitin skeleton. (Online version in colour.)
Table 2.
Detected amino acids by HPLC of the moulted cuticle KOH hydrolysate and from decellularized animals. Amino acid analyses of fibroin (B. mori), collagen (P. americana) and resilin (S. gregaria) are included for comparison.
| amino acids |
moulted cuticle | decellularized animal | fibroin [32] B. mori |
collagen [33] P. americana |
resilin [34] S. gregaria |
|
|---|---|---|---|---|---|---|
| ASP | aspartic acid | 5.25 | 3.76 | 1.30 | 5.00 | 12.50 |
| GLU | glutamic acid | 5.49 | 3.93 | 1.00 | 9.10 | 6.20 |
| SER* | Serine | 7.79 | 17.04 | 12.10 | 3.90 | 8.00 |
| HIS | histidine | 0.14 | 0.09 | 0.20 | 0.40 | 1.70 |
| GLY* | glycine | 56.02 | 35.49 | 44.50 | 32.00 | 26.90 |
| THR | threonine | 1.01 | 3.49 | 0.90 | 2.40 | 3.70 |
| ARG | arginine | 0.46 | 0.94 | 0.50 | 4.50 | 6.50 |
| ALA | alanine | 5.82 | 15.62 | 29.30 | 7.80 | 9.45 |
| TYR* | tyrosine | 7.00 | 4.40 | 5.20 | — | 4.00 |
| MET | methionine | 0.06 | 0.33 | 0.10 | — | — |
| VAL | valine | 0.47 | 1.07 | 2.20 | 2.20 | 3.10 |
| PHE | phenylalanine | 1.77 | 2.54 | 0.60 | 1.30 | 4.40 |
| ILE | isoleucine | 0.50 | 1.07 | 0.70 | 1.60 | 1.90 |
| LEU | leucine | 5.07 | 6.77 | 0.50 | 3.50 | 2.60 |
| LYS | lysine | 0.55 | 0.27 | 0.30 | 2.00 | 0.85 |
| CYS | cysteine | — | — | 0.20 | — | — |
| PRO | proline | 1.61 | 2.00 | 0.30 | 12.00 | 8.20 |
| HYP | hydroxyproline | — | — | — | 10.70 | — |
| HYL | hydroxylysine | — | — | — | 1.60 | — |
For protein identification, the epicuticular structures were exposed to 8 M urea, 6 M guanidine hydrochloride, guanidinium thiocyanate (TriFast) and 20% sodium dodecyl sulfate (SDS) for at least one week at 60°C. However, these treatments did not alter the epicuticular pattern (figure 4a), indicating a robust protein structure. Interestingly, the common covalent cross-links, such as disulfide bonds or dityrosine amino acids residues, were not observed in the samples by UV–Vis spectroscopy (electronic supplementary material, figure S10), suggesting a non-covalent association.
After KOH hydrolysis, the remaining procuticle skeletons (figure 4c) were further hydrolysed by 6 M HCl and analysed by ESI-MS (electronic supplementary material, figure S11) that confirmed the presence of chitin. Additionally, SEM (figure 4c) and Cryo-SEM (figure 5) studies revealed a regular distribution of pore channels within the chitin skeleton. The pore channels showed different sizes and morphologies. Most channels were small with a diameter of about 200 nm and vertically interpenetrated the entire procuticle. Some pore channels appeared to be thicker (diameter of about 2 µm) but branched into smaller channels close to the epicuticle.
Figure 5.
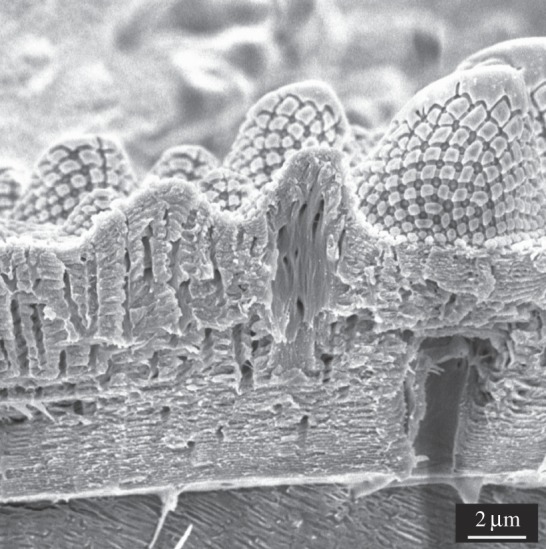
Cryo-SEM image of the cuticle with visible pore channels.
In summary, the chemical analyses revealed a multi-layered cuticle structure, consisting of a topmost lipid-rich envelope, a protein-rich epicuticle and a chitin-rich procuticle (figure 6). The homogeneous lipid-rich layer, covering the entire surface, encompassed aliphatic hydrocarbons such as steroids, triglycerides, fatty acids, wax esters and terpenes. The characteristic morphological surface features of the cuticle consisted of a protein-rich layer, dominated by a glycine-rich structural protein. The inner cuticle layer consisted of a lamellar chitin skeleton that was interpenetrated by numerous pore channels.
Figure 6.
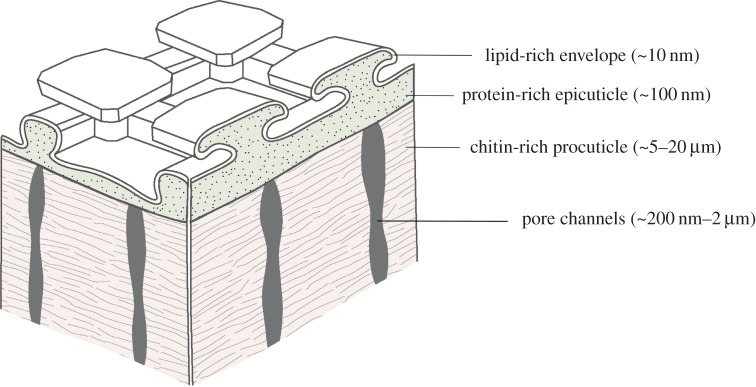
Model of the multi-layered cuticle of Collembola. (Online version in colour.)
3. Discussion
Our presented study revealed that the cuticle of T. bielanensis, showing the characteristic collembolan ornamentations, consisted of a cross-sectional layer structure known from arthropods, namely, a chitin-rich procuticle, a chitin-free but protein-rich epicuticle and an envelope composed of a lipid mixture. The reported results confirm earlier investigations on basic features of the collembolan cuticle [24–26] and on cuticle differentiation [33].
The chitin-rich procuticle forms the basis of the exoskeleton and provides rigidity and mechanical protection to the body [23]. The numerous nanostructured pore channels enable material transport [25,34]. Moreover, these channels may indicate a preliminary stage of a tracheal system that particularly allows T. bielanensis, the European giant springtail, to respire by gaseous diffusion through the cuticle surface. Nevertheless, the channels do not interpenetrate the entire cuticle, which can be considered a physiological advantage in preventing high transpirations rates and penetration of microorganisms.
The protein-rich epicuticle covers the chitin layer. The epicuticle contains the distinctive surface features of Collembola in displaying a comb-like mesh covering the entire body. Cavities inside the epicuticular mesh allow for respiration [12,13]. The nanoscopic tubercles at the intersections of the mesh structure prevent liquids wetting these cavities facilitating respiration under wet conditions and preventing suffocation in temporarily rain-flooded habitats. In a recent study, the durability of these epicuticular structures was demonstrated by sand blast experiments [12]. We found that proteins with high amounts of glycine, tyrosine and serine formed the epicuticular structures. The composition of the amino acid mixture resembled that of known structural proteins such as fibroin, collagen or resilin [30–32], which often combine stiffness and toughness [35]. Thus, it can be reasonably assumed that the durability of patterned epicuticle of T. bielanensis to withstand wear in soil habitats results from epicuticular protein structures.
As the outermost layer, a lipid mixture of fatty acids, wax esters and terpenes envelopes the epicuticular structures, forming the first protective barrier of the animal. Wax esters support the non-wetting properties due to their hydrophobic characteristics [6]. The thin lipid layer enables gas exchange, but hardly protects against transpiration and desiccation [36]. Therefore, Collembola, with some exceptions, depend on humid surroundings as given in soil habitats. Furthermore, the collembolan cuticle is exposed to microorganisms. Some of the lipids detected, such as fatty acids and terpenes, can be assumed to afford the non-fouling characteristics of the cuticle surface due to their intrinsic antibacterial effect. Free fatty acids, for example, were reported to interact with the cell membrane of bacteria causing growth inhibition or direct killing [37–39]. Furthermore, it was shown that free fatty acids with medium or short chain lengths can inhibit fungal development [40]. Likewise, terpenes are considered defence substances of plants and insects, and can therefore be expected to protect the cuticle surface against parasitic or pathogenic microorganisms [41].
The unique hierarchical topography of the layered cuticle reflects the adaptation of Collembola to their soil habitats, enabling the permeability to gases, minimizing liquid wettability and fouling by microorganisms and resisting abrasion. Those remarkable cuticle characteristics were found to be achieved by a rather typical arthropod cuticle surface chemistry. Thus, our data highlight the importance of topographical features of the cuticle for its functional characteristics.
4. Material and methods
4.1. Animals
Tetrodontophora bielanensis species were collected in the wooded mountains of Saxony near Dresden, southeastern Germany. The animals were kept as laboratory colonies in large Petri dishes using soil, litter, decaying wood and moss from their original habitat as substrate and food source. The substrate was wetted regularly to maintain humid conditions. Tetrodontophora bielanensis species survived up to six months in captivity, preferably at 12–14°C, which was maintained by storage in wine cooler board. During this time, animals were collected regularly for experiments. Additionally, moulted cuticles of the animals were collected from the Petri dishes and used as cuticle samples. Moulting occurred at three to four week intervals.
4.2. Electron imaging
SEM studies were performed using a XL30 ESEM-FEG microscope (Philips) in the usual HighVac mode at voltages of 5 kV. Cryo-SEM studies were performed by a Zeiss Supra 40VP with an Emitech K1250× cryo transfer device in the usual HighVac mode at voltages of 5 kV. The animals were prepared by freezing and subsequent air-drying without any fixation. Samples were coated with a 15 nm gold layer (BALZERS SCD 050 Sputter Coater) to avoid surface charging effects. TEM studies were carried out using an EM 912 Omega (Carl Zeiss SMT). The samples were fixed, stained and subsequently sliced into ultrathin sections as described in detail by Helbig et al. [12].
4.3. Sample preparation for chemical analysis
4.3.1. Lipid layer analysis
Moulted cuticles (30–40 specimens) were collected, washed in distilled water, air-dried and subsequently extracted in a hexane/dichloromethane (1 : 1) solvent mixture for 30 min at 60°C. The extract was concentrated by solvent evaporation. Complete animals (20 specimens) were shock frozen, air-dried for 24 h at room temperature (RT) and extracted under the same conditions. The hexane/dichloromethane extracts were used for TLC and GC-MS analysis.
4.3.2. Amino acid analysis
Extracted moulted cuticles were treated directly with 2.5 M KOH solution for 0.5–24 h at RT [42]. Extracted animal samples were decellularized to remove cellular proteins by placing animals in 0.5% TritonX (Sigma-Aldrich) solution for one week. Afterwards, the decellularized animals were washed in distilled water several times to remove TritonX. The decellularized animals were treated with 2.5 M KOH solution for 0.5–24 h at RT. The KOH solution with the hydrolysed cuticle proteins were used for HPLC analysis.
4.3.3. Chitin analysis
Remaining cuticle samples treated with 2.5 M KOH were further hydrolysed in 6 M HCl for 4 days at 80°C. The obtained samples were filtered with 0.4-µm filter and freeze dried. The solid remnant was dissolved in methanol for ESI-MS analysis.
4.4. Time of flight secondary ion mass spectrometry
For TOF-SIMS analysis, animals were shock frozen and air-dried for 24 h at RT. For extract analysis, animal samples were prepared as described in §4.3.1. The extract was coated on glass surfaces and analysed. Measurements were performed using an ION TOF TOF-SIMS V instrument equipped with a Bi liquid metal ion gun. Analysis was carried out as described in detail by Nygren et al. [43]. All image analyses were performed within the Ion-Tof Ion image software (v. 3.1, Ion-Tof, GmbH, Münster, Germany).
4.5. Thin layer chromatography
Hexane/dichloromethane extracts of moulted cuticles and complete animals were prepared as described in §4.3.1. A mixture of 30 mg lipids in 2 ml of dichloromethane was used as a lipid standard, containing phosphatidylcholine, cholesterol, glyceryltrioleate, palmitic acid, stearyl palmitate and cholesteryl palmitate. TLC was performed using TLC sheets with a 0.2 mm silica gel layer (ALUGRAM, Machery-Nagel). Three solvent systems were used stepwise on one TLC plate for lipid separation (adapted from [44]):
methanol : chloroform : acetic acid (50 : 30 : 16),
hexane : diethyl ether : acetic acid (70 : 2 : 0.2) and
hexane.
System (1) ran until the solvent line migrated to the first third of the plate. Subsequently, the plate was dried and placed in the solvent system (2) until the solvent line migrated to the second third of the plate. Solvent system (3) ran the complete plate until 1 cm above the end of the plate. All solvents were purchased by Sigma-Aldrich. Subsequent staining of the lipids was carried out with amido black staining (Sigma-Aldrich) [45].
4.6. Gas chromatography mass spectrometry
Hexane/dichloromethane extracts of moulted cuticles and complete animals were prepared as described in §4.3.1. Measurements were performed using an Agilent Technologies 6890 N GC System equipped with a 5973 Mass Selective Detector. GC separation was conducted with a temperature programme from 110°C (1 min) to 340°C (30 min) at a rate of 10°C min−1, respectively. One microlitre of the sample was injected for analysis. The mass spectrometer was operated in the electron impact ionization mode with ionization energy of 70 eV. Data evaluation was performed using LIPID MAPS Lipidomics Gateway database.
4.7. High-performance liquid chromatography
KOH hydrolysates of extracted moulted cuticles and extracted animals were prepared as described in §4.3.2 and used for amino acid analysis. HPLC analysis was performed by amino acid sample derivatization using o-phthalaldehyde for primary amino acids and 9-fluorenylmethyl chloroformate for secondary amino acids [46]. Measurements were performed by analytical HPLC Zorbax Eclipse-AAA column (4.6 × 150 mm, 3.5 µm, Agilent Technologies, USA) for 40 min and a flow rate of 0.8 ml min−1 for the analytical column. A linear gradient of 0.1 M phosphate buffer (pH7.8) and acetonitrile/methanol/water 45/45/10 was used as the mobile phase. A two-pump system (Agilent Technologies 1100 Series, USA) equipped with a UV/Vis detector/spectrophotometer in line with fluorescence detector both having a 1 cm path length cell was used for analysis of the amino acid composition.
Supplementary Material
Acknowledgement
We thank Dr Armin Springer for assistance on the preparation of the TEM samples and Uta Reuter for the TEM investigations. The Tascon GmbH is thanked for TOF-SIMS measurements and data evaluation. Prof. Christian Hannig and Marco Reich are gratefully acknowledged for helpful advices on lipid analysis concerning TLC and GC-MS. We are grateful to Ljubow Rößler for GC-MS measurements and Dr Ingmar Bauer and Dr Susanne Machill for supporting discussion on GC-MS data evaluation. Marcus Günther is acknowledged for assistance on the Cryo-SEM imaging. Special thanks go to Dr Ralf Helbig for helpful discussions on the project and for improving the manuscript.
Funding statement
This work was supported by Deutsche Forschungsgemeinschaft (DFG WE 2539/17-1).
References
- 1.Kirschner CM, Brennan AB. 2012. Bio-inspired antifouling strategies. Annu. Rev. Mater. Res. 42, 211–229. ( 10.1146/annurev-matsci-070511-155012) [DOI] [Google Scholar]
- 2.Liu K, Jiang L. 2012. Bio-inspired self-cleaning surfaces. Annu. Rev. Mater. Res. 42, 231–263. ( 10.1146/annurev-matsci-070511-155046) [DOI] [Google Scholar]
- 3.Barthlott W, Neinhuis C. 1997. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202, 1–8. ( 10.1007/s004250050096) [DOI] [Google Scholar]
- 4.Neinhuis C, Barthlott W. 1997. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 79, 667–677. ( 10.1006/anbo.1997.0400) [DOI] [Google Scholar]
- 5.Ensikat HJ, Ditsche-Kuru P, Neinhuis C, Barthlott W. 2011. Superhydrophobicity in perfection: the outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2, 152–161. ( 10.3762/bjnano.2.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch K, Ensikat H-J. 2008. The hydrophobic coatings of plant surfaces: epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 39, 759–772. ( 10.1016/j.micron.2007.11.010) [DOI] [PubMed] [Google Scholar]
- 7.Neinhuis C, Koch K, Barthlott W. 2001. Movement and regeneration of epicuticular waxes through plant cuticles. Planta 213, 427–434. ( 10.1007/s004250100530) [DOI] [PubMed] [Google Scholar]
- 8.Patankar NA. 2004. Mimicking the lotus effect: influence of double roughness structures and slender pillars. Langmuir 20, 8209–8213. ( 10.1021/la048629t) [DOI] [PubMed] [Google Scholar]
- 9.Koch K, Dommisse A, Barthlott W, Gorb SN. 2007. The use of plant waxes as templates for micro- and nanopatterning of surfaces. Acta Biomater. 3, 905–909. ( 10.1016/j.actbio.2007.05.013) [DOI] [PubMed] [Google Scholar]
- 10.Koch K, Bhushan B, Barthlott W. 2009. Multifunctional surface structures of plants: an inspiration for biomimetics. Prog. Mater. Sci. 54, 137–178. ( 10.1016/j.pmatsci.2008.07.003) [DOI] [Google Scholar]
- 11.Verho T, Bower C, Andrew P, Franssila S, Ikkala O, Ras RHA. 2011. Mechanically durable superhydrophobic surfaces. Adv. Mater. 23, 673–678. ( 10.1002/adma.201003129) [DOI] [PubMed] [Google Scholar]
- 12.Helbig R, Nickerl J, Neinhuis C, Werner C. 2011. Smart skin patterns protect springtails. PLoS ONE 6, e25105 ( 10.1371/journal.pone.0025105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hensel R, Helbig R, Aland S, Braun H-G, Voigt A, Neinhuis C, Werner C. 2013. Wetting resistance at its topographical limit: the benefit of mushroom and serif T structures. Langmuir 29, 1100–1112. ( 10.1021/la304179b) [DOI] [PubMed] [Google Scholar]
- 14.Nickerl J, Helbig R, Schulz H-J, Werner C, Neinhuis C. 2013. Diversity and potential correlations to the function of Collembola cuticle structures. Zoomorphology 132, 183–195. ( 10.1007/s00435-012-0181-0) [DOI] [Google Scholar]
- 15.Bellinger PF, Christiansen KA, Janssens F. 1996–2014. Checklist of the Collembola of the world See http://www.collembola.org (accessed 12 August 2013).
- 16.Rusek J. 1998. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 7, 1207–1219. ( 10.1023/A:1008887817883) [DOI] [Google Scholar]
- 17.Davies WM. 1927. Memoirs: on the tracheal system of Collembola, with special reference to that of Sminthurus viridis, Lubb. Q. J. Microsc. Sci. 71, 15–30. [Google Scholar]
- 18.Zinkler D. 1966. Vergleichende Untersuchungen zur Atmungsphysiologie von Collembolen (Apterygota) und anderen Bodenkleinarthropoden. Z. Vgl. Physiol. 52, 99–144. ( 10.1007/BF00343157) [DOI] [Google Scholar]
- 19.Hinton H. 1961. How some insects, especially the egg stages, avoid drowning when it rains. Proc. S. Lond. Entomol. Nat. Hist. Soc. 1960, 138–154. [Google Scholar]
- 20.Hale WG, Smith AL. 1966. Scanning electron microscope studies of cuticular structures in the genus Onychiurus (Collembola). Rev. Ecol. Biol. Sol. 3, 343–354. [Google Scholar]
- 21.Hensel R, Helbig R, Aland S, Voigt A, Neinhuis C, Werner C. 2013. Tunable nano-replication to explore the omniphobic characteristics of springtail skin. NPG Asia Mater. 5, e37 ( 10.1038/am.2012.66) [DOI] [Google Scholar]
- 22.Hensel R, Finn A, Helbig R, Braun H-G, Neinhuis C, Fischer W-J, Werner C. 2014. Biologically inspired omniphobic surfaces by reverse imprint lithography. Adv. Mater. 26, 2029–2033. ( 10.1002/adma.201305408) [DOI] [PubMed] [Google Scholar]
- 23.Neville AC. 1975. Biology of the arthropod cuticle, 4th edn Berlin, Germany: Springer. [Google Scholar]
- 24.Noble-Nesbitt J. 1963. The fully formed intermoult cuticle and associated structures of Podura aquatica (Collembola). Q. J. Microsc. Sci. 104, 253–270. [Google Scholar]
- 25.Krzysztofowicz A, Klag J, Komorowska B. 1972. The fine structure of the cuticle in Tetrodontophora bielanensis (Waga), Collembola. Acta Biol. Cracoviensia Ser. Zool. 15, 113–119. [Google Scholar]
- 26.Eisenbeis G, Wichard W. 1987. Atlas on the biology of the soil arthropods. Berlin, Germany: Springer. [Google Scholar]
- 27.Noble-Nesbitt J. 1963. Transpiration in Podura aquatica L. (Collembola, Isotomidae) and the wetting properties of its cuticle. J. Exp. Biol. 40, 681–700. [Google Scholar]
- 28.Ghiradella H, Radigan W. 1974. Collembolan cuticle: wax layer and antiwetting properties. J. Insect Physiol. 20, 301–306. ( 10.1016/0022-1910(74)90062-6) [DOI] [PubMed] [Google Scholar]
- 29.Brasse G. 2005. Neue Naturstoffe aus Collembolen. Doctoral Thesis. Technische Universität Carolo-Wilhelmia, Braunschweig, Germany. [Google Scholar]
- 30.Lotz B, Colonna Cesari F. 1979. The chemical structure and the crystalline structures of Bombyx mori silk fibroin. Biochimie 61, 205–214. ( 10.1016/S0300-9084(79)80067-X) [DOI] [PubMed] [Google Scholar]
- 31.Francois J, Herbage D, Junqua S. 1980. Cockroach collagen: isolation, biochemical and biophysical characterization. Eur. J. Biochem. 112, 389–396. ( 10.1111/j.1432-1033.1980.tb07217.x) [DOI] [PubMed] [Google Scholar]
- 32.Bailey K, Weis-Fogh T. 1961. Amino acid composition of a new rubber-like protein, resilin. Biochim. Biophys. Acta 48, 452–459. ( 10.1016/0006-3002(61)90043-9) [DOI] [PubMed] [Google Scholar]
- 33.Moussian B. 2010. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 40, 363–375. ( 10.1016/j.ibmb.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 34.Locke M. 1961. Pore canals and related structures in insect cuticle. J. Biophys. Biochem. Cytol. 10, 589–618. ( 10.1083/jcb.10.4.589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lintz ES, Scheibel TR. 2013. Dragline, egg stalk and byssus: a comparison of outstanding protein fibers and their potential for developing new materials. Adv. Funct. Mater. 23, 4467–4482. ( 10.1002/adfm.201300589) [DOI] [Google Scholar]
- 36.King P, Pugh P, Fordy M, Love N, Wheeler S. 1990. A comparison of some environmental adaptations of the littoral collembolans Anuridella marina (Willem) and Anurida maritima (Guérin). J. Nat. Hist. 24, 673–688. ( 10.1080/00222939000770461) [DOI] [Google Scholar]
- 37.McGaw L, Jäger A, Staden JV. 2002. Antibacterial effects of fatty acids and related compounds from plants. S. Afr. J. Bot. 68, 417–423. [Google Scholar]
- 38.Benkendorff K, Davis AR, Rogers CN, Bremner JB. 2005. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Bio. Ecol. 316, 29–44. ( 10.1016/j.jembe.2004.10.001) [DOI] [Google Scholar]
- 39.Desbois AP, Smith VJ. 2010. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 85, 1629–1642. ( 10.1007/s00253-009-2355-3) [DOI] [PubMed] [Google Scholar]
- 40.Koidsumi K. 1957. Antifungal action of cuticular lipids in insects. J. Insect Physiol. 1, 40–51. ( 10.1016/0022-1910(57)90022-7) [DOI] [Google Scholar]
- 41.Gershenzon J, Dudareva N. 2007. The function of terpene natural products in the natural world. Nat. Chem. Biol. 3, 408–414. ( 10.1038/nchembio.2007.5) [DOI] [PubMed] [Google Scholar]
- 42.Brunner E, et al. 2009. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 168, 539–547. ( 10.1016/j.jsb.2009.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nygren H, Börner K, Hagenhoff B, Malmberg P, Månsson J. 2005. Localization of cholesterol, phosphocholine and galactosylceramide in rat cerebellar cortex with imaging TOF-SIMS equipped with a bismuth cluster ion source. Biochim. Biophys. Acta 1737, 102–110. ( 10.1016/j.bbalip.2005.10.004) [DOI] [PubMed] [Google Scholar]
- 44.Schuh TJ. 2002. An introduction to lipid analysis in the cell biology laboratory. Am. Biol. Teach. 64, 122–129. ( 10.1662/0002-7685(2002)064[0122:AITLAI]2.0.CO;2) [DOI] [Google Scholar]
- 45.Plekhanov AY. 1999. Rapid staining of lipids on thin-layer chromatograms with amido black 10B and other water-soluble stains. Anal. Biochem. 271, 186–187. ( 10.1006/abio.1999.4127) [DOI] [PubMed] [Google Scholar]
- 46.Henderson JW, Ricker RD. 2000. Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Agilent Technologies, Technical Note 5980-1193E. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.