Abstract
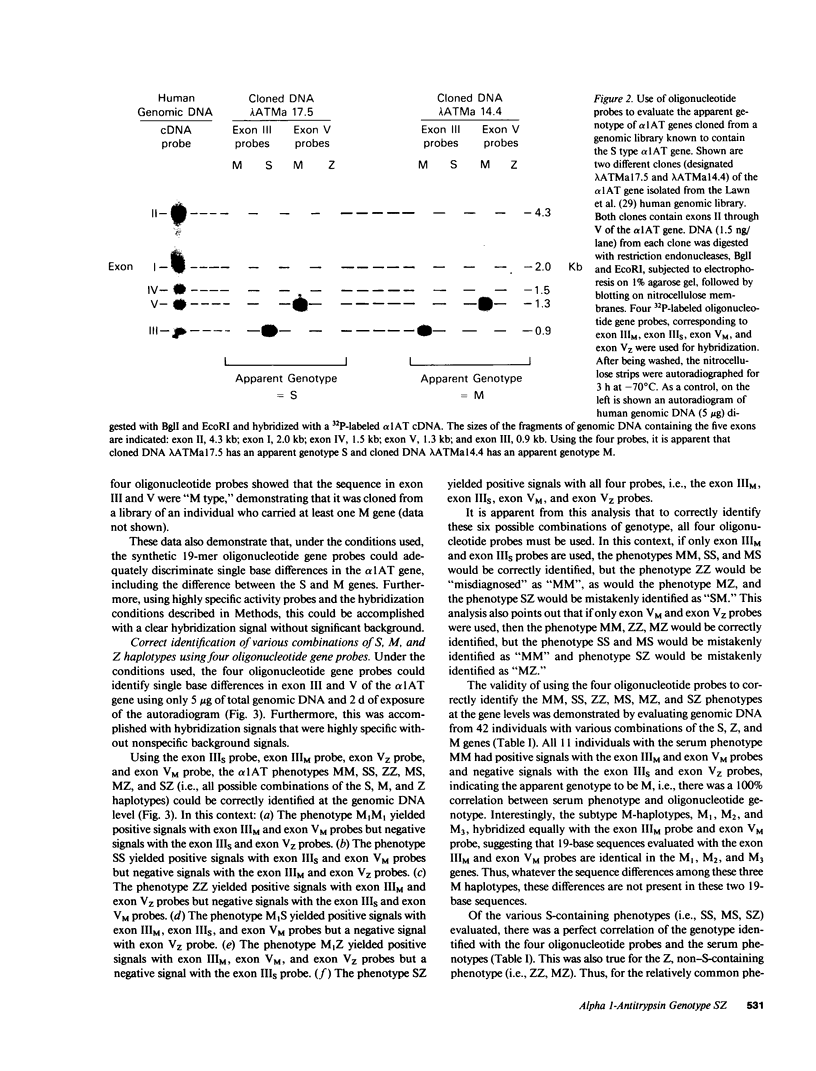
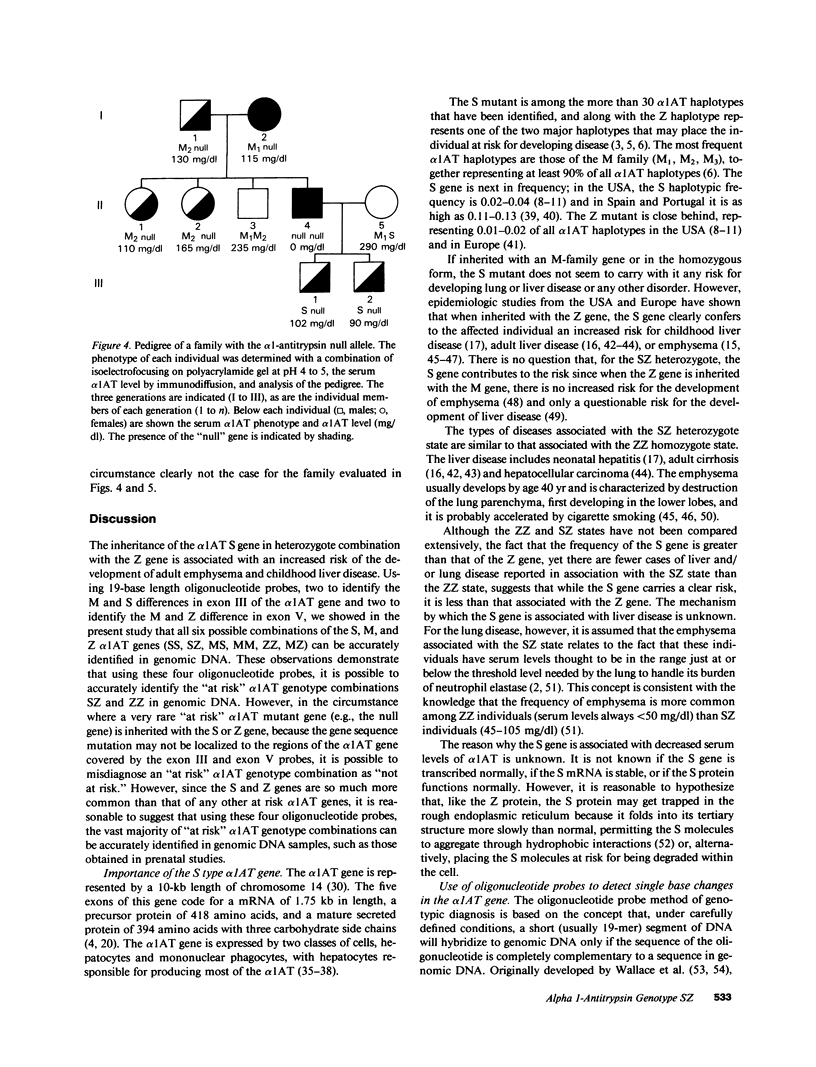
Alpha 1-antitrypsin (alpha 1AT), a 52,000-mol-wt serum glycoprotein produced by hepatocytes and mononuclear phagocytes, functions as the major inhibitor of neutrophil elastase. The alpha 1AT haplotype S is associated with childhood liver disease and/or adult emphysema when inherited with the Z haplotype to give the phenotype SZ. To accurately identify the SZ phenotype at the level of genomic DNA, four 32P-labeled 19-mer synthetic oligonucleotide probes were prepared; two to identify the M and S difference in exon III, and two to identify the M and Z difference in exon V. These probes were hybridized with various cloned DNAs and genomic DNAs cut with the restriction endonucleases BgII and EcoRI; the genomic DNAs represented all six possible phenotype combinations of the M, S, and Z haplotypes (MM, MS, MZ, SS, ZZ, and SZ). Using the four probes to evaluate 42 samples of genomic DNA, the "at risk" SZ and ZZ phenotypes were correctly identified in all cases, as were the "not at risk" phenotypes SS, MS, MM, and MZ, demonstrating that both exon III and exon V directed probes are necessary to properly identify all of the major "at risk" alpha 1AT genes. However, when used to evaluate a very rare family carrying a null allele, these four oligonucleotide probes misidentified the "at risk" null-null and S null phenotypes as "not at risk" MM and SM combinations. These observations indicate that oligonucleotide gene probes yielded reliable and accurate assessment of "at risk" alpha 1AT genotypes in almost all situations, but in the context of prenatal diagnosis and genetic counseling this approach must be used with caution and in combination with family studies so as not to misidentify rare genotypes that may be associated with a risk for disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Toksoz D., Marshall C. J., Verlaan-de Vries M., Veeneman G. H., van der Eb A. J., van Boom J. H., Janssen J. W., Steenvoorden A. C. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. 1985 Jun 27-Jul 3Nature. 315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- Bruce R. M., Cohen B. H., Diamond E. L., Fallat R. J., Knudson R. J., Lebowitz M. D., Mittman C., Patterson C. D., Tockman M. S. Collaborative study to assess risk of lung disease in Pi MZ phenotype subjects. Am Rev Respir Dis. 1984 Sep;130(3):386–390. doi: 10.1164/arrd.1984.130.3.386. [DOI] [PubMed] [Google Scholar]
- Campra J. L., Craig J. R., Peters R. L., Reynolds T. B. Cirrhosis associated with partial deficiency of alpha-1 antitrypsin in an adult. Ann Intern Med. 1973 Feb;78(2):233–238. doi: 10.7326/0003-4819-78-2-233. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Chan C. H., Steer C. J., Vergalla J., Jones E. A. Alpha1-antitrypsin deficiency with cirrhosis associated with the protease inhibitor phenotype SZ. Am J Med. 1978 Dec;65(6):978–986. doi: 10.1016/0002-9343(78)90750-7. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Dente L., Cortese R. Cell-specific expression of a transfected human alpha 1-antitrypsin gene. Cell. 1985 Jun;41(2):531–540. doi: 10.1016/s0092-8674(85)80026-x. [DOI] [PubMed] [Google Scholar]
- Conner B. J., Reyes A. A., Morin C., Itakura K., Teplitz R. L., Wallace R. B. Detection of sickle cell beta S-globin allele by hybridization with synthetic oligonucleotides. Proc Natl Acad Sci U S A. 1983 Jan;80(1):278–282. doi: 10.1073/pnas.80.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans J., Viau M., Gouaillard C. Pi M4: an additional Pi M subtype. Hum Genet. 1980;55(1):119–121. doi: 10.1007/BF00329137. [DOI] [PubMed] [Google Scholar]
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Craig J. R., Dunn A. E., Peters R. L. Cirrhosis associated with partial deficiency of alpha-1-antitrypsin: a clinical and autopsy study. Hum Pathol. 1975 Jan;6(1):113–120. doi: 10.1016/s0046-8177(75)80112-2. [DOI] [PubMed] [Google Scholar]
- Dykes D. D., Miller S. A., Polesky H. F. Distribution of alpha 1-antitrypsin variants in a US white population. Hum Hered. 1984;34(5):308–310. doi: 10.1159/000153485. [DOI] [PubMed] [Google Scholar]
- Eriksson S. Studies in alpha 1-antitrypsin deficiency. Acta Med Scand Suppl. 1965;432:1–85. [PubMed] [Google Scholar]
- Evans H. E., Bognacki N. S., Perrott L. M., Glass L. Prevalence of of alpha 1-antitrypsin Pi types among newborn infants of different ethnic backgrounds. J Pediatr. 1977 Apr;90(4):621–624. doi: 10.1016/s0022-3476(77)80384-3. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Fagerhol M. K., Hauge H. E. Serum Pi types in patients with pulmonary diseases. Acta Allergol. 1969 May;24(2):107–114. doi: 10.1111/j.1398-9995.1969.tb03760.x. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K. Serum Pi types in Norwegians. Acta Pathol Microbiol Scand. 1967;70(3):421–428. doi: 10.1111/j.1699-0463.1967.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Fisher R. L., Taylor L., Sherlock S. alpha-1-antitrypsin deficiency in liver disease: the extent of the problem. Gastroenterology. 1976 Oct;71(4):646–651. [PubMed] [Google Scholar]
- Frants R. R., Noordhoek G. T., Eriksson A. W. Separator isoelectric focusing for identification of alpha-1-antitrypsin (Pi M) subtypes. Scand J Clin Lab Invest. 1978 Sep;38(5):457–462. doi: 10.3109/00365517809108451. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Hunninghake G. W., Fells G. A., Zimmerman R. L., Keogh B. A., Crystal R. G. Evaluation of the protease-antiprotease theory of human destructive lung disease. Bull Eur Physiopathol Respir. 1980;16 (Suppl):27–40. doi: 10.1016/b978-0-08-027379-2.50005-3. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Klein H. G., Holland P. V., Crystal R. G. Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981 Nov;68(5):1158–1165. doi: 10.1172/JCI110360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Northemann W., Schmelzer E., Gross V., Gauthier F., Heinrich P. C. Synthesis of alpha 1-antitrypsin in rat-liver hepatocytes and in a cell-free system. Eur J Biochem. 1982 Aug;126(1):189–195. doi: 10.1111/j.1432-1033.1982.tb06765.x. [DOI] [PubMed] [Google Scholar]
- Gishen P., Saunders A. J., Tobin M. J., Hutchison D. C. Alpha 1-antitrypsin deficiency: the radiological features of pulmonary emphysema in subjects of Pi type Z and Pi type SZ: a survey by the British Thoracic Association. Clin Radiol. 1982 Jul;33(4):371–377. doi: 10.1016/s0009-9260(82)80297-3. [DOI] [PubMed] [Google Scholar]
- Goedde H. W., Hirth L., Benkmann H. G., Pellicer A., Pellicer T., Stahn M., Singh S. Population genetic studies of serum protein polymorphisms in four Spanish populations. II. Hum Hered. 1973;23(2):135–146. doi: 10.1159/000152565. [DOI] [PubMed] [Google Scholar]
- Hutchison D. C. A survey of alpha 1-antitrypsin deficiency by the British Thoracic Association. Bull Eur Physiopathol Respir. 1980;16 (Suppl):315–319. doi: 10.1016/b978-0-08-027379-2.50033-8. [DOI] [PubMed] [Google Scholar]
- Hutchison D. C., Tobin M. J., Cook P. J. Alpha 1 antitrypsin deficiency: clinical and physiological features in heterozygotes of Pi type SZ. A survey by the British Thoracic Association. Br J Dis Chest. 1983 Jan;77(1):28–34. doi: 10.1016/0007-0971(83)90003-7. [DOI] [PubMed] [Google Scholar]
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Fagerhol M. Properties of isolated human alpha1-antitrypsins of Pi types M, S and Z. Eur J Biochem. 1978 Feb 1;83(1):143–153. doi: 10.1111/j.1432-1033.1978.tb12078.x. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Markham A. F., Chapman C. R., Youssoufian H., Waber P. G. Quantification of the close association between DNA haplotypes and specific beta-thalassaemia mutations in Mediterraneans. Nature. 1984 Jul 12;310(5973):152–154. doi: 10.1038/310152a0. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Golbus M. S., Wallace R. B., Itakura K., Woo S. L. Prenatal diagnosis of alpha 1-antitrypsin deficiency by direct analysis of the mutation site in the gene. N Engl J Med. 1984 Mar 8;310(10):639–642. doi: 10.1056/NEJM198403083101007. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Christopherson M. J. Alpha1-antitrypsin: further genetic heterogeneity revealed by isoelectric focusing. Am J Hum Genet. 1978 Jul;30(4):359–365. [PMC free article] [PubMed] [Google Scholar]
- Lai E. C., Kao F. T., Law M. L., Woo S. L. Assignment of the alpha 1-antitrypsin gene and a sequence-related gene to human chromosome 14 by molecular hybridization. Am J Hum Genet. 1983 May;35(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Dirksen H., Sundström G., Eriksson S. Lung function studies in asymptomatic individuals with moderately (Pi SZ) and severely (Pi Z) reduced levels of alpha1-antitrypsin. Scand J Respir Dis. 1976;57(6):267–280. [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Gaidulis L., Klotz S. D. A new deficient variant of alpha1-antitrypsin (MDUARTE). Inability to detect the heterozygous state by antitrypsin phenotyping. Am Rev Respir Dis. 1976 Jan;113(1):31–36. doi: 10.1164/arrd.1976.113.1.31. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Martin J. P., Sesboue R., Charlionet R., Ropartz C., Pereira M. T. Genetic variants of serum alpha1-antitrypsin (Pi types) in Portuguese. Hum Hered. 1976;26(4):310–314. doi: 10.1159/000152819. [DOI] [PubMed] [Google Scholar]
- Morse J. O., Lebowitz M. D., Knudson R. J., Burrows B. Relation of protease inhibitor phenotypes to obstructive lung diseases in a community. N Engl J Med. 1977 May 26;296(21):1190–1194. doi: 10.1056/NEJM197705262962102. [DOI] [PubMed] [Google Scholar]
- Morse J. O. alpha1-antitrypsin deficiency (first of two parts). N Engl J Med. 1978 Nov 9;299(19):1045–1048. doi: 10.1056/NEJM197811092991905. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Markham A. F., Kazazian H. H., Jr Direct detection of the common Mediterranean beta-thalassemia gene with synthetic DNA probes. An alternative approach for prenatal diagnosis. J Clin Invest. 1983 Mar;71(3):775–779. doi: 10.1172/JCI110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. C., Brennan S. O., Lewis J. H., Carrell R. W. Mutation of antitrypsin to antithrombin. alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983 Sep 22;309(12):694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- Palmer P. E., Gherardi G. J., Baldwin J. M., Wolfe H. J. Adult liver disease in SZ phenotype alpha-1-antitrypsin deficiency. Ann Intern Med. 1978 Jan;88(1):59–60. doi: 10.7326/0003-4819-88-1-59. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. A., Eradio B., Dew T. A. Antitrypsin phenotypes in St. Louis. JAMA. 1975 Feb 10;231(6):609–612. [PubMed] [Google Scholar]
- Pirastu M., Kan Y. W., Cao A., Conner B. J., Teplitz R. L., Wallace R. B. Prenatal diagnosis of beta-thalassemia. Detection of a single nucleotide mutation in DNA. N Engl J Med. 1983 Aug 4;309(5):284–287. doi: 10.1056/NEJM198308043090506. [DOI] [PubMed] [Google Scholar]
- Rosatelli C., Falchi A. M., Tuveri T., Scalas M. T., Di Tucci A., Monni G., Cao A. Prenatal diagnosis of beta-thalassaemia with the synthetic-oligomer technique. Lancet. 1985 Feb 2;1(8423):241–243. doi: 10.1016/s0140-6736(85)91026-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sproule B. J., Cox D. W., Hsu K., Salkie M. L., Herbert F. A. Pulmonary function associated with the Mmalton deficient variant of alpha 1-antitrypsin. Am Rev Respir Dis. 1983 Feb;127(2):237–240. doi: 10.1164/arrd.1983.127.2.237. [DOI] [PubMed] [Google Scholar]
- Studencki A. B., Conner B. J., Impraim C. C., Teplitz R. L., Wallace R. B. Discrimination among the human beta A, beta S, and beta C-globin genes using allele-specific oligonucleotide hybridization probes. Am J Hum Genet. 1985 Jan;37(1):42–51. [PMC free article] [PubMed] [Google Scholar]
- Studencki A. B., Wallace R. B. Allele-specific hybridization using oligonucleotide probes of very high specific activity: discrimination of the human beta A- and beta S-globin genes. DNA. 1984;3(1):7–15. doi: 10.1089/dna.1.1984.3.7. [DOI] [PubMed] [Google Scholar]
- Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976 Jun 10;294(24):1316–1321. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Schold M., Johnson M. J., Dembek P., Itakura K. Oligonucleotide directed mutagenesis of the human beta-globin gene: a general method for producing specific point mutations in cloned DNA. Nucleic Acids Res. 1981 Aug 11;9(15):3647–3656. doi: 10.1093/nar/9.15.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Ewing C., Wessels M., Lieberman J., Gaidulis L. Molecular abnormality of PI S variant of human alpha1-antitrypsin. Am J Hum Genet. 1977 May;29(3):233–239. [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]









