Abstract
Background
Mechanical ventilation is a common and often life-saving intervention in intensive care medicine. About 35% of all patients in intensive care are mechanically ventilated; about 15% of these patients develop a ventilation-associated pneumonia. The goal of ventilation therapy is to lessen the work of respiration and pulmonary gas exchange and thereby maintain or restore an adequate oxygen supply to the body's tissues. Mechanical ventilation can be carried out in many different modes; the avoidance of ventilation-induced lung damage through protective ventilation strategies is currently a major focus of clinical interest.
Method
This review is based on pertinent articles retrieved by a selective literature search.
Results
Compared to conventional lung-protecting modes of mechanical ventilation, the modern modes of ventilation presented here are further developments that optimize lung protection while improving pulmonary function and the synchrony of the patient with the ventilator. In high-frequency ventilation, tidal volumes of 1–2 mL/kgBW (body weight) are given, at a respiratory rate of up to 12 Hz. Assisted forms of spontaneous respiration are also in use, such as proportional assist ventilation (PAV), neurally adjusted ventilatory assist (NAVA), and variable pressure-support ventilation. Computer-guided closed-loop ventilation systems enable automated ventilation; according to a recent meta-analysis, they shorten weaning times by 32%.
Conclusion
The currently available scientific evidence with respect to clinically relevant endpoints is inadequate for all of these newer modes of ventilation. It appears, however, that they can lower both the invasiveness and the duration of mechanical ventilation, and thus improve the care of patients who need ventilation. Randomized trials with clinically relevant endpoints must be carried out before any final judgments can be made.
In a prospective cohort study, Esteban et al. found that about 35% of all patients in intensive care receive mechanical ventilation (1). It has been estimated that, in the USA, mechanical ventilation is given in about 2.8% of all hospitalizations (i.e., about 790 000 patients per year) (2). The treatment cost of ventilated patients is an estimated $27 billion per year, corresponding to about 12% of the total treatment cost of all hospitalized patients (2).
Although mechanical ventilation is often life-saving, in that it lessens the work of respiration and enables adequate pulmonary gas exchange for the oxygenation of the body's tissues, it can also cause lung damage, or worsen it if already present (3). This phenomenon is called ventilation-induced lung damage, and its main mechanisms are:
high tidal volumes causing overexpansion of the lungs (volutrauma),
high airway pressure (barotrauma),
cyclical collapse and reopening of atelectatic alveolar regions (atelectrauma) (4).
These three types of physical injury lead to a pulmonary inflammatory reaction called "biotrauma,” which often extends beyond the pulmonary parenchyma. It can take the form of a systemic inflammatory reaction, potentially ending in multiple organ system failure.
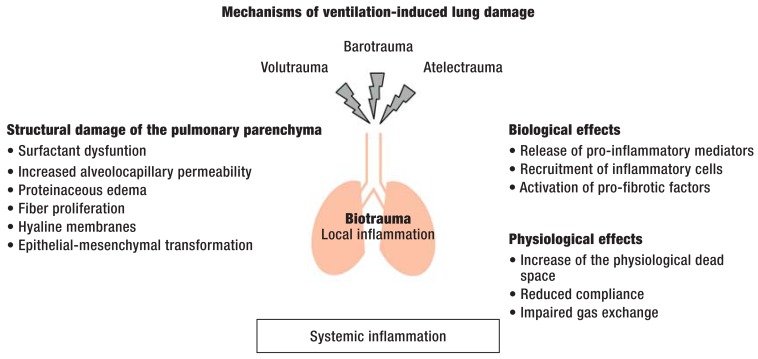
The main pathophysiological mechanisms of ventilation-induced lung damage are shown in the Figure. A further problem is the development of ventilation-associated pneumonia, most often caused by limited mucociliary clearance of the respiratory tract; this is a central challenge in intensive care medicine today. Craven et al. recently reported that about 15% of all ventilated patients develop ventilation-associated pneumonia (5). Modern modes of mechanical ventilation are, therefore, intended to lessen the invasiveness and duration of ventilation to the greatest possible extent in order to prevent such complications. A protective ventilation strategy is important even for patients who do not suffer from any underlying lung disease, as such patients are also exposed to the risk of ventilation-induced lung damage. A meta-analysis by Serpa Neto et al. (6) revealed that, in ventilated patients without any underlying lung disease, the use of a lung-protective mode of ventilation with low tidal volumes significantly lessened not only the frequency of lung damage (relative risk [RR], 0.33; number needed to treat [NNT], 11), but also mortality (RR 0.64, NNT 23).
Figure.
Local and systemic effects of mechanical ventilation – The use of high tidal volumes (volutrauma) and high airway pressures (barotrauma) and the cyclical collapse and reopening of alveolar territories (atelectrauma) can lead to the development of ventilation-induced lung damage. The pulmonary parenchyma sustains structural injury, and pro-inflammatory and pro-fibrotic mediators may be released and/or activated. This pulmonary inflammatory reaction is called biotrauma. Impaired alveolocapillary integrity can also result in a systemic inflammatory reaction, leading to multiple organ system failure. The physiological effects of ventilation-induced lung damage include an increase of the physiological dead space, reduced pulmonary compliance, and impaired pulmonary gas exchange.
Although the newer modes of ventilation discussed here have already been the subject of intensive experimental and clinical research, no evidence-based treatment recommendations can be enunciated at present, as no relevant randomized and controlled clinical trials have yet been carried out.
Method
We selectively searched the PubMed and Cochrane Library databases for pertinent articles with the key words "mechanical ventilation,” "acute respiratory distress syndrome,” "ventilator induced lung injury,” and "new modes of mechanical ventilation.” We subjectively chose studies for discussion here on the basis of our clinical experience. Extracorporeal lung-replacement techniques are explicitly excluded from the discussion; for more information on this topic, the reader is referred to the relevant literature (7).
Conventional lung-protective ventilation strategy
For some years now, a lung-protective ventilation strategy with low tidal volumes (4–8 mL/kgBW in relation to the patient's ideal body weight) and low inspiratory plateau pressures (< 30 cm H2O) has been the gold standard for the mechanical ventilation of patients with the acute respiratory distress syndrome (ARDS) (8). Nonetheless, the mortality of ARDS patients has remained consistantly high at 40% despite the widespread use of this type of lung-protective ventilation (9). Many modifications have been proposed, but the current state of the evidence remains inadequate overall. In particular, the use of positive end-expiratory pressure (PEEP) has been a matter of controversy for years. A meta-analysis of randomized, controlled clinical trials by Briel et al. revealed that the use of high PEEP levels (Day 1: 15.3 cmH2O vs. 9.0 cmH2O) improves oxygenation, at least in the short term (arterial partial pressure of oxygen on Day 1: 96 mmHg [high PEEP] vs. 83 mmHg [low PEEP]), while also lessening the need for emergency measures (13.7% [high PEEP] vs. 21.3% [low PEEP]) (10). The emergency measures for the treatment of refractory hypoxemia that are referred to here included, for example, ventilation in the prone position, the use of inhaled nitric oxide, and extracorporeal membrane oxygenation (ECMO) (10). For patients with moderate or severe ARDS, treatment with high PEEP has been found to improve survival (mortality with low vs. high PEEP, 39.1% vs. 34.1%; RR 0.90, 95% confidence interval [CI] 0.81–1.00, p = 0.049) (10). Moreover, in the treatment of severe ARDS, both early muscle relaxation of short duration (11) and ventilation in the prone position (12) have been shown to lessen mortality.
High-frequency ventilation
High-frequency oscillatory ventilation (HFOV) is based on the administration of relatively small tidal volumes (1–2 mL/kg BW) at high respiratory rates (up to 12 Hz). The special ventilators that are needed for this generally allow the user to set the respiratory rate, the inspiratory-to-expiratory time ratio, the fraction of oxygen in inspired air, and the mean airway pressure. HFOV is thought to improve gas exchange in comparison with conventional ventilation as a consequence of the higher mean airway pressure combined with lower peak airway pressure. In addition to altering the distribution of respiratory gas flow, this can improve the recruitment of initially collapsed lung areas and prevent alveolar collapse in other areas (13).
Theoretically, HFOV is an ideal mode of ventilation for protecting the lungs, because it lowers mechanical stress on the pulmonary parenchyma through the combination of low tidal volumes, high mean airway pressure, and lower peak airway pressure. While early clinical trials revealed a benefit for HFOV in comparison to conventional ventilation—in particular, short-term improvements in oxygenation—two recently published randomized, controlled trials revealed no effect (14) and an adverse effect (15) of HFOV: in the second of these trials, the mortality among ARDS patients treated with HFOV was higher than in the control group (47% vs. 35%) (15). The reason for these findings is currently under discussion. It is thought that the hemodynamic side effects of elevated intrathoracic pressure lead to an increased need for vasopressor drugs, sedation, and relaxation in patients being treated with HFOV. The current state of the evidence does not permit a recommendation for the routine use of HFOV in patients with ARDS.
Proportional pressure-support ventilation
In conventional pressure-support ventilation (PSV), the patient's inspiratory efforts are detected by a pressure or flow trigger and supported by an assistive pressure at a set level. In contrast, proportional pressure-support techniques provide an assistive pressure that is proportional to the patient's inspiratory effort. The first technique of this type, called proportional assist ventilation (PAV), was introduced in hospitals in 1992 (16). The principle of PAV is based on the separate setting of compensatory factors for the elastic and resistive components of the respiratory system (compliance and resistance). This creates pressure support that is proportional to the patient's inspiratory effort. The optimal setting of PAV is complicated by the need for the user to estimate or measure the elastic and resistive properties of the lungs. To circumvent this difficulty and make PAV more user-friendly, a modified version called PAV+ was developed in which the elastance and resistance of the respiratory system are continuously and automatically measured and the corresponding gains are automatically adjusted. This guarantees that resistance and compliance are compensated for to the desired extent (a preset percentage) (17). Clinical trials have not yet revealed any improvement in outcome-relevant variables with the use of PAV+. It was shown in a randomized crossover trial in which patients were ventilated at night with either PAV+ or conventional PSV that those receiving PAV+ had significantly fewer wake-up reactions (9 vs. 16) and asynchrony events (24 vs. 53) (18).
In contrast to other types of pressure-assisted spontaneous respiration, neurally adjusted ventilatory assist (NAVA) detects the patient's inspiratory effort not by measuring pressure or flow in the ventilator, but rather by measuring the electrical activity of the diaphragm by means of a probe placed in the esophagus (19). The pressure support delivered to the patient is proportional to the detected diaphragmatic activity, with a gain that can be adjusted by the user. Numerous small-scale clinical trials of NAVA in various groups of patients have revealed benefits, including, in particular, improved synchrony of the patient with the ventilator (20). There have not yet been any large-scale randomized clinical trials of NAVA to study outcome-relevant endpoints.
Faulty adjustment of the gain in NAVA, or of the compensatory factors in PAV and PAV+, can lead to the so-called runaway phenomenon, in which the ventilator continues to supply inspiratory pressure when the patient has already gone into the expiratory phase. The risk of this is particularly high in patients with impaired respiratory drive or complex disturbances of pulmonary mechanics.
Variable pressure-support ventilation
The intrinsic variability of tidal volume and respiratory rate in healthy individuals is lowered by many diseases (21). Variable or "noisy” pressure-support ventilation (PSV) is based on the idea of combining the positive effects of variable ventilation (22) with those of assisted spontaneous respiration (23). While proportional pressure-support techniques already enable a certain amount of respiratory variability, corresponding to the intrinsic variability of the patient's breathing, the application of a variable respiratory pattern (external variability) can induce or restore optimal variability (24). Animal experiments have revealed a benefit for variable PSV in comparison with controlled ventilation and conventional PSV (25) and PAV (26). It is thought that the intermittent application of higher airway pressures recruits collapsed areas of the lungs, while a redistribution of pulmonary perfusion to better-ventilated areas is the main reason for improved gas exchange (27). A clinical pilot study has shown that variable PSV is safe and easy to apply (28). A randomized, multicenter trial is now in progress whose goal is to compare weaning times under "noisy” and conventional PSV, in order to evaluate outcome-relevant improvements (29) The clinical utility of variable PSV cannot yet be definitively assessed, as no large-scale trials with clinically relevant endpoints have yet been published.
Closed-loop modes of mechanical ventilation
The number of ventilated patients is rising, while the necessary treatment personnel (physicians and nurses) is in increasingly short supply; automatic modes of ventilation might alleviate the difficulty of providing adequate ventilatory care in routine intensive-care medicine. The concept of adaptive support ventilation (ASV) is based on closed-loop control of the ventilator: the user sets basic parameters, such as
the patient's ideal body weight,
the desired minute volume,
-
safety limits, including
maximal and minimal tidal volume,
maximal pressure,
maximal respiratory rate.
On the basis of automated measurements of the resistance and compliance of the respiratory system, an algorithm is used to compute the values of tidal volume and respiratory rate that optimize the work of respiration (30). Unlike the modes of assisted spontaneous respiration discussed above, ASV can be applied either as controlled ventilation (for patients without any respiratory effort) or as assisted ventilation (for patients who still have spontaneous respiratory activity). In a randomized clinical trial, ASV was shown to shorten the duration of ventilation in comparison to conventional ventilation in patients being weaned from a ventilator after cardiac surgery (165 vs. 485 minutes) (31). Experimental studies in animals indicate that ASV lessens ventilation-induced diaphragmatic dysfunction (32). The algorithm still appears to be suboptimally developed for patients with complex respiratory disturbances, such as ARDS: unfavorable combinations of tidal volume and respiratory rate that might damage the lungs have been observed (33). In a further development of the technique, called IntelliVent-ASV, capnography and pulse oximetry are integrated into the algorithm's calculations along with the above-mentioned parameters. Initial observations of clinical use appear promising (34), but no randomized, controlled clinical trials have yet been carried out.
In contrast to ASV/IntelliVent-ASV, SmartCare is a special mode of ventilation for automated weaning. Based on conventional pressure-supported spontaneous respiration in the PSV mode, SmartCare involves continuous and automatic measurement of respiratory rate, tidal volume, and expiratory CO2 concentration. These measurements are made every 2 to 5 minutes and used for automatic adjustment of the support pressures to keep the patient in the respiratory comfort zone. When certain criteria that have been preset in the algorithm are fulfilled, SmartCare automatically conducts a trial of spontaneous respiration. For optimal matching of the mode of ventilation with the patient, the following additional information is needed as well:
the type of airway (endotracheal tube or tracheal canula),
body weight,
whether the patient has chronic obstructive pulmonary disease (COPD),
any neurological condition affecting respiratory drive,
the moisturization system used (active moisturization or ventilation filter).
Once these patient characteristics have been entered, the algorithm calculates the limits of tidal volume, respiratory rate, and expiratory carbon dioxide. Information about the type of airway and moisturization system is used to define the level of pressure support at which a trial of spontaneous respiration should be initiated. SmartCare should not be used in patients with neurological impairment of the central regulation of breathing, patients with severe broncho-spasm, or highly agitated patients (35). In a randomized, controlled clinical trial, Schädler et al. found no overall difference in ventilation times between patients treated with SmartCare and a control group receiving conventional ventilation (36), although ventilation times were significantly lower in a subgroup of patients who had undergone cardiac surgery (24 vs. 35 hours).
In a current systematic Cochrane review of the use of closed-loop ventilation systems, such as ASV and SmartCare, a combined analysis of 15 randomized, controlled clinical trials with a total of 1173 patients revealed that the time until the patient could be weaned from the ventilator was 32% shorter with these systems than with conventional modes of ventilation (a statistically significant difference) (37). Nonetheless, the available evidence is highly heterogeneous, and outcomes when conventional weaning protocols are used depend to a large extent on the staffing of the intensive care unit (38).
Overview
The aims of modern strategies of ventilation are to improve gas exchange and pulmonary mechanics, protect the lungs from the adverse effects of mechanical ventilation, and shorten the overall duration of ventilation by reducing the invasiveness of ventilation as rapidly as possible. These strategies include controlled modes of ventilation, such as HFOV, and modes involving assisted spontaneous respiration, such as PAV, PAV+, PSV, "noisy” PSV, and NAVA. ASV and IntelliVent-ASV can be used as either controlled or assisted ventilation. The use of SmartCare might possibly simplify and accelerate weaning from the ventilator. The modes of ventilation discussed in this review have been implemented to date only in the ventilator machines of certain manufacturers; most users will not yet be able to use all of them in their own intensive care units. The Table contains an overview of the modes of ventilation presented here.
Table. Overview of commonly used modern modes of ventilation, based on the authors' clinical and scientific experience.
| Mode | Principle | Advantages | Disadvantages |
| HFOV |
|
|
|
| PAV |
|
|
|
| PAV+ |
|
|
|
| NAVA |
|
|
|
| variable ("noisy”) PSV |
|
|
|
| ASV |
|
|
|
| IntelliVent-ASV |
|
|
|
| SmartCare |
|
|
|
HFOV, "high-frequency oscillatory ventilation”; ARDS, "adult respiratory distress syndrome”; PAV, "proportional assist ventilation”;
PAV+, "proportional assist ventilation with load-adjusted gain factors”; NAVA, "neurally adjusted ventilatory assist”;
PSV, "pressure-support ventilation”; ASV, "adaptive support ventilation”
A further problem is the still inadequate evidence base for the use of modern types of ventilation. While numerous animal experiments and small-scale clinical trials have been conducted, no definitive assessment can yet be made of the clinical utility of these newer modes of ventilation with respect to outcome-relevant endpoints. It is not possible to date either to judge these strategies individually or to assess their relative merits compared to one another.
Key Messages.
Innovative modes of ventilation are aimed at improving lung protection and patient comfort.
These techniques are intended to prevent ventilation-induced lung damage by reducing the invasiveness and duration of ventilation.
The new modes of ventilation include methods of controlled ventilation, such as HFOV, and assisted spontaneous respiration, such as PAV, PAV+, NAVA, PSV, and noisy PSV. ASV/IntelliVent-ASV are special cases: they can be applied either as controlled ventilation or as assisted spontaneous respiration.
Adequate evidence is not yet available for the superiority of these new modes of ventilation to conventional ones with respect to clinically relevant outcome parameters.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Dr. Spieth has three patents on the use of variable modes of ventilation.
Prof. Koch has three patents on the use of variable modes of ventilation. She also receives third-party funding from Dräger Medical AG for a study involving electrical impedance tomography.
Prof. Gama de Abreu has three patents on the use of variable modes of ventilation. He received one-time licensing fees from Dräger Medical AG when that company acquired the patent for variable pressure-support ventilation. He has also served as a paid consultant for Dräger Medical AG, providing advice on the use of electrical impedance tomography. He has received lecture honoraria and reimbursement of meeting participation fees and travel expenses from Dräger Medical AG and Novalung GmbH. He receives third-party funding from Firma Dräger Medical AG for ventilator machines.
References
- 1.Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 2.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 3.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 4.Plataki M, Hubmayr RD. The physical basis of ventilator-induced lung injury. Expert Rev Respir Med. 2010;4:373–385. doi: 10.1586/ers.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craven DE, Lei Y, Ruthazer R, Sarwar A, Hudcova J. Incidence and outcomes of ventilator-associated tracheobronchitis and pneumonia. AJM. 2013;126:542–549. doi: 10.1016/j.amjmed.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 7.Müller T, Bein T, Philipp A, Graf B, Schmid C, Riegger G. Extracorporeal pulmonary support in severe pulmonary failure in adults—a treatment rediscovered. Dtsch Arztebl Int. 2013;110:159–166. doi: 10.3238/arztebl.2013.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ARDS Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Phua J, Badia JR, Adhikari NKJ, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 10.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 11.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 12.Guerin C, Reignier J, Richard JC, et al. Prone Positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 13.Ip T, Mehta S. The role of high-frequency oscillatory ventilation in the treatment of acute respiratory failure in adults. Current Opinion in Critical Care. 2012;18:70–79. doi: 10.1097/MCC.0b013e32834f1805. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson ND, Cook DJ, Guyatt GH, et al. High-Frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 15.Young D, Lamb SE, Shah S, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 16.Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis. 1992;145:121–129. doi: 10.1164/ajrccm/145.1.121. [DOI] [PubMed] [Google Scholar]
- 17.Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilation–proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Medicine. 2006;32:692–699. doi: 10.1007/s00134-006-0110-0. [DOI] [PubMed] [Google Scholar]
- 18.Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048–1054. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- 19.Sinderby C, Navalesi P, Beck J, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5:1433–1436. doi: 10.1038/71012. [DOI] [PubMed] [Google Scholar]
- 20.Navalesi P, Colombo D, Corte Della F. NAVA ventilation. Minerva Anestesiol. 2010;76:346–352. [PubMed] [Google Scholar]
- 21.Tobin MJ, Chadha TS, Jenouri G, Birch SJ, Gazeroglu HB, Sackner MA. Breathing patterns 2. Diseased subjects. Chest. 1983;84:286–294. doi: 10.1378/chest.84.3.286. [DOI] [PubMed] [Google Scholar]
- 22.Spieth PM, Carvalho AR, Pelosi P, et al. Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. Am J Respir Crit Care Me. 2009;179:684–693. doi: 10.1164/rccm.200806-975OC. [DOI] [PubMed] [Google Scholar]
- 23.Gama de Abreu M, Spieth PM, Pelosi P, et al. Noisy pressure support ventilation: a pilot study on a new assisted ventilation mode in experimental lung injury. Crit Care Med. 2008;36:818–27. doi: 10.1097/01.CCM.0000299736.55039.3A. [DOI] [PubMed] [Google Scholar]
- 24.Spieth PM, Carvalho AR, Güldner A, et al. Effects of different levels of pressure support variability in experimental lung injury. Anesthesiology. 2009;110:342–350. doi: 10.1097/ALN.0b013e318194d06e. [DOI] [PubMed] [Google Scholar]
- 25.Spieth PM, Carvalho AR, Güldner A, et al. Pressure support improves oxygenation and lung protection compared to pressure-controlled ventilation and is further improved by random variation of pressure support. Crit Care Med. 2011;39:746–755. doi: 10.1097/CCM.0b013e318206bda6. [DOI] [PubMed] [Google Scholar]
- 26.Spieth PM, Güldner A, Beda A, et al. Comparative effects of proportional assist and variable pressure support ventilation on lung function and damage in experimental lung injury. Crit Care Med. 2012;40:2654–2661. doi: 10.1097/CCM.0b013e3182592021. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho AR, Spieth PM, Güldner A, et al. Distribution of regional lung aeration and perfusion during conventional and noisy pressure support ventilation in experimental lung injury. Journal of Applied Physiology. 2011;110:1083–1092. doi: 10.1152/japplphysiol.00804.2010. [DOI] [PubMed] [Google Scholar]
- 28.Spieth PM, Güldner A, Huhle R, et al. Short-term effects of noisy pressure support ventilation in patients with acute hypoxemic respiratory failure. Crit Care. 2013;17 doi: 10.1186/cc13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiss T, Güldner A, Bluth T, et al. Rationale and study design of ViPS - variable pressure support for weaning from mechanical ventilation: study protocol for an international multicenter randomized controlled open trial. Trials. 2013;14 363 doi: 10.1186/1745-6215-14-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin N Am. 2001;7:425–440. doi: 10.1016/s1078-5337(05)70049-6. [DOI] [PubMed] [Google Scholar]
- 31.Gruber PC, Gomersall CD, Leung P, et al. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology. 2008;109:81–87. doi: 10.1097/ALN.0b013e31817881fc. [DOI] [PubMed] [Google Scholar]
- 32.Jung B, Constantin JM, Rossel N, et al. Adaptive support ventilation prevents ventilator-induced diaphragmatic dysfunction in piglet: an in vivo and in vitro study. Anesthesiology. 2010;112:1435–1443. doi: 10.1097/ALN.0b013e3181d7b036. [DOI] [PubMed] [Google Scholar]
- 33.Dongelmans DA, Paulus F, Veelo DP, Binnekade JM, Vroom MB, Schultz MJ. Adaptive support ventilation may deliver unwanted respiratory rate-tidal volume combinations in patients with acute lung injury ventilated according to an open lung concept. Anesthesiology. 2011;114:1138–1143. doi: 10.1097/ALN.0b013e31820d8676. [DOI] [PubMed] [Google Scholar]
- 34.Arnal JM, Garnero A, Novonti D, et al. Feasibility study on full closed-loop control ventilation (IntelliVent-ASV™) in ICU patients with acute respiratory failure: a prospective observational comparative study. Crit Care. 2013;17 R196. doi: 10.1186/cc12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burns KEA, Lellouche F, Lessard MR. Automating the weaning process with advanced closed-loop systems. Intensive Care Medicine. 2008;34:1757–1765. doi: 10.1007/s00134-008-1154-0. [DOI] [PubMed] [Google Scholar]
- 36.Schädler D, Engel C, Elke G, et al. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. Am J Respir Crit Care Med. 2012;185:637–644. doi: 10.1164/rccm.201106-1127OC. [DOI] [PubMed] [Google Scholar]
- 37.Rose L, Schultz MJ, Cardwell CR, Jouvet P, McAuley DF, Blackwood B. Automated versus non-automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children. Cochrane Database Syst Rev. 2013;6 doi: 10.1002/14651858.CD009235.pub2. CD009235. [DOI] [PubMed] [Google Scholar]
- 38.Burns KE, Lellouche F, Lessard MR, Friedrich JO. Automated weaning and spontaneous breathing trial systems versus non-automated weaning strategies for discontinuation time in invasively ventilated postoperative adults. Cochrane Database Syst Rev. 2014;2 doi: 10.1002/14651858.CD008639.pub2. CD008639. [DOI] [PMC free article] [PubMed] [Google Scholar]