Abstract
The biomass and net primary productivity (NPP) of 5‐ to 15‐year‐old Shisham (Dalbergia sissoo Roxb.) forests growing in central Himalaya were estimated. Allometric equations were developed for all above‐ and below‐ground components of trees and shrubs for each stand. Understorey forest floor biomass and litter fall were also estimated in forest stands. The biomass (dry matter), forest floor biomass (standing crop litter), tree litter fall and NPP of trees and shrubs increased with increasing age of the forest stand, whereas the dry matter and herb NPP decreased significantly (P < 0·001) with increasing age of the forest. Total forest biomass and NPP ranged from 58·7 (5‐year‐old stand) to 136·1 t ha–1 (15‐year‐old stand) and 12·6 (5‐year‐old stand) to 20·3 t ha–1 year–1 (15‐year‐old stand), respectively. Of these values, tree biomass accounted for 85·7 (5‐year‐old stand) to 90·1 % (15‐year‐old) of total forest biomass, and tree NPP for 72·2 (5‐year‐old) to 82·3 % (15‐year‐old) of total forest NPP. The biomass accumulation ratio (BAR) of the bole component (bole wood + bole bark) increased with increasing age of the forest stand. The bole BAR was 5·8 (5‐year‐old stand) to 7·9 (15‐year‐old stand). However, total BAR of the forest stand ranged from 5·5 (5‐year‐old) to 7·5 (15‐year‐old).
Key words: Dalbergiasissoo Roxb, biomass, net primary productivity, litter input, forest floor biomass, turnover rate, Tarai belt, Central Himalaya, India
INTRODUCTION
Shisham (Dalbergia sissoo Roxb.) is an important tree species belonging to the family Papilionaceae. It is a medium‐ to large‐sized, gregarious deciduous tree, attaining a height of approx. 30 m and a girth of approx. 2·5 m in a favourable climate (sub‐tropical and tropical zones). According to Champion and Seth (1968), the Shisham tree is a characteristic species of Khair–Sissoo (Acacia catechu–Dalbergia sissoo) primary seral type forest. Shisham occurs naturally throughout the sub‐Himalayan tract and outer Himalayan valley from the Indus to Assam, usually at elevations of about 900 m, but sometimes occurring up to 1500 m. Shisham is found either in a pure forest stand or together with other species, commonly Khair (Acacia catechu). Shisham is a good example of a pioneer species in the riverain succession of the Gangetic alluvial plains in India. Shisham usually regenerates naturally on recently laid down terraces of rivers, on freshly exposed soils, road cuttings, fresh embankment and landslips where drainage is good and there is sufficient soil aeration. However, Shisham shows remarkable variation in growth pattern and yield per unit area because it is adapted to a wide range of ecological habitats which thus influence its dry matter production.
Shisham is a very hardy species and produces valuable timber. Trees shed leaves from October to February; new leaves appear between February and April. Shisham can be propagated both by seeds and vegetative parts. Stump planting of Shisham (i.e. planting approx. 5 cm of stem and 20 cm of root) is known to be the best method of artificial regeneration.
Shisham is amongst the principal tree species commonly recommended for plantation programmes in dry regions for soil and water conservation as well as for fuel wood production. This species can survive at sites where nitrogen levels are low and also on saline and alkaline soils. Its adaptability, drought resistance, hardiness and nitrogen‐fixing properties, as well as its multipurpose nature, make it suitable for afforestation and reforestation in many parts of the country. Thus, this species is environmentally and socio‐economically acceptable to the people of India.
Leith and Whittaker (1975) pointed out that if forest biomass is measured and analysed in its proper context as part of production, an overall picture of ecosystem functioning can be gained. However, the biomass and productivity of tree species varies from place to place due to variation in climate, soil, temperature and rainfall. Biomass and net primary productivity (NPP) have been studied in temperate and tropical forest vegetation types (Ogawaet al., 1965; Ovington, 1965; Whittaker, 1966; Newbould, 1967; Attiwill and Ovington, 1968; Satoo, 1968; Whittaker and Woodwell, 1968; Whittaker and Likens, 1975; Chaturvediet al., 1984; Gurumurtiet al., 1987; Sharmaet al., 1988; Swaminath, 1988; Pal and Raturi, 1990). Bargaliet al. (1992), Lodhiyalet al. (1995) and Lodhiyal and Lodhiyal (1997) have made detailed studies of the biomass and productivity of exotic plantations in the central Himalayan Tarai belt (Tarai is a region of high water and nutrient availability). Teller (1968) pointed out that forest floor biomass plays a significant role in the structure and functioning of forest ecosystems by acting as a nutrient reservoir for the intra‐system cycling processes and improves the infiltration rate and water holding capacity of soils. Litter fall is another important component of nutrient recycling in a forest, and its inputs depend on a variety of factors such as species, age groups, canopy cover, weather conditions and biotic factors. Observations on litter input in different forests have been made by Carlisleet al. (1966), Attiwillet al. (1978), Merriamet al. (1982), Rawat and Singh (1988) and Lodhiyal and Lodhiyal (1997), but there is no detailed information about the structure and function of Shisham forests in relation to annual productivity and dry matter transfer in the Tarai belt of the central Himalayan mountains.
The main objectives of this study were: (1) to determine the biomass and net annual (primary) productivity in Shisham forests of different ages; and (2) to assess whether these forests perform better than conventional exotic plantations (i.e. poplar and eucalypt) and natural forests of the region. In this respect we adopted an ecological approach by considering dry matter flows in terms of quantity and seasonal periodicity.
MATERIALS AND METHODS
Description of study sites
The three study sites were located between 28°43′ and 29°37′N, and 79°20′ and 79°23′E at an altitude of 250 m in Tarai in the district of Udham Singh Nagar (about 80 km from Kumaun University, Nainital) of the Indo‐Gangetic plains in the south of the Outer Shiwalik range of central Himalaya, India.
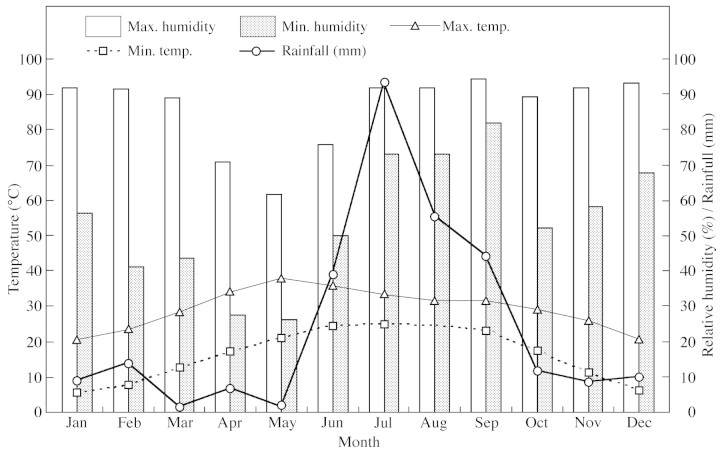
The climate of the study area is sub‐tropical and monsoonal. There are three seasons per year: winter (November to February), summer (April to mid‐June), and a rainy season (mid‐June to mid‐September). The months of October and March are transitional periods and are known as autumn and spring, respectively. The average monthly rainfall ranges from 0·5 mm in February (minimum) to 109·9 mm in July (maximum) (Fig. 1). The average minimum and maximum temperatures range from 4·5 °C (January) to 25·4 °C (July), and from 17·3 °C (December) to 37·0 °C (May), respectively (Fig. 1).
Fig. 1. Ombrothermic diagram based on meteorological data for 1996 and 1997 for the study area.
The slope of the Tarai area is not less than 2·5 m per km. The fertile soil is fine‐textured, alluvial, loamy and free from boulders. Because the Tarai is recharged by water seepage from higher elevations it has a high water table and thus a high moisture and nutrient content, contributing to the high productivity of the site.
The Tarai belt is rich in natural resources. The natural vegetation in this region is mainly alluvial savannah woodland type 3/I51, with some pockets of the moist Tarai sal forest type 3C/C2c (Champion and Seth, 1968). The area consists of luxuriant growth of tall grasses with scattered growth of deciduous forest trees.
The total area planted with Shisham trees was 47 ha. Of this area, 5‐, 10‐ and 15‐year‐old forest stands occupied 14, 16 and 17 ha, respectively. These 5‐, 10‐ and 15‐year‐old forests were planted in 1991, 1986 and 1981, respectively, after clear felling of species indigenous to the area.
Biomass
Sampling design.
In all three stands trees were similarly spaced (4 m × 4 m), giving a density of 625 trees ha–1. A 1 ha plot was sampled in each forest stand. The sample plot was divided into four replicate sub‐plots of 50 × 50 m2 (0·25 ha), and 100 trees in each sub‐plot (total of 400 trees in each forest stand) were measured. The height and diameter [diameter at breast height (dbh), 1·37 m] of trees were measured by Ravi’s Multimeter and Tree Callipers, respectively. All the trees measured in each forest stand were divided into three diameter classes: 10·0–15·0, 16·0–21·0 and 22·0–27·0 cm in 5‐year‐old stands; 15·0–19·0, 20·0–24·0 and 25·0–29·0 cm in 10‐year‐old stands; and 21·0–24·0, 25·0–28·0 and 29·0–32·0 cm in 15‐year‐old forest stands. The average height of trees and of four shrubs (Lantana camara, Murraya koenighii, Clerodendrum viscosum and Pogostemone benghalense; 25 individuals of each shrub giving a total of 100 individuals of all four shrub species from each sub‐plot) was 8·0, 12·0 and 18·0 m and 2·3, 2·5 and 2·6 m, respectively, in 5‐, 10‐ and 15‐year‐old forest stands.
Individual trees and shrubs were selected at random from each sub‐plot of each forest stand. To estimate understorey density, shrub and herb individuals were sampled in 50 quadrats of 2 × 2 m and 1 × 1 m, respectively in each forest stand.
To estimate the biomass of Shisham forest, the selective harvest technique was adopted (Ovington, 1962; Newbould, 1967). Twelve trees in each forest stand (three trees in each sub‐plot, four from each dbh class) were harvested. Harvested trees were cut into 1–2 m logs. Above‐ (bole wood, bole bark, branches, twigs, leaves and reproductive parts, i.e. inflorescences, flowers and fruits) and below‐ground (stump root, lateral roots and fine roots) components were assessed. The roots (stump root and lateral roots) were excavated from a 2 m3 volume of soil for each harvested tree in each forest stand. The fine roots (roots < 5 mm diameter) and associated mycorrhizae were sampled by removing three randomly distributed 25 × 25 × 25 cm blocks of soil around harvested trees in each dbh class of each sub‐plot (total of 12 from each forest stand).
The fresh weight of all components was determined in the field using a heavy weight spring balance and pan balance. Samples of approx. 500 g (fresh weight) of each tree component from each forest stand were taken separately to the laboratory and oven‐dried at 60 °C to constant weight. Using the fresh/dry weight ratio, the dry weight of each tree was estimated. Regression equations were developed for different tree components. The data were subjected to regression in the form Y = a + bX, where Y is the dry weight of a component (kg), and X is the dbh above ground level (cm). The mean diameter value for each diameter class was used in the regression equation of the different components to obtain an estimate of mean biomass. This value was then multiplied by tree density in that diameter class. The total biomass of trees for each forest stand was calculated by summing the biomass of each diameter class.
Ten individuals of each shrub species differing in height and diameter were harvested and roots were recovered to a depth of 50 cm. The harvested material was separated into foliage, stem and roots. A regression equation was developed for each component to estimate shrub biomass. For herbaceous biomass, herbs were harvested at their peak (in the rainy season; September 1997) from ten 50 × 50 cm quadrats. The harvested material was divided into above‐ and below‐ground components. Fresh and dry weights were determined for each shrub and herb component.
The total vegetation biomass was obtained by summing biomass values of trees, shrubs and herbs for each forest stand.
Net primary productivity
After the selecting a permanent sample plot (area: 1 ha) in each forest stand, 100 trees in each sub‐plot were marked with white paint (at breast height, 1·37 m from the base) in September 1996 to assess diameter and height increments at annual intervals. The height and diameter of marked trees were measured again in September 1997. The mean diameter and height increments for each diameter class were then calculated.
The net primary productivity of the different tree components [bole wood, bole bark, branches, twigs, leaves and reproductive parts (above‐ground parts), and stump root, lateral roots and fine roots (below‐ground parts)] was calculated for each forest stand using the allometric equations of Rawat and Singh (1988) and Lodhiyal and Lodhiyal (1997).
The net increase in biomass (ΔB = B2 – B1) yielded annual biomass accumulation. The sum of the ΔB values for the different components yielded net biomass accretion in the trees. Values for litter fall in the forests were added to the respective components (leaf and twigs). Considerable mortality of fine roots occurs in actively growing trees. However, a basic knowledge of the control of fine root production requires a better understanding of soil ecosystems (Nadelhoffer and Raich, 1992). The biomass of fine roots could not be estimated repeatedly during the course of the present study so we followed the methods of Kalela (1954), Orlov (1968) and Ogino (1977). We assumed that fine root mortality was equivalent to one‐fifth of leaf litter fall; but present estimates of fine root production may be a gross underestimation (Harriset al., 1980; Vogt et al., 1982, 1986; Fogel, 1983; Lodhiyalet al., 1995; Lodhiyal and Lodhiyal, 1997), and may differ from the real value as Nadelhoffer and Raich (1992) found no correlation between fine root production and leaf litter fall for a large data set. However, it must be pointed out that all methods, including those based on related biomass estimations of fine roots, are subject to uncertainties and possible biases (Lauenrothet al., 1986; Salaet al., 1988).
The net production value of each component was summed across diameter classes to give total net production of trees in each forest stand.
Twenty individuals of each dominant shrub species (Lantana camara, Murraya koenighii, Clerodendrum viscosum and Pogostemone benghalense) differing in height and diameter were marked with white paint and their basal diameter was measured in September 1996. After 1 year the marked individuals were re‐measured. Increases in biomass of these shrubs were calculated using regression equations, and to this value was added the foliage biomass (assuming 100 % turnover of leaves in 1 year) to obtain net annual production. The average production of a species multiplied by density yielded the total production of that species. Summing net production values for all species gave total shrub production for a site.
The biomass (above‐ and below‐ground) of herbs at all sites was determined during their peak growth period in September 1996. This value was assumed to equal to net herb production. The sum of net production values of trees, shrubs and herbs yielded the total net primary productivity of vegetation in each Shisham forest stand.
Litter fall
Litter fall was studied for a 1 year period from July 1996 to June 1997. The litter input was measured by randomly placing 20 litter traps (five litter traps for each dbh class in each sub‐plot) on the forest floor in each forest stand. Each trap was 50 × 50 cm with 15 cm high wooden sides fitted with a nylon net bottom. Litter was collected at monthly intervals on each sampling date and taken to the laboratory in polyethylene bags. Samples were then sorted into leaf, wood, reproductive parts and other components. The wood component comprised bark and twigs. Samples of separated components were cleaned and weighed after oven drying at 60 °C to constant weight. After weighing, the litter components were ground and retained for nutrient analysis.
Forest floor biomass
Forest floor litter data were collected using 15 quadrats (1 m × 1 m) placed randomly in each forest stand once in each season, i.e. rainy, winter and summer. In each quadrat, all components were categorized into: (a) fresh leaf litter; (b) partially and decomposed litter; (c) wood litter (including twigs, bark and branches); (d) miscellaneous litter (consisting of inflorescences, flowers and fruits, litter parts of shrubs); and (e) herbaceous litter (living and dead), following Rawat and Singh (1988), Lodhiyal (1990), Bargaliet al. (1992), Lodhiyalet al. (1995) and Lodhiyal and Lodhiyal (1997). In each quadrat all herbaceous standing shoots (alive and dead) were harvested at ground level (Green, 1959; Line, 1959; Lodhiyal, 1990; Lodhiyalet al., 1995; Lodhiyal and Lodhiyal, 1997). Forest floor material was collected carefully to avoid soil contamination. Collected material was taken to the laboratory in polyethylene bags, cleaned of soil particles, and weighed after oven drying at 60 °C to constant weight.
Turnover of litter
The turnover rate (K) of litter was calculated following Jennyet al. (1949), Olson (1963) and Lodhiyal and Lodhiyal (1997): K = A/(A + F) where A is the annual increment of litter (i.e. annual litter fall) and F is the biomass of the litter at steady state. Turnover time (t, years) is the reciprocal of turnover rate and is expressed as t = 1/K. In the present study, F was the standing crop of partially and decomposed litter during the winter season and A was the annual tree litter fall plus shrub litter (equal to foliage biomass) plus herb litter (equal to peak above‐ground herb biomass).
Soil samples were collected in September 1996, January 1997 and May 1997 from each forest stand (nine samples in each sub‐plot, three samples from each depth class) using a soil corer placed randomly at three depths 0–10, 10–20 and 20–30 cm. Soil texture, bulk density, moisture content, water holding capacity and soil porosity were determined according to Misra (1968). The pH of each soil sample was determined using a digital pH meter, the nitrogen concentration using a micro‐Kjeldahl technique (Peach and Tracy, 1956), phosphorus by spectrophotometry and potassium by flame photometry (Jackson, 1958).
RESULTS AND DISCUSSION
Stand structure and soil characteristics
Table 1 summarizes stand structure and physicochemical properties of the soil studied in the three Shisham forest stands. The basal area increased significantly (P < 0·01) from 16·9 (5‐year‐old stand) to 34·7 m2 ha–1 (15‐year‐old stand) with age of the forest stand. The soil parameters such as sand, clay, porosity, moisture percentage (in rainy and winter seasons), soil pH and soil nutrient (NPK) concentration decreased (the last not significantly); however, other soil parameters increased with increasing age of the forest (Table 1).
Table 1.
Stand structure and soil characteristics of Shisham (Dalbergia sissoo Roxb.) forests of different ages in central Himalaya
| Age of Shisham forests (years) | |||
| Parameters | 5 | 10 | 15 |
| Altitude (m) | 250 | 250 | 250 |
| Area of forest stand (ha) | 14 | 16 | 17 |
| Tree density (ha–1) | 625 | 625 | 625 |
| Basal area (m2 ha–1) | 16·9 | 23·3 | 34·7 |
| Bulk density of soil (g cm–3) | 1·02 | 1·06 | 1·11 |
| Soil texture (%, in 0–30 cm) | |||
| Sand | 25·2 | 24·3 | 23·1 |
| Silt | 38·1 | 40·0 | 41·9 |
| Clay | 36·7 | 35·7 | 35·0 |
| Porosity (%, in 0–30 cm) | 62 | 61 | 58 |
| Soil moisture (%, in 0–30 cm) | |||
| Rainy season | 28·4 | 27·0 | 26·5 |
| Winter | 17·5 | 16·5 | 15·2 |
| Summer | 11·8 | 12·5 | 12·8 |
| Water holding capacity (%, in 0–30 cm) | 86·7 | 87·1 | 87·6 |
| Soil pH | 6·6 | 6·5 | 6·4 |
| Nutrient conc. of soil (%) | |||
| N | 0·155 ± 0·30 | 0·155 ± 0·31 | 0·151 ± 0·43 |
| P | 0·019 ± 0·26 | 0·013 ± 0·26 | 0·012 ± 0·39 |
| K | 0·064 ± 0·38 | 0·061 ± 0·32 | 0·056 ± 0·40 |
Biomass
The allometric equations, variables and parameters relating biomass of different tree components to dbh are presented in Table 2, while the allometric equations developed for estimating biomass of the different shrub components based on basal diameter are given in Table 3. Diameter at breast height was selected as the independent variable because of the ease and accuracy involved in making these measurements.
Table 2.
Relationship between the biomass of tree components (Y, kg per tree) and diameter at breast height (X, cm) for Shisham (Dalbergia sissoo Roxb.) forests
| Age of Shisham forests (years) | ||||
| Components | 5 | 10 | 15 | |
| Bole wood | a | –0·5672 | 1·6532 | –4·9071 |
| b | 2·3707 | 3·7616 | 4·3190 | |
| r 2 | 0·995 | 0·994 | 0·981 | |
| Bole bark | a | –0·1147 | 0·3029 | –0·8850 |
| b | 0·4725 | 0·7095 | 0·7716 | |
| r 2 | 0·995 | 0·994 | 0·982 | |
| Branch | a | –0·0777 | 0·2397 | –0·7306 |
| b | 0·3435 | 0·5402 | 0·6476 | |
| r 2 | 0·993 | 0·994 | 0·981 | |
| Twig† | a | –0·0463 | 0·1102 | –0·3821 |
| b | 0·1593 | 0·2568 | 0·3245 | |
| r 2 | 0·994 | 0·994 | 0·981 | |
| Leaf‡ | a | –0·1231 | 0·1475 | –0·4092 |
| b | 0·2862 | 0·3377 | 0·3245 | |
| r 2 | 0·994 | 0·994 | 0·940 | |
| Reproductive parts§ | a | –0·0154 | 0·0195 | –0·9809 |
| b | 0·0357 | 0·0680 | 0·1296 | |
| r 2 | 0·996 | 0·993 | 0·827* | |
| Stump root¶ | a | –0·1121 | 0·2963 | –0·6018 |
| b | 0·4985 | 0·6754 | 0·7163 | |
| r 2 | 0·995 | 0·994 | 0·986 | |
| Lateral roots|| | a | –0·0432 | –0·3068 | –0·1004 |
| b | 0·1854 | 0·3154 | 0·3573 | |
| r 2 | 0·994 | 0·981 | 0·979 | |
| Fine roots** | a | –0·0288 | 0·0338 | –0·5149 |
| b | 0·0671 | 0·1087 | 0·1534 | |
| r 2 | 0·994 | 0·993 | 0·941 |
The equation used was Y = a + bX. All equations are significant at P < 0·01 except those denoted by an asterisk which are significant at P < 0·05. † Shoots of larger dimension without leaves. ‡ Current shoots bearing leaves. § Includes flowers and fruits. ¶ Main root bearing 30 cm above‐ground stem part. || Lateral branches of stump root (main root) with a diameter > 5 mm. ** Root originates from lateral roots with a diameter < 5 mm and associated mycorrhizae.
Table 3.
Allometric relationship between biomass of shrub components (Y, kg per tree) and basal diameter (D, cm) for four shrub species under Shisham (Dalbergia sissoo) forests in the Tarai belt of central Himalaya
| Species | Components | Intercept (a) | Slope (b) | r 2 |
| 5‐year‐old stand | ||||
| Lantana | Foliage | 1·8624 | 1·0542 | 0·970 |
| camara | Stem | 1·7506 | 3·1195 | 0·995 |
| Roots | 1·7608 | 2·1074 | 0·997 | |
| Murraya | Foliage | 1·2647 | 1·6441 | 0·998 |
| koenighii | Stem | 1·2448 | 6·6278 | 0·996 |
| Roots | 1·2700 | 3·9169 | 0·998 | |
| Clerodendrum | Foliage | 1·0662 | 3·4817 | 0·982 |
| viscosum | Stem | 1·1838 | 12·6674 | 0·981 |
| Roots | 1·0561 | 6·2988 | 0·993 | |
| Pogostemone | Foliage | 1·4407 | 1·8659 | 0·996 |
| benghalense | Stem | 1·4574 | 5·4921 | 0·993 |
| Roots | 1·4574 | 3·5306 | 0·993 | |
| 10‐year‐old stand | ||||
| Lantana | Foliage | 1·6972 | 1·3303 | 0·992 |
| camara | Stem | 1·4594 | 5·0578 | 0·865* |
| Roots | 1·7222 | 1·8654 | 0·991 | |
| Murraya | Foliage | 1·2557 | 1·5577 | 0·988 |
| koenighii | Stem | 1·2719 | 7·7734 | 0·969 |
| Roots | 1·8716 | 0·8279 | 0·434 n.s. | |
| Clerodendrum | Foliage | 1·1549 | 2·6947 | 0·970 |
| viscosum | Stem | 1·1783 | 14·9055 | 0·966 |
| Roots | 1·4324 | 2·1308 | 0·916 | |
| Pogostemone | Foliage | 1·3724 | 2·0368 | 0·982 |
| benghalense | Stem | 1·3204 | 9·3897 | 0·981 |
| Roots | 1·3575 | 3·3033 | 0·987 | |
| 15‐year‐old stand | ||||
| Lantana | Foliage | 1·6782 | 1·4005 | 0·988 |
| camara | Stem | 1·7844 | 3·8373 | 0·957 |
| Roots | 1·6285 | 2·1831 | 0·927 | |
| Murraya | Foliage | 1·3247 | 1·5179 | 0·977 |
| koenighii | Stem | 1·2685 | 7·3875 | 0·955 |
| Roots | 0·6652 | 4·8477 | 0·845* | |
| Clerodendrum | Foliage | 1·1080 | 2·9784 | 0·959 |
| viscosum | Stem | 1·1938 | 12·1948 | 0·928 |
| Roots | 1·1391 | 4·3298 | 0·956 | |
| Pogostemone | Foliage | 1·5405 | 1·6191 | 0·935 |
| benghalense | Stem | 1·5586 | 7·2454 | 0·975 |
| Roots | 1·7561 | 1·9661 | 0·824* |
All equations are significant at P < 0·01 except those with an asterisk which are significant at P < 0·05. n.s., Not significant.
As evident from r2‐values in Table 2, the relationships between biomass and dbh were found to be quite satisfactory. Biomass was not calculated using the X2 h method (where h is height) because the resulting r2‐values were not significantly better than those obtained using dbh (X). Therefore, the regression model Y = a + bX was used for computation of forest biomass.
Total biomass of trees ranged from 50·3 ± 2·46 (5‐year‐old stand) to 122·7 ± 3·14 t ha–1 (15‐year‐old stand), of which above‐ and below‐ground parts represented 83–84 and 16–17 %, respectively (Table 4). Shrub biomass ranged from 5·6 ± 1·54 (5‐year‐old stand) to 11·0 ± 2·04 t ha–1 (15‐year‐old stand), of which stem, foliage and roots accounted for about 54–61, 13–15 and 26–30 %, respectively (Table 4). The herb biomass ranged from 2·4 ± 2·44 (15‐year‐old stand) to 2·8 ± 1·76 t ha–1 (5‐year‐old stand). Of this, the above‐ground component accounted for 76–79 %. Herb biomass decreased with increasing age of the forest stand (Table 4). The vegetation biomass ranged from 58·7 ± 1·92 (5‐year‐old stand) to 136·1 ± 2·54 t ha–1 (15‐year‐old stand). Of this, the tree layer accounted for 86–90 %, shrubs for 8–10 % and herbs for 2–5 % (Table 4).
Table 4.
Component‐wise biomass (t ha–1) in tree, shrub and herb layers at different ages of Tarai Shisham forests in Central Himalaya
| Age of Shisham forests (years) | |||
| Vegetation | 5 | 10 | 15 |
| Tree layer | 50·3 ± 2·46 | 93·8 ± 2·92 | 122·7 ± 3·14 |
| % Allocation in | |||
| bole* | 64·3 | 66·0 | 66·0 |
| branch† | 11·4 | 11·8 | 12·6 |
| leaf | 6·4 | 5·0 | 4·2 |
| reproductive part | 0·8 | 1·0 | 1·2 |
| coarse roots‡ | 15·5 | 14·6 | 14·2 |
| fine roots | 1·6 | 1·6 | 1·8 |
| Shrub layer | 5·6 ± 1·54 | 9·7 ± 1·98 | 11·0 ± 2·04 |
| % Allocation in | |||
| above‐ground parts§ | 69·7 | 74·2 | 72·7 |
| below‐ground parts | 30·3 | 25·8 | 27·3 |
| Herb layer | 2·8 ± 1·76 | 2·6 ± 2·14 | 2·4 ± 2·44 |
| % Allocation in | |||
| above‐ground parts | 78·9 | 78·1 | 75·8 |
| below‐ground parts | 21·1 | 21·8 | 24·2 |
| Total vegetation | 58·7 ± 1·92 | 106·1 ± 2·35 | 136·1 ± 2·54 |
* Bole wood + bole bark, which accounted for 10·0–10·7 % of the values. † Branch + twig, which accounted for 3·6–4·2 % of the values. ‡ Stump root (main root) + lateral roots (lateral branches of main root), which accounted for 4·2–4·8 % of the values. § Stem + foliage, which accounted for 13·4–16·1 % of the values.
Values for above‐ground biomass in the present study are comparable with those of aspen–maple–birch, Sal, Shisham, eucalypt and poplar forest stands studied elsewhere (Crow, 1978; Singh, 1979; Sharmaet al., 1988; Raizada and Srivastava, 1989; Lodhiyal, 1990; Lodhiyal and Lodhiyal, 1997) (Table 5). The present values fall within the range reported for eucalypt stands (54–319 t ha–1; Attiwill, 1979; Frederick et al., 1985a, b; Tandonet al., 1988; Bargaliet al., 1992), for 30‐year‐old stands of Shisham forest (141–172 t ha–1; Lodhiyal, 2000), for 9‐ to 10‐year‐old Populus deltoides plantations (105–152 t ha–1; Singh, 1989), for 8‐year‐old poplar plantations (134 t ha–1; Lodhiyal et al., 1995), and for Pinus roxburghii forests (113–283 t ha–1; Chaturvedi, 1983), but are much lower than values reported for 100+‐year‐old oak forests (263–301 t ha–1; Negiet al., 1983; Rawat, 1983) (Table 5). Biomass production is higher in young Shisham forests than in older natural forests of the region.
Table 5.
Comparisons of biomass distribution (%) in above‐ground tree components of certain forests and plantations around the world
| % Allocation | ||||||||||
| Vegetation | Location | Age (years) | Density | Above‐ground biomass (t ha–1) | Bole | Branch | Twig | Foliage | Reproductive parts | References |
| Shorea robusta | India | >100 | – | 70·2 | 43·9 | 51·0 | – | 5·1 | – | Singh (1979) |
| Aspen–maple–birch | USA | – | – | 95·4 | 79·5 | 17·6 | – | 2·9 | – | Crow (1978) |
| Oak forest | India | >100 | – | 301·5 | 51·0 | 44·4 | – | 4·6 | – | Rawat (1983) |
| Oak forest | India | >100 | – | 263·2 | 51·7 | 40·5 | – | 7·8 | – | Negi et al. (1983) |
| Eucalyptus obliqua | Australia | 51 | 865 | 298·2 | 91·2 | 6·5 | – | 2·3 | – | Attiwill (1979) |
| E. grandis | India | 5 | 1650 | 97·6 | 83·8 | 6·8 | 4·6 | 4·6 | – | Tandon et al. (1988) |
| E. grandis | India | 10 | 689 | 275·1 | 92·0 | 3·9 | 2·0 | 2·0 | Tandon et al. (1988) | |
| E. saligna | Australia | 8 | 829 | 129·8 | 80·9 | 13·4 | 1·2 | 4·4 | 0·08 | Frederick et al. (1985b) |
| E. regnans | Australia | 10 | 1075 | 319·0 | 82·1 | 13·0 | – | 4·8 | – | Frederick et al. (1985a) |
| Pinus roxburghii | India | – | – | 113·0–283·0 | 76·1 | 19·4 | – | 4·5 | – | Chaturvedi (1983) |
| Eucalyptus hybrid | India | 10 | 1223 | 21·9 | 62·1 | 15·4 | 8·8 | 13·7 | – | Pandey et al. (1987) |
| Eucalyptus hybrid | India | 1 | 2000 | 0·5 | 50·0 | 10·4 | 2·1 | 35·4 | – | Bargali et al. (1992) |
| Eucalyptus hybrid | India | 2 | 2000 | 3·2 | 75·2 | 5·6 | 1·9 | 17·2 | – | Bargali et al. (1992) |
| Eucalyptus. hybrid | India | 3 | 2000 | 18·5 | 77·7 | 7·0 | 1·8 | 14·5 | – | Bargali et al. (1992) |
| Eucalyptus hybrid | India | 5 | 2000 | 54·1 | 80·8 | 6·6 | 1·6 | 10·2 | 0·7 | Bargali et al. (1992) |
| Populus deltoides Marsh | India | 14 | – | 44·5 | 69·9 | 13·7 | – | 7·4 | – | Raizada and Srivastava (1989) |
| P. deltoides (I C) | India | 10 | – | 105·4 | 74·4 | 13·0 | – | 12·6 | – | Singh (1989) |
| P. deltoides Marsh | India | 9 | – | 151·6 | 71·9 | 16·0 | – | 12·1 | – | Singh (1989) |
| P. deltoides Marsh | India | 1 | 666 | 7·4 | 56·7 | 17·6 | 5·4 | 20·3 | – | Lodhiyal and Lodhiyal (1997) |
| P. deltoides Marsh | India | 2 | 666 | 28·0 | 59·6 | 18·6 | 6·1 | 15·7 | – | Lodhiyal and Lodhiyal (1997) |
| P. deltoides Marsh | India | 3 | 666 | 49·4 | 65·2 | 16·0 | 5·9 | 12·9 | – | Lodhiyal and Lodhiyal (1997) |
| P. deltoides Marsh | India | 4 | 666 | 89·3 | 67·2 | 15·2 | 5·4 | 12·2 | – | Lodhiyal and Lodhiyal (1997) |
| P. deltoides Marsh | India | 5 | 400 | 67·6 | 69·4 | 14·8 | 4·3 | 11·5 | – | Lodhiyal et al. (1995) |
| P. deltoides Marsh | India | 8 | 400 | 134·3 | 74·7 | 12·6 | 4·0 | 8·6 | – | Lodhiyal et al. (1995) |
| Dalbergia sissoo | India | 3 | 10000 | 41·39 | 38·6 | 29·2 | 12·0 | – | – | Tewari (1994) |
| D. sissoo | India | 5 | – | 82·0 | 40·6 | 34·9 | – | 6·5 | – | Sharma et al. (1988) |
| D. sissoo | India | 24 | 467 | 16·16 | 65·5 | 14·2 | 2·6 | – | – | Sharma et al. (1988) |
| Tarai Shisham | Uttaranchal (India) | 5 | 625 | 41·8 | 64·4 | 7·8 | 3·6 | 6·4 | 0·8 | Present study |
| (D. sissoo) forests | Uttaranchal (India) | 10 | 625 | 78·6 | 66·1 | 8·0 | 3·8 | 4·9 | 1·0 | Present study |
| (D. sissoo) forests | Uttaranchal (India) | 15 | 625 | 103·1 | 66·0 | 8·4 | 4·2 | 4·2 | 1·2 | Present study |
The proportion of total biomass found in roots was 16–17 %, which is lower than values reported for Populus deltoides plantations (19–21 %; Lodhiyal et al., 1995) and Eucalyptus signata and E. umbra forests (43 %; Westman and Rogers, 1977), but is higher than values reported for temperate forests (8–15 %; Whittaker and Woodwell, 1971; Nihlgard, 1972; Larsenet al., 1976) and the 10–12 % reported for eucalypt forests (Feller, 1980). The differences in root contribution may be related to the method of estimating root biomass, which presents great sampling difficulty (Lodhiyal et al., 1995) and is often omitted in biomass studies.
Analysis of variance showed significant (P < 0·01) variations in total tree biomass and its components among plantations of different ages. The contribution of bole wood, branches, twigs, reproductive parts, lateral roots and fine roots increased with age while that of the remaining parts decreased.
Forest floor biomass
Turnover rate ranged from 0·77 (15‐year‐old stand) to 0·81 kg year–1 (5‐year‐old stand), while turnover time was 1·23 (5‐year‐old stand) to 1·29 years (15‐year‐old stand) (Table 6). The replacement of litter from forest floor was 77–81 % each year. The turnover time increased with increasing age of the stand (Table 6). The turnover time of litter on the forest floor was greater than 1 year.
Table 6.
The average forest floor biomass (t ha–1, across seasons) and turnover of litter (rate and time) in Shisham forests of different ages in central Himalaya
| Age of Shisham forests (years) | |||
| Components | 5 | 10 | 15 |
| Forest floor litter (t ha–1) | 4·8 ± 2·69 | 5·6 ± 3·01 | 6·6 ± 3·37 |
| % Allocation in fresh leaf litter | 8·9 | 9·3 | 9·8 |
| Partially and more decomposed litter | 22·9 | 27·8 | 30·0 |
| Wood litter | 6·5 | 12·5 | 18·8 |
| Miscellaneous litter* | 24·6 | 21·1 | 19·1 |
| Herbaceous litter† | 37·0 | 29·2 | 22·3 |
| Turnover rate (kg year–1) | 0·81 | 0·78 | 0·77 |
| Turnover time (t, year) | 1·23 | 1·28 | 1·29 |
* Includes reproductive parts of trees and litter parts of shrubs. † Includes living and dead herbaceous material, which accounted for 3·8–6·4 % of the values.
The biomass of litter on the forest floor increased with age of the forest stand. However, the herbaceous biomass (both alive and dead) showed the reverse trend. The same trend was observed for Populus deltoides plantations (Lodhiyalet al., 1995) and Eucalyptus hybrid plantations (Bargaliet al., 1992). In the present study, forest floor biomass was lower than that reported for Pinus stands (13–110 t ha–1; Ovington, 1965), oak forests (9·6–12·6 t ha–1; Monket al., 1970; Reiners and Reiners, 1970), Eucalyptus saligna (12·4 t ha–1; Richards and Charley, 1977), Eucalyptus obliqua (18·3 t ha–1; Attiwill et al., 1978), Eucalyptus regnans (47·5 t ha–1; Feller, 1980) and Pinus roxburghii forests (9·6–13·6 t ha–1; Chaturvedi and Singh, 1987a) but is within the range reported for 1‐ to 30‐year‐old Dalbergia sissoo forests (2·5–7·3 t ha–1; Lodhiyal, 2000), tropical deciduous forests (1·5–8·9 t ha–1; Singh and Misra, 1978; Singh, 1979) and eucalypt plantations (Bradstock, 1981; Frederick et al., 1985b), and close to values reported for Populus deltoides plantations (2·8–6·4 t ha–1; Lodhiyal, 1990), Eucalyptus hybrid plantations (4·0–6·7 t ha–1; Bargaliet al., 1992) and oak forest (Rawat and Singh, 1988) in adjacent areas. The smaller biomass on the forest floor indicates a high rate of decomposition under the warm and humid conditions. Similar findings were also reported for deciduous poplar plantations in adjacent areas (Lodhiyalet al., 1995; Lodhiyal and Lodhiyal, 1997).
The turnover rate of forest floor litter biomass in the present study sites is higher than values reported for Cassia siamea, Dalbergia sissoo and Gmelina arborea forests (0·58, 0·60 and 0·73, respectively; Pacholi, 1997). The turnover time of litter is close to the value of 1·25 years reported for Eucalyptus grandis (Turner and Lambert, 1983). The present values are lower than the 2·7–14·3 years reported for temperate forests (Reiners and Reiners, 1970; Goszet al., 1972; Rochow, 1975), 3·5 years for Eucalyptus obliqua (Attiwillet al., 1978) and 1·4–1·5 years for central Himalayan forests (Rawat and Singh, 1988). Thus, present findings show that Shisham forest floor litter biomass is more dynamic than that of temperate deciduous forests, exotic eucalypt plantations and central Himalayan oak forests.
Litter input
Leaves are a major component of the total litter input. Leaf fall began in October and culminated in January the following year. Total litter fall ranged from 2·7 (5‐year‐old stand) to 5·1 t ha–1 year–1 (15‐year‐old stand) (Table 7). Of this, leaf litter accounted for 67–74 %, within the range reported for natural forests of central Himalaya (60–80 %; Singh and Singh, 1992) and for temperate forests (40–84 %; Rodin and Bazilevick, 1967). However, wood litter represented 3–7 %, which is much lower than the value reported for various forests around the world (10–36 %; Bray and Gorham, 1964; Killingbeck and Wali, 1978; Singh and Singh, 1992) but close to values reported for Tarai poplar plantations (2–5 %; Lodhiyalet al., 1995).
Table 7.
Litter production (t ha–1 year–1) in Shisham (Dalbergia sissoo) forests of Tarai area of central Himalaya
| Age of Shisham forests (years) | |||
| Litter components | 5 | 10 | 15 |
| Leaf litter | 2·02 (74·3) | 3·04 (72·0) | 3·44 (66·9) |
| Wood litter* | 0·09 (3·3) | 0·21 (5·0) | 0·36 (7·0) |
| Reproductive litter† | 0·03 (1·1) | 0·09 (2·1) | 0·18 (3·5) |
| Other litter‡ | 0·58 (21·3) | 0·88 (20·9) | 1·16 (22·6) |
| Total | 2·72 (100) | 4·22 (100) | 5·14 (100) |
* Includes barks, twigs and branches. † Includes inflorescences, pods and fruits of trees and shrubs. ‡ Includes the leaf litter of shrubs and herbs.
Our estimates of leaf litter turnover (2·0–3·4 t ha–1 year–1) are towards the higher end of the range (1·3–4·2 t ha–1 year–1) reported for poplar (Populus tremuloides and Populus tristis; Crow, 1974; van Cleave and Noonan, 1975; Zavitkovski, 1981), but the present estimates are lower than those reported for Eucalyptus regnans and many deciduous forests (5·7–7·8 t ha–1 year–1; Ashton, 1975; Singh and Mishra, 1978; Singh, 1989; Lodhiyalet al., 1995a), dry deciduous forests (8–10 t ha–1 year–1; Jennyet al., 1949), bamboo/mixed broad leaf forests of eastern Himalaya (9·6 t ha–1 year–1; Toky and Ramakrishnan, 1983a), moist tropical forest (10·5 t ha–1 year–1; Golleyet al., 1975) and tropical forests (8–12 t ha–1 year–1; Procter et al., 1983).
Net primary productivity
Total NPP (t ha–1 year–1) of Shisham forests at different ages is given in Table 8. NPP in the tree layer ranged from 9·1 (5‐year‐old stand) to 16·7 t ha–1 year–1 (15‐year‐old stand). Above‐ground parts account for 78 (15‐year‐old stand) to 81 % (5‐year‐old stand) and below‐ground parts for 19 (10‐year‐old stand) to 22 % (15‐year‐old stand) (Table 8). The net primary productivity of the shrub layer was 0·7 (5‐year‐old stand) to 1·3 t ha–1 year–1 (15‐year‐old stand). Of this, foliage and roots accounted for 14–17 % and 25–31 %, respectively (Table 8). The productivity of the herb layer was 2·4 (15‐year‐old stand) to 2·8 t ha–1 year–1 (5‐year‐old stand). Above‐ground parts accounted for 76 (15‐year‐old stand) to 79 % (5‐year‐old stand) and below‐ground parts for 21 (5‐year‐old stand) to 24 % (15‐year‐old stand) (Table 8).
Table 8.
Component wise net primary productivity (t ha–1 year–1) in trees, shrubs and herbs of Shisham forests in central Himalaya
| Age of Shisham forests (years) | |||
| Vegetation | 5 | 10 | 15 |
| Tree layer | 9·1 ± 1·25 | 12·5 ± 1·76 | 16·7 ± 2·02 |
| % Allocation in | |||
| bole* | 61·5 | 63·2 | 60·4 |
| branch† | 11·1 | 11·2 | 11·4 |
| leaf | 6·6 | 4·8 | 4·8 |
| reproductive part | 1·0 | 1·6 | 1·8 |
| coarse roots‡ | 14·3 | 12·8 | 15·6 |
| fine roots | 5·5 | 6·4 | 6·0 |
| Shrub layer | 0·7 ± 0·74 | 1·2 ± 0·85 | 1·3 ± 0·98 |
| % Allocation | |||
| above‐ground§ | 71·4 | 75·0 | 69·2 |
| below‐ground | 28·6 | 25·0 | 30·8 |
| Herb layer | 2·8 ± 1·42 | 2·6 ± 1·68 | 2·4 ± 1·92 |
| % Allocation | |||
| above‐ground | 78·9 | 78·1 | 75·8 |
| below‐ground | 21·1 | 21·9 | 24·2 |
| Total vegetation | 12·6 ± 1·14 | 16·3 ± 1·43 | 20·3 ± 1·64 |
* Bole wood + bark, which accounted for 8·9–10·4 % of the values. † Branch + twigs (current shoots bearing leaves), which accounted for 3·3–4·0 % of the values. ‡ Stump root (main root) + lateral roots (lateral branches of main root), which accounted for 3·2–7·2 % of the values. § Stem + foliage, which accounted for 14·3–16·7 % of the values.
The total NPP in vegetation ranged from 12·6 (5‐year‐old stand) to 20·3 t ha–1 year–1 (15‐year‐old stand), of which the tree layer accounted for 72 (5‐year‐old stand) to 82 % (15‐year‐old stand), the shrub layer for 5 (5‐year‐old stand) to 7 % (10‐year‐old stand) and the herb layer for 12 (15‐year‐old stand) to 22 % (5‐year‐old stand) (Table 8).
The NPP: foliar standing crop (FSC) ratio ranged from 2·6 (10‐year‐old stand) to 3·3 (15‐year‐old stand). The production efficiency of Dalbergia sissoo leaves is similar to that (2·5 kg kg–1 FSC) reported for a eucalypt plantation (Bargaliet al., 1992) and a Pinus roxburghii forest (Singh and Singh, 1992), but is higher than that reported for poplar plantations (Lodhiyalet al., 1995) in an adjacent area of central Himalaya. The greater efficiency of Shisham forests seems to be largely due to their longer growing period; it could also be due to higher photosynthetic efficiency (Singh and Singh, 1992). The production of herbaceous vegetation was greater than that of shrubs, and decreased significantly (P < 0·01) with the age of the forest (see Table 8). Similar observations of herbaceous production have been reported for exotic poplar plantations in the region (Lodhiyalet al., 1995). Comparisons with other forests and plantations around the world show that the NPP of the present Shisham forests (13–20 t ha–1 year–1) is slightly lower than that reported for an 8‐year‐old fast‐growing exotic eucalypt plantation (23·4 t ha–1 year–1; Bargaliet al., 1992), and for a 100+‐year‐old natural Sal (Shorea robusta) forest in an adjacent area (22 t ha–1 year–1; Singh and Singh, 1987); however, it was much lower than the value of 32·4 t ha–1 year–1 reported for a 4‐year‐old high density poplar plantation (Lodhiyal and Lodhiyal, 1997) (Table 9). NPP values in the study forests are higher than the 12–15 t ha–1 year–1 reported for many mature stable temperate forests of favourable environments, and fall within the normal range of NPP (10–20 t ha–1 year–1) of world forests growing in favourable climates (Whittaker, 1975).
Table 9.
Total net primary productivity (NPP) of Shisham forests compared with other forests and plantations around the world
| Vegetation | Location | NPP (t ha–1 year–1) | References |
| Camelia japonica | Japan | 29·4 | Kan et al. (1965) |
| Cryptomeria japonica | Japan | 10·9 | Tadaki et al. (1965) |
| Pine forest | USA | 11·0 | Whittaker (1966) |
| Tropical rain forest | Thailand | 28·6 | Kira et al. (1967) |
| Conifer forests | USSR | 7·0–10·0 | Rodin and Bazilevick (1967) |
| Tropical rain forest | Java | 24·3 | Warner (1970) |
| Tropical moist forest | – | 11·4 | Golley et al. (1975) |
| Dry deciduous forest | India | 14·6–15·7 | Singh (1979) |
| Sal old growth forest | India | 16·0–18·9 | Singh and Singh (1979) |
| Tropical rain forest | Malaysia | 19·2 | Bullock (1981) |
| Shisham plantation (24 years old) | India | 7·2 | Sharma et al. (1988) |
| Rianj dominated forest | India | 15·5 | Rana et al. (1989) |
| Chir‐pine forest | India | 17·3 | Rana et al. (1989) |
| Eucalyptus plantation (8 years old) | India | 23·4 | Bargali et al. (1992) |
| Low density poplar plantation (8 years old) | India | 24·5 | Lodhiyal et al. (1995) |
| High density poplar plantation (4 years old) | India | 32·4 | Lodhiyal and Lodhiyal (1997) |
| Gmelinaarborea forest | India | 10·0 | Pacholi (1997) |
| Cassia siamea forest | India | 18·8 | Pacholi (1997) |
| Dalbergia sissoo forest | Bihar(India) | 22·3 | Pacholi (1997) |
| Tarai Shisham forests | Uttaranchal (India) | 12·6–20·3 | Present study |
In this context, productivity of Shisham can be increased substantially by planting trees at a higher density with the help of better silvicultural management input (Lodhiyal, 2000). Comparison with other deciduous forests in India suggests that fine root production in the present study is perhaps an underestimation. A value of fine root production of 0·5 (5‐year‐old stand) to 1·0 t ha–1 year–1 (15‐year‐old stand) gives ratios of fine root production to above‐ground production of 13 (10‐year‐old stand) to 15 (5‐year‐old stand), compared with ratios of 5 and 11 reported for Shorea robusta and Populus deltoides, respectively (Singh and Singh, 1992; Lodhiyalet al., 1995). According to Nadelhoffer and Raich (1992), the ratio of fine root to above‐ground production is influenced by the soil status; these authors argued that there is still uncertainty with regards to understanding of soil systems. Moreover, little fine root production in our study indicates the higher fertility of sites and is similar to values reported for fast‐growing exotic plantations in adjacent areas (Singh, 1989; Lodhiyalet al., 1995).
Biomass accumulation ratio
The biomass accumulation ratio (BAR; biomass/NPP) is an expression of the quantity of biomass retained per unit of net production. BAR has been used to characterize the production conditions in communities (Whittaker, 1966; Whittaker and Woodwell, 1969; Lodhiyal and Lodhiyal, 1997). BAR is a measure of accumulation of primarily persistent material, a characteristic largely dependent on the age of the dominant species and environmental harshness. BAR ranges between 5·5 and 7·5 for Dalbergia sissoo forests (Table 10); this is higher than the values of 0·9–5·9 reported for eucalypt plantations (Bargaliet al., 1992), and falls within the range of 4·9–7·7 reported for Populus deltoides plantations (Lodhiyalet al., 1995). The high BAR suggests that Shisham forests produce more dry matter than eucalypt plantations, and a similar amount to poplar plantations growing in an adjacent area of central Himalaya.
Table 10.
Biomass accumulation ratio (BAR, biomass/ total NPP of tree) in different tree components of Tarai Shisham forests
| Age of forests (years) | |||
| Component | 5 | 10 | 15 |
| Bole wood | 2·96 | 4·17 | 4·11 |
| Bole bark | 0·60 | 0·79 | 0·74 |
| Branch | 0·43 | 0·60 | 0·62 |
| Twig | 0·20 | 0·29 | 0·31 |
| Leaf | 0·35 | 0·37 | 0·31 |
| Reproductive parts | 0·04 | 0·07 | 0·09 |
| Stump root | 0·62 | 0·75 | 0·69 |
| Lateral roots | 0·23 | 0·34 | 0·35 |
| Fine roots | 0·08 | 0·12 | 0·13 |
| Total | 5·51 | 7·50 | 7·35 |
Dry matter transfer
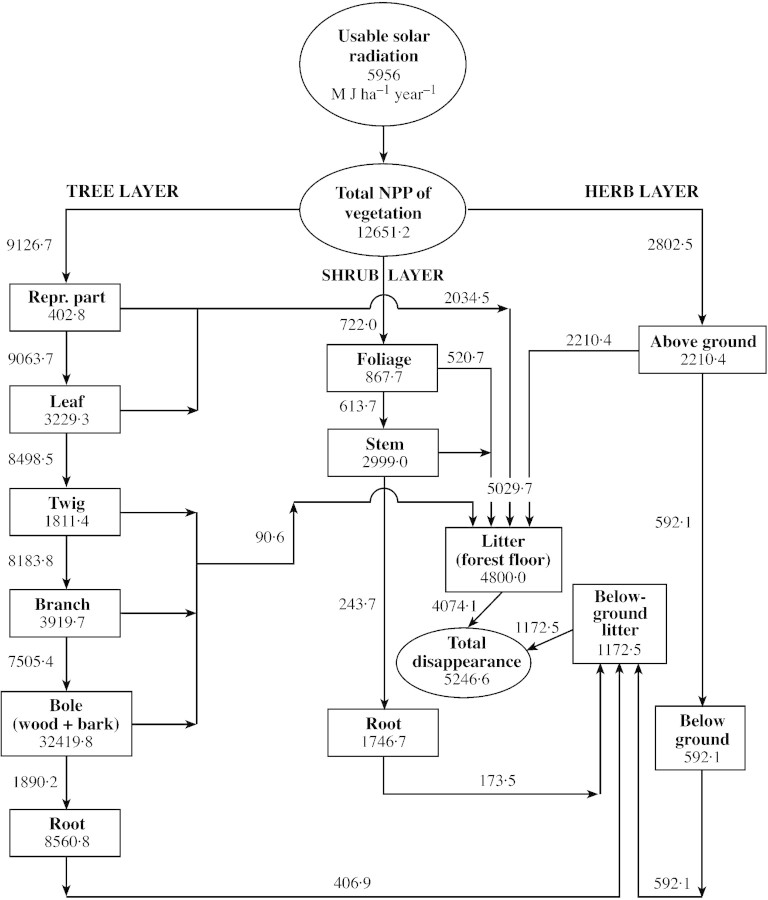
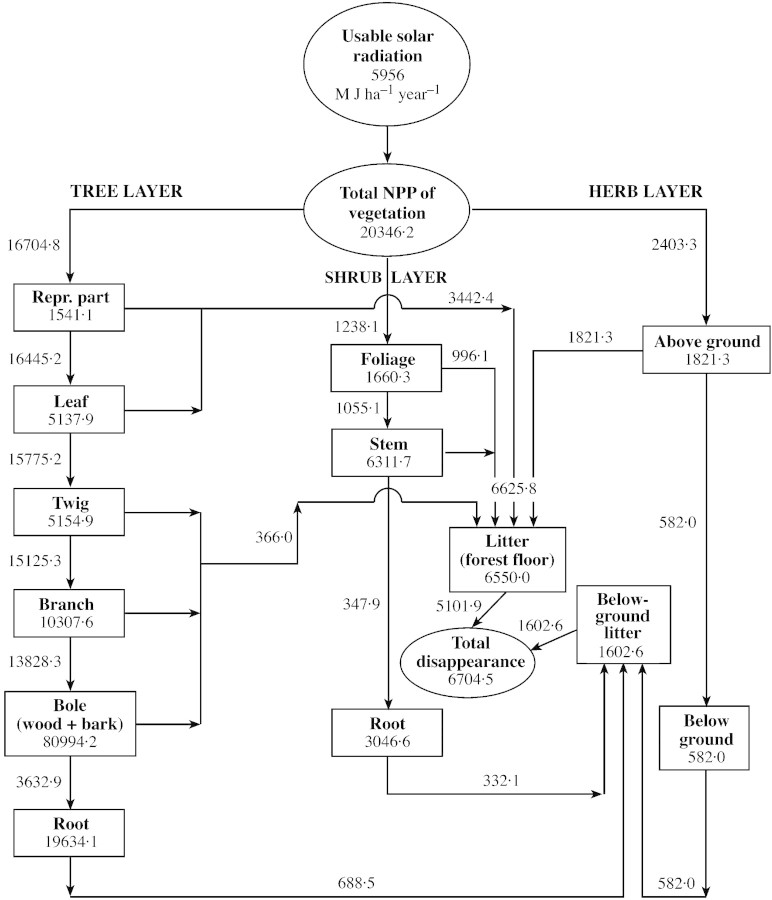
A compartment model of dry matter flow in 5‐ and 15‐year‐old forests is given in Figs 2 and 3. The mean annual incident solar insolation is 5956 M J ha–1 year–1. Of this, about 2978 M J ha–1 year–1 (50 % of the total) is considered photosynthetically active.
Fig. 2. Compartment model for dry matter distribution in 5‐year‐old Shisham (Dalbergia sissoo Roxb) forest. Rectangles represent compartments for standing crop of dry matter, arrows represent net flux rate. The three ovals represent total annual amounts of usable solar radiation (M J ha–1 year–1), total net production (kg ha–1 year–1) and total disappearance (kg ha–1 year–1). Compartment values are in kg ha–1 and turnover rate in kg ha–1 year–1.
Fig. 3. Compartment models for dry matter distribution in 15‐year‐old Shisham (Dalbergia sissoo Roxb.) forest. Rectangles represent compartments for standing crop of dry matter, arrows represent net flux rate. The three ovals represent total annual amounts of usable solar radiation (M J ha–1 year–1), total net production (kg ha–1 year–1) and total disappearance (kg ha–1 year–1). Compartment values are in kg ha–1 and turnover rate in kg ha–1 year–1.
The biomass of the vegetation is 58·7 × 103 kg ha–1 in the 5‐year‐old stand and 136·1 × 103 kg ha–1 in the 15‐year‐old stand. Trees account for 86 %, shrubs 9 % and herbs 5 % of the total dry matter (biomass) of vegetation in the 5‐year‐old forest; these figures are 90 %, 8 % and 2 %, respectively, in the 15‐year‐old forest. In the tree layer, the above‐ground ratio of photosynthetic : non‐photosynthetic tissue ranged from 0·52 (15‐year‐old stand) to 0·08 (5‐year‐old stand), and the root : shoot ratio ranged from 0·19 (15‐year‐old stand) to 0·20 (5‐year‐old stand). These ratios are similar to those of natural forests (Chaturvedi and Singh, 1987b; Rawat and Singh, 1988) and eucalypt plantations (Bargaliet al., 1992), and are quite similar to those of poplar plantations (Lodhiyalet al., 1995).
Net primary production is 12 651 kg ha–1 year–1 in the 5‐year‐old stand and 20 346 kg ha–1 year–1 in the 15‐year‐old stand. Tree, shrub and herb layers accounted for 72 %, 6 % and 22 % of NPP in the 5‐year‐old stand, and 82 %, 6 % and 12 % in the 15‐year‐old stand, respectively.
In the tree layer, the highest fraction of net production is by bole wood (51 %) followed by bole bark (10 %) in the 5‐year‐old stand, and bole wood (52 %) followed by bole bark (9 %) in the 15‐year‐old stand. Leaves accounted for about 6 % in the 5‐year‐old stand and 4 % in the 15‐year‐old stand.
The apportioning of net production in shrubs follows the order: stem (52 %) > roots (33 %) > foliage (15 %) in the 5‐year‐old stand, and stem (57 %) > roots (28 %) > foliage (15 %) in the 15‐year‐old stand. However, the order: foliage > stem > roots was reported for the shrub layer in pine (Chaturvedi and Singh, 1987b) and oak forests (Rawat and Singh, 1988) and in poplar plantations (Lodhiyalet al., 1995).
In the herb layer, the proportion of NPP by above‐ground components (76 % in 15‐year and 79 % in 5‐year‐old stands) is much higher than that in central Himalayan forests (62–64 %; Chaturvedi and Singh, 1987b; Rawat and Singh, 1988), but is similar to that of poplar plantations (80 %; Lodhiyalet al., 1995). The maximum production of photosynthetically active parts of the different vegetational layers was in the order: herbs > shrubs > trees.
The restitution of biomass through litter formation is 5030 (5‐year‐old stand) to 6626 kg ha–1 year–1 (15‐year‐old stand). Of the total litter fall from the tree layer, leaf litter constitutes about 74 % in the 5‐year‐old stand and 67 % in the 15‐year‐old stand. The present values are similar to those of natural forests (67–77 %; Chaturvedi and Singh, 1987a; Rawat and Singh, 1988) and are higher than those of a eucalypt plantation (55 %; Bargaliet al., 1992) but lower than those of a poplar plantations (94–96 %; Lodhiyalet al., 1995).
In terms of dry matter, biomass restitution is 23 (15‐year‐old stand) to 25 % (5‐year‐old stand) of the total annual production of trees and 18 (5‐year‐old stand) to 19 % (15‐year‐old stand) of the total vegetation. Our estimates are lower than those for poplar (Lodhiyalet al., 1995) and eucalypt plantations (Bargaliet al., 1992) and natural forests of oak (Rawat and Singh, 1988) and pine (Chaturvedi and Singh, 1987a).
The mean standing crop of litter on the forest floor ranged from 4800 (5‐year‐old stand) to 6550 kg ha–1 (15‐year‐old stand), which is 5·3 (15‐year‐old stand) to 9·5 % (5‐year‐old stand) of the tree biomass and 4·9 (15‐year‐old stand) to 8·6 % (5‐year‐old stand) of the total vegetation.
Decomposition of litter at the soil surface, as indicated by turnover rate, is 4074 (5‐year‐old stand) to 5102 kg ha–1 year–1 (15‐year‐old stand) of the total litter input. This amounts to 77·0 (15‐year‐old stand) to 81·0 % (5‐year‐old stand) of the total litter input. Compared with this, in the present 5‐ and 15‐year‐old forests, the annual weight loss in leaf litter as determined by litter bags was 93 (15‐year‐old stand) to 94 % (5‐year‐old stand) (Lodhiyal, 2000). At the end of the annual cycle, 956 (5‐year‐old stand) to 1524 kg ha–1 year–1 (15‐year‐old stand) litter remains under composed, and is carried over to the next year. According to Lodhiyalet al. (1995), little mortality of main roots occurs in actively growing trees and shrubs, but considerable mortality occurs in fine roots. Orlov (1968) and Ogino (1977) found that mortality of fine roots is equivalent to one‐fifth of leaf litter; we made the same assumption here. However, our present estimates of fine root production may be a gross underestimation (Fogel, 1983; Vogtet al., 1986). Since herbaceous vegetation at all three sites is annual, root mortality was assumed to be 100 %. The root mortality of trees, shrubs and herbs amounts to 407, 174 and 592 kg ha–1 year–1, respectively, in the 5‐year‐old forest, and 689, 332 and 582 kg ha–1 year–1 in the 15‐ year‐old Shisham forest.
Received: 6 February 2001; Returned for revision: 3 April 2001; Accepted: 14 September 2001.
References
- Ashton DH.1975. Studies of litter in Eucalyptus regnans forests. Australian Journal of Botany 23: 413–433. [Google Scholar]
- Attiwill PM.1979. Nutrient cycling in an Eucalyptus obliqua (L’ Herit) forest. III. Growth, biomass and net primary production. Australian Journal of Botany 27: 439–458. [Google Scholar]
- Attiwill PM, Ovington JD.1968. Determination of forest biomass. Forest Science 14: 3–15. [Google Scholar]
- Attiwill PM, Guthrie HB, Leuning R.1978. Nutrient cycling in an Eucalyptus obliqua (L’ Herit) forest. I. Litter production and nutrient return. Australian Journal of Botany 26: 76–91. [Google Scholar]
- Bargali SS, Singh SP, Singh RP.1992. Structure and function of an age series of eucalypt plantations in central Himalaya. I. Dry matter dynamics. Annals of Botany 69: 405–411. [Google Scholar]
- Bray JR, Gorham E.1964. Litter production in forests of World. Advances in Ecological Research 2: 101–157. [Google Scholar]
- Bradstock R.1981. Biomass in an age series of Eucalyptus grandis plantations. Australian Journal of Forest Research 11: 111–127. [Google Scholar]
- Bullock JA.1981. In: Reichle DE. Dynamic properties of forest ecosystems. Cambridge: Cambridge University Press. [Google Scholar]
- Carlisle A, Brown AHF, White ET.1966. Litter fall, leaf litter production and effects of defoliation by Tortrix viridiana in a sessile oak (Quercus petracea) woodland. Journal of Ecology 54: 65–68. [Google Scholar]
- Champion HG, Seth SK.1968. A revised survey of forest types of India. Delhi: Government of India. [Google Scholar]
- Chaturvedi OP.1983. Biomass structure, productivity and nutrient cycling in Pinus forest. PhD Thesis, Kumaun University, Nainital, India. [Google Scholar]
- Chaturvedi OP, Singh JS.1987. A quantitative study of the forest floor biomass, litter fall and nutrient return in a Pinus roxburghii forest in Kumaun Himalaya. Vegetatio 71: 97–106. [Google Scholar]
- Chaturvedi OP, Singh JS.1987. The structure and function of pine forest in central Himalaya: Dry matter dynamics.Annals of Botany 60: 237–252. [Google Scholar]
- Chaturvedi AN, Sharma, SC, Ramje Srivastava.1984. Water consumption and biomass production in some forest trees. Commonwealth Forestry Review 63: 217–223. [Google Scholar]
- Crow TR.1974. Temporal and spatial patterns of pre‐treatment litter production in site 1 and the control area. In: Rudolph TD, ed. Enterprise radiation forest U.S. AEC Publications TID‐26113, 105–113. [Google Scholar]
- Crow TR.1978. Biomass and production in three contiguous forests in northern Wisconsin. Ecology 54: 265–273. [Google Scholar]
- Feller MC.1980. Biomass and nutrient distribution in two eucalypt forest ecosystems. Australian Journal of Ecology 5: 309–333. [Google Scholar]
- Fogel R.1983. Root turnover and productivity of coniferous forests. Plant and Soil 71: 75–85. [Google Scholar]
- Frederick DJ, Madgwick HAI, Jurgenson MF, Oliver GR.1985. Dry matter content and nutrient distribution in an age series of Eucalyptus regnans plantations in New Zealand. New Zealand Journal of Forestry Science 15: 158–179. [Google Scholar]
- Frederick DJ, Madgwick HIAI, Olnerand GR, Jurgensen MF.1985. Dry matter and nutrient content of 8‐year‐old Eucalyptus saligna growing of Tahoka Forest. New Zealand Journal of Forestry Science 15: 251–254. [Google Scholar]
- Golley FB, McGinnis JT, Clements RG, Child GI, Duever MJ.1975. Mineral cycling in a tropical moist forest ecosystem. Athens: Georgia Press. [Google Scholar]
- Gosz JR, Likens GE, Bormann FH.1972. Nutrient content of litter fall on Hubbard Brook Experimental Forest, New Hampshire. Ecological Monographs 43: 173–191. [Google Scholar]
- Green JP.1959. The measurement of herbage production. In: Ivins JD, ed. The measurement of green land productivity. London, 62–68. [Google Scholar]
- Gurumurti K, Dhawan M, Rawat PS.1987. Biomass production technique for decentralized energy planning. Urja Update 4: 3–16. [Google Scholar]
- Harris WF, Santantonio D, McGinty D.1980. The dynamic below ground ecosystem. In: Waring RH, ed. Forest: fresh perspectives from ecosystem analysis. Corvallis: Oregon State University Press, 119–130. [Google Scholar]
- Jackson ML.1958. Soil chemical analysis. Englewood Cliffs, N.J.: Prentice Hall. [Google Scholar]
- Jenny H, Gessel SP, Bingham FT.1949. Comparative study of decomposition rates of organic matter in temperate and tropical regions. Soil Science 68: 419–432. [Google Scholar]
- Kalela EK,1954. Mantysie menipuidenja‐puastojen. Jurrisuhtelsta. Acta Forestalia Fennica 61: 1–16. [Google Scholar]
- Kan MH, Saito, Shidei T.1965. Studies of the productivity of evergreen broad leaved forests. Bulletin Kyoto University of Forestry37: 55–75. [Google Scholar]
- Khan SA.1957. Attempt to regenerate Shisham in conjunction with cultivation of cotton in Khanewal irrigated plantation. Pakistan Journal of Forestry 7: 39–43. [Google Scholar]
- Killingbeck KT, Wali MK.1978. Analysis of North Dakota Gallery forest: Nutrient trace element and productivity relations. Oikos 30: 29–60. [Google Scholar]
- Kira T, Ogawa H, Yoda K, Ogino K.1967. Comparative ecological studies on three main types of forest vegetation in Thailand. IV. Dry matter production, with special reference to the Khao Chong rain forest. Nature and Life in South East Aria 5: 149–174. [Google Scholar]
- Larsen HS, Carter MC, Gooding JM, Hyink DM.1976. Biomass and nitrogen distribution in four 13‐year‐old loblolly pine plantations in hilly coastal plain of Alabama. Canadian Journal of Forest Research 10: 92–101. [Google Scholar]
- Lauenroth WK, Hunt HW, Swift DM, Singh JS.1986. Reply to Vogt et al.Ecology 67: 580–582. [Google Scholar]
- Leith H, Whittaker RL.1975. Primary productivity of biosphere. New York: Springer‐Verlag. [Google Scholar]
- Line C.1959. The value of herbage estimates in animal production estimates. In: Ivins JD, ed. The measurement of grassland productivity. London, 71–78. [Google Scholar]
- Lodhiyal LS.1990. Structure and functioning of Poplar plantations in Tarai belt of Kumaun Himalaya. PhD Thesis, Kumaon University, Nainital, India. [Google Scholar]
- Lodhiyal LS, Lodhiyal N.1997. Variation in biomass and net primary productivity in short rotation high density Central Himalayan poplar plantations. Forest Ecology and Management 98:167–179. [Google Scholar]
- Lodhiyal LS, Singh RP, Singh SP.1995. Structure and function of an age series of poplar plantations in central Himalaya. I. Dry matter dynamics. Annals of Botany 76: 191–199. [Google Scholar]
- Lodhiyal N.2000. Structure and functioning of Shisham (Dalbergia sissoo Roxb) forests in Tarai and Bhabar belt of Kumaon Himalaya. PhD Thesis, Kumaon University, Nainital, India. [Google Scholar]
- Merriam G, Duwryer L, Wegner J.1982. Litter fall in two Canadian deciduous woods: Quantity, quality and timing. Holarctic Ecology 50: 1–19. [Google Scholar]
- Misra R.1968. Ecology work book. Calcutta: Oxford and IBH Publishing. [Google Scholar]
- Monk CD, Child GD, Nicholson SA.1970. Biomass, litter and leaf surface area estimates of an Oak Hickory forest. Oikos 21: 138–141. [Google Scholar]
- Nadelhoffer KJ, Raich JW.1992. Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 73: 1139–1147. [Google Scholar]
- Negi KS, Rawat YS, Singh JS.1983. Estimation of biomass and nutrient storage in a Himalayan moist temperate forest. Canadian Journal of Forest Research 13: 1185–1196. [Google Scholar]
- Newbould PJ.1967. Methods for estimating the primary production of forests. IBH handbook No. 2. Oxford and Edinburgh: Blackwell Scientific Publications, 172. [Google Scholar]
- Nihlgard B.1972. Plant biomass, primary production and distribution of chemical elements in beech and a planted spruce forest in South Sweden. Oikos 23: 69–81. [Google Scholar]
- Ogawa H, Yoda K, Ogino K, Kira T.1965. Comparative ecological studies on three main types of forest vegetation in Thailand. II. Plant biomass. Nature and Life in South East Aria 4: 49–80. [Google Scholar]
- Ogino K.1977. A beech forest in Ashia: Biomass, its enrichment and net production. In: Shidei T, Kira T, eds. Primary productivity of terrestrial communities. Tokyo, Japan: University of Tokyo Press, 172–186. [Google Scholar]
- Olson JS.1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44: 322–331. [Google Scholar]
- Orlov AJ.1968. Development and life duration of pine feeding roots. In: Gholarov MS, Kovda Va, Novich Kovalvanova LN, Rodin LE, Sveshnika VM, eds. Productivity studies in root systems and rhizosphere organisms. USSR, Leningrad, 139–145. [Google Scholar]
- Ovington JD.1962. Quantitative ecology and the woodland ecosystem concept. Advances in ecological Research. Vol. 1. New York: Academic Press. [Google Scholar]
- Ovington JD.1965. Organic production, turnover and mineral cycling. Biological Review 40: 295–336. [Google Scholar]
- Pal M, Raturi DP.1990. Biomass production and organic matter partitioning. I. Dalbergia sissoo Roxb. grown in energy plantation. Journal of Tropical Forestry 6: 120–121. [Google Scholar]
- Pacholi RK.1997. Biomass productivity and nutrient cycling in Cassia siamea, Dalbergia sissoo and Gmelina arborea plantations. PhD Thesis, Kumaon University, India. [Google Scholar]
- Pandey MC, Tandon VN, Rawat HS.1987. Organic matter production and distribution of nutrient in Eucalyptus hybrid plantation ecosystem in Karnataka. Indian Forester 114: 713–724. [Google Scholar]
- Peach K, Tracy MV.1956. Modern methods of plant analysis Vol. I. Berlin: Springer‐Verlag. [Google Scholar]
- Proctor J, Anderson JM, Chal P, Vallack HW.1983. Ecological studies in four contrasting lowland rain forests in Gunug Mulu National Park, Sarawak. II. Litter fall, litter standing crop and preliminary observations on herbivory. Journal of Ecology 71: 261–283. [Google Scholar]
- Raizada A, Srivastava MM.1989. Biomass yield and biomass equations for Populus deltoides Marsh. Indian Journal of Forestry 12: 56–61. [Google Scholar]
- Rana BS, Singh SP, Singh RP.1989. Biomass and net primary productivity in Central Himalayan forests along an altitudinal gradient. Forest Ecology and Management 27: 199–218. [Google Scholar]
- Rawat YS.1983. Plant biomass, net primary production and nutrient cycling in oak forest. PhD Thesis, Kumaun University, Nainital, India. [Google Scholar]
- Rawat YS, Singh JS.1988. Structure and function of oak forest in central Himalaya: Dry matter dynamics. Annals of Botany 62: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners WA, Reiners NM.1970. Energy and nutrient dynamics of forest floors in three Minnesota forests. Journal of Ecology 58: 497–519. [Google Scholar]
- Richards BN, Charley JL.1977. Carbon and nitrogen flux through native forest floors. CSIRO Symposium on Nutrient Cycling in Indigenous Forest Ecosystems. Perth: CSIRO, 65–82. [Google Scholar]
- Rochow JJ.1975. Mineral nutrient pool and cycling in a Missouri forest. Journal of Ecology 63: 985–994. [Google Scholar]
- Rodin LE, Bazilevick NI.1967. Production and mineral cycling in terrestrial vegetation. Edinburgh: Oliver and Boyd. [Google Scholar]
- Sala OE, Biondi ME, Lauenroth WK.1988. Bias in estimates of primary production: an analytical solution. Ecological Modelling 44: 43–55. [Google Scholar]
- Satoo T.1968. Primary production in relation to woodlands of Pinus densiflora In: Young HE, ed. Primary productivity and mineral cycling in natural ecosystems. Maine: University of Maine Press, 53–80. [Google Scholar]
- Sharma DC, Taneja PL, Bisht APS.1988. Biomass, productivity and nutrient cycling in a Dalbergia sissoo plantation. Indian Forester 114: 261–268. [Google Scholar]
- Singh B.1979. Ecologyof Pinus patula planted in Darjeeling Himalaya. PhD Thesis, Banaras Hindu University, Varanasi, India. [Google Scholar]
- Singh B.1989. A study of silvicultural practices, biomass productivity and nutrient cycling (NPK) in Poplar plantation of Sub‐Himalayan tract of U.P. State, India. PhD Thesis, Kumaun University, Nainital, India. [Google Scholar]
- Singh B, Mathur HN, Joshi P.1980. Effect of tree shade on grassland production in the moist subtropical region of northern India. Indian Journal of Forestry 3: 345–348. [Google Scholar]
- Singh JS, Singh SP.1987. Forest vegetation of the Himalaya. Botanical Reviews 53: 82–192. [Google Scholar]
- Singh JS, Singh SP.1992. Forest of Himalaya; Structure, functioning and impact of man. Nainital, India: Gyanodaya Prakashan. [Google Scholar]
- Singh KP, Misra R.1978. Structure and functioning of natural, modified and silvicultural ecosystems of eastern Uttar Pradesh. MAB Technical Report, Banaras Hindu University, Varanasi, India (unpublished). [Google Scholar]
- Swaminath MH.1988. Studies on response of fast growing forestry species for biomass production under irrigation. My Forest 24: 117–123. [Google Scholar]
- Tadaki Y, Ogata N, Nagatoma Y.1965. The dry matter productivity in several stands of Cryptomeria japonica in Kyushu. Bull Government Forest Extension, Meguro, Tokyo 173: 45–66. [Google Scholar]
- Tandon VN, Pande MC, Singh Rajinder.1988. Biomass estimations and distribution of nutrients in five different aged Eucalyptus grandis plantation ecosystems in Kerala State. Indian Forester 114: 184–199. [Google Scholar]
- Teller HL.1968. Impact of forest land use on floods. Unasylva 22: 18–20. [Google Scholar]
- Tewari DN.1994. A monogragh of Dalbergia sissoo Roxb. India: I.B.D. Dehra Dun. [Google Scholar]
- Toky OP, Ramakrishnan PS.1983. Secondary succession following slash and burn agriculture in north‐eastern India. I. Biomass, litter fall and productivity. Journal of Ecology 71: 735–745. [Google Scholar]
- Turner J, Lambert MJ.1983. Nutrient cycling within a 27‐year‐old Eucalyptus grandis plantation in New South Wales. Forest Ecology and Management 6: 155–168. [Google Scholar]
- van Cleave K, Noonan LL.1975. Litter fall and nutrient cycling in the forest floor of birch and aspen stands in interior Alaska. Canadian Journal of Forest Research 5: 626–639. [Google Scholar]
- Vogt KA, Grier CC, Meier CE, Edmonds RL.1982. Mycorrhizal role in net primary productivity production and nutrient cycling in Abies annabalis ecosystems in western Washington. Ecology 63: 370–380. [Google Scholar]
- Vogt KA, Grier CC, Grower ST, Sprugel DG, Volt DJ.1986. Over estimation of net root production: a real or imaginary problem. Ecology 67: 577–579. [Google Scholar]
- Warner H.1970. Soil respiration, litter fall and productivity of tropical rain forest Journal of Ecology 58: 543–547. [Google Scholar]
- Westman WE, Rogers RW.1977. Biomass and structure of a sub‐tropical eucalypt north, North Stradbroke island. Australian Journal of Botany 25: 171–191. [Google Scholar]
- Whittaker RH.1966. Forest dimensions and production in the great smoky Mountains. Ecology 47: 103–121. [Google Scholar]
- Whittaker RH.1975. Community and ecosystems. 2nd edn. New York: MacMillan. [Google Scholar]
- Whittaker RH, Likens GE. 1975 The biosphere and Man. In: Leigh H, Whittaker RH, eds. Primary productivity of biosphere. New York: Springer‐Verlag. [Google Scholar]
- Whittaker RH, Woodwell GM.1968. Dimension and production relations of trees and shrubs in the Brookhaven forest, New York. Journal of Ecology 56: 1–25. [Google Scholar]
- Whittaker RH, Woodwell GM.1969. Structure production of oak‐pine forest at Brookhaven, New York. Journal of Ecology 57: 155–174. [Google Scholar]
- Whittaker RH, Woodwell GM.1971. Measurement of net primary production in forests. In: Whittaker RH, Woodwell GM, Productivity of forest ecosystems. Paris: UNESCO, 159–175. [Google Scholar]
- Zavitkovski J.1981. Structure and seasonal distribution of litter fall in young plantations of Populus ‘Tristis’. Plant and Soil 60: 321–504. [Google Scholar]