Abstract
A gradient of development consisting of successive zones of cell division, cell elongation and cell maturation occurs along the longitudinal axis of elongating leaf blades of tall fescue (Festuca arundinacea Schreb.), a C3 grass. An increase in specific leaf weight (SLW; dry weight per unit leaf area) in the maturation region has been hypothesized to result from deposition of secondary cell walls in structural tissues. Our objective was to measure the transverse cell wall area (CWA) associated with the increase in SLW, which occurs following the cessation of leaf blade elongation at about 25 mm distal to the ligule. Digital image analysis of transverse sections at 5, 15, 45, 75 and 105 mm distal to the ligule was used to determine cell number, cell area and protoplast area of structural tissues, namely fibre bundles, mestome sheaths and xylem vessel elements, along the developmental gradient. Cell diameter, protoplast diameter and area, and cell wall thickness and area of fibre bundle cells were calculated from these data. CWA of structural tissues increased in sections up to 75 mm distal to the ligule, confirming the role of cell wall deposition in the increase in SLW (r2 = 0·924; P ≤ 0·01). However, protoplast diameter of fibre cells did not decrease significantly as CWA increased, although mean thickness of fibre cell walls increased by 95 % between 15 and 105 mm distal to the ligule. Therefore, secondary cell wall deposition in fibre bundles of tall fescue leaf blades resulted in continued radial expansion of fibre cells rather than in a decrease in protoplast diameter.
Key words: Festuca arundinacea Schreb., tall fescue, secondary cell wall deposition, specific leaf weight (SLW), structural cell wall area (CWA), fibre cell protoplast diameter, digital image analysis, sclerenchyma fibre bundle development
INTRODUCTION
During elongation growth of grass leaf blades, tissue is displaced from the leaf base by cell division and elongation. Progressive cell differentiation can be observed in cell files along the developmental gradient from the base of the leaf blade to the tip. In tall fescue (Festuca arundinacea Schreb.), specific leaf weight (SLW; dry weight per unit leaf area) first decreases along this gradient in the basal portion of the leaf blade as newly divided cells are displaced through the epidermal cell elongation zone, then increases following the cessation of elongation (MacAdam and Nelson, 1987). The decrease in SLW associated with elongation is due to a decrease in the density of transverse cell walls per unit leaf area. The increase in SLW following cessation of cell elongation is thought to result from the deposition of secondary cell walls in structural tissues (MacAdam and Nelson, 1987).
In standard descriptions of the development of sclerenchyma fibre cells, the secondary wall is laid down after cell expansion has ceased, and secondary wall deposition occurs at the expense of protoplast transverse area (Brett and Waldron, 1996). However, in the maturation region of tall fescue, where increase in SLW is most likely due to deposition of secondary cell walls, leaf width also continues to increase (MacAdam and Nelson, 1987). This suggests that all cells, including those with secondary walls, must still be capable of radial expansion. There is evidence that differentiation of relatively mature fibre cells contributes to leaf curvature of maize (Zea mays L.) via shortening of cells (Hay et al., 2000). In sorghum [Sorghum bicolor (L.) Moench], both proto‐ and metaxylem elements in leaves affected by salinity were narrower than those in controls. This reduced water deposition in expanding cells and could thus reduce the rate of cell growth (Baum et al., 2000). These studies suggest that secondary growth in herbaceous plants may contribute to both form and function in ways not generally recognized.
In this study we sought to document the correlation between secondary cell wall deposition and the increase in SLW in the maturation region of tall fescue by direct measurement of the transverse cell wall area of structural tissue. We used these data to determine the relationship between wall thickness and protoplast diameter of fibre bundle cells along the developmental gradient of elongating leaf blades.
MATERIALS AND METHODS
Plant growth conditions
Leaf blades used in this study were from vegetatively propagated plants of a single genotype of tall fescue (Festuca arundinacea Schreb.), designated low yield per tiller (LYT) in previous studies (Vassey, 1986; MacAdam and Nelson, 1987). Three vegetative tillers were transplanted into plastic pots (11 cm diameter, 15 cm deep) containing a sphagnum peat moss–vermiculite potting mixture (Terra‐Lite Redi‐Earth Peat‐Lite; Scotts‐Sierra Horticultural Products Co., Marysville, OH, USA). Plants were grown for 15 weeks in a controlled‐environment chamber. Mean photosynthetic photon flux density at canopy height was 490 µmol m–2 s–1, photoperiod was 14 h, day/night temperature was a constant 22 °C and relative humidity was 70 %. Plants were watered daily to field capacity, and fertilized once a week with 100 ml per pot of Hoagland’s No. 2 nutrient solution (Epstein, 1972).
Leaf growth measurements
The tips of elongating leaves used in this study had emerged from the sheaths of older leaves; the length of these sheaths was about 60 mm. The overall length of the elongating leaves was less than half that of the blade of the next‐oldest leaf. At this stage of development cell division and cell elongation are both continuing at the base of the leaf blade and a ligule has differentiated approx. 1 mm distal to the point of attachment of the leaf to the shoot apex. The leaf elongation rate is nearly constant (Schnyder and Nelson, 1988), and the sheath of the elongating leaf has not begun to elongate.
The abaxial epidermis is exposed in rolled, elongating leaves of tall fescue as they emerge from the sheaths of older leaves. Epidermal cell lengths of leaves used in this study were determined from replicas of the abaxial epidermis of eight elongating leaves selected for uniform length. The full length of elongating leaves was exposed by removing older leaf sheaths, and leaves were excised from the plant at the point of attachment to the shoot apex. The ligule was marked using the tip of a needle dipped in a 1 % (w/v) aqueous solution of acid fuchsin. To form the replica, a thin coating of a 4 % (w/v) solution of polyvinylformaldehyde (Formvar; Ted Pella Co., Redding, CA, USA) in chloroform was spread along the leaf using a glass rod and allowed to dry for approx. 5 s. A strip of clear Cellophane adhesive tape was applied along the length of the leaf, the replica was lifted off, and the Cellophane tape and replica were applied to a glass microscope slide (Schnyder et al., 1990). Formvar is routinely used to make replicas for electron microscopy, and accurately reproduces surface features without distortion (Schaefer and Harker, 1942). The lengths of five intercostal epidermal cells were measured at successive 5 mm intervals distal to the ligule on each replica.
The pattern of change in SLW of elongating tall fescue leaf blades had been established in earlier replicated studies (MacAdam and Nelson, 1987; Schnyder and Nelson, 1988). For this study, SLW of segments along the elongating leaf blade was determined using just six leaves selected for uniform length to verify previous data for SLW. Each leaf blade was cut into 10‐mm‐long segments from the ligule to the tip, and the width at the middle of each segment was determined with an ocular micrometer. Segments were pooled by location along the leaf blade, dried for 48 h at 60 °C, allowed to cool under desiccation, and dry weights were determined.
Measurement of structural cell wall area
Transverse segments 1 mm long and the full width of the leaf blade were excised from three elongating leaves at successive 5 mm intervals from the ligule to 105 mm distal to the ligule. Segments were fixed overnight in a solution of 4 % (v/v) formaldehyde and 1 % (v/v) glutaraldehyde in 0·2 m Na‐phosphate buffer, pH 7·2 (Burns and Bretschneider, 1981).
Segments were dehydrated in an ethanol series and infiltrated for two 3 h periods with catalysed glycol methacrylate (JB‐4; Polysciences, Warrington, PA, USA). Segments were infiltrated for an additional 10 min in one change of catalysed JB‐4 saturated with acid fuchsin to aid orientation. Segments were rinsed in clear, catalysed JB‐4, then transferred to fresh, catalysed, activated JB‐4 and allowed to polymerize at room temperature. After hardening, blocks were fixed to wooden dowels with epoxy resin and sectioned.
Differential shrinking and swelling of tissues within a gradient of development is a potential problem in a study such as this. More recent observations of tissue from the same genotype of tall fescue fixed in glutaraldehyde and osmium tetroxide and embedded in Spurr’s (1969) resin show the same relative dimensions of fibre and other cells as found in this study (see photomicrographs in MacAdam et al., 1992).
Transverse sections 5 µm thick were cut using a rotary microtome equipped with a glass knife. Sections were stained for 1 min in a 1 % (w/v) aqueous solution of acid fuchsin, then rinsed, dried and stained for 1 min in a 0·05 % (w/v) solution of toluidine blue O in benzoate buffer, pH 4·4 (O’Brien and McCully, 1981).
Segments at 5, 15, 45, 75 and 105 mm were chosen for measurements of structural cell wall area (CWA). There is no distinct midrib in tall fescue leaf blades, and ridges containing major (with xylem) or minor (without xylem) veins alternate across the width of the leaf blade, with a minor vein usually located at each margin. After a preliminary determination of ridge number and type (major vs. minor vein) across the leaf blade at each location, the same two adjacent ridges from a given leaf were photographed at each location and used to measure structural CWA. Selected ridges were located at least two ridges away from the margin of each leaf.
In a C3 grass such as tall fescue, secondary cell walls are deposited in various tissues, including fibre cells, xylem elements, along the inner wall of the mestome (inner bundle) sheath, and along the outer wall of the abaxial (lower) epidermis (Hanna et al., 1973). Individual micrographs were made of each sclerenchyma fibre bundle in adjacent major and minor veins at each location. Sections were photographed using a Zeiss Photomicroscope III with a 63× objective, and final magnification was determined from photomicrographs of a stage micrometer taken using the same objective. Micrographs were printed at a final magnification of 994× for image analysis, and Digital Paintbrush (The Computer Colorworks, Sausalito, CA, USA) was used as a planimeter and digitizer.
Total area was calculated for each of the three sclerenchyma fibre bundles in each vein, namely the adaxial or upper fibre bundle, the abaxial or lower fibre bundle, and the vascular fibre bundle, located between the metaxylem vessel elements. The area of each protoplast of all cells within each fibre bundle was also measured and total protoplast area was subtracted from total fibre bundle area to determine CWA. No air spaces were noted between cells of fibre bundles.
Secondary cell wall deposition has been determined by other means, such as by measuring the thickness of the double wall between two fibre cells in internodes of reproductive tillers of darnel (Lolium temulentum L.). In this case, the thickness of secondary walls of fibre cells increased linearly with distance distal to the node (Juniper et al., 1981). However, this approach would not have detected change in protoplast diameter or cell number during leaf development. More automated methods such as image skeletonization (Travis et al., 1993) have been used to estimate CWA, and such an approach would allow more samples to be examined. However, it is not readily adapted to measurement of irregular wall shapes such as the inner wall of the mestome sheath.
To determine mean wall thickness for cells within a given fibre bundle, we first calculated mean fibre cell area (total fibre bundle area divided by the number of fibre cells in that bundle). Cell area was used to calculate mean fibre cell diameter, assuming a circular shape, which is generally representative. Similarly, protoplast diameter was calculated from mean protoplast area, again assuming a circular shape. Protoplast radius was subtracted from fibre cell radius to give mean cell wall thickness. Dimensions of CWAs for proto‐ and metaxylem vessels were calculated similarly.
The CWA of the thickened inner wall of the mestome sheath was measured directly. At the light microscope level, the thickened outer wall of the abaxial epidermis could not be distinguished from cuticular wax so the contribution of this tissue was not estimated. Of the cells that developed lignified secondary cell walls, those adaxial to the mestome sheath were included in the adaxial fibre bundle and those abaxial to the mestome sheath were included in the abaxial fibre bundle. The total of the measured structural CWAs for a leaf blade at a given location distal to the ligule was estimated by multiplying all measured structural CWAs for one major and one minor ridge by the number of ridges of that type occurring across the leaf blade at that location.
RESULTS
At 5 mm distal to the ligule, epidermal cells were approx. one‐tenth of their final length (Fig. 1). Lignified cell walls appear green when stained with toluidine blue (O’Brien et al., 1964), and at 5 mm distal to the ligule only walls of protoxylem vessel elements of major ridges were stained green (Fig. 2A). As epidermal cells elongated, tissue was also displaced through the basal 25 mm of the leaf blade by continuing basal leaf growth (Fig. 1). Near the centre of the elongation zone, at 15 mm distal to the ligule for this genotype, cell elongation is at its maximal rate (MacAdam et al., 1989). At this location, cells in fibre bundles had thin walls but some thickening could be observed at cell junctions (Fig. 2B).
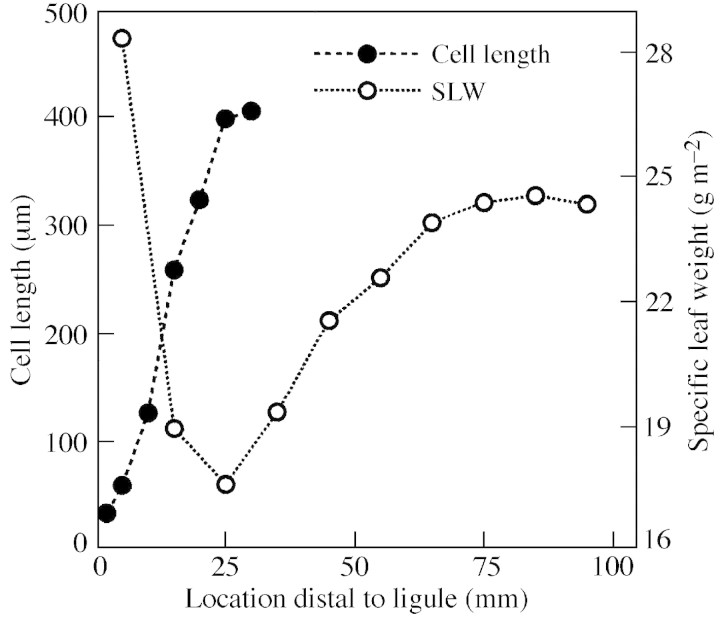
Fig. 1. Epidermal cell length and specific leaf weight (SLW) of elongating tall fescue leaf blades of the LYT genotype. Specific leaf weight is dry weight per unit leaf area. For this study, SLW was calculated for a pooled sample of six leaves. Cell length LSD0·05 = 127 µm, n = 8.
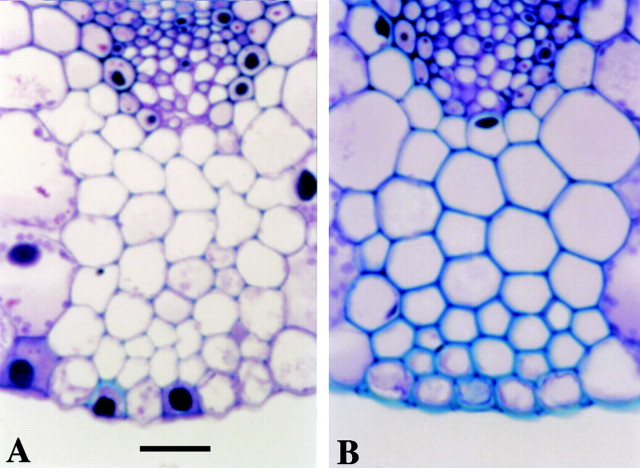
Fig. 2. Photomicrographs of transverse sections of a major and minor ridge at 5 (A), 15 (B), 45 (C) and 75 (D) mm distal to the ligule of an elongating leaf blade. The arrow in A indicates a lignified protoxylem element; arrows in B indicate fibre bundles with thickened cell wall junctions. Veins in minor ridges do not develop metaxylem vessel elements. b, Abaxial fibre bundle; d, adaxial fibre bundle; m, mestome sheath; v, vascular fibre bundle; M, metaxylem element; P, protoxylem element. Bar = 50 µm.
Specific leaf weight increased about 40 % between 25 mm, where epidermal cell elongation ceased, and 85 mm distal to the ligule (Fig. 1). When leaf blade tissue was displaced to 45 mm (Fig. 2C), well into the region of increasing SLW, green staining of secondary cell walls was visible throughout the vascular bundle and the bundle sheath extension that connects the mestome sheath with the adaxial and abaxial bundle fibres in major veins (Metcalfe, 1960). At 75 mm distal to the ligule (Fig. 2D), metaxylem elements and all fibre bundles had thickened, green‐stained cell walls. Staining was consistent with the assumption that protoxylem elements have lignified cell wall thickenings in meristematic tissue, and that lignification occurs in secondary cell walls of fibre cells as successive wall layers are deposited following cessation of cell elongation.
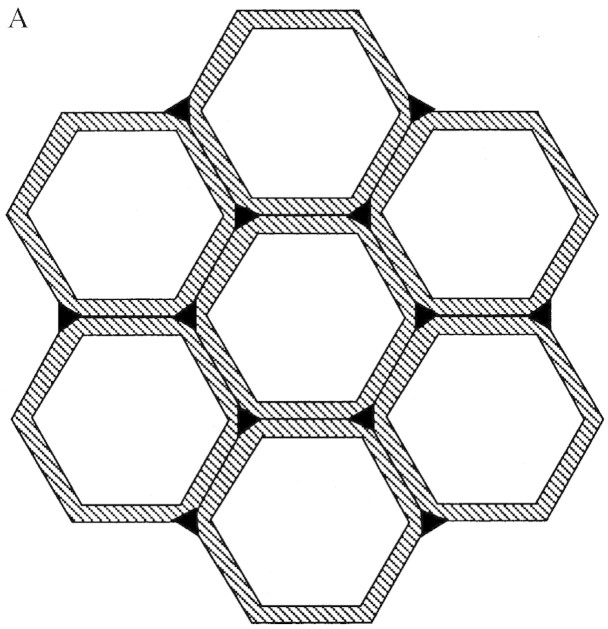
The hexagonal appearance of fibre cells in the abaxial bundle sheath at 45 mm distal to the ligule (Fig. 3A) resulted from wall thickening at cell junctions, and protoplast shape appeared similar to wall shape. As more secondary cell wall was deposited in fibre cells as they were displaced to 75 mm distal to the ligule (Fig. 3B), some fibre cell protoplasts appeared more circular, while the shared walls within fibre bundles remained pentagonal or hexagonal. The overall change in appearance and mean increase in diameter of fibre bundle cells from 45 to 75 mm is also illustrated in Fig. 4. Between 75 and 105 mm, there was little change in either SLW (Fig. 1) or in the appearance of fibre cell walls, so a section at 105 mm was not included in Fig. 2.
Fig. 3. Photomicrographs of transverse sections of the abaxial fibre bundle and the lower half of the mestome sheath from the same major ridge of one elongating leaf blade at 45 (A) and 75 (B) mm distal to the ligule. Bar = 20 µm.
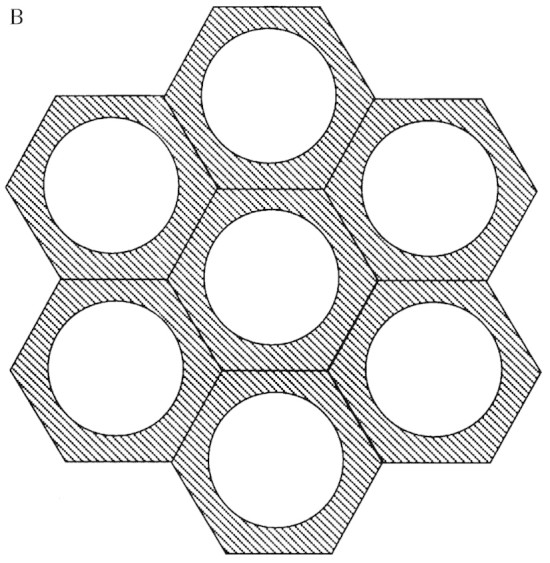
Fig. 4. Using weighted means of cells in sclerenchyma fibre bundles, fibre cell area was 76 µm2 and fibre protoplast area was 47 µm2 at 45 mm distal to the ligule (A) while fibre cell area was 89 µm2 and protoplast area was 50 µm2 at 75 mm distal to the ligule (B). Increase in cell wall thickness occurred primarily by increase in the outer diameter of fibre cells rather than by a decrease in diameter of fibre protoplasts.
Total structural CWA in transverse sections (Fig. 5) increased between 5 and 75 mm distal to the ligule (P = 0·02), with the greatest incremental change occurring between 45 and 75 mm distal to the ligule. The regression coefficient (r2) of SLW with structural CWA was 0·924 (P ≤ 0·01). The largest absolute increase in CWA was in abaxial fibre bundles of major veins, while the largest percentage increase occurred in CWA of minor veins. Leaf width continued to increase beyond the distal margin of the elongation zone, which was probably due to continued radial cell growth. However, in a study of elongating leaf blades, the contribution of radial cell expansion to leaf width is confounded by the tapering of leaf width due to a decrease in vein number towards the leaf tip.
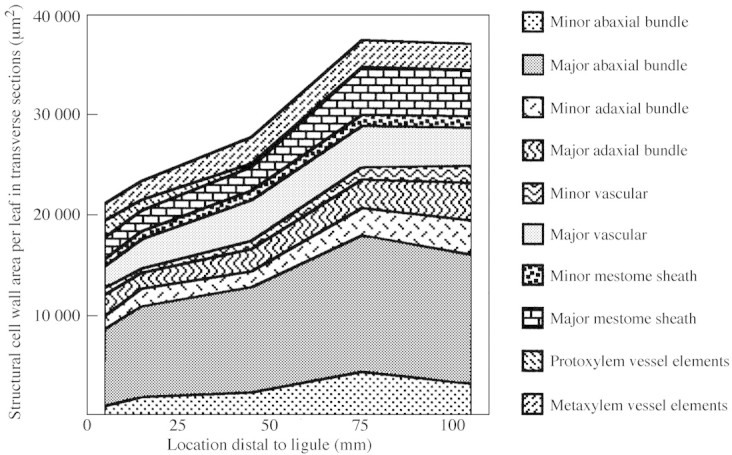
Fig. 5. Transverse structural cell wall area (CWA) per leaf at five locations along the elongating leaf blade and CWA of the contributing structural tissues. Data for individual structural tissues from one major and one minor ridge from each leaf were multiplied by the number of ridges of that type occurring across the width of that leaf to estimate total structural CWA. LSD0·05 = 10 790 µm2 to compare total structural CWA at different locations along the leaf blade (n = 3).
From 75 to 105 mm the cell wall area of individual bundle sheaths remained constant or increased. The decrease in total structural CWA of major and minor abaxial fibre bundle fractions (Fig. 5) resulted from a decrease in the number of cells in these fibre bundles (Table 1). This decrease in cell number reflects the normal ontogeny of tall fescue leaf blades, which taper toward the leaf tip. Because the number of veins across the leaf decreases gradually towards the tip, data for wall thickness and protoplast diameter are presented on a per‐cell basis (Table 1).
Table 1.
Mean wall thickness (µm), protoplast diameter (µm), and number of cells in sclerenchyma fibre bundles at five locations along elongating leaf blades
| Location distal to the ligule (mm) | ||||||||
| Sclerenchyma fibre bundle | 5 | 15 | 45 | 75 | 105 | P | LSD0.05 (n = 3) | |
| Major veins—number across leaf: | 10 | 10 | 10 | 9.7 | 9.3 | |||
| Fibre cell number | ||||||||
| Adaxial bundle | 95 | 82 | 72 | 61 | 67 | 0.33 | – | |
| Vascular bundle | 139 | 206 | 203 | 172 | 158 | 0.40 | – | |
| Abaxial bundle | 356 | 334 | 289 | 259 | 236 | 0.02 | 67 | |
| Wall thickness (µm) | ||||||||
| Adaxial bundle | 0.90 | 0.80 | 1.07 | 1.44 | 1.60 | 0.00 | 0.22 | |
| Vascular bundle | 0.71 | 0.72 | 1.19 | 1.15 | 1.13 | 0.02 | 0.29 | |
| Abaxial bundle | 0.80 | 0.89 | 1.06 | 1.49 | 1.59 | 0.00 | 0.20 | |
| Protoplast diameter (µm) | ||||||||
| Adaxial bundle | 7.50 | 7.85 | 8.17 | 9.04 | 9.38 | 0.46 | – | |
| Vascular bundle | 6.67 | 5.31 | 4.10 | 5.27 | 5.53 | 0.25 | – | |
| Abaxial bundle | 7.94 | 9.20 | 10.10 | 9.80 | 9.05 | 0.25 | – | |
| Minor veins—number across leaf: | 9 | 8.6 | 8.6 | 8.6 | 8.6 | |||
| Fibre cell number | ||||||||
| Adaxial bundle | 63 | 75 | 71 | 92 | 96 | 0.45 | – | |
| Vascular bundle | 45 | 35 | 42 | 51 | 52 | 0.52 | – | |
| Abaxial bundle | 52 | 84 | 93 | 93 | 69 | 0.15 | – | |
| Wall thickness (µm) | ||||||||
| Adaxial bundle | 0.77 | 0.77 | 0.82 | 1.12 | 1.34 | 0.00 | 0.22 | |
| Vascular bundle | 0.77 | 0.54 | 0.86 | 1.16 | 1.44 | 0.02 | 0.46 | |
| Abaxial bundle | 0.79 | 0.79 | 0.87 | 1.54 | 1.65 | 0.00 | 0.32 | |
| Protoplast diameter (µm) | ||||||||
| Adaxial bundle | 7.77 | 9.37 | 7.99 | 7.77 | 7.08 | 0.43 | – | |
| Vascular bundle | 6.18 | 6.52 | 6.53 | 5.53 | 5.62 | 0.21 | – | |
| Abaxial bundle | 8.27 | 8.58 | 8.30 | 8.50 | 7.93 | 0.99 | – | |
| Means of major and minor vein fibre cells | ||||||||
| Mean fibre cell number per leaf | 750 | 816 | 770 | 728 | 678 | 0.17 | – | |
| Weighted mean wall thickness (µm) | 0.79 | 0.80 | 1.04 | 1.34 | 1.44 | |||
| Mean wall thickness (µm) | 0.79 | 0.75 | 0.98 | 1.32 | 1.46 | 0.00 | 0.22 | |
| Weighted mean protoplast diameter (µm) | 7.55 | 7.92 | 7.73 | 7.94 | 7.61 | |||
| Mean protoplast diameter (µm) | 7.39 | 7.81 | 7.53 | 7.65 | 7.43 | 0.97 | – |
Mean cell number is a sum of all fibre cells across the leaf (n = 3). Weighted means were calculated using the number of each type of cell per fibre bundle, and were not substantially different from statistical means.
The weighted mean thickness of sclerenchyma fibre bundle cell walls, calculated using the number of each type of cell per fibre bundle, nearly doubled between 15 and 105 mm distal to the ligule, from 0·80 to 1·44 µm (Table 1). Contrary to the expectation that secondary cell wall thickening would occur at the expense of the diameter of fibre cell protoplasts, there was no consistent decrease in mean fibre cell protoplast diameter as walls thickened following cessation of elongation at 25 mm distal to the ligule. As leaf tissue was displaced from 15 to 105 mm distal to the ligule, the 0·64 µm (80 %) increase in weighted mean wall thickness was primarily achieved by an increase in overall cell diameter rather than by a decrease in protoplast diameter: sclerenchyma fibre cell diameter increased from 9·52 to 10·49 µm (10 %), while protoplast diameter only decreased from 7·92 to 7·61 µm (4 %).
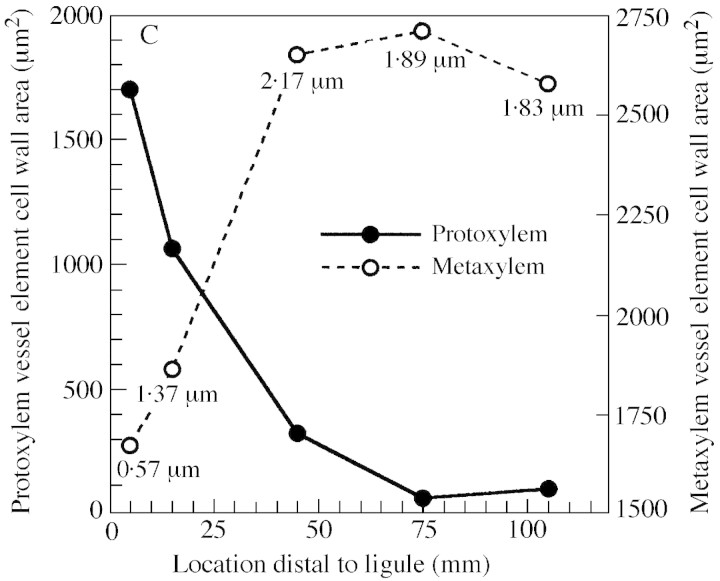
The lignified annular wall thickenings of protoxylem vessel elements in tall fescue leaf blades resemble a coiled spring (Fig. 6A). As these cells were displaced through the elongation zone their longitudinal walls continued to elongate with adjacent tissues and distance between annular wall thickenings increased (Fig. 6B). There was progressively less probability of sectioning through a wall‐thickening of a protoxylem element in transverse section as location distal to the ligule increased (Fig. 6C). In contrast, metaxylem cells gained thick secondary walls and walls increased in cross‐sectional area between 5 (Fig. 2A) and 45 mm (Fig. 2C). Both xylem CWA and mestome sheath inner wall area are included in calculations of total transverse structural cell wall area.
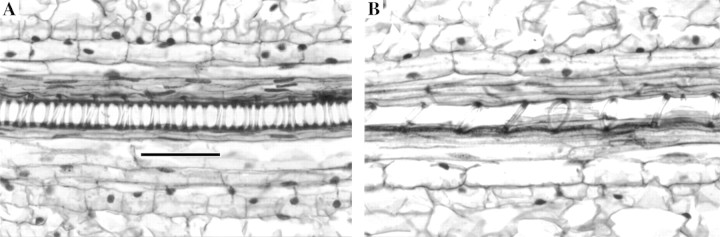
Fig. 6. Development of vessel elements in the elongating leaf blade. Longitudinal sections of protoxylem elements at 6 (A) and 15 (B) mm distal to the ligule. Elongation increases the distance between annular wall thickenings of protoxylem vessels. Bar = 50 µm. C, Transverse CWA per leaf of xylem vessel elements at five locations distal to the ligule. Mean metaxylem wall thickness at each location is indicated numerically on the figure; metaxylem cell wall thickness LSD0·05 = 0·90 µm (n = 3).
DISCUSSION
These data demonstrate that synthesis of secondary cell walls in structural tissues of elongating tall fescue leaf blades takes place between 15 and 75 mm distal to the ligule, in the same region where increase in specific leaf weight occurs. This work confirms, therefore, that structural cell wall deposition accounts for the majority of the increase in SLW. In addition, but more surprisingly, deposition of secondary cell walls was found to increase the transverse area of fibre cells rather than to decrease significantly fibre cell protoplast area. This increase in cell diameter probably contributes to the continuing increase in leaf width following cessation of longitudinal growth observed in tall fescue leaf blades by MacAdam and Nelson (1987) and Maurice et al. (1997).
In rapidly expanding plant cells, the middle lamella and its adjacent primary cell wall must expand to accommodate both longitudinal and radial growth, while in the standard definition, a secondary cell wall is deposited after expansion of the primary cell wall has ceased (Esau, 1977). However, the progress of secondary cell wall development in fibre cells in our study resembled collenchyma cell development, in which cell wall thickening begins at cell junctions and results in radial expansion that continues following the cessation of cell elongation (Vian et al., 1993). Our results suggest that as cell elongation stops and wall component synthesis shifts, by definition, from primary to secondary cell wall deposition, radial expansion of fibre cells in tall fescue leaf blades continues despite evidence of lignification in these tissues.
While it is often assumed that lignification occurs only after cell wall growth has ceased, this is apparently not the case. Using polyclonal antibodies that detect uncondensed lignin, Müsel et al. (1997) reported the presence of lignin throughout the primary walls of rapidly expanding maize coleoptile parenchyma cells. Furthermore, in elongating maize internodes, where cell wall carbohydrates were accumulating in the basal 40 % of internodes, lignin content increased from 35 to 59 mg g–1, or to about 80 % of the maximum lignin content of 73 mg g–1 in these tissues (calculations from data of Scobbie et al., 1993). Thus, neither deposition of secondary cell walls nor lignification should continue to be considered mutually exclusive with cell wall expansion.
ACKNOWLEDGEMENTS
The authors thank P. R. Beuselinck and J. P. Gustafson (USDA‐ARS University of Missouri) for access to microscopes, D. W. Sisson (Utah State University) for statistical analyses, and R. J. Mueller (Utah State University) for helpful comments on the manuscript. Contribution of the Missouri Agriculture Experiment Station, Journal Series No. 12 845.
Received: 5 June 2001; Returned for revision: 26 August 2001; Accepted: 21 September 2001.
References
- Baum SF, Tran PN, Silk WK.2000. Effects of salinity on xylem structure and water use in growing leaves of sorghum. New Phytologist 146: 119–197. [Google Scholar]
- Brett CT, Waldron KW.1996. Physiology and biochemistry of plant cell walls. 2nd edn. London: Chapman and Hall. [Google Scholar]
- Burns WA, Bretschneider A.1981. Thin is in: plastic embedding of tissue for light microscopy. Chicago: American Society of Clinical Pathologists. [Google Scholar]
- Epstein E 1972. Mineral nutrition of plants: principles and perspectives. New York: Wiley. [Google Scholar]
- Esau K 1977. Anatomy of seed plants. 2nd edn. New York: Wiley. [Google Scholar]
- Hanna WW, Monson WG, Burton GW.1973. Histological examination of fresh forage leaves after in vitro digestion. Crop Science 13: 90–102. [Google Scholar]
- Hay JO, Moulia B, Lane B, Freeling M, Silk WK.2000. Biomechanical analysis of the Rolled (Rld) leaf phenotype of maize. American Journal of Botany 87: 625–633. [PubMed] [Google Scholar]
- Juniper BE, Lawton JR, Harris PJ.1981. Cellular organelles and cell‐wall formation in fibres from the flowering stem of Lolium temulentum L. New Phytologist 89: 609–619. [DOI] [PubMed] [Google Scholar]
- MacAdam JW, Nelson CJ.1987. Specific leaf weight in zones of cell division, elongation and maturation in tall fescue leaf blades. Annals of Botany 59: 369–376. [Google Scholar]
- MacAdam JW, Nelson CJ, Sharp RE.1992. Peroxidase activity in the leaf elongation zone of tall fescue. 1. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiology 99: 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAdam JW, Volenec JJ, Nelson CJ.1989. Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiology 89: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice I, Gastal F, Durand J‐L.1997. Generation of form and associated mass deposition during leaf development in grasses: a kinematic approach for non‐steady growth. Annals of Botany 80: 673–683. [Google Scholar]
- Metcalfe CR 1960. Anatomy of the monocotyledons. I. Gramineae. London and Oxford: Clarendon Press. [Google Scholar]
- Müsel G, Schindler T, Bergfeld R, Ruel K, Jacquet G, Lapierre C, Speth V, Schopfer P.1997. Structure and distribution of lignin in primary and secondary cell walls of maize coleoptiles analyzed by chemical and immunological probes. Planta 201: 146–159. [Google Scholar]
- O’Brien TP, McCully ME.1981. The study of plant structure. Principles and selected methods. Melbourne: Termarcarphi Pty Ltd. [Google Scholar]
- O’Brien TP, Feder N, McCully ME.1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 368–373. [Google Scholar]
- Schaefer VJ, Harker D.1942. Surface replicas for use in the electron microscope. Journal of Applied Physics 13: 427–433. [Google Scholar]
- Schnyder H, Nelson CJ.1988. Diurnal growth of tall fescue leaf blades. I. Spatial distribution of growth, deposition of water, and assimilate import in the elongation zone. Plant Physiology 86: 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H, Seo S, Rademacher IF, Kühbauch W.1990. Spatial distribution of growth rates and of epidermal cell lengths in the elongation zone during leaf development in Lolium perenne L. Planta 181: 423–431. [DOI] [PubMed] [Google Scholar]
- Scobbie L, Russell W, Provan GJ, Chesson A.1993. The newly extended maize internode: a model for the study of secondary cell wall formation and consequences for digestibility. The Journal of the Science of Food and Agriculture 61: 217–225. [Google Scholar]
- Spurr AR 1969. A low‐viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Murison SD, Chesson A 1993. Estimation of plant cell wall thickness and cell size by image skeletonization. Journal of Agricultural Science, Cambridge 120: 279–287. [Google Scholar]
- Vassey TL 1986. Morphological, anatomical and cytohistological evaluations of terminal and axillary meristems of tall fescue. PhD Thesis, University of Missouri, Columbia, USA. [Google Scholar]
- Vian B, Roland J‐C, Reis D.1993. Primary cell wall texture and its relation to surface expansion. International Journal of Plant Science 154: 1–9. [Google Scholar]