Abstract
Grevillea beadleana (Proteaceae) is an endangered species known from five populations in northern New South Wales, Australia. The reproductive ecology of G. beadleana was compared in two populations with a ten‐fold difference in the number of plants. Grevillea beadleana was found to be self‐compatible in both populations and an examination of pollen viability and stigma maturation revealed that the species is protandrous. Flowering within inflorescences is acropetallous. In the first season plants in the largest population produced approx. ten‐fold more inflorescences than those in the smaller population and, although the number of flowers per inflorescence did not vary significantly between populations the first season, the larger population produced more fruit per inflorescence than the smaller population. However, fruit to flower ratios were less than 0·2 in both seasons and populations. In both populations the number of fruit was significantly greater at the proximal end of the inflorescence, where flowers open first, compared with medial and distal positions. Several bird species were observed visiting flowers, although few birds were recorded foraging at plants in the smaller population. Within both populations, birds tended to make more within‐ than between‐plant visits. Self‐compatibility, acropetally and proximal fruit‐set, combined with the predominantly within‐plant movement of honeyeaters, suggests inbreeding may be common within both populations of G. beadleana. Pollination and fruiting success are discussed for G. beadleana and breeding systems among rare and common taxa in Grevillea are reviewed.
Key words: Grevillea beadleana, pollination ecology, endangered species, breeding system, stigmatic receptivity, pollen viability, fruit‐set, floral visitors, Proteaceae
INTRODUCTION
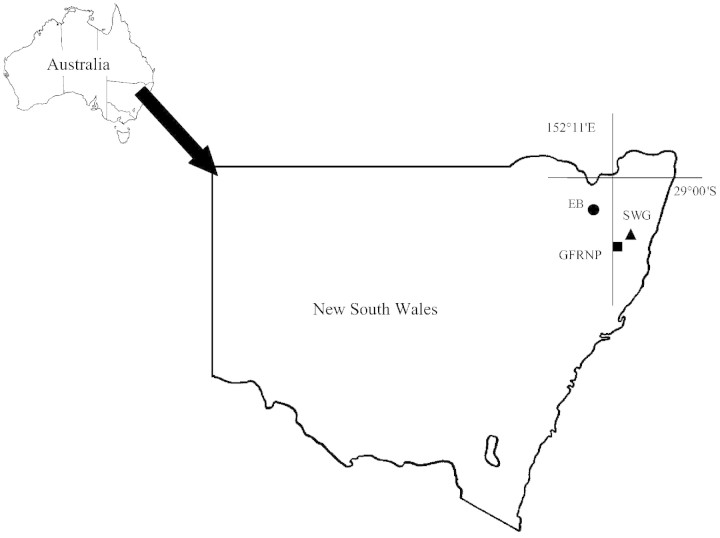
Grevillea (Proteaceae) contains 362 species, 357 of which are found in Australia (Makinson, 2000). A significant number of species and subspecies in Grevillea are rare or threatened. Makinson (2000) lists at least 84 taxa as such and a further 86 taxa as poorly known. Grevillea beadleana McGillivray is an endangered species found, at the time of the study, in three disjunct locations in northern New South Wales (NSW) (Fig. 1). (Two additional populations have been found recently.) These three populations contain vastly different numbers of individuals (approx. > 10 000, 600 and ten individuals), thereby providing an opportunity to compare reproductive systems and fecundity in populations differing in size.
Fig. 1. Locations of the study populations in northern New South Wales: Eastern Binghi (EB; circle); Guy Fawkes River National Park (GFRNP; square); and south‐west Grafton (SWG; triangle).
Species within the Proteaceae utilize a variety of animal vectors (mammals, insects and birds) to disperse pollen (Carolin, 1961; Collins and Rebelo, 1987). Breeding systems vary within and among genera and also within some species in the Proteaceae (e.g. Whelan and Burbridge, 1980; Vaughton, 1988; Stocket al., 1989; Goldingay and Whelan, 1990; Lamontet al., 1993; Richardsonet al., 2000). Several studies have been undertaken into breeding systems within Grevillea and the pattern emerging is that most species are self‐compatible (see Table 1).
Table 1.
Breeding system and putative pollinators of some Grevillea species
| Grevillea species | Breeding system | Floral visitors | Conservation status | Source |
| G. acanthifolia ssp. stenomera | Self‐compatible | None seen | Rare | Smith (1997) |
| G. banksii | Self‐compatible | Common | Herscovitch and Martin (1990) | |
| G. beadleana | Self‐compatible | Birds, honeybees | Endangered | Smith (1997) |
| G. macleayana (G. barklyana ssp. macleayana) | Self‐compatible | Birds | Rare | Harriss and Whelan 1993; Vaughton (1996) |
| G. caleyi | Self‐compatible? | Birds | Endangered | Scott et al. (1995) |
| G. huegelii | Self‐compatible | Common | Chivell and Carthew, unpub. cited in Goldingay and Carthew (1998) | |
| G. laspicula | Self‐incompatible | Birds?, honeybees? | Endangered | Hoebee and Young (2001) |
| G. lavandulacea | Self‐compatible | Common | Chivell and Carthew, unpub. cited in Goldingay and Carthew (1998) | |
| G. leucopteris | Self‐compatible | Insects, honey possums | Common | Lamont (1982) |
| G. linearifolia | Self‐compatible | Common | Hermanutz et al. (1998) | |
| G. longifolia | Self‐compatible | Rare | Hermanutz et al. (1998) | |
| G. mucronulata | Partially self‐compatible | Birds, honeybees | Common | Hermanutz et al. (1998); Richardson et al. (2000) |
| G. muricata | Self‐compatible | Rare | Chivell and Carthew, unpub. cited in Goldingay and Carthew (1998) | |
| G. oleoides | Partially self‐compatible | Common | Hermanutz et al. (1998) | |
| G. paradoxa | Self‐compatible | Birds | Common | Olde and Marriott (1995) |
| G. robusta | Self‐compatible? self‐incompatible | Birds and mammals? | Common | Brough (1933); Kalinganire et al. (2000) |
| G. sericea | Self‐compatible | Insects? | Common | Olde and Marriott (1995) |
| G. scapigera | Self‐incompatible | Insects | Endangered | Rossetto et al. (1995); Rossetto (pers. comm., 2000) |
| G. sphacelata | Partially self‐compatible. Self‐incompatible | Honeybees | Common | Hermanutz et al. (1998) Richardson et al. (2000) |
| G. triternata | Self‐compatible? | Insects? | Common | Olde and Marriott (1995) |
| G. wilsonii | Self‐compatible | Common | Collins and Grey (1988) |
Nomenclature and conservation status follows Makinson (2000).
Effective management of endangered plant species requires specific knowledge of life history traits including, for example, breeding systems and response to disturbance, as well as information on processes threatening the species. In this study we examined the breeding system and seasonal variation in fruit production within and between one small and one large population of G. beadleana. The temporal aspects of stigma receptivity and pollen longevity were investigated in relation to protandry (the occurrence of anther dehiscence prior to stigma receptivity) and breeding system. We also identified floral visitors and putative pollinators.
MATERIALS AND METHODS
Species
Grevillea beadleana is a spreading shrub that grows up to 2·5 m tall and up to 2·5 m across (McGillivray, 1993). Individuals are often killed outright by fire, although re‐sprouting is common in the population at Guy Fawkes River National Park (Gross, unpubl. res., 2001). Evidence suggests that seed germination is stimulated by fire (Streat, 1996) and non‐fire events involving seed scarification (Gross, unpubl. res., 2001). Floral examination revealed that flowers are zygomorphic, bisexual, and have a protruding style and terminal pollen‐presenter surrounding the stigma (Smith, 1997). As with many other proteaceous species, pollen is dehisced onto the pollen presenter before anthesis. Flowers are red and produce nectar (Smith, 1997).
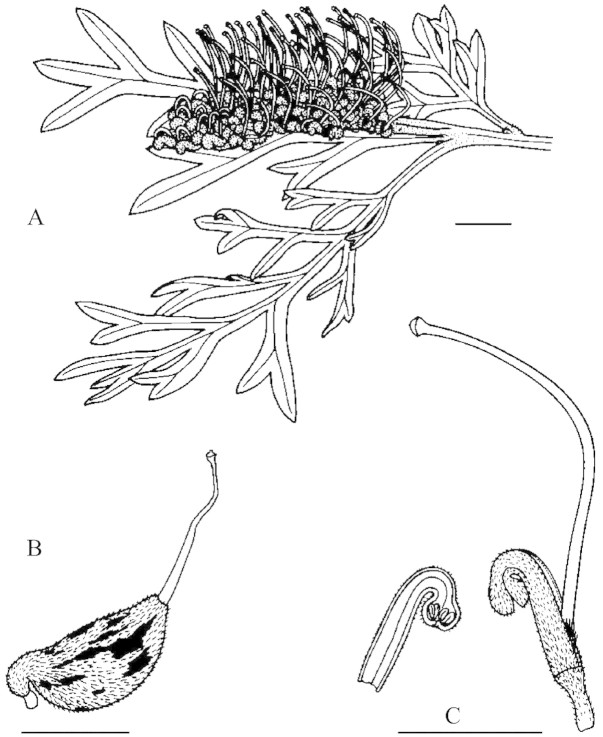
Inflorescences are the terminal toothbrush type, with five to 70 pairs of flowers on a rachis approx. 35–50 mm long (Fig. 2). Flowering is acropetallous in inflorescences such that a floral rachis may contain, at any one time, developing fruits, flowers and buds. Flowers can become fully open at any time of the day or night. In this study, inflorescences with at least one open flower were designated ‘mature’, while those with no open flowers were designated ‘immature’. Inflorescences were designated ‘nearly mature’ when their hooked styles protruded well beyond the tepals; these flowers generally opened within approx. 5 d.
Fig. 2. Inflorescence and leaf shape of Grevillea beadleana (A); fruit with persistent style (B); and position of the pollen presenter and anthers on an individual flower (C). Bars = 10 mm.
Study sites
The three disjunct populations of G. beadleana are separated by at least 75 km (Fig. 1). One population occurs in Guy Fawkes River National Park (GFRNP) (30°05′S, 152°19′E; 950 m asl) and consists of approx. 685 plants, spread over 3·5 ha on the exposed rim and scree slopes of Guy Fawkes River Gorge (Streat, 1996). Plants throughout approx. 90 % of the area were included in this study. The second study population is located on private property at Eastern Binghi (EB) (29°12′S, 151°45′E; 650 m asl). In EB, our study was undertaken over an area of approx. 25 ha, where the species is patchily distributed. There are over 10 000 individuals within the whole area (Streat, 1996). The segment of the EB population studied grows in a sheltered position adjacent to Oakey and Catarrh Creeks. The species also extends to several of the rocky knolls in the area. The third population is located south‐west of Grafton and at the time of the study contained ten plants. This population had to be excluded from our study because only one of the plants produced flower buds during the study and these shrivelled before flowering.
Spatial distribution within both study populations is strongly clumped, although population structure differs markedly at each site. In 1996, the GFRNP population consisted primarily of seedlings (79 %), with similar numbers of intermediate (10 %) and adult (11 %) individuals (Streat, 1996). Conversely, seedlings constituted only 0·05 % of the EB population, with most plants categorized as intermediate (21 %) or adult (79 %). Streat (1996) concluded that the differences in population structure between the two sites corresponded to differences in fire frequency. The population at GFRNP has been subjected to frequent fires and was partially destroyed following bushfires in 1994. The EB population had not been exposed to fire for at least 12 years at the time of the study.
In G. beadleana, flowering begins in early spring and ceases in late autumn (Smith, 1997). Fieldwork was conducted on the two populations between spring 1995 and autumn 1996 (season 1) and spring 1996 and autumn 1997 (season 2). At both sites plants grow in a freely draining sandy‐loam soil of granitic origin which is acidic, rich in silica, iron and aluminium, and poor in calcium, magnesium, phosphorus and nitrate (Benson, 1992). Summer rainfall predominates (Nov.–Feb.) and it is estimated that GFRNP receives more rain (approx. 1000 mm per annum) than EB (approx. 800 mm per annum) (Benson, 1992).
Breeding system
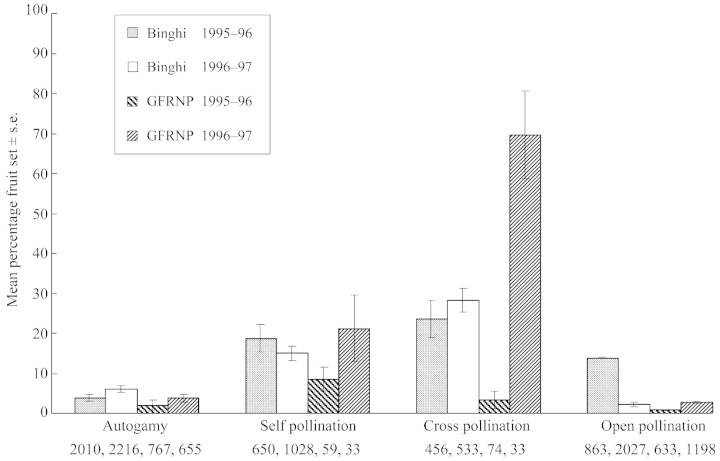
Breeding system work was conducted in both populations in each flowering season using mature and immature inflorescences. To determine whether the species is able to produce fruit autogamously, immature inflorescences on plants were individually enclosed within bags of fine nylon‐organza (floral sample sizes for all treatments are shown in Fig. 3) and left in place until fruit‐drop. Bags excluded airborne pollen, invertebrates and vertebrates from the inflorescences. To assess self‐compatibility, other immature inflorescences were individually bagged at the same time as the autogamy treatment was initiated, on the same plants where possible. After opening, these flowers were hand‐pollinated by manually pressing self‐pollen already present on the pollen presenter into the stigmatic region using sufficient pressure to ensure most of the pollen remained on the presenter. Hands were cleaned with 70 % ethanol between inflorescences to avoid unintentional cross‐pollination of flowers. In addition, the styles of unopened flowers in these inflorescences were cut to prevent autogamy which would confound fruit counts. In the third treatment, mature inflorescences were chosen for cross‐pollination, and spent and open flowers on these inflorescences were excluded by clipping immediately prior to bagging. Inflorescences were bagged and left overnight. The following day self‐pollen was removed from newly opened flowers with a cotton bud. For each inflorescence in the cross‐pollination treatment, donor pollen was collected from one plant in a different clump of plants at least 5 m from the recipient. Donor pollen was then applied to the stigmatic region by rubbing it with pollen‐laden donor presenters. The styles of unopened flowers were also clipped at this time to avoid confounding the fruit counts. Cross pollinations were repeated within 3 d after pollination using donor pollen from the same source. In the fourth treatment, levels of open pollination were assessed in both populations over two flowering seasons using ten to 26 inflorescences on eight to 26 individuals. These inflorescences were tagged and then left un‐manipulated. Resultant fruit‐set for each treatment was determined between 6 and 10 weeks after treatment application. Generally, four inflorescences per plant were chosen for the four pollination treatments and each inflorescence had only one treatment type applied to it. A shortage of inflorescences at the appropriate stage of development at GFRNP in the second season meant that treatments were spread over many more individuals.
Fig. 3. Fruit‐set in G. beadleana after four breeding system treatments were applied to each of 21–26 individuals at Eastern Binghi (Binghi) and seven to 13 individuals at Guy Fawkes River National Park (GFRNP). Numbers under treatments are the number of flowers treated.
Floral resources and fruit‐set
The number of mature inflorescences per fertile individual was scored at EB in the first season (n = 47 plants) and at GFRNP in both seasons (n = 33 and 18 plants, respectively, in the first and second seasons). The number of flowers per inflorescence was counted on those inflorescences selected for breeding system work (EB season 1: n = 42; season 2: n = 104; GFRNP season 1: n = 23; season 2: n = 54; see above). Fruit to flower ratios were measured by counting all flowers and, later, all fruits on open‐pollinated infructescences.
Flower position and fruit‐set
The ability of a fertilized flower to develop fruit may be influenced by the position of the flower on the inflorescence (Stephenson, 1981). The numbers of fruit in proximal (first third of inflorescence length), medial (second third) and distal positions (last third) on a rachis were counted on at least two to five inflorescences on 16–29 individuals per population. Data were collected over both seasons in EB and in season 1 in GFRNP. Individual plants and infructescences were selected haphazardly (e.g. sterile plants and plants on remote rock shelves were not included).
Determination of stigma receptivity
Glasshouse‐grown plants propagated from cuttings obtained from both sites provided the plant material used for examination of stigma receptivity. Simultaneous anthesis was induced in seven flowers by tripping (stroking once) mature flower buds about to open. As the retention of self‐pollen is purported to retard stigmatic opening in Banksia (Vaughton and Ramsey, 1991), self‐pollen on presenters was removed with a cotton bud to allow the stigmatic region to mature. Pollen presenters were harvested at 24, 36, 48, 60, 72, 120 and 144 h after anthesis and immediately placed in labelled Eppendorf tubes containing the fixative, formalin propionic acid in 70 % ethanol (90 : 5 : 5 FPA).
Samples were removed from FPA, placed into 70 % ethanol, then washed in a graded ethanol series, beginning at 70 % through 80 %, 90 %, 95 % and twice in 100 % ethanol, each for 10 min. All specimens were air dried and affixed to double‐sided tape, placed on stubs and sputter‐coated with 50 nm gold at 2·4 kV. Scanning electron micrographs were taken using a JEOL JSM‐5800LV at 20 kV.
Pollen viability
Pollen viability in flowers ranging from those newly opened to flowers that had been open for 3 d was assayed to determine whether self‐pollen is viable when stigmas are receptive. Three nearly mature inflorescences were taken from plants at GFRNP and kept in the laboratory in vases containing water and sucrose. Inflorescences were checked at 12 h intervals and the anthesis of each flower was recorded to identify pollen age. The harvested flowers provided pollen samples ranging from 0 h old, to over 72 h old, at 12 hourly age intervals. Three to four samples from each time interval were examined.
Pollen viability tests were performed using a 0·5 % solution of 2,3,5‐triphenyl tetrazolium chloride (TCC) in 12 % sucrose, after Cook and Stanley (1960). Slides were left for 3 d to allow thorough infiltration of TCC into the pollen exine. Under 200 × magnification, the first 200 grains viewed on a microscope slide were assessed as viable or inviable. Pollen grains stain red in the presence of reductases, indicating enzyme activity; therefore red grains were scored as viable. Pollen killed in FPA and soaked in the TCC solution provided a standard against which unviable pollen could be compared. Inviable pollen does not take up the stain.
Avian floral visitors
Birds were observed at both sites during each reproductive season. Most observations were made from dawn (approx. 0600 h) to approx. 0900 h, except on two occasions when times were extended to late morning and late afternoon. A total of 8·5 h and 10 h was spent in observation at EB during the first and second reproductive seasons, respectively. Less time, 3·0 h and 3·25 h, was spent in direct observation at GFRNP during seasons 1 and 2, respectively, although avian visitors observed at other times were recorded. Several clumped plants (two to five plants) were placed under observation at EB. The sparse distribution of plants at GFRNP prevented observation of more than two shrubs at once. Avian visitors to plants were viewed using binoculars from hide positions amid rocky outcrops at a distance of 5–10 m. The following data were recorded: bird identity; the identity of each plant under observation; the number of plants visited per bird (inter‐plant visits were calculated as the number of plants visited per bird per visitation event minus one; Smith, 1997); the number of intra‐plant visits (i.e. number of inflorescences visited per plant); the number of probes per bird per inflorescence; and the amount of time (in seconds) spent per bird at each plant. Honeyeaters not foraging at G. beadleana but observed in the vicinity were also recorded. Bird species were identified using Slateret al. (1986).
Data analysis
All analyses were undertaken using the Statgraphics™ statistical package. Fixed model multi‐factor ANOVAs were used to investigate the effects of site, season and treatment on fruit‐set in breeding system work and to investigate inflorescence numbers against site and season. Log transformations of these data were required for homoscedasticity. Flower numbers and fruit to flower ratios were each analysed with Kruskal–Wallis non‐parametric ANOVA (H). Positional data were analysed with a fixed model multi‐factor analysis of variance (ANOVA). A square‐root transformation was required for homoscedasticity of positional data. Unplanned comparisons between means were explored when F‐ratios were significant using Tukey’s Honestly Significantly Difference (HSD) method (Sokal and Rohlf, 1981). The interaction between season and position was not significant (F2,594 = 0·93, P = 0·39), therefore EB data from both seasons were pooled and compared with season 1 data from GFRNP. Differences between means for pollen viability were assessed using a Student’s t‐test. Bird visitation data were analysed using Kruskal–Wallis non‐parametric ANOVA.
RESULTS
Breeding system
Results of the autogamy and self‐pollination treatments provide strong evidence of self‐compatibility in G. beadleana both with and without mechanical assistance from pollen vectors (Fig. 3). Treatment type had different effects on fruit set in some site and year combinations (Table 2). Generally, fruit set increased when pollen was added to flowers, and flowers were more likely to set fruit if outcross pollen rather than self‐pollen was used. This difference may have arisen because flowers in the outcross treatment received two applications of pollen and/or may reflect the fact that the species is preferentially outbreeding. Notably, within sites the lowest fruit set generally occurred in the autogamy and open‐control flowers and fruit set did not vary significantly between these treatments (Fig. 3).
Table 2.
Analysis of log proportion of fruits produced in autogamy, selfing, outcrossing and open pollination treatments on G. beadleana plants in EB and GFRNP in 1995–96 and 1996–97 seasons
| Source | Sums of squares | d.f. | Mean square | F‐ratio | P‐value |
| Main effects | |||||
| Site | 0·74 | 1 | 0·74 | 1·51 | 0·22 |
| Year | 1·53 | 1 | 1·53 | 3·13 | 0·08 |
| Treatment | 89·14 | 3 | 29·71 | 60·93 | 0·00001 |
| Interactions | |||||
| Site × year | 6·97 | 1 | 6·97 | 14·29 | 0·0002 |
| Site × treatment | 6·68 | 3 | 2·22 | 4·57 | 0·0041 |
| Year × treatment | 6·56 | 3 | 2·18 | 4·48 | 0·0046 |
| Site × year × treatment | 6·05 | 3 | 2·01 | 4·14 | 0·0072 |
| Residual | 94·12 | 193 | 0·49 | ||
| Total | 264·34 | 208 |
Floral resources and fruit set
Only 61 % of plants at GFRNP flowered in either season and the number of inflorescences per plant did not vary significantly between seasons (Table 3). Seasonal data were pooled and the mean number of inflorescences per fertile plants was 4·6 ± 0·9 (n = 31). At EB, where inflorescence data were only collected in the second season, 100 % of plants scored produced inflorescences and the mean number of inflorescences per plant was 51·2 ± 6·8 (n = 47). This ten‐fold difference in mean number of inflorescences between sites was highly significant (F1,65 = 171·40, P = 0·001). Counts of flowers per inflorescence varied significantly between seasons for EB but not for GFRNP (Table 3). Within seasons and between sites, the number of flowers on inflorescences did not vary significantly (H = 0·42, P = 0·51) in the first season, but in the second season plants at EB produced almost twice as many flowers per inflorescence than those at GFRNP (Table 3); this difference was significant (H = 52·08, P = 0·0001). Infructescences produced an average of only approx. four fruits (Table 2) across sites and seasons, although a significant difference was detected at EB between seasons (Table 2; F2,334 = 2·99, P = 0·05). Fruit to flower ratios were also low (Table 3) and varied significantly within sites between seasons (EB: H = 9·94, P = 0·002; GFRNP: H = 5·65, P = 0·017) and between sites in season 1 (H = 8·14, P = 0·004) but not in season 2 (H = 1·18, P = 0·27).
Table 3.
Seasonal and site effects on inflorescence number, flower number and fruit/flower ratio in G. beadleana*
| Site | ||||
| Eastern Binghi | Guy Fawkes River National Park | |||
| Character | Season 1† | Season 2† | Season 1† | Season 2† |
| Number of inflorescences per plant | 51·2 ± 6·8 (47)b | – | 4·1 ± 1·0 (20)a | 5·4 ± 1·8 (11)a |
| Number of flowers per inflorescence | 68·4 ± 5·8 (42)a | 90·8 ± 2·8 (104)b | 62·1 ± 3·8 (23)a | 53·5 ± 2·5 (54)a |
| Fruit/flower | 0·1 ± 0·05 (21)a | 0·02 ± 0·005 (26)b | 0·007 ± 0·003 (10)c | 0·02 ± 0·004 (21)b |
* Each value is the mean number or ratio ± standard error, with the sample size shown in parentheses. † Season 1 = 1995–96; Season 2 = 1996–97. Within characters, values followed by different superscript letters differ significantly at P < 0·05.
Flower position and fruit set
In a multi‐factor ANOVA of year and fruit position for EB, no significant difference in fruit production on fertile inflorescences was found between seasons (F1,594 = 1·49, P = 0·22) (Table 4). A significant effect was found for fruit position (F2,594 = 19·82, P = 0·001), but as interaction between season and position was not significant (F2,594 = 0·93, P = 0·39), EB data from both seasons were pooled and compared with the season 1 data from GFRNP (Table 4). Site and interaction were not significant (site: F1,547 = 0·12, P = 0·73; interaction: F2,547 = 0·99, P = 0·37) when pooled data from the first and second seasons at EB were compared with data from the first season at GFRNP, but a significant difference was found for fruit position (F2,547 = 15·49, P = 0·001). Tukey’s HSD comparison procedure was undertaken on amalgamated site data and a significant effect was found between distal and proximal fruit positions (contrast between means = 0·121) and between medial and proximal positions (contrast between means = 0·08). Distal and medial positions did not bear significantly different numbers of fruit (Table 4).
Table 4.
The effect of location and season on the number of fruits occupying the distal, medial and proximal thirds ofG. beadleana infructescences*
| Location, season (n) | Distal | Medial | Proximal | Total fruit on fertile rachis (range) |
| EB 1995–96 (24, 120) | 1·1 ± 0·1 | 0·9 ± 0·1 | 1·9 ± 0·1 | 4·0 ± 0·2A (1–13) |
| EB 1996–97 (16, 80) | 0·9 ± 0·1 | 0·8 ± 0·1 | 1·6 ± 0·2 | 3·4 ± 0·2B (1–14) |
| GFRNP 1995–96 (29, 137) | 0·6 ± 0·08 | 0·9 ± 0·1 | 1·9 ± 0·1 | 3·7 ± 0·1B (1–11) |
| Combined (69, 337) | 0·8 ± 0·06b | 0·9 ± 0·08b | 1·8 ± 0·1a |
* Each value is the mean ± standard error. Numbers in parentheses under Location, season indicate plant number and number of infructescences scored, respectively. EB, Eastern Binghi; GFRNP, Guy Fawkes River National Park. Values followed by different superscript letters differ significantly at P < 0·05.
Stigma receptivity
The stigmatic region of G. beadleana is positioned centrally on the pollen presenter, is apical and is covered with pollen at anthesis (Fig. 4A). Pollen is triporate with a sculptured exine (Fig. 4B). Stigmatic papillae protrude from the stigmatic region 24 h post‐anthesis and a small amount of stigmatic exudate is present, covering some of the outer papillae (Fig. 5A). The stigma did not appear to be receptive even at 36 h post‐anthesis, as papillae were situated close together and the stigmatic groove remained closed (Fig. 5B). Opening of the stigmatic groove became evident 48 h after anthesis (Fig. 5C). As the pollen presenters aged, the stigmatic papillae separated further forming a definite slit or groove (Figs 4F and 5D). Figure 5G illustrates the way a pollen grain may be held in position, lodged between the stigmatic papillae. It is not known whether this pollen grain had germinated.
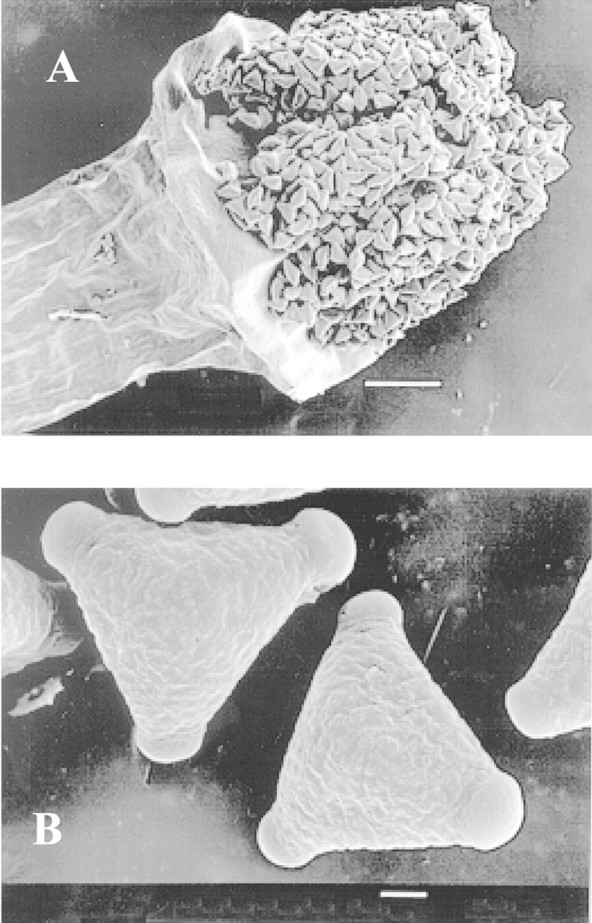
Fig. 4. A, The apical stigmatic region of Grevillea beadleana is centrally located on the pollen presenter. Pollen covers the entire stigmatic surface at anthesis. Bar = 200 µm. B, Pollen is triporate with a highly sculptured exine. Bar = 10 µm.
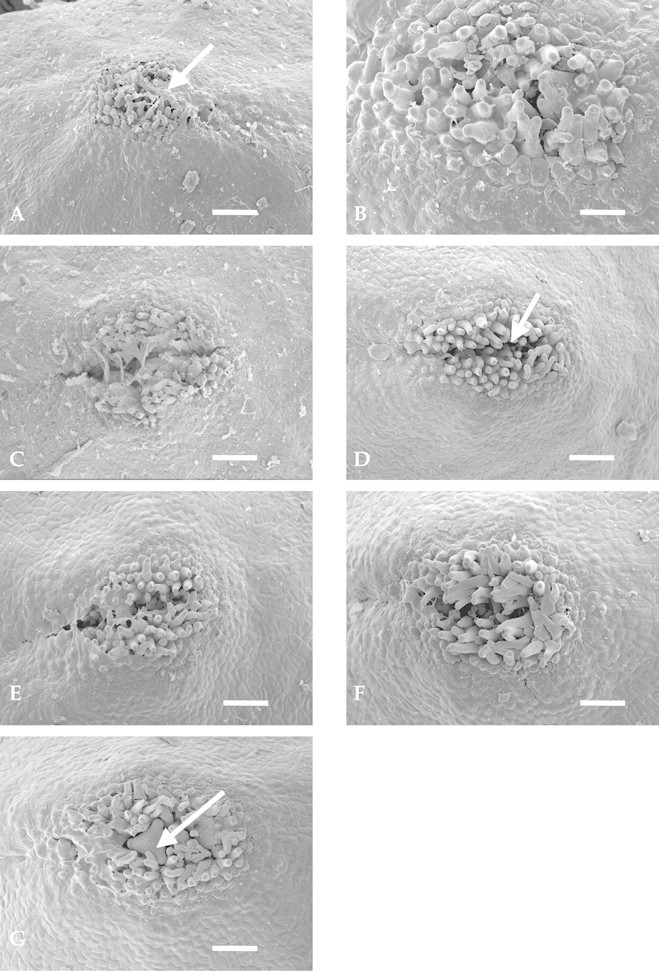
Fig. 5. A, Grevillea beadleana pollen presenter 24 h post‐anthesis. Arrow shows stigmatic exudate at the right of the stigmatic papillae. Stigma is not ripe as papillae have yet to grow and separate. The stigmatic groove is not yet evident. Bar = 71·25 µm. B, At 36 h after anthesis stigmatic papillae are still positioned close together, but stigmatic exudate appears less abundant than at 24 h. Bar = 60·27 µm. C, At 48 h after anthesis papillae have begun to separate revealing the slit‐like stigmatic groove. Stigmatic exudate remains attached to the papillae (centre). Bar = 65·99 µm. D, At 60 h post‐anthesis the stigmatic papillae have separated further and there is less evidence of stigmatic exudate. Arrow points to stigmatic groove. Bar = 30 µm. E, Stigmatic region 72 h post‐anthesis. The stigmatic groove appears to be lengthening as well as widening. Exudate covers some of the papillae (centre left) which have yet to extend and separate. Bar = 59·37 µm. F, The stigmatic papillae have separated fully after 120 h, exposing the underlying receptive surface of the stigma. The groove has widened to approx. 50 µm, exposing the stigmatic surface underneath. Bar = 51·43 µm. G, Arrow indicates a pollen grain that is held close to the opening of the stigmatic area by widely separated stigmatic papillae. It is not known whether the pollen grain has germinated. Bar = 53·98 µm.
Pollen viability
Although pollen viability decreased with pollen age over 3 d, the difference between the highest and lowest values was not statistically significant (P > 0·05). At anthesis (0 h), the mean amount of viable pollen approached 100 % (99·5 ± 0·3 %) and remained high until pollen age exceeded 24 h (94·8 ± 2·2 %). The amount of viable grains per pollen presenter decreased steadily to 84·5 ± 7·77 % in pollen aged between 60 and 72 h. Pollen may have been viable before anthesis and after 72 h, but this was not tested.
Avian floral visitors
A total of five bird species was observed foraging at G. beadleana inflorescences at EB over the two flowering seasons (Table 5), with the yellow‐tufted honeyeater, Lichenostomus melanops (Latham), being the most frequently recorded visitor. Two crimson rosellas, Platycerus elegans (Gmelin), were observed ripping flowers from inflorescences. The principal avian floral visitor to the species at GFRNP was the eastern spinebill, Acanthorhynchus tenuirostris (Latham) (Table 5), and, although four other species were recorded, sightings were infrequent in both flowering seasons. Foraging was observed to begin at different times during the early morning hours but most bird activity generally occurred between 0630 and 0800 h. Nectar foraging did not occur on mornings following wet days or nights. On these mornings, birds tended to remain in trees surrounding the grevilleas, and appeared to hawk (capture insects in mid‐flight) or forage for insects on leaves or bark. The paucity of bird sightings at GFRNP in season 1 prevented statistical comparisons of season 1 and season 2 data.
Table 5.
Bird species observed near and foraging at G. beadleana populations at Eastern Binghi (EB) and Guy Fawkes River National Park (GFRNP) over two reproductive seasons
| Location | Reproductive season | Common name | Scientific name | Visits by species/ total species visiting |
| EB | 1995–1996 | Yellow‐tufted honeyeater* | Lichenostomus melanops | 10/13 |
| White‐naped honeyeater* | Melithreptus lunatus | 3/13 | ||
| Brown‐headed honeyeater | Melithreptus brevirostris | – | ||
| Fuscous honeyeater | Lichenostomus flavescens | – | ||
| 1996–1997 | Yellow‐tufted honeyeater* | Lichenostomus melanops | 6/10 | |
| Noisy friarbird* | Philemon corniculatus | 2/10 | ||
| Spiny‐cheeked honeyeater* | Acanthogenys rufogularis | 1/10 | ||
| Crimson rosella* | Platycerus elegans | 1/10 | ||
| Red wattle bird | Anthochaera carunculata | – | ||
| GFRNP | 1995–1996 | Eastern spinebill* | Acanthorhynchus tenuirostrus | 1/1 |
| 1996–1997 | Eastern spinebill* | Acanthorhynchus tenuirostrus | 4/7 | |
| Red wattle bird* | Acanthorhynchus carunculata | 1/7 | ||
| White‐naped honeyeater* | Melithreptus lunatus | 1/7 | ||
| White‐cheeked honeyeater* | Phylidonyris nigra | 1/7 |
* Birds that foraged at G. beadleana. Authorities for Latin names follow the Reader's Digest Complete Book of Australian Birds (1990).
No statistical difference in bird visitation was detected between years at EB (Table 6; H = 2·44, P = 0·12). These data were pooled and compared with season 2 data from GFRNP; it was found that birds visited more plants at EB than GFRNP, and this difference was significant (H = 7·01, P = 0·01). Birds visited statistically more inflorescences per plant in EB in season 1 than in season 2 (H = 12·54, P = 0·001). No statistical difference was found in any of the measured parameters between sites in season 2 (Table 6). Inter‐plant visits were low compared with intra‐plant movement at both sites, indicating that the majority of movement by avian foragers is within plants. Inter‐plant visits did not vary significantly among sites or seasons (Table 6).
Table 6.
Comparison of bird visitation patterns at G. beadleana at two sites over two reproductive seasons*
| Site | |||
| EB | GFRNP | ||
| 1995–1996 | 1996–1997 | 1996–1997 | |
| Number of plants per bird visit | 3·0 ± 0·45a (17) | 1·8 ± 0·38a (9) | 1·13 ± 0·13b (8) |
| Number of inter‐plant visits per bird | 2·0 ± 0·42a (18) | 0·88 ± 0·38a (9) | 0·13 ± 0·13a (8) |
| Number of intra‐plant visits per bird | 3·56 ± 0·37a (51) | 1·64 ± 0·24b (17) | 1·63 ± 0·26b (8) |
| Amount of time spent at each plant (s) | 259·4 ± 49·81a (17) | 59·55 ± 25·93b (9) | 17·63 ± 5·08b (8) |
| Number of probes per inflorescence | 4·90 ± 0·29a (120) | 6·14 ± 0·70a (27) | 4·25 ± 0·74a (12) |
* Each value is the mean ± standard error, with the sample size shown in parentheses. Values within rows followed by different superscripts differ significantly at P < 0·05. EB, Eastern Binghi; GFRNP, Guy Fawkes River National Park.
DISCUSSION
Grevillea beadleana was found to be self‐compatible, a trait common to most Grevillea taxa studied to date, including common and rare species (Table 1). Protandry, which occurs frequently within the Proteaceae (Goldingay and Carthew, 1998), was also confirmed to operate in G. beadleana. Temporal separation of male and female reproductive function (dichogamy) may reduce the possibility of self‐fertilization in hermaphroditic species that also have little spatial separation (herkogamy) between male and female reproductive structures. In G. beadleana, pollen was viable at anthesis and on day 2 when stigmas on pollen‐cleaned presenters were fully receptive. This suggests that weak dichogamy occurs in this species unless stigma maturation is delayed by the retention of self‐pollen. However, autogamy may be unavoidable even then, as some self‐pollen may remain on the stigmatic surface. Furthermore, as Brunet and Charlesworth (1995) note, when dealing with inflorescences with sequentially opening flowers, selfing may be more frequent in early‐opening flowers on an inflorescence than in later‐opening ones. This is because receptive stigmas in early‐opening flowers are likely to coincide with the period of maximum pollen viability in flowers in the same inflorescence or on the same plant. Unless pollen is transported to another plant, geitonogamous self‐pollinations therefore become more likely when plants are visited.
As proximal flowers are the first to open in G. beadleana and are likely to be pollinated before flowers at other positions on the inflorescence, fertilized flowers and developing fruits may have a temporal and spatial advantage in the competition for maternal resources (Stephenson, 1981), thereby becoming resource sinks (e.g. Gross, 1993). Most fruit set occurred at the proximal end of inflorescences in G. beadleana which suggests that self‐pollinations are likely to have occurred (Brunet and Charlesworth, 1995) followed by subsequent sinking of resources to fruits at proximal positions (Stephenson, 1981). Several hypotheses regarding patterns of fruit set have been examined in the Proteaceae (e.g. Ayre and Whelan, 1989; Goldingay and Whelan, 1993; Harriss and Whelan, 1993; Matthewset al., 1999), and Ayre and Whelan (1989) conclude that the factors limiting fruit set may differ among sites, species and with time. Furthermore, preferential fruit set does not always occur in proximal positions in the Proteaceae, as found here, but has been recorded in medial (Harriss and Whelan, 1993) and distal positions (Goldingay and Whelan, 1993). This suggests that fruit set is also influenced by the probing patterns of pollinators at inflorescences.
Self‐compatibility and proximal fruit set, combined with the predominantly within‐plant movement of honeyeaters, suggest inbreeding may commonly occur within both populations of G. beadleana studied. Mating systems and fitness assays would assist in determining whether each population is predominantly self‐pollinated or outbreeding.
In general, self‐pollinations may be a common event in the toothbrush grevillea group, in which flowering is often acropetallous (Makinson, 2000). Unusually high selfing rates may thus prevail, and this has indeed been found in the toothbrush‐flowered Grevillea barklyana F. Muell ex Benth. (Ayreet al., 1994). In addition, Lamont (1982) notes that for G. leucopteris Meisn. autogamy and geitonogamy probably account for most seed set. The ability to self‐pollinate and preferentially set proximal fruit that concurrently may be derived from self‐pollinations could be features that induce high levels of inbreeding in the toothbrush grevilleas, contributing to their rare or threatened status. Almost 70 % of the 54 toothbrush grevilleas surveyed by Makinson (2000) are rare or threatened.
Unravelling the causative factors associated with the decline or rarity of a species has led workers to look for patterns in key characters among rare species (e.g. geographical distribution, niche breadth and population size; Rabinowitz, 1981). To this we add the features described above for toothbrush grevilleas. While many rare species are predominantly self‐compatible, suggesting that self‐compatibility is a derived condition which is selected for under conditions where outbreeding is not possible or is unreliable, Kunin and Gaston (1993) note that the self‐compatibility trait may instead be basal and may precipitate inbreeding which then induces rarity and a decline in numbers. We postulate that the latter may be operating in the toothbrush grevilleas because of the likelihood (see above) that many fruits arise from selfing events. Further work is required to understand the cause and effect properties of self‐compatibility and rarity/decline in this genus. In addition, we postulate here that the preponderance of self‐compatibility in species‐rich Grevillea may have assisted speciation by allowing breeding and establishment after long‐distance dispersal events.
The smaller number of inflorescences on plants at GFRNP compared with those at EB was expected as the older, larger plants at EB are capable of supporting more inflorescences. Many of the individuals sampled at GFRNP in season 1 were classified by Streat (1996) as intermediates, most of which had not begun flowering. Many of these plants were in their first reproductive season when sampled 1 year later for the present study. The fire frequency at the GFRNP population is currently one fire in 6 years (Gross, unpubl. res., 2001) which has resulted in a comparatively young population and has prevented the majority of plants from reaching maturity or increasing their size and hence their reproductive output. Inflorescences at EB contained significantly more flowers than those at GFRNP in one of the two survey seasons which suggests that resource levels may vary significantly between years (e.g. moisture availability).
The greater number of inflorescences per plant at EB should result in the production of more seeds per plant per reproductive season than in the population at GFRNP. This is the case at the population level, but not for individual inflorescences. Fecundity (fruit per fertile infructescence) was similar in both populations (approx. four fruits per infructescence), despite the greater numbers of flowers on inflorescences at EB. However, based on two seeds per fruit, extrapolation of the mean number of fruit per infructescence by the mean number of inflorescences per plant yields approx. 348–412 seeds per plant at EB (seasons 1 and 2) and only approx. 20 seeds per plant at GFRNP (season 1). This represents an upper limit as not all fruits yield two seeds and not all plants flowered in GFRNP. A follow‐up survey of fruit production on 20 fertile plants in January 2001 at GFRNP revealed that plants are still producing approx. four fruits per rachis (3·79 ± 0·13) but infructescence production per plant was higher in 2001 (approx. ten infructescences per plant) than in either study season 1 or 2 (10·57 ± 0·26, n = 20 plants). This increase in rachis production was expected as many plants had increased in size since the study began in 1995. Hence, current seed yields at GFRNP are likely to be double that of the predicted yields for the first study season.
In some proteaceous species, floral abundance and the availability of floral rewards is an important factor known to influence avian foraging behaviour (Ford and Paton, 1982; McFarland, 1996). Birds are opportunistic and tend to feed at plants that offer the most reward for the least energy expenditure (Collinset al., 1984). In addition, honeyeaters are known to forage on a range of plant families, genera and species at any one time, and do not rely on a single plant species for food (Hopper, 1980). Thus, for honeyeaters, food availability is more important at the plant community level, rather than at the plant species level.
Compared with EB, bird observations at GFRNP were few, possibly reflecting differences in floral resources within the plant community or within the G. beadleana population. Stephenson (1981) considered that the production of excess flowers on inflorescences is a method of attracting pollinators due to increased advertisement and increased abundance of floral rewards. Inflorescences with fewer flowers produced by plants at GFRNP may possibly signify fewer rewards. In addition, the smaller population at GFRNP may not produce sufficient numbers of inflorescences to entice birds out of the forest and onto the unprotected gorge rim. The exposed position of the population may reduce its attractiveness as a foraging ground. Large birds of prey such as wedge‐tailed eagles (Aquila audax Latham) were frequently observed hovering above the gorge in search of food. The shrub layer was not well developed near most mature plants, so protective cover is lacking. Exposure to predators may be one factor that limits the foraging activities of small nectarivorous birds at this site. Autogamous pollination then, may provide a ‘fail‐safe’ mechanism within the species to ensure reproduction when pollinators are absent or in short supply.
CONCLUSIONS
Plants at EB produced approx. 20‐fold more fruit than those at GFRNP as the older, larger plants at EB produced more inflorescences and infructescences than plants in the smaller population. Few avian floral visitors were observed in the smaller population, although infructescences in both the small and large populations produced approx. four fruits per rachis in all years. The predominantly within‐plant movement of birds combined with self‐compatibility, the success of autogamous and self‐pollinations, incomplete protandry, acropetally and proximal fruit production suggest that both populations may be primarily inbreeding. It is thus important to determine the genetic structure of the population and the proportion of the population that is derived from selfed seeds and/or sib‐matings from genetically similar individuals. In addition, determining whether outcrossing or selfing is more likely in flowers that open first in a self‐compatible toothbrush Grevillea inflorescence, than in those that open later, would be informative. Overall, however, we consider that the primary conservation issue for G. beadleana is to ensure that individuals in small populations are allowed to grow to a size at which they can produce many inflorescences per plant. Additional inflorescences may increase fruit production per plant and may encourage greater pollinator visitation. Older, larger plants are important components of the population and could be promoted by a fire‐free interval of at least 10 years.
ACKNOWLEDGEMENTS
We thank Peter Garlick from the University of New England (UNE) for assistance with Scanning Electron Micrographs, the former Australian Nature Conservation Agency and the New South Wales National Parks and Wildlife Service (NSW NPWS) for providing project funds to C. L. Gross. G. Vaughton (UNE) is thanked for discussions and for comments on some of the data presented here when in thesis format; D. Mackay provided the illustration; Andrew Steed, Danny Corcoran and Mal Dwyer (all NSW NPWS) are thanked for their practical assistance; and the following are thanked for their field assistance: D. Mackay, P. Lisle (UNE), C. Macgregor (UNE), Banjo Smith, Alice Howe, Bill Forsythe and Trevor Williams. Sarah Caldwell generously provided some of our glasshouse plants and the McWhinney family gave us access to their property.
Received: 19 February 2001; Returned for revision: 23 April 2001; Accepted: 26 September 2001.
References
- Ayre D, Whelan RJ.1989. Factors controlling fruit‐set in hermaphroditic plants: studies within the Australian Proteaceae. Trends in Ecology and Evolution 4: 267–272. [DOI] [PubMed] [Google Scholar]
- Ayre D, Whelan RJ, Reid A.1994. Unexpectedly high levels of selfing in the Australian shrub Grevillea barklyana (Proteaceae). Heredity 72: 168–174. [Google Scholar]
- Benson JS.1992. The distribution, abundance and conservation status of Grevillea beadleana (Proteaceae): an endangered species. Cunninghamia 2: 503–521. [Google Scholar]
- Brough P.1933. The life history of Grevillea robusta Cunn. Proceedings of the Linnean Society of New South Wales 58: 33–73. [Google Scholar]
- Brunet J, Charlesworth D.1995. Floral sex allocation in sequentially blooming plants. Evolution 49: 70–79. [DOI] [PubMed] [Google Scholar]
- Carolin RE.1961. Pollination of the Proteaceae. Australian Museum Magazine September:371–374. [Google Scholar]
- Collins BG, Grey J.1988. Pollination and seed set in Grevillea wilsonii In: Singh MB, Troiani LF, eds. Pollination ’88 Parkville, Melbourne: School of Botany, University of Melbourne, 67–70. [Google Scholar]
- Collins BG, Rebelo T.1987. Pollination biology of the Proteaceae in Australia and southern Africa. Australian Journal of Ecology 12: 387–421. [Google Scholar]
- Collins BG, Newland C, Briffa P.1984. Nectar utilisation and pollination by Australian honeyeaters and insects visiting Calothamnus quadrifidus (Myrtaceae). Australian Journal of Ecology 9: 353–65. [Google Scholar]
- Cook SA, Stanley RG.1960. Tetrazolium chloride as an indicator of pine pollen germinability. Silvae Genetica 9: 134–136. [Google Scholar]
- Ford HA, Paton DC.1982. Partitioning of nectar sources in an Australian honeyeater community. Australian Journal of Ecology 7: 149–159. [Google Scholar]
- Goldingay RL, Carthew SM.1998. Breeding and mating systems of Australian Proteaceae. Australian Journal of Botany 46: 421–437. [Google Scholar]
- Goldingay RL, Whelan RJ.1990. Breeding system and tests for pollen‐limitation in two species of Banksia Australian Journal of Botany 38: 63–71. [Google Scholar]
- Goldingay R, Whelan RJ.1993. Influence of pollinators on fruit positioning in the Australian shrub Telopea speciosissima (Proteaceae). Oikos 68: 501–509. [Google Scholar]
- Gross CL.1993. The reproductive ecology of Canavalia rosea (Fabaceae) on Anak Krakatau, Indonesia. Australian Journal of Botany 41: 591–599. [Google Scholar]
- Harriss F, Whelan RJ.1993. Selective fruit abortion in Grevillea barklyana (Proteaceae). Australian Journal of Botany 41: 499–509. [Google Scholar]
- Hermanutz L, Innes D, Denham A, Whelan R.1998. Very low fruit:flower ratios in Grevillea (Proteaceae) are independent of breeding system. Australian Journal of Botany 41: 591–599. [Google Scholar]
- Herscovitch JC, Martin ARH.1990. Pollen‐pistil interactions in Grevillea banksii II. Pollen tube ultrastructure and interactions, and the results of field experiments. Grana 29: 5–17. [Google Scholar]
- Hoebee SE, Young AG.2001. Low neighbourhood size and high interpopulation differentiation in the engendered shrub Grevillea iaspicula McGill (Protaeceae). Heredity 86: 489–496. [DOI] [PubMed] [Google Scholar]
- Hopper SD.1980. Bird and mammal pollen vectors in Banksia communities at Cheyne Beach, Western Australia. Australian Journal of Botany 28: 61–75. [Google Scholar]
- Kalinganire A, Harwood CE, Slee MU, Simons AJ.2000. Floral structure, stigma receptivity and pollen viability in relation to protandry and self‐incompatibility in Silky Oak (Grevillea robusta A. Cunn.). Annals of Botany 86: 133–148. [Google Scholar]
- Kunin WE, Gaston KJ.1993. The biology of rarity: patterns, causes and consequences. Trends in Ecology and Evolution 8: 260–263. [DOI] [PubMed] [Google Scholar]
- Lamont BB.1982. The reproductive biology of Grevillea leucopteris (Proteaceae) including reference to its glandular hairs and colonising potential. Flora 172: 1–20. [Google Scholar]
- Lamont BB, Klinkhammer PGL, Witkowski ETF.1993. Population fragmentation may reduce fertility to zero in Banksia goodii – a demonstration of the Allee effect. Oecologia 94: 446–450. [DOI] [PubMed] [Google Scholar]
- McFarland DC.1996. Aggression and nectar use in territorial non‐breeding New Holland honeyeaters Phylidonyris novaehollandiae in eastern Australia. Emu 96: 181–188. [Google Scholar]
- McGillivray DJ.1993. New names in Grevillea (Proteaceae). Melbourne: Melbourne University Press. [Google Scholar]
- Makinson RO.2000. Grevillea Flora of Australia 17A: 1–460. [Google Scholar]
- Matthews ML, Gardner J, Sedgley M.1999. The proteaceous pistil: morphological and anatomical aspects of the pollen presenter and style of eight species across five genera. Annals of Botany 83: 385–399. [Google Scholar]
- Olde PM, Marriot NR.1995. The Grevillea Book Volume 3. Portland, Oregon, USA: Timber Press. [Google Scholar]
- Rabinowitz D.1981. Seven forms of rarity. In Synge H, ed. The biological aspects of rare plant conservation New York: John Wiley and Sons Ltd, 205–217. [Google Scholar]
- 30Reader's Digest Complete Book of Australian Birds 1990. Sydney: Surry Hills. [Google Scholar]
- Richardson MBG, Ayre DJ, Whelan RJ.2000. Pollinator behaviour, mate choice and the realised mating systems of Grevillea mucronulata and Grevillea sphacelata Australian Journal of Botany 48: 357–366. [Google Scholar]
- Rossetto M, Weaver PK, Dixson KW.1995. Use of RAPD analysis in devising conservation strategies for the rare and endangered Grevillea scapigera (Proteaceae). Molecular Ecology 4: 321–329. [DOI] [PubMed] [Google Scholar]
- Scott J, Marshall A, Auld TD.1995. Conservation research statement and recovery plan for Grevillea caleyi R. Br. Australian Nature Conservation Programme. Endangered Species Project No. 456. [Google Scholar]
- Slater P, Slater P, Slater R.1986. The Slater field guide to Australian birds. Revised Edition. Sydney: Lansdowne. [Google Scholar]
- Smith JA.1997. The reproductive ecology of the endangered plant Grevillea beadleana (Proteaceae). BSc Hons Thesis, University of New England, Armidale. [Google Scholar]
- Sokal RR, Rohlf FJ.1981. Biometry, 2nd edn. New York: W.H. Freeman and Co. [Google Scholar]
- Stephenson AG.1981. Flower and fruit abortion: proximate causes and ultimate functions. Annual Review of Ecology and Systematics 12: 253–279. [Google Scholar]
- Stock WD, Pate JS, Kuo J, Hansen AP.1989. Resource control of seed‐set in Banksia laricina C. Gardener (Proteaceae). Functional Ecology 3: 453–460. [Google Scholar]
- Streat J.1996. Aspects of the ecology of Grevillea beadleana (Proteaceae). Masters of Natural Resources Thesis, University of New England, Armidale. [Google Scholar]
- Vaughton G.1988. Pollination and seed‐set in Banksia spinulosa: evidence for autogamy. Australian Journal of Botany 36: 633–642. [Google Scholar]
- Vaughton G.1996. Pollination disruption by European honeybees in the bird‐pollinated shrub Grevillea barklyana (Proteaceae). Plant Systematics and Evolution 200: 89–100. [Google Scholar]
- Vaughton G, Ramsey M.1991. Floral biology and inefficient pollen removal in Banksia spinulosa var. neoanglica (Proteaceae). Australian Journal of Botany 39: 167–177. [Google Scholar]
- Whelan RJ, Burbridge AH.1980. Flowering phenology, seed‐set and bird pollination of five Western Australian Banksia species. Australian Journal of Ecology 5: 1–7. [Google Scholar]