Abstract
Aluminium (Al) toxicity in rye (Secale cereale L.), an Al‐resistant crop, was examined by measuring root elongation and cytoplasmic free activity of calcium ([Ca2+]cyt) in intact root apical cells. Measurement of [Ca2+]cyt was achieved by loading a Ca2+‐sensitive fluorescent probe, Fluo‐3/AM ester, into root apical cells followed by detection of intracellular fluorescence using a confocal laser scanning microscope. After 20 min of exposure to 50 µm Al (pH 4·2) a slight increase in [Ca2+]cyt of root apical cells was observed, while the response of [Ca2+]cyt to 100 µm Al (pH 4·2) was faster and larger ([Ca2+]cyt increased by 46 % in 10 min). Increases in [Ca2+]cyt were correlated with inhibition of root growth, generally measurable after 2 h. Addition of 400 µm malic acid (pH 4·2) largely ameliorated the effect of 100 µm Al on [Ca2+]cyt in root apical cells and protected root growth from Al toxicity. These results suggest that an increase in [Ca2+]cyt in root apical cells in rye is an early effect of Al toxicity and is followed by the secondary effect on root elongation.
Key words: Aluminium toxicity, Secale cereale L., rye, cytoplasmic Ca2+, root growth
INTRODUCTION
Plant roots are sensitive to Al3+ ions that occur in solution in acidic soils. Disturbance of [Ca2+]cyt homeostasis is generally believed to be the primary trigger of Al toxicity (Bennet and Breen, 1991; Rengel, 1992a, b; Kochian, 1995). A recent study on wheat by Zhang and Rengel (1999) has provided direct evidence in support of this hypothesis, showing a close correlation between the Al‐induced increase in [Ca2+]cyt in root apical cells and inhibition of root growth.
Resistance to Al varies among cereal species, normally in the order: rye ≥ triticale > wheat > barley (Aniol and Gustafson, 1984). Although the Al‐induced increase in [Ca2+]cyt of wheat root apical cells is one of the primary toxic effects of Al (Zhang and Rengel, 1999), it is not known whether such an effect occurs in rye which is more resistant to Al. In this study, we examined the dynamic changes of [Ca2+]cyt in intact root apical cells, and the elongation of rye roots subjected to different levels of Al. Exogenous malic acid was also applied to investigate whether the Al‐induced disturbance in [Ca2+]cyt, and thus the adverse effect on root growth, could be avoided. This study increases understanding of Al toxicity mechanisms in rye and the resistance of this crop to Al stress.
MATERIALS AND METHODS
Root culture and treatments
Rye (Secale cereale L. ‘Bevy’) seeds were surface‐sterilized in 1 % (v/v) NaOCl for 5 min, rinsed thoroughly with deionized water, and germinated on filter paper saturated with 0·2 mm CaCl2 solution (pH 4·2) at 20 °C in the dark for 2 d before measurement of root elongation and [Ca2+]cyt in root apical cells. For the measurements, roots were first grown in an aerated control solution (0·2 mm CaCl2, pH 4·2) before being subjected to the treatments: 0, 50 or 100 µm Al3+ alone, or a combination of 100 µm Al3+ and 400 µm malic acid (0·2 mm CaCl2, pH 4·2). Al3+ was diluted to the required concentration from a 5 mm AlCl3 stock in 0·1 mm HCl.
Loading of Fluo‐3/AM into root cells
When measuring activities of [Ca2+]cyt using a fluorescent imaging technique, the essential step is the loading of Ca2+‐sensitive fluorescent probes into plant cells. A Ca2+‐sensitive fluorescent dye, acetoxymethyl ester (AM) of Fluo‐3, was purchased from Molecular Probes (Eugene, OR, USA). The procedures followed to load this dye into rye root cells were similar to those described previously for wheat by Zhanget al. (1998) and Zhang and Rengel (1999). Intact rye roots (1–2 cm long and straight in shape) were incubated in a solution containing 20 µm Fluo‐3/AM, 50 mm sorbitol and 0·2 mm CaCl2 (pH 4·2) at 4 °C for 2 h in the dark. This low‐temperature incubation inhibited hydrolysis of Fluo‐3/AM ester by extracellular esterases but had less effect on diffusion of the dye across the plasma membranes of root cells. The dye‐loaded roots were then incubated in an aerated 0·2 mm CaCl2 solution at 20 °C for 2 h in the dark, allowing the accumulated Fluo‐3/AM ester to be hydrolysed by the intracellular esterases, releasing membrane‐impermeable Ca2+‐sensitive Fluo‐3 into the cytoplasm.
Confocal laser scanning microscopy
Intact rye roots were mounted in a Plexiglas chamber (2 ml) with a clean cover slip attached to the bottom and were flushed with control solution for about 10 min prior to confocal imaging using a Bio‐Rad MRC 1000 unit attached to a Nikon Diaphot inverted microscope with Apolo X20 (NA, 0·8) lens. Intracellular fluorescence of cortical cells (30–60 µm from the root surface) of the root apex (about 0·5 mm from the root tips) was excited with a 488 nm argon–krypton laser, and emission signals above 515 nm were collected using BHS filter block. The fluorescence intensity of root cells was calculated using Bio‐Rad image‐processing software and was expressed in pixel numbers on a scale ranging from 0 to 255.
Intracellular fluorescence was first collected from the root cells bathed in the control solution, followed by the treatment solutions containing 50 or 100 µm AlCl3 or a combination of 100 µm AlCl3 and 400 µm malic acid. The same apical cells of six to eight roots of each treatment were scanned consecutively every 10 or 20 min for up to 80 min.
Measurement of root elongation
Intact rye roots were flushed with the control solution in a Plexiglas chamber, and root elongation was recorded hourly with an eye piece (10×). Once the elongation rate had stabilized, the roots were perfused with aerated solutions containing different Al treatments (0·2 mm CaCl2, pH 4·2). To examine any possible effect of Fluo‐3/AM ester on root growth, the elongation rate of dye‐loaded roots was also measured; it proved to be similar to that of dye‐free roots (data not shown).
RESULTS AND DISCUSSION
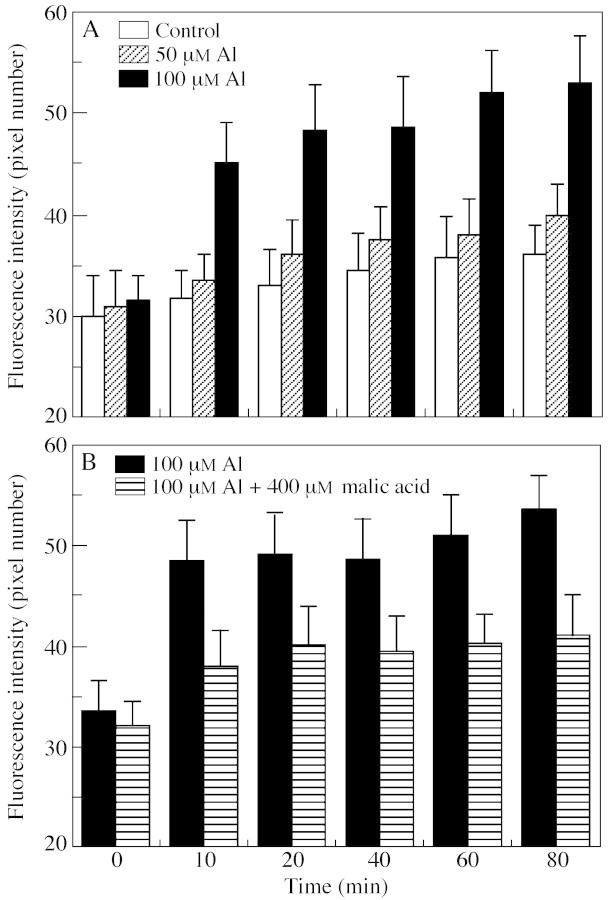
When intact rye roots were exposed to 50 µm Al, [Ca2+]cyt fluorescence of the apical cells was slightly increased after 20 min and then remained mostly unchanged in relation to the controls during the period of Al exposure (up to 80 min; Figs 1 and 3A). Treatment with 100 µm Al, however, induced a fast and significant response of [Ca2+]cyt in the apical cells: [Ca2+]cyt fluorescence increased by 46 % within 10 min (Figs 2 and 3A). The intensity of the fluorescence continued to increase in parallel with the duration of the 100 µm Al treatment. This contrasts with a previous study in which a concentration as low as 2·6 µm Al induced an increase in [Ca2+]cyt of intact root apical cells in an Al‐sensitive wheat line, with the magnitude of the increase being proportional to Al concentration (Zhang and Rengel, 1999).
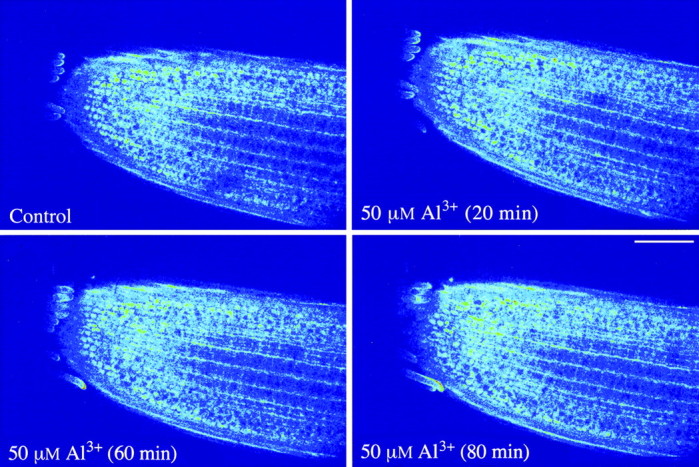
Fig. 1. Confocal images of Fluo‐3 fluorescence showing a slight increase in the intensity (i.e. increased cytoplasmic Ca2+ activity) in rye root apical cells in the presence of 50 µm AlCl3 (pH 4·2). Pseudocolour is used to enhance visualization of Fluo‐3 distribution and indicate fluorescence intensity, where dark is minimum and white is maximum intensity. Bar = 100 µm.
Fig. 3. Al‐induced changes in Fluo‐3 fluorescence intensity in rye root apical cells treated with different levels of Al (A) and a combination of Al and malic acid (B). Values are means ± s.e. of six to eight roots.
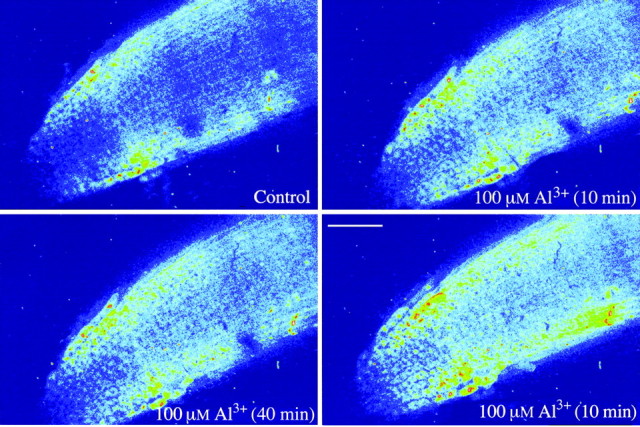
Fig. 2. Confocal images of Fluo‐3 fluorescence showing a dramatic increase in the cytoplasmic Ca2+ activity in rye root apical cells in the presence of 100 µm AlCl3 (pH 4·2). See Fig. 1 for a description of pseudocolour to indicate the fluorescence intensity. Bar = 100 µm.
Elevation of [Ca2+]cyt in root apices of both wheat and rye was detectable after about 10 min of exposure to Al, but was slower than that found in wall‐free protoplasts derived from wheat roots (<2 min; Lindberg and Strid, 1997). The slower response of [Ca2+]cyt to Al in intact wheat and rye roots could be due to the interaction of cationic Al species with the cell wall. Al‐induced increase in [Ca2+]cyt of root apical cells was also observed by Nichol and Oliveira (1995) in barley, but they only studied the long‐term (2 d) effect of Al (50 µm) in excised Al‐sensitive barley roots. On the other hand, a decrease in [Ca2+]cyt in tobacco cell cultures in the presence of Al was reported by Joneset al. (1998). However, the decrease may not necessarily be representative of Al‐induced changes in [Ca2+]cyt in root cells since the morphology and physiology of cell cultures could be different from those of intact root cells.
Although exposure to 100 µm Al alone induced a dramatic increase in [Ca2+]cyt in rye root apical cells (Figs 2 and 3A), the [Ca2+]cyt activity was only marginally increased over the period of measurements when the roots were treated with 100 µm Al plus 400 µm malic acid (Fig. 3B), indicating a maintenance of cell Ca2+ homeostasis in the root apex, the critical site of Al toxicity. The interactive effect of Al and malate on [Ca2+]cyt was probably due to the fact that malate binds to Al, making the malate‐Al complex non‐toxic (Delhaize and Ryan, 1995; Matsumoto, 2000).
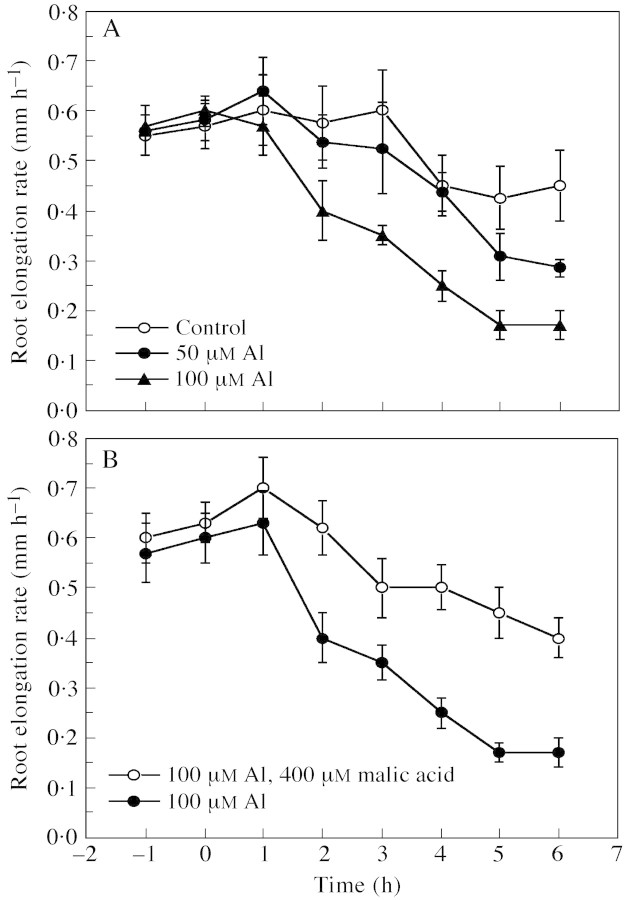
The response of root growth to Al treatments was closely correlated to that of [Ca2+]cyt in root apical cells, despite a time lag between the two. After 2 h of exposure to 100 µm Al there was a significant decrease in the rate of root elongation, while root growth in the treatment with 50 µm Al was mostly unaffected (Fig. 4A). As the Al treatments continued, the toxic effect of 100 µm Al became more severe, and growth of roots treated with 50 µm Al was also reduced significantly after 5 h of exposure. However, the reduction of root elongation due to 100 µm Al was largely ameliorated when the culture solution was supplemented with 400 µm malic acid (Fig. 4B).
Fig. 4. The rate of rye root elongation after exposure to different levels of Al (A) and a combination of Al and malic acid (B). Values are means ± s.e. of eight to ten roots.
The increase in [Ca2+]cyt in the presence of Al might disrupt Ca2+‐dependent metabolic processes, which are directly or indirectly involved in regulation of cell division and elongation (Rengel, 1992a, b; Delhaize and Ryan, 1995). For instance, an increase in [Ca2+]cyt is a prerequisite for synthesis of callose (1,3‐β‐glucan) in plant cells (Kauss, 1985). Indeed, Al is known to induce callose synthesis in wheat root tips, which is positively related to inhibition of wheat root growth (Zhanget al., 1994). These observations are in agreement with the findings of the present study that the increase in [Ca2+]cyt in intact rye root apical cells is dependent upon Al concentration, and that high [Ca2+]cyt is accompanied by severe reduction of root elongation.
In summary, the primary effects of Al stress on rye and wheat are similar, i.e. Al first induces an increase in [Ca2+]cyt of root apical cells, which then disrupts Ca2+‐dependent metabolic processes (e.g. regulation of cell division and elongation) and leads to inhibition of root growth. The greater Al resistance of rye compared with wheat is reflected in the higher Al concentrations required to cause a comparable toxicity effect.
ACKNOWLEDGEMENTS
We thank Dr Tim Colmer for valuable discussions and Mr John Murphy for help with confocal microscopy. This project was funded by the Smart Bequest, University of Western Australia.
Supplementary Material
Received: 14 June 2001; Returned for revision: 22 July 2001; Accepted: 1 October 2001.
References
- AniolA, Gustafson JP.1984. Chromosome location of genes controlling aluminum tolerance in wheat, rye and triticale. Canadian Journal of Genetic Cytology26: 701–705. [Google Scholar]
- BennetRJ, Breen CM.1991. The aluminium signal: new dimensions to mechanisms of aluminium tolerance. Plant and Soil 149: 87–94. [Google Scholar]
- DelhaizeE, Ryan PR.1995. Aluminum toxicity and tolerance in plants. Plant Physiology 107: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JonesDL, Kochian LV, Gilroy S.1998. Aluminum induces a decrease in cytosolic calcium concentration in BY‐2 tobacco cell cultures. Plant Physiology 116: 81–89. [Google Scholar]
- KaussH.1985. Callose biosynthesis as a Ca2+ regulated process and possible relations to the induction of other metabolic changes. Journal of Cell Science Supplement 2: 89–103. [DOI] [PubMed] [Google Scholar]
- KochianLV.1995. Cellular mechanisms of aluminium toxicity and tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 46: 237–260. [Google Scholar]
- LindbergS, Strid H.1997. Aluminum induces rapid changes in cytosolic pH and free calcium and potassium concentrations in root protoplasts of wheat (Triticum aestivum). Physiologia Plantarum 99: 405–414. [Google Scholar]
- MatsumotoH.2000. Cell biology of aluminum toxicity and tolerance in higher plants. International Review of Cytology 200: 1–46. [DOI] [PubMed] [Google Scholar]
- NicholBE, Oliveira LA.1995. Effects of aluminum on the growth and distribution of calcium in roots of an aluminum‐sensitive cultivar of barley (Hordeum vulgare). Canadian Journal of Botany 73: 1849–1858. [Google Scholar]
- RengelZ.1992a Disturbance of cell Ca2+ homeostasis as a primary trigger of Al toxicity syndrome. Plant, Cell and Environment 15: 931–938. [Google Scholar]
- RengelZ.1992b Role of calcium in aluminium toxicity. New Phytologist 121: 499–513. [DOI] [PubMed] [Google Scholar]
- ZhangG, Hoddinott J, Taylor GJ.1994. Characterisation of 1,3‐β‐d‐glucan (callose) synthesis in roots of Triticum aestivum in response to aluminum toxicity. Journal of Plant Physiology 144: 229–234. [Google Scholar]
- ZhangWH, Rengel Z.1999. Aluminium induces an increase in cytoplasmic calcium in intact wheat root apical cells. Australian Journal of Plant Physiology 26: 401–409. [Google Scholar]
- ZhangWH, Rengel Z, Kuo J.1998. Determination of intracellular Ca2+ in cells of intact wheat roots: loading of acetoxymethyl ester of Fluo‐3 under low temperature. The Plant Journal 15: 147–151. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.