Abstract
The pollination process in Trigonidium obtusum Lindl. (Epidendroideae: Maxillariinae) is documented. The flowers are pollinated by sexually excited drones of Plebeia droryana (Meliponinae). When attempting to copulate either with sepals or petals, these bees slip on the waxy perianth surface and become trapped in the funnel‐like flower tube. Bees trying to escape from the flowers may instead access the space between the column and lip, fixing the pollinarium on their scutellum. Pollinarium‐bearing bees may pollinate the flowers when repeating the above‐mentioned steps, leaving pollinia on the concave stigmatic surface, thus effecting pollination. Recently removed pollinaria are too broad to enter the stigma but they begin to dehydrate and within 40 min of removal are small enough to fit the stigmatic cavity. This mechanism prevents insect‐mediated self‐pollination and promotes cross‐pollination. Preliminary evidence based on experiments with cultivated plants suggests that they are self‐compatible but that fruit set is pollinator‐dependent. The data obtained are discussed in a phylogenetic context. It is suggested that the pseudocopulatory syndrome in Trigonidium could have evolved from rewardless (food advertising) ancestors. Pseudocopulation in the context of the long flowering period of this orchid species (about 7 months) is understandable since the eusocial Plebeia bees produce fertile individuals several times a year.
Key words: Trigonidium, Maxillaria, Maxillariinae, pollination, pseudocopulation, Plebeia, Meliponinae
INTRODUCTION
Orchid flowers are notorious for their complex morphology and the often remarkable relationships they establish with their pollinators. Among the terrestrial species of the subfamily Orchidoideae, most European and African Ophrys species and several Australian orchid genera have developed a remarkable pollination system, termed ‘pseudocopulation’ (Kullenberg, 1961; Stoutamire, 1974, 1975, 1981; Dafni and Bernhardt, 1990). These orchids are pollinated by deceived male Hymenoptera (wasps or bees) which attempt to copulate with ‘dummy‐like’ structures of the labellum (median petal). In doing so, the insects pick up pollinaria, whose pollen content will be spread in subsequent flower visits. Insects are attracted by flower volatiles which may mimic female pheromones (Borg‐Karlson and Tengo, 1986), but ‘dummy‐like’ labellar structures also visually mimic female Hymenoptera. Visual (dots, calli, tubercles) and tactile (hairs) cues often reinforce the insect‐like flower appearance (Kullenberg, 1961). These latter features may help position the insect correctly for the fixation of pollinaria. Whereas pseudocopulation has frequently been documented in European–African Ophrys species and Australian orchids (Kullenberg, 1961; Stoutamire, 1974, 1975; Dafni and Bernhardt, 1990), the situation is very different for neotropical Orchidaceae. Pseudocopulation has been mentioned for a few epidendroid epiphytic South American orchid genera such as Trichoceros, Telipogon (both Ornithocephalinae) and Trigonidium (Maxillariinae) (Van der Pijl and Dodson, 1966). More recently, pseudocopulation has been reported for Tolumnia henekenii (Oncidiinae, referred to as Oncidium henekenii) (Dod, 1976). Nevertheless, these reports are sketchy and somewhat confusing, lacking the wealth of detail and evidence of the above‐mentioned studies (Kullenberg, 1961; Stoutamire, 1974, 1975, 1981). For instance, there is not a single published photograph or drawing that demonstrates that the putative pollinators of Telipogon, Trichoceros and Trigonidium species really pick up the pollinaria of the orchids they visit; this is a minimum requirement for considering them as pollinators.
Trigonidium obtusum Lindl was considered pseudocopulatory by Van der Pijl and Dodson (1966), based on an earlier report by Kerr and Lopez (1962). However, these latter authors mentioned the attraction of male Plebeia droryana (Meliponinae) (as Trigona droryana) to flowers of Trigonidium obtusum, without documenting removal or deposition of pollinaria (Kerr and Lopez, 1962). Yet Plebeia males were considered very small to perform pollination (Kerr and Lopez, 1962, p. 339). Van der Pijl and Dodson (1966) reproduced a drawing by Professor Sakagami, showing a few bees apparently scratching the distal part of the sepals, a strange behaviour for pseucocopulatory pollination since the gynostemium remains hidden in a funnel‐like cavity made up of the sepals and petals (see below for details). The lack of any ‘dummy‐like’ structure in Trigonidium flowers, together with the fact that the previous reports were poorly documented (Van der Pijl and Dodson, 1966), led later authors to question the pseudocopulatory nature of Trigonidium flowers (Singer and Cocucci, 1999; Van der Cingel, 2001).
Recently, we had the opportunity to document, in detail, the pollination process in Trigonidium obtusum in south‐eastern Brazil. Our observations indicated that these flowers are, indeed, pseudocopulatory. However, the pollination process, as a whole, is remarkably different from that of previously published reports (Kullenberg, 1961; Stoutamire, 1974, 1975, 1981). The aim of this contribution is two‐fold: (1) to confirm the pseudocopulatory nature of T. obtusum; and (2) to present the first well‐documented report on pseudocopulation in neotropical Orchidaceae.
MATERIALS AND METHODS
Observations were conducted on the campus and in the glasshouses of Campinas State University (Unicamp, São Paulo State, south‐eastern Brazil; approx. 22°44′45″S and 47°06′33″W, altitude approx. 670 m). Five vigorous plants of Trigonidium obtusum Lindl. have been in cultivation at Unicamp since early 1999. These plants were collected on the Ilha do Cardoso (south‐eastern Brazil) (voucher: G. Machado s. n. 1999, UEC). Observations at the campus were planned after we detected several Plebeia droryana (Friese 1900) workers and drones [the putative pollinators of T. obtusum according to Van der Pijl and Dodson (1966)] visiting nearby vegetation (especially Baccharis–Compositae–shrubs). In 2000, the flowering plants cultivated at Unicamp were kept outside the glasshouse and suspended in a few trees nearby. Observations were made between 15 Nov. and 21 Dec. Additional observations were made between 1 Feb. and 12 Mar. 2001. The observation period, as a whole, was from 0800 h to 1600 h, totalling about 30 observation hours. During the whole observation period, 25 flowers were produced. Pollinator behaviour at the flowers was recorded through field notes and photographs. A few insects were captured, pinned and vouchers deposited at the Laboratorio de Abelhas, São Paulo State University.
Morphological features of fresh flowers were recorded using a binocular stereomicroscope with a camera lucida attachment. Since flowers are produced in small numbers and each flower takes a considerable time to develop, the breeding system was studied gradually. Thus, priority was given to detecting whether plants are pollinator‐dependent (i.e. if pollination is needed for the flowers to set fruits) and self‐compatible. In 1999, all the flowers produced were left untouched, and were thus considered as controls for the presence/absence of automatic self‐pollination. Plants were subsequently kept in a glasshouse where insects had no access to them. In 2000, plants in the Unicamp glasshouses were used for pollination observations (see above). In addition, 18 fresh, intact flowers at the living orchid collection of the Instituto de Botânica de São Paulo (IBt) were manually self‐pollinated and tagged. Potential pollinators were not excluded from these flowers, but the shape and size of both the stigmatic cavity and the pollinarium (see below for details) prevent further pollinations occurring in flowers that have already been pollinated. If the whole pollinarium is left in the stigmatic cavity (as it was during hand pollinations), the stigmatic cavity is completely filled and further deposition of pollinaria is prevented.
Throughout this paper, the taxonomic and morphological concepts of Dressler (1993) are followed.
RESULTS AND DISCUSSION
Plant features
Trigonidium obtusum is an epiphyte, often forming large clumps (presumably large clones). The plants branch sympodially, producing large, flattened, bifoliate pseudobulbs and lateral, solitary flowers on long pedicels (13–20 cm), numbering one to six per pseudobulb. Flowers are produced gradually, each pseudobulb having only one fully developed flower at a time. The flowers are upright, erect and funnel‐like in shape (Fig. 1A and B), measuring about 40 mm in length. Readers interested in details of the size and shape of perianth parts are referred to Hoehne (1953). The lip is articulated at the base of the column (Fig. 1C). The column is erect and the anther is incumbent (Fig. 1D). The anther holds a pollinarium made up of four flattened, slightly clavate, entire pollinia, hyaline caudicles and a saddle‐like, semilunar viscidium (Fig. 1E and F). The anther cap is remarkable since it shows an indument of hyaline short hairs (Fig. 1G and H). The stigmatic surface is markedly concave (Fig. 1D). All these aforementioned features are common in subtribe Maxillariinae (Dressler, 1993). In spite of the characteristic ‘funnel‐like’ flower shape, floral and vegetative features are essentially the same as those of several Brazilian Maxillaria species. After examining several Brazilian bifoliate Maxillaria species cultivated either at Unicamp or at IBt, we were not able to find a set of morphological characters (other than flower shape) clearly separating these Maxillaria species from the bifoliate, large‐flowered species of Trigonidium (such as T. obtusum and T. egertonianum). Recently obtained molecular evidence (M. Whitten, pers. comm.) supports this observation. Thus, from a phylogenetic point of view, Trigonidium may be ‘nested’ within a broadly defined Maxillaria. Dressler (1993) pointed out that generic circumscriptions are unconvincing in subtribe Maxillariinae as a whole.
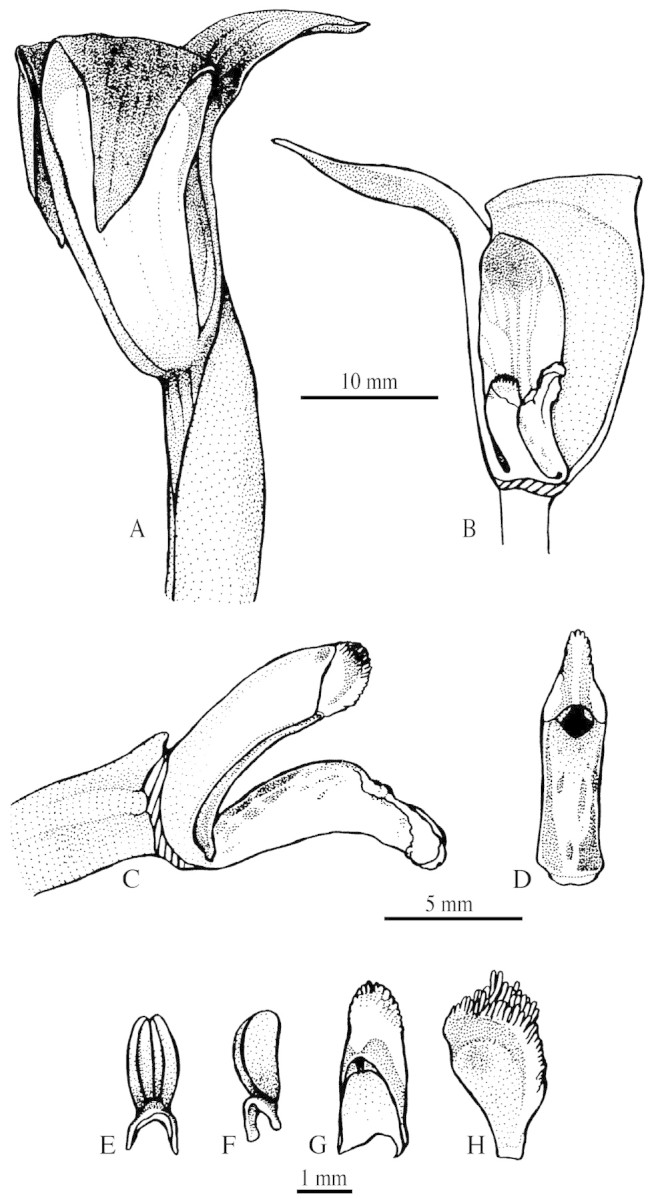
Fig. 1. Floral features of Trigonidium obtusum Lindl. A, Flower in lateral view. B, Longitudinal section through flower showing the column and lip at the bottom of the flower. C, Column in lateral view with lip hinged at its base. D, Column in frontal view. E and F, Dorsal (E) and lateral (F) views of a pollinarium. G and H, Ventral (G) and lateral (H) views of an anther cap. stg, Stigmatic surface.
During the hottest hours of the day (1100–1500 h), the flowers emit a sweet, pleasant fragrance, vaguely reminiscent of lemon. Preliminary analysis of flower volatiles (M. Gomes‐Reis and A. Marsaioli, pers. comm.) have shown that pentadecane is the main component of the fragrance. Pentadecane is also an important component of the floral fragrance in some Maxillaria species, such as M. nigrescens (Kaiser, 1993).
Cultivated flowers withered 4 d after opening, although Kerr and Lopez (1962) reported floral life‐spans of up to 10 d. Such differences may reflect different cultivation conditions. Flower development takes 10‐plus days, but flowers are produced from September to late April. The fruit is a capsule bearing numerous dust‐like fusiform seeds.
Pollination mechanism and preliminary data on the breeding system in Trigonidium obtusum
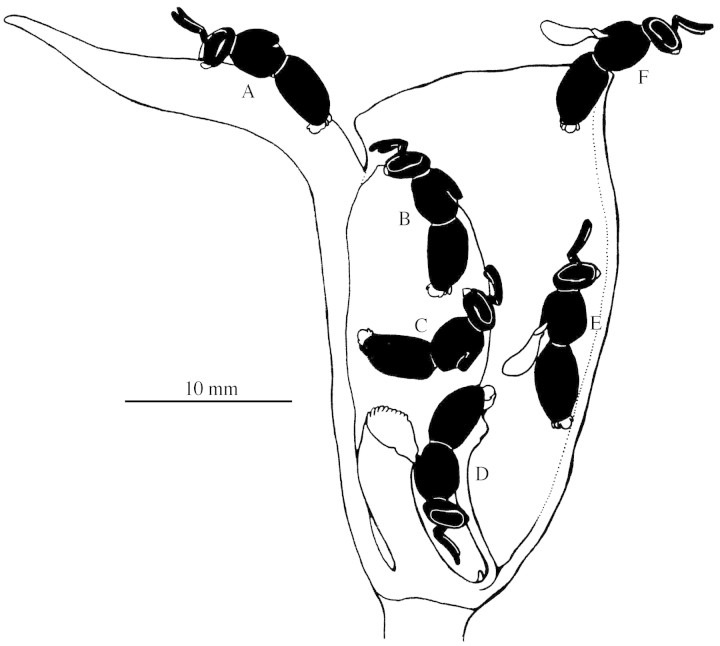
The only observed pollinators are males of the bee Plebeia droryana (Apidae: Meliponinae). Male bees show a patrolling behaviour between 1000 and 1130 h. During this period no flower visits were observed, but several drones were seen perching in nearby vegetation. All these bees show clearly extruded genitalia, strongly suggesting pre‐mating behaviour. Flower visitations begin around 1130 h and continue until 1500 h. Bees approach the flowers and hover over them for a few seconds (Fig. 2A), then land on either the sepals or petals and erratically attempt copulation (Figs 2B, C and 3A, B). Two different ‘strategies’ of flower behaviour were detected: in one flower type, bees are attracted exclusively by the petals (Figs 2C and 3B) while in the other type bees are attracted by the sepals (Figs 2B and 3A). Each flower type is fixed, each plant producing only one kind of flower throughout the whole flowering period. The whole perianth surface is waxy (Fig. 2A–E) and in both flower types the bees slip down and become trapped in the funnel‐like flower cavity (Fig. 3C). The two flower types are morphologically identical, differing only in the way they attract pollinators. In the flower type with attractive petals, the insects are trapped almost immediately. In flowers with attractive sepals, since these perianth parts are inflexed, bees may spend several seconds (15 or more) on the flower sepals before falling into the flower cavity. In both flower types, the insects are retained in the flower cavity for 5–30 s. Pseudocopulatory behaviour is best perceived in the flower type with attractive sepals (Fig. 2B). The coexistence of the two flower ‘morphs’ can be explained from an ecological point of view: the two ‘morphs’ may be part of the attraction strategy. Since pollination relies on bee deception, the coexistence of the two flower types would hinder the process of bee learning and recognition, ultimately increasing the chances of cross‐pollination. An analogous strategy was reported for European Ophrys sphegodess populations (Ayasseet al., 2000): variations in fragrance composition occur either in flowers of the same or different inflorescences. This strategy takes advantage of the learning behaviour of bees to promote cross‐pollination, since bees may avoid visiting the same flower but not another conspecific one (Ayasseet al., 2000). We suggest that in T. obtusum the coexistence of two flower types, each one with different attractive parts, has a similar function in promoting cross‐pollination, but further research on volatile composition (and the probable variations) is needed.

Fig. 2. The flower and its pollinators. A, Bee arriving at a flower. B, Bees attempting to copulate with the lateral sepals. C, Bee attempting copulation with petal (one lateral sepal has been removed). D, Bee attempting to copulate with a previously landed drone. E, Bee leaving a flower with a pollinarium attached to its scutellum. F, Detail of bee with a pollinarium on its scutellum.
Fig. 3. Diagram of the interactions between the flower and its bee pollinator. A and B, Bees attempting copulation with sepals (A) or petals (B). C, Bee falling into the flower tube. D, Trying to escape, the bee accesses the space between the lip and column, fixing the pollinarium on its scutellum. E, The bee leaves the lip, removing the pollinarium. F, The bee eventually leaves the flower.
The sexual excitement of the bees was apparent through their extruded genitalia and their repeated attempts to copulate, evidenced through spasmodic abdominal movements. In both kind of flowers (Fig. 2B and D), the simultaneous presence of several male bees (one–five) was noticed. Quite often, in the flower type with attractive sepals, arriving males tried to copulate with drones which had already landed (Fig. 2D). This behaviour has been reported previously by Kerr and Lopez (1962). In our opinion, such behaviour supports the pseudocopulatory nature of T. obtusum flowers and may be triggered by the absence of ‘female‐like’ flower parts.
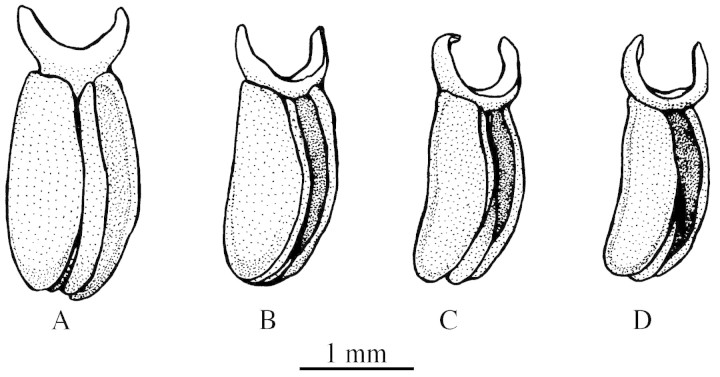
The trapped bees try to escape from the flowers by climbing the sepals, but escape is made even more difficult by the slippery waxy sepal surface. As an alternative way to escape from the flower, bees enter the cavity between the column and the lip (Fig. 3D). The lip and the bottom of the flower are light yellow or cream‐coloured and the bees may perceive them as a way out. The lip is articulated at the base of the column and bends down when the bee goes between the lip and the column, recovering its position after the insect has fully entered the space in between. When leaving the lip, the bee presses its scutellum against the anther, fixing the pollinarium. The semilunar viscidium embraces the scutellum and the whole anther (including the anther cap) is removed (Fig. 3E). The anther cap falls immediately, exposing the pollinarium (Figs 2E and 3E). The process of removing pollinaria is essentially the same as that in Maxillaria species (Singer and Cocucci, 1999). During our observations, we noticed that bees with recently fixed pollinaria sometimes re‐visited the same flower a second time. This behaviour, however, does not promote self‐pollination. At the time of pollinarium removal, the pollinia are broader than the stigmatic cavity. Dehydration is necessary before the pollinia can be deposited (Fig. 4). Observations with the help of a dissecting binocular stereomicroscope indicated that fresh pollinia reduce to an adequate size to allow pollination 40 min after removal (Fig. 4). This mechanism clearly inhibits self‐pollination and promotes cross‐pollination. Analogous mechanisms have been reported to occur in many (Euglossine‐pollinated) Stanhopeinae orchids (Van der Pijl and Dodson, 1966) and in at least one Pleurothallis species (Singer and Cocucci, 1999), and are particularly important when the pollinator may return to previously visited flowers.
Fig. 4. Dehydration of a pollinarium. A pollinarium which has just been removed (A), after 15 min (B), 30 min (C) and 40 min (D).
Pollination occurs when a pollinarium‐carrying bee is trapped in a flower and repeats the steps described above. When the bee leaves the lip, and the pollinia are sufficiently dehydrated, they are caught in the concave stigmatic cavity, thus effecting pollination.
Unpollinated flowers wither after 4 d of blooming, but are attractive to pollinators only during the first 3 d. Kerr and Lopez (1962) reported longer flower lifespans, but asserted that the flowers were attractive for only 3 d.
The pollination process in T. obtusum is unique among orchid pseudocopulatory mechanisms since sexual attraction of Plebeia males alone does not result in pollination. Bees are trapped in the funnel‐like flower tube and need to enter the space between the lip and column in order to pick up pollinaria and deposit them on subsequent flower visits. In the Ophrys species and most Australian pseudocopulatory orchids, correct positioning of Hymenoptera males on the lip (mostly interacting with ‘dummy‐like’ structures) may be sufficient for the insects to pick up pollinaria or effect pollination (Kullenberg, 1961; Stoutamire, 1974, 1975; Peakall, 1990). In Australasian Pterostylis species, irritable, motile lips have been reported to press the male dipteran pollinators against the column, resulting in pollinarium fixation (Dafni and Bernhardt, 1990). However, the pseudocopulatory nature of Pterostylis species has been questioned (Adams and Lawson, 1993).
In 1999, none of the 41 untouched flowers in the glasshouse set fruits. In 2000, 94·4 % (17/18) of the manually self‐pollinated flowers set fruits. Although these data are preliminary, they clearly suggest that plants are self‐compatible but pollinator‐dependent (unable to set fruits in the absence of pollinators). No fruits were recorded during observations at Unicamp, yet fruits are commonly produced at IBt. At IBt many plants are cultivated outdoors and Plebeia visits could also be confirmed (see below).
Although pentadecane is the main component of the fragrance, three bioassays with strips of filter paper soaked with pure, liquid pentadecane, failed to attract any Plebeia bees. These bioassays were performed in mid‐March 2000, from 1100 to 1400 h, at Unicamp. On this occasion, several Plebeia workers and males were observed in the nearby vegetation. This suggests that further and more refined volatile analysis is needed to identify the flower volatiles necessary for pollinator attraction.
Evolutionary considerations
Since anatomical, morphological and molecular evidence suggests a close relationship between Trigonidium and several Maxillaria species (Holtzmeieret al., 1998; M. Whitten, pers. comm.), a brief discussion of pollination data on Maxillaria seems appropriate. Most reports concerning Maxillaria pollination were reviewed by Van der Pijl and Dodson (1966). These reports involve bees of the subtribes Bombini, Euglossini and Meliponini. A few Maxillaria species show floral cues suggesting hummingbird pollination (Van der Pijl and Dodson, 1966). More recent reports (Braga, 1977; Singer and Cocucci, 1999) have demonstrated that a few Brazilian species are pollinated by wasps and Trigona (Meliponinae) bees. Several Ecuadorian Maxillaria species of section Grandiflora offer pads of ‘pseudopollen’ to their bee pollinators (Davieset al., 2000). ‘Pseudopollen’ is a pollen‐like, farinaceous substance offered in cushions or pads on the surface of the lip. It consists of detachable moniliform hairs containing protein and starch (and, to a lesser degree, lipids) (Davieset al., 2000). Pollinators of these Maxillaria are supposed to collect this pseudopollen, but published reports are somewhat preliminary (Van der Pijl and Dodson, 1966).
Several Brazilian Maxillaria species (e.g. M. picta) do not offer any reward to pollinators (Singer and Cocucci, 1999). These flowers are pollinated by deceived Meliponini workers of the genera Trigona and Parthamona. These flowers are extremely fragrant and show long (approx. 12–15 d) life spans. That bee pollinators visit the flowers only for a few days may suggest learned avoidance (Singer and Cocucci, 1999).
A few Brazilian Maxillaria species, such as M. brasiliensis and M. cerifera, offer pads of wax on their labella. Once again, these flowers are pollinated by Meliponinae worker bees which store the wax on their corbiculae (R. B. Singer, unpubl. res.). This material may be used in nest building. It is noteworthy that according to the studies of Illg (1977), M. brasiliensis may be apomictic, although pollinator‐dependent; i.e. apomixis is triggered by pollen‐tube development.
Some small‐flowered Maxillaria species (species once placed in the genus Ornithidium or Pseudomaxillaria) offer small nectar droplets on their lip surface. Preliminary evidence suggests that a few Brazilian species (e.g. M. parviflora) may be pollinated by nectar‐seeking Plebeia workers (R. B. Singer, unpubl. res.) but further observations are needed.
Interestingly, flower and vegetative features suggest that Trigonidium could be closely related to the bifoliate, ‘food‐fraud’ Maxillaria species. If this relationship were confirmed, a shift between deceptive (food‐fraud) and pseudocopulatory species may become apparent. A similar shift was verified by Stoutamire (1983) for several Australian Caladenia (Orchidoideae) species: whereas some Australian Caladenia species are ‘deceptive’ (food advertising) flowers, other species are pseudocopulatory. Yet, hybrids between species of both flower kinds have been recorded (Stoutamire, 1983). ‘Deceptive’ (reward lacking) orchids are, in my opinion, well suited as putative ancestors of pseudocopulatory species. Deceptive orchids show a set of characters which may provide a suitable preadaptive context, such as showy, large floral displays (either big flowers or a large number of flowers), strong fragrance and often long‐lived flowers.
Another suggestive case is that of pollination of the japanese Cymbidium pumilum (Epidendroideae: Cimbidiinae). This reward‐lacking orchid is pollinated either by workers or drones of Apis cerana japonica which fix pollinaria on the scutellum (Sasakiet al., 1991). It is possible that flower volatiles mimic a pheromone of swarm aggregation, since bees (either workers or drones) tend to aggregate on the inflorescences (Sasakiet al., 1991).
Among orchids of subtribe Maxillariinae (as circumscribed in Dressler, 1993), pseudocopulation has been suggested to occur in three genera: Trigonidium, Mormolyca and Cyrtidiorchis (as Cyrtidium) (Van der Pijl and Dodson, 1966). For the two latter genera, such an affirmation is, as yet, purely speculative. More research on the pollination mechanism is needed to confirm whether flowers of the whole Trigonidium genus are pollinated by pseudocopulation. Remarkably, plants of T. obtusum cultivated at the Instituto de Botânica de São Paulo are, as here reported, often visited and (eventually) pollinated by Plebeia males. However, at the same time, several cultivated plants of the smaller and unifoliate T. acuminatum were also in bloom. These flowers were never visited by local Plebeia bees and did not set fruits. This suggests that a different pollinator may be involved. Although Trigonidium acuminatum is distributed from Rio de Janeiro State to Venezuela, it often occurs sympatrically with T. obtusum.
Finally, it is worth noting that T. obtusum is pollinated by eusocial bees. All previous reports of orchid pollination through pseudocopulation involve solitary wasps or bees (Kullenberg, 1961; Stoutamire, 1974, 1975, 1981; Peakall, 1990). In all of these, newly emerged males temporally precede the females by about a month, so the flowers exploit the ‘naivety’ of the males who focus their attention on the flowers until the females emerge (Kullenberg, 1961). Consequently, pollination and fruit set are limited to a brief period of time preceding female emergence. The pollination biology of T. obtusum is also remarkable in that flowers are produced over several months. This does not seem compatible with the pseudocopulatory syndrome since deceived males would quickly learn to recognize those flowers. The pollination system in T. obtusum is tied to its pollinator biology. Eusocial bees (such as Plebeia) live in hives containing many individuals (Kerr and Lopez, 1962). A Plebeia hive may contain about 2000–3000 individuals (I. Alves dos Santos, pers. comm.). Fertile individuals (drones and queens) are produced not once, but several times during the year. In the temperate to tropical Brazilian climate, colonies may be active all year round. Thus, males are normally produced in great numbers, from unfertilized (therefore haploid) eggs. In other words, ‘naive’ males and virgin females will be present several times during the year. This fact may have made the flowering and pollination strategy of Trigonidium obtusum possible.
CONCLUSIONS
Pollination through pseudocopulation is demonstrated here for T. obtusum. Observations in the field are required to assess: (1) pollinator behaviour in natural populations; (2) the natural proportions of the two flower types; and (3) fruiting success under natural conditions. Additional information is required on the breeding system and volatile composition. Several disciplines such as morphology and molecular biology are increasing the understanding of phylogenetic relationships among the Maxillariinae. Generic rearrangements are to be expected in the near future. It is hoped that multidisciplinary work will help us understand not only the phylogenetic affinities of Trigonidium, but also how pseudocopulation evolved in subtribe Maxillariinae.
ACKNOWLEDGEMENTS
I thank Fabio de Barros and Fabio Pinheiro (Instituto de Botânica, São Paulo) for use of cultivated Trigonidium plants and assistance in many ways. Mariza Reis‐Gomes, A. Marsaioli (both Instituto de Química, Unicamp) and Mark W. Whitten (University of Florida) kindly shared unpublished data. S. Koehler, V. Bittrich (both Unicamp, São Paulo State) and I. Alves dos Santos (Laboratório de Abelhas, USP) helped improve earlier drafts of this manuscript. The suggestions of Dr S. Corbet and Dr A. Lack considerably improved the manuscript. Alain François is thanked for English improvements.
Supplementary Material
Received: 28 August 2001; Returned for revision: 21 September 2001; Accepted: 17 October 2001.
References
- AdamsPB, Lawson SD.1993. Pollination in Australian orchids: A critical assessment of literature 1882–1992. Australian Journal of Botany 41: 553–575. [Google Scholar]
- AyasseM, Schiestl FP, Paulus HF, Lofstedt C, Hansson B, Ibarra F, Francke W.2000. Evolution of reproductive strategies in the sexually deceptive orchid Ophrys sphegodes: how does flower‐specific variation of odor signals influence reproductive success? Evolution 54: 1995–2006. [DOI] [PubMed] [Google Scholar]
- Borg‐KarlsonAK, Tengo J.1986. Odor mimetism? Journal of Chemical Ecology 12: 1927–1941. [DOI] [PubMed] [Google Scholar]
- BragaPIS.1977. Aspectos biológicos das Orchidaceae de uma campina da Amazônia Central. Acta Amazonica 2 (suppl.). [Google Scholar]
- DafniA, Bernhardt P.1990. Pollination of terrestrial orchids of Southern Australia and the Mediterranean region. Systematics, ecological and evolutionary implications. Evolutionary Biology 24: 193–253. [Google Scholar]
- DaviesKL, Winters C, Turner MP.2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- DodDD.1976. Oncidium henekenii Bee orchid pollinated by bee. American Orchid Society Bulletin: 792–795. [Google Scholar]
- DresslerRL.1993. Phylogeny and classification of the orchid family. Portland, Oregon: Dioscorides Press. [Google Scholar]
- HoehneFC.1953. Flora Brasilica. Fasc 10, Vol XII–VII. São Paulo: Instituto de Botânica. [Google Scholar]
- HoltzmeierMA, Stern WL, Judd WS.1998. Comparative anatomy and systematics of Senghas’s cushion species of Maxillaria (Orchidaceae). Botanical Journal of the Linnean Society 127: 43–82. [Google Scholar]
- IllgRD.1977. Sobre a reprodução em Maxillaria brasiliensis Brieg. et Illg e M cleistogama Brieg. et Illg (Orchidaceae). Revista Brasileira de Biologia 37: 267–279. [Google Scholar]
- KaiserR.1993. The scent of orchids. Olfactory and chemical investigations. The Netherlands: Roche Ed. [Google Scholar]
- KerrWE, Lopez CR.1962. Biologia da reprodução de Trigona (Plebeia) droryana F. Smith. Revista Brasileira de Biologia 22: 335–341. [Google Scholar]
- KullenbergB.1961. Studies in Ophrys pollination. Zoologiska Bidrag fran Uppsala 34–1: 1–340. [Google Scholar]
- PeakallR.1990. Responses of male Zaspilothynnus trilobatus Turner wasps to females and the sexually deceptive orchid it pollinates. Functional Ecology 4: 159–167. [Google Scholar]
- SasakiM, Ono M, Asada S, Yoshida T.1991. Oriental orchid (Cymbidium pumilum) attracts drones of the Japanese honeybee (Apis cerana japonica) as pollinators. Experientia 47: 1229–1231. [DOI] [PubMed] [Google Scholar]
- SingerRB, Cocucci AA.1999. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 14: 47–56. [Google Scholar]
- StoutamireWP.1974. Australian terrestrial orchids, thynnid wasps and pseudocopulation. American Orchid Society Bulletin: 13–19. [Google Scholar]
- StoutamireWP.1975. Pseudocopulation in Australian orchids. American Orchid Society Bulletin: 226–235. [Google Scholar]
- StoutamireWP.1981. Pollination studies in Australian terrestrial orchids. National Geographic Society Research Report 13: 591–598. [Google Scholar]
- StoutamireWP.1983. Wasp‐pollinated species of Caladenia (Orchidaceae) in South‐western Australia. Australian Journal of Botany 31: 383–394. [Google Scholar]
- Van der CingelNA.2001. An atlas of orchid pollination. America, Africa, Asia and Australia. Rotherdam: Balkema Publishers. [Google Scholar]
- Van der PijlL, Dodson CH.1966. Orchid flowers. Their pollination and evolution. Coral Gables, Florida: University of Miami Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.