Abstract
Young trees 0·03–1·7 m high of three coexisting Betula species were investigated in four sites of varying soil fertility, but all in full daylight, to separate nutrient and plant size controls on leaf dry mass per unit area (MA), light‐saturated foliar photosynthetic electron transport rate (J) and the fraction of plant biomass in foliage (FL). Because the site effect was generally non‐significant in the analyses of variance with foliar nitrogen content per unit dry mass (NM) as a covariate, NM was used as an explaining variable of leaf structural and physiological characteristics. Average leaf area (S) and dry mass per leaf scaled positively with NM and total tree height (H) in all species. Leaf dry mass per unit area also increased with increasing H, but decreased with increasing NM, whereas the effects were species‐specific. Increases in plant size led to a lower and increases in NM to a greater FL and total plant foliar area per unit plant biomass (LAR). Thus, the self‐shading probably increased with increasing NM and decreased with increasing H. Nevertheless, the whole‐plant average MA, as well as MA values of topmost fully exposed leaves, correlated with NM and H in a similar manner, indicating that scaling of MA with NM and H did not necessarily result from the modified degree of within‐plant shading. The rate of photosynthetic electron transport per unit dry mass (JM) scaled positively with NM, but decreased with increasing H and MA. Thus, increases in MA with tree height and decreasing nitrogen content not only resulted in a lower plant foliar area (LAR = FL/MA), but also led to lower physiological activity of unit foliar biomass. The leaf parameters (JM, NM and MA) varied threefold, but the whole‐plant characteristic FL varied 20‐fold and LAR 30‐fold, indicating that the biomass allocation was more plastically adjusted to different plant internal nitrogen contents and to tree height than the foliar variables. Our results demonstrate that: (1) tree height and NM may independently control foliar structure and physiology, and have an even greater impact on biomass allocation; and (2) the modified within‐plant light availabilities alone do not explain the observed patterns. Although there were interspecific differences with respect to the statistical significance of the relationships, all species generally fit common regressions. However, these differences were consistent, and suggested that more competitive species with inherently larger growth rates also more plastically respond to N and H.
Key words: Betula nana, Betula pendula, Betula pubescens, construction cost, leaf density, leaf dry mass per unit area, leaf size, nitrogen content, photosynthetic electron transport rate, plasticity, tree height
INTRODUCTION
Leaf dry mass per unit area (MA) and the fractional investment of the biomass in foliage (FL) are important plant parameters which are strongly linked to plant growth rates (Evans 1972; Poorter and Remkes, 1990; Körner, 1991; Cornelissenet al., 1996, 1998). The total leaf area per unit plant biomass (LAR) is given by FL/MA, and, thus, both a large FL and a small MA allow formation of an extensive foliar area, thereby enhancing plant potential for light interception and photosynthesis. Therefore, plant growth rates often vary directly with LAR (Ingestad and McDonald, 1989; Poorter 1990; Cornelissenet al., 1996, 1998; Wanget al., 1998).
Along gradients of nutrient availability, the biomass requirements for light harvesting conflict with those of nutrient uptake and, accordingly, the significance of a large foliar area is greater at high nutrient availabilities and less at low nutrient availabilities. To cope with a broad variability in soil conditions, plants often possess a large capacity for plastic modification of biomass allocation and total leaf area. Increased nitrogen supply invariably leads to a larger LAR (Karlsson and Nordell, 1987; Küpperset al., 1988; Thompsonet al., 1988; Ingestad and McDonald, 1989; Bowler and Press, 1996; Wanget al., 1998). Yet, it is still unclear to what extent these changes result from allocation (FL) and from alterations in leaf structure (MA). There are consistent positive relationships between FL and nutrient availability (Ingestad and Lund, 1979; Karlsson and Nordell, 1987; Ingestad and McDonald, 1989; Wanget al., 1998; Poorter and Nagel, 2000), but the evidence of nutrient availability effects on MA is contrasting. Strong interspecific negative correlations between MA and foliage nitrogen content per unit dry mass (Turner, 1994; Reichet al., 1995; Niinemets, 1999) are often found, but the relationships are weaker in other studies (Reich and Walters, 1994). In addition, increases in nitrogen availability have resulted in a lower whole‐plant average MA (Juriket al., 1982; Waringet al., 1985; Karlsson and Nordell, 1987; Hirose, 1988; Thompsonet al., 1988; Latham, 1992; Bowler and Press, 1996; Grubbet al., 1996; Wanget al., 1998; Meziane and Shipley, 1999) but, in some cases, MA is invariable (Karlsson and Nordell, 1989; Kolbet al., 1990; Fredeenet al., 1991; Latham, 1992; Ackerly and Bazzaz, 1995; Grubbet al., 1996; Simset al., 1998b; Rosatiet al., 2000) or even increases (Latham 1992; Simset al., 1998a) with increasing nitrogen availability.
Apart from the possible nutrient effects, MA scales positively with light availability (Latham, 1992; Ackerly and Bazzaz, 1995; Niinemets, 1998; Niinemets and Kull, 1998; Rosatiet al., 2000). Given that the increase in foliar area as the result of improved nutrition inevitably leads to a greater within‐plant shading (Ackerly and Bazzaz, 1995), a decrease in MA in plants at higher nutrient availability may be a consequence of lower quantum flux densities within these plants. In addition, MA is also positively related to plant size (Steeleet al., 1989; Niinemets, 1997a; Niinemetset al., 1999). Because the plants at higher nitrogen availability are generally larger, plant size effects on MA may level off or over‐rule the influences of enhanced shading, providing an explanation of insignificant or positive effects of improved N nutrition on MA.
We studied biomass allocation and leaf morphology in young trees in four sites of contrasting fertility to: (1) determine the extent to which leaf nitrogen availability controls MA, foliar photosynthetic characteristics and the fraction of plant biomass in foliage (FL); and (2) understand whether these effects can be ascribed to modifications in intra‐plant shading and/or differences in plant size. We also analysed (3) foliar construction cost in terms of unit glucose investment for a unit foliar biomass production (Vertregt and Penning de Vries, 1987; Poorter, 1994) to determine whether the carbon cost of leaves varies as a function of foliar nitrogen. We expected that in less fertile habitats, where carbon availability limits growth to a lesser extent than in more fertile patches, the content of carbon‐rich protective chemicals such as lignin and condensed phenolics increases (Waringet al., 1985; Chapin, 1989; Chapinet al., 1990) thereby leading to a greater foliar glucose cost (e.g. Niinemets, 1997b).
Three species of Betula were used for the analyses. Betula nana L. is a very shade‐intolerant shrub species (height 0·4–1·2 m) with characteristic polycormic growth and with leaves 0·4–2 cm2 in area on short, 0·5–1 mm long petioles, such that the leaves are seemingly directly attached to the stem. B. nana is widespread in tundra heaths (for further details, see Jonasson, 1981; de Grootet al., 1997), and as a relic of the last glaciation is restricted to raised bogs in Estonia (Dierssen, 1977). B. pubescens Ehrh. and B. pendula Roth. are closely related shade‐intolerant tree species with leaves 5–20 cm2 in area typically on 0·4–2 cm long petioles. Both species are pioneering trees, but B. pendula colonizes drier habitats than B. pubescens, which often dominates on wet soils (Atkinson, 1992). In Estonia, the maximum height of B. pendula is 35 m and that of B. pubescens 25 m (Masing, 1990). Because all the studied species coexisted in the most nutrient‐limited wet habitat, but the competitive potential of B. nana was apparently lower at greater nutrient availabilities, we suggested that foliage characteristics adjust less plastically to improved nutrient availability in this than in the other species. The hypothesis that species responsiveness to nutrient availability depends on species growth potentials was posed by Coomes and Grubb (2000), but conclusive data to test the hypothesis are unavailable. Given that species success in nutrient‐limited open environments is mainly driven by root characteristics, foliar variables of coexisting species may largely differ in these habitats. In contrast, in nutrient‐rich systems, there is strong evidence of a functional convergence of foliage structural and physiological characteristics of coexisting species (Niinemets and Kull, 1994; Ackerly and Reich, 1999).
We used foliar nitrogen content per unit dry mass (NM) as an estimate of internal plant nutrient availability. Plant nutrient contents depend not only on soil nutrient availability, but also on the efficiency and rate of nutrient uptake, and on nutrient partitioning within plant, as well as on the growth rates of the plant (Chapinet al., 1986; Hilbert, 1990; Oren and Sheriff, 1995). Thus, internal N concentrations control nutrient‐related modifications in foliage structure and fractional biomass allocation, rather than soil‐available N concentrations (Hirose, 1988; Levinet al., 1989; Hilbert, 1990; Tan and Hogan, 1998). Total tree height (H) was employed as a second independent variable to account for plant size related allocation and morphology differences.
MATERIALS AND METHODS
Study sites
Betula nana L. (16 plants with average ± s.e. total height, H, of 0·39 ± 0·05 m), B. pubescens Ehrh. (43 trees, H = 0·70 ± 0·08 m) and B. pendula Roth. (six trees, H = 0·58 ± 0·15 m) were studied at a nutrient‐poor site on Sphagnum peat in Männikjärve raised bog, Endla State Nature Reserve, Estonia (58°52′N, 26°15′E). The peat layers were extensive—up to 8 m in the centre of the bog, and in general more than 4 m at the margins (Veber, 1974). The sparse tree layer (200 trees/ha) with an average height of approx. 1–2 m and an average age of 50–100 years was dominated by Pinus sylvestris L. and B. pubescens. Excavations of the entire root systems at the site demonstrated that the roots mostly occupied the upper peat horizons of 5–15 cm, and did not penetrate the dense peat layers. The average characteristics of the upper peat layers are reported in Table 1. Fertilizer experiments in temperate raised bogs have demonstrated that plant growth in these habitats is strongly curtailed by low soil nutrient availability. The data demonstrate that nitrogen and phosphorus are in especially short supply (Paavilainen, 1980; Damman, 1986; Finér, 1992).
Table 1.
Site average (± s.d.)* chemical characteristics of the upper soil horizon and average foliar chemical data
| Soil | Foliage | ||||||||||
| Site | pH (H2O) | pH (KCl) | Soluble P (mg/g) | Total P (mg/g) | Total N (%) | C/N molar ratio | n | N (%) | P (%) | P/N molar ratio | n |
| Endla | 3·28 ± 0·10a | 2·64 ± 0·27a | 0·088 ± 0·022a | 0·57 ± 0·31a | 1·36 ± 0·32a | 42 ± 9a | 13 | 1·65 ± 0·32c | 0·143 ± 0·005b | 0·039 ± 0·006a | 62 |
| Viimsi‐1 | 3·35 ± 0·18a | 2·56 ± 0·24a | 0·067 ± 0·030a | 0·78 ± 0·27a | 1·46 ± 0·54a | 39 ± 11a | 8 | 3·17 ± 0·21a | 0·166 ± 0·015b | 0·025 ± 0·002b | 11 |
| Viimsi‐2 | 3·15 ± 0·13a | 2·42 ± 0·09a | 0·072 ± 0·018a | 0·83 ± 0·15a | 1·96 ± 0·21a | 29·2 ± 3·3ab | 3 | 3·07 ± 0·18a | 0·30 ± 0·11a | 0·045 ± 0·019ab | 8 |
| Voore† | n.d. | 3·60 ± 0·28b | n.d. | 0·98 ± 0·15a | 0·110 ± 0·015b | 13·8 ± 3·5b | 2 | 2·25 ± 0·46b | 0·26 ± 0·08a | 0·055 ± 0·029ab | 10 |
* Means followed by the same letter are not significantly different (P > 0·05 according to one‐way ANOVA followed by Bonferroni test).
† Soil chemical data are from Reintam (1970). Foliage chemical data were averages of the current study (B. pendula) and those of Kõlli and Kährik (1970) [Corylus avellana L., Fraxinus excelsior L., Picea abies (L.) Karst., Rhamnus cathartica L., and Rubus idaeus L.].
Betula pubescens was also investigated at two adjacent fell sites in the Viimsi peninsula. Both sites were previously dominated by Picea abies (L.) Karst. and were on acidic, sandy, podsolic soil with a raw humus horizon (Table 1), with the mineral soil layers starting at approx. 20 cm depth. The first fell site (Viimsi‐1, 59°32′N, 24°52′E) was 2 years old, and first and second year seedlings (24 trees, H = 0·134 ± 0·023 m) were investigated at this site. The second clearing (Viimsi‐2, 59°33′N, 24°51′E) was 5 years old, and 2–4‐year‐old saplings (20 trees, H = 0·67 ± 0·07 m) were sampled. In both the bog and the fell sites, the light availability at the top of the sampled plants was always more than 90 % of that in a completely open location.
We also included another set of trees of B. pendula (five trees, H = 1·10 ± 0·21 m) sampled in Voore, Estonia (58°44′N, 26°45′E; Kull and Niinemets, 1993). The 30–35 m tall, and approx. 100 year‐old forest was dominated by P. abies, and had a vigorous deciduous shrub understory. The soil was a brown pseudopodzol with a mull type A horizon (Table 1). A thorough description of this study site is provided by Frey (1977). The light conditions above each sapling were assessed by hemispherical photography (Kull and Niinemets, 1993). The trees included in the current study grew in recent clearings or at the edges of the forest, and received at least 85 % of above‐canopy irradiance.
Overall, the trees of different species that were sampled in various sites were of similar size. According to an ANOVA (analysis of variance), mean tree height was lower than in other cases for the first and second year seedlings of B. pubescens sampled in Viimsi‐1 (P < 0·001), and was not statistically different between other site–species pairs (P > 0·05).
Plant and foliar sampling: foliage morphological parameters
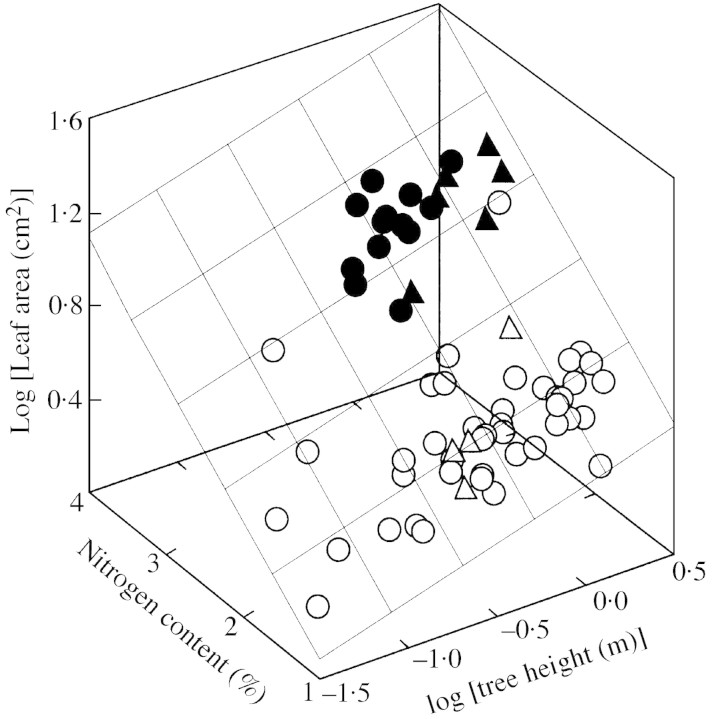
Plant sampling was conducted in July 1999 at Endla, September 1999 at Viimsi and August 1989 in Voore. There were no signs of foliar senescence during plant sampling. In Endla and Viimsi, the trees were excavated carefully to yield a reliable estimate of both below‐ and above‐ground plant biomass. All leaves were harvested, and ten leaves from each plant were randomly taken for foliar area measurements. The remaining leaves, stem, coarse (diameter >1 mm) and fine roots were dried at 75 °C for at least 48 h, and weighed. The sample leaves were scanned by a Hewlett–Packard scanner at a resolution of 1200 d.p.i., converted to 1‐bit bitmap, and the area of the sample leaves was measured by self‐developed computer software. The sample leaves were also dried and weighed, and the leaf dry mass per unit area (MA) was calculated. Total foliar area per plant was determined as the ratio of total leaf dry mass to MA. Leaf area ratio (LAR, cm2 g–1) was further computed as the ratio of total plant foliar area to total plant dry mass.
Given that the foliar characteristics of the plants excavated in Endla and Viimsi were averages for the entire plants, within‐plant shading was likely to be considerable, and may have a critical affect on the correlations. To clarify the contribution of intra‐plant shading to the relationships observed, we sampled the uppermost leaves of 35 separate plants in Endla. Because the top leaves were fully exposed, foliar characteristics could be studied without the interfering self‐shading effects. Although we are not quantifying the degree of within‐plant shading this way, the finding of the same correlations with the leaf characteristics of the uppermost leaves as with the whole‐plant average leaf characteristics indicates that these correlations cannot be attributed to modified within‐plant shading alone. In Voore, only the topmost four to seven exposed leaves were sampled. For the top leaves, the circumference of each leaf was digitized, and the area was calculated as described by Niinemetset al. (1999).
Foliage and soil chemical analyses
Samples for soil analyses were taken from the A (A0) horizon, where most of the roots of the sampled seedlings and saplings were found (5–10 cm for Endla, 5–15 cm for Viimsi and 3–20 cm for Voore). A sample for chemical analysis consisted of all leaves per plant (excavated plants in Endla and Viimsi) or of the uppermost completely exposed leaves in Endla (35 separate plants) and Voore (five separate plants).
For most samples, nitrogen and carbon contents were estimated by gas chromatography after combustion of the sample at >1000 °C in oxygen (elemental analyzer CHN‐O‐Rapid; Foss Heraeus GmbH, Hanau, Germany). Phosphorus contents were determined by inductively coupled plasma emission spectroscopy (Integra XMP; GBC Scientific Instruments, Melbourne, Australia) after digestion of pulverized leaves in 65 % HNO3. For some samples, standard Kjeldahl digestion was applied, and N content was determined by the indophenol method and P content by the molybdenum blue method (Grimshawet al., 1989). Extensive comparisons of samples from different sites by various analytical routines indicated that the different methods gave similar results for both N and P with the differences ≤1 %. Ash content was estimated after the combustion of the sample in a muffle furnace at 500 °C for 3 h, and mineral content (A) calculated taking the fraction of minerals in ash as equal to 0·67 (Vertregt and Penning de Vries, 1987).
For the Endla and Viimsi sites, soil pH (1 g dry soil per 5 ml solution) was measured in distilled water (pHH2O) and in 1 m KCl solution (pHKCl). Soluble P [ammonium lactate–acetate‐soluble phosphorus (AL‐method, Swedish standard SS 02 83 10; Egneret al., 1960)] was determined by the molybdenum blue method after extraction with an aqueous solution (pH = 3·75) consisting of lactic (0·1 m) and acetic (0·3 m) acids, and ammonium acetate (0·1 m). The detailed soil characteristics and methods for chemical analyses of soils in the Voore site are reported by Kõlli and Kährik (1970), Reintam (1970) and Kull and Niinemets (1993).
Leaf construction cost
Mineral‐free carbon content (CA, g g–1) was found as CA = Cm/(1 – A), where Cm is total leaf carbon content per unit dry mass, and an estimate of leaf construction cost [G, g glucose (g dry mass)–1] was computed as (Vertregt and Penning de Vries, 1987; Poorter, 1994; Niinemets, 1997b):
G = (5.077CA – 1.041)(1 – A). (1)
The primary assumption of eqn (1) is that the source of foliar nitrogen is ammonium or nitrogen in organic form, or that all nitrate is reduced in the leaf (Poorter, 1994; Niinemets, 1997b). Given that ammonium and amino acids are the predominate nitrogen source in bogs and raw humus soils (Chapinet al., 1986; Hobbieet al., 2000), we consider this assumption justified.
Estimations of foliage photosynthetic electron transport rate
In Endla (Table 1), chlorophyll fluorescence measurements were conducted in the uppermost leaves with a pulse‐amplitude modulated fluorometer PAM‐2000 (Heinz Walz GmbH, Effeltrich, Germany). Natural illumination was used for most measurements, but on cloudy days, the internal halogen lamp of the PAM‐2000 was used as the source of actinic light. The leaf was held in a beam irradiance of 1200–1600 µmol m–2 s–1, which was saturating for photosynthetic electron transport in the studied species. Pulses of white light of 8000 µmol m–2 s–1 were applied to determine the maximum fluorescence yield of light‐adapted sample (Fm′) as detailed by Niinemetset al. (1998b). From these measurements, the effective quantum yield of the light‐adapted sample (ΦPSII) was computed as (Fm′ – F)/Fm′, where F is the fluorescence yield in actinic light (Schreiberet al., 1994). Assuming that both Photosystems I and II absorb equal amounts of light, an estimate of the whole chain electron transport rate (J) was computed as described by Gentyet al. (1989):
J = 0·5ΦPSIIΘQ. (2)
where Q is the quantum flux density and Θ is the leaf absorptance. A constant value of 0·84 was used for Θ in the current study. Average (± s.d.) leaf temperature was 27·7 ± 3·1 °C during the fluorescence measurements and no attempt was made to standardize the observed values of J to a common temperature.
Statistical analysis
The maximum height of the excavated plants was only 1·7 m, but the height range of the studied plants was more than 50‐fold, and significant effects of tree height were found on both foliar morphological and plant allocation parameters. Because tree height and foliar nitrogen content were not strongly correlated (Fig. 1), the effects of total tree height and N could be studied independently. To improve the normality of the residuals, tree height, average leaf area, average dry mass and leaf area ratio were transformed to logarithms before the statistical analysis was performed. Simple and multiple linear regression analyses were employed to examine the dependencies of foliar and plant characteristics on total tree height and foliar nitrogen content. The species and site effects were investigated either by analyses of variance (Site and Species as main effects) when logH and N effects were non‐significant or by analyses of covariance (logH and N as covariates), and the means were separated by the Bonferroni test. Preliminary analysis indicated that the Site as well as Site × N and Site × logH interactions were generally non‐significant (P > 0·05), indicating that Species, logH and N were the primary determinants of foliar characteristics. Given the low explaining power, the Site effects as well as the third‐order interactions were suppressed in the final ANOVA model.
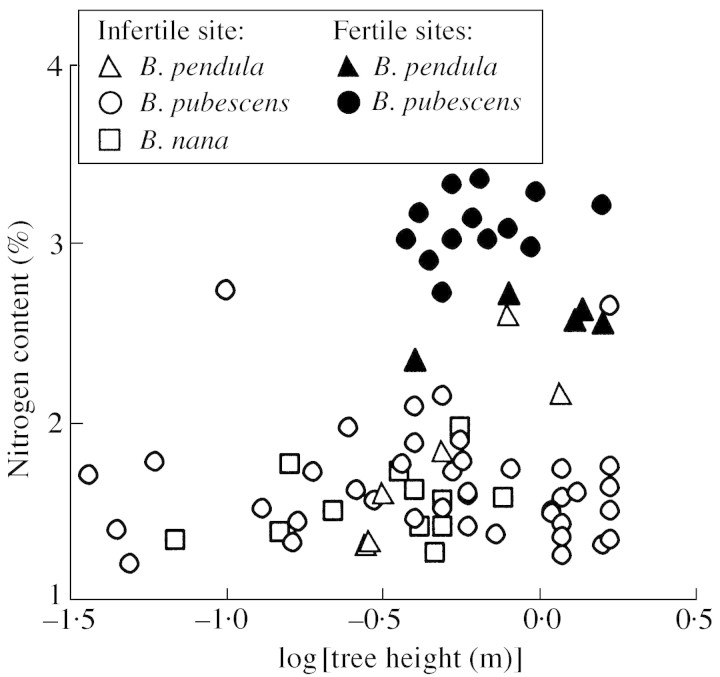
Fig. 1. Scatter plot of foliar nitrogen content per unit dry mass vs. total tree height. The data were sampled from the bog (poor site) and from the clear fell sites in Viimsi (B. pubescens) and forest edges in Voore (B. pendula, Table 1). Average foliar nutrient contents (Table 1) provided the primary criterion to distinguish the sites on the basis of nutrient availability (fertile vs. infertile). The same symbols are used for the Viimsi data because (1) the primary difference between the Viimsi sites (Viimsi‐1, and Viimsi‐2, Table 1) was only the age of the clear fells, and (2) the sites did not differ in measured soil characteristics and foliar nutrient contents. The linear correlation is non‐significant (r = 0·21, P > 0·06 for all data pooled).
Because of differences in species abundance and dispersal patterns at the various sites, different numbers of plants were available for each species–site pair. In particular, the number of plants of B. pubescens was considerably larger than that of other species. This lack of balance may have potentially altered interspecific comparisons and the conclusions with respect to the significance of the pooled regressions. To test for these possibilities, we constructed another dataset by randomly removing the data of B. pubescens to give a total of 16 observations for this species. Although this procedure altered the statistical significance of various differences and relationships, all multiple correlations were qualitatively the same for this and for the entire dataset, indicating that the pooled regressions were not affected by the lack of balance in the data.
RESULTS
Differences in the soil nutrient availability between the sites
The measured soil characteristics between the bog (Endla) and the fell sites (Viimsi‐1 and Viimsi‐2) were not statistically different. However, the upper soil horizons at the Voore site were significantly less acidic, with lower C/N molar ratio compared with the other sites (Table 1). Nevertheless, foliar nitrogen and phosphorus contents (Table 1) suggested that the nutrient availability was significantly larger at the fell sites than in the bog, possibly because of intensive soil mineralization following felling, or because the seedlings and saplings had access to deeper soil mineral horizons at these sites.
We observed a large within‐site range in foliar nutrient contents, especially in the bog where foliar N contents ranged from 1·21 % to 2·74 %. Non‐quantitative observations suggested that these differences were tightly related to the microheterogeneity in topography (e.g. the hummock–hollow gradient characteristic of the bogs, Titus and Wagner, 1984), and accordingly, in the water table level. In general, leaves with greater nutrient contents were sampled at higher locations, where the soil mineralization rates were likely to be higher.
According to Wassenet al. (1995), foliar P/N molar ratios >0·032 indicate primary N deficiency, and P/N ratios <0·02 indicate P deficiency in peat and raw humus soils. Given that the lowest P/N ratio observed in our study was 0·025 (Table 1), we conclude that the vegetation was primarily N‐limited in all sites.
Average leaf area and mass in relation to species, tree size and foliar nitrogen content
Average leaf area was positively correlated with foliar nitrogen content per unit dry mass (NM) in all species (Table 2, r2 = 0·49, P < 0·001 for all data pooled). Taller trees also had larger leaves such that total tree height and S were positively related (Table 2, r2 = 0·08, P < 0·002 for all data pooled). Given that logH and NM were essentially independent (Fig. 1), inclusion of both explaining variables into a multiple regression generally resulted in a higher fraction of explained variance than the separate regressions (Table 2, Fig. 2).
Table 2.
Species‐specific regressions between foliar structural characteristics, and foliar nitrogen content and total tree height
| Nitrogen content (NM, %) | Tree height [log(H, m)] | Multiple regression† | |||||||||||
| Species | Dependent variable* | Intercept | P | Slope | r 2 | P | Intercept | P | Slope | r 2 | P | r 2 | P |
| B. nana | logS | –1·24 | 0·005 | 0·681 | 0·42 | 0·02 | 0·139 | 0·06 | 0·687 | 0·70 | 0·001 | 0·83 | 0·001 |
| B. pendula | logS | –0·607 | 0·05 | 0·704 | 0·87 | 0·001 | 1·10 | 0·001 | 1·02 | 0·62 | 0·005 | 0·87 | 0·001‡ |
| B. pubescens | logS | 0·0469 | 0·4 | 0·317 | 0·69 | 0·001 | 0·822 | 0·001 | 0·191 | 0·12 | 0·001 | 0·83 | 0·001 |
| B. nana | logM | –16·3 | 0·001 | 1·41 | 0·41 | 0·02 | –13·5 | 0·001 | 1·24 | 0·50 | 0·005 | 0·65 | 0·001 |
| B. pendula | logM | –15·1 | 0·001 | 1·71 | 0·83 | 0·001 | –10·9 | 0·001 | 2·41 | 0·56 | 0·01 | 0·83 | 0·002‡ |
| B. pubescens | logM | –13·9 | 0·001 | 0·951 | 0·84 | 0·001 | –11·7 | 0·001 | 0·0687 | 0·00 | 0·6 | 0·86 | 0·001 |
| B. nana | M A | 68·9 | 0·02 | 10·7 | 0·04 | 0·5 | 96·7 | 0·001 | 26·3 | 0·34 | 0·02 | 0·40 | 0·02§ |
| B. pendula | M A | 85·4 | 0·001 | –6·05 | 0·12 | 0·3 | 71·9 | 0·001 | –2·83 | 0·01 | 0·7 | 0·32 | 0·2 |
| B. pubescens | M A | 112·5 | 0·001 | –16·8 | 0·38 | 0·001 | 81·5 | 0·001 | 26·1 | 0·38 | 0·001 | 0·70 | 0·001 |
| B. nana | log(LAR) | 1·13 | 0·05 | 0·143 | 0·04 | 0·6 | 1·19 | 0·001 | –0·210 | 0·16 | 0·2 | 0·36 | 0·3 |
| B. pubescens | log(LAR) | 0·835 | 0·001 | 0·230 | 0·37 | 0·001 | 1·32 | 0·001 | –0·479 | 0·38 | 0·001 | 0·56 | 0·001 |
| B. nana | F L | –0·0567 | 0·7 | 0·159 | 0·20 | 0·2 | 0·140 | 0·02 | –0·0461 | 0·04 | 0·5 | 0·27 | 0·4 |
| B. pubescens | F L | 0·00884 | 0·8 | 0·0749 | 0·34 | 0·001 | 0·138 | 0·001 | –0·233 | 0·46 | 0·001 | 0·46 | 0·001 |
* logS = logarithm of average leaf size (cm2), logM = logarithm of average leaf dry mass (g), MA = leaf dry mass per area (g m–2), log(LAR) = logarithm of leaf area ratio (cm2 g–1), FL = fraction of foliar biomass in leaves (g g–1), FL = LAR × MA.
† Both nitrogen content and logH included as explaining variables.
‡ LogH was not statistically significant in the multiple regression (P > 0·4).
§NM was not statistically significant in the multiple regression (P > 0·9).
Fig. 2. Dependence of leaf size on foliar nitrogen content and on total tree height in Betula nana, B. pendula and B. pubescens. All data were fitted by a single multiple linear regression: logS = –0·190 + 0·358logH + 0·447NM (r2 = 0·60, P < 0·001 for both logH and NM, P > 0·1 for the intercept). Symbols as in Fig. 1.
First ignoring the possible interactions, both the one‐way analyses of variance (Table 3) and covariance (common slope model with logH and NM as covariates) suggested that at a common height and foliar nitrogen content, B. nana had the smallest leaves, B. pubescens intermediate, and B. pendula the largest leaves (P < 0·001; Table 3). Thus, interspecific variability was an important determinant of leaf size. However, further analysis of the species effects by a separate slope model indicated that the interaction term Species × N was also significant (P < 0·001, separate slope analysis of co‐variance), as well as the interaction term Species × logH (P < 0·05). These significant interactions were indicative of lower responsiveness of leaf area in B. pubescens to NM, and logH (lower slope values, Table 2).
Table 3.
Averages (± s.e.) of foliage structural and chemical characteristics and foliar construction cost: results of one‐way analyses of variance*
| Species (site) | Leaf size (cm2)† | MA (g m–2) | LAR (cm2 g–1)† | N (%) | C (%) | G (g glu g–1) |
| B. nana (Endla) | 0·90 ± 0·19d | 84·1 ± 3·1a | 21·3 ± 2·7c | 1·56 ± 0·06c | 49·66 ± 0·19b | 1·500 ± 0·010b |
| B. pendula (Endla) | 5·1 ± 1·8bc | 74·8 ± 3·1a | 11·7 ± 3·1c | 1·89 ± 0·20c | 49·33 ± 0·33b | 1·485 ± 0·017b |
| B. pendula (Voore) | 16·8 ± 2·4a | 69·6 ± 3·4ab | n.d. | 2·57 ± 0·06b | n.d. | n.d. |
| B. pubescens (Endla) | 4·72 ± 0·44b | 84·8 ± 2·5a | 18·7 ± 2·5c | 1·65 ± 0·05c | 48·69 ± 0·20b | 1·451 ± 0·010b |
| B. pubescens (Viimsi‐1) | 6·3 ± 0·6b | 49·4 ± 1·2c | 92·6 ± 4·9a | 3·17 ± 0·12a | 54·7 ± 0·7a | 1·762 ± 0·034a |
| B. pubescens (Viimsi‐2) | 12·2 ± 1·0a | 56·7 ± 2·1bc | 35·8 ± 2·7b | 3·08 ± 0·05a | 54·14 ± 0·15a | 1·739 ± 0·008a |
MA = leaf dry mass per unit area, LAR = leaf area ratio (total leaf area per unit total plant mass), G = leaf construction cost (eqn. 1), n.d. = not determined.
* Each individual species‐site pair was used as a factor level. Means followed by the same letter are not significantly different at P < 0·05.
† The variable was transformed to a logarithm before statistical comparisons.
The species ranking was also identical in terms of average leaf dry mass (Table 3). As with the leaf area, the responsiveness of leaf dry mass to NM and logH was lower in B. pubescens than in the other species (P < 0·03 for Species × N and P < 0·05 for Species × logH interaction).
Effects of tree size and nitrogen content on leaf dry mass per unit area (MA)
Increases in the area of individual leaves with NM were larger than increases in the dry mass, such that MA scaled negatively with nitrogen content per unit dry mass (Fig. 3A, Table 2). Similarly, lower responsiveness of leaf area than leaf mass to logH led to a positive relationship between MA and logH (Fig. 4A, Table 2). The scaling of MA with NM was significant only in B. pubescens, and scaling with logH in B. nana and B. pubescens (Table 2). Nevertheless, the species effect (P > 0·6) as well as Species × N (P > 0·4) and Species × logH (P > 0·8) interactions were non‐significant, indicating that despite the data scatter in B. nana and B. pendula, they adhered the same overall trend (Fig. 4A).
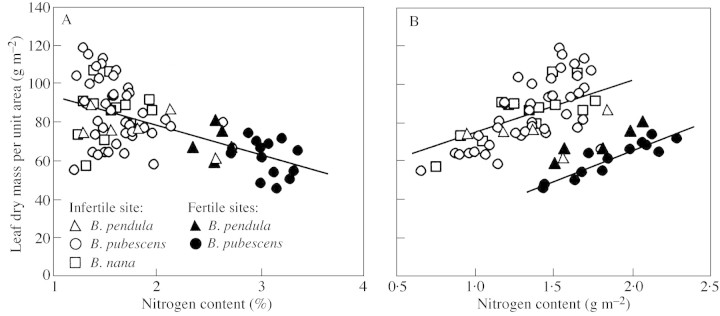
Fig. 3. Correlations between leaf dry mass per unit area (MA) and leaf nitrogen content per unit dry mass (A) and N per unit area (B). In A, all data were fitted by a single linear regression (r2 = 0·33, P < 0·001). In B, separate regressions were fitted for all sample points from the infertile site (upper regression line, r2 = 0·31, P<0·001) and for B. pubescens from the fertile site (lower regression line, r2 = 0·85, P<0·001). Symbols as in Fig. 1.
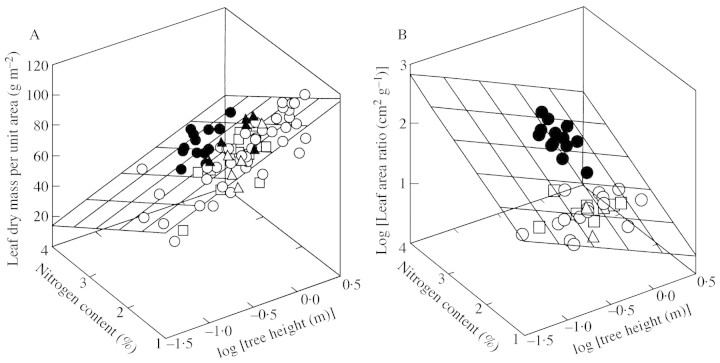
Fig. 4. Effects of leaf nitrogen content and total tree height on MA (A) and on leaf area ratio (B). The regression surfaces were fitted through all data: MA = 123·4 + 23·2logH – 18·8NM (r2 = 0·64) for A, and log(LAR) = 0·622 – 0·340LogH + 0·277NM for B (r2 = 0·49). All regression coefficients were significant at P < 0·001. Symbols as in Fig. 1.
All plants included in the study were exposed to more than 85 % of the light in a completely open location. However, the values of MA depicted in Figs 3A and 4A were obtained either as an average of all leaves per plant or as the average of uppermost exposed leaves. To test the possibility that the negative scaling of MA with NM resulted from greater self‐shading because of a larger foliar area in plants with better N nutrition, we also computed the regressions separately for the whole plant average MA and for the uppermost MA values. In both sets of data, MA was negatively correlated with NM and positively with logH. For all species pooled, r2 = 0·55 (P < 0·001 for both independent variables) for the uppermost leaves, and r2 = 0·61 (P < 0·001) for the whole plant averages. Thus, increased self‐shading within the canopy did not provide the only explanation for the strong negative relationships between MA and NM (Table 2, Fig. 4A).
Leaf nitrogen content per unit area (NA = NM·MA) and MA were positively related (Fig. 3B). However, because of the differences in NM and a negative relationship between MA and NM (Table 2, Figs 3A and 4A), the intercept of the MA vs. NA relationship was larger in the leaves with lower NM (P < 0·001 for the site effect, Fig. 3B). Despite NM being independent of tree height (Fig. 1), positive effects of logH on MA (Fig. 4A) also led to a positive correlation between NA and logH (r2 = 0·43, P < 0·001).
Dependence of leaf area ratio (LAR) and fraction of plant biomass in leaves (FL) on tree size and nitrogen content
Leaf area ratio (total foliar area per total plant dry mass) was negatively related to logH and positively to NM in B. pubescens, but not in B. nana (Table 2, Fig. 4B). However, covariance analysis demonstrated that the values of LAR of B. nana overlapped with those observed in B. pubescens. Both interaction terms, Species × logH and Species × N, were non‐significant (P > 0·6 for both) according to the separate slope ANCOVA model, and the main effect, Species, was insignificant according to the common slope model (P > 0·08). Thus, despite the very different plant architecture, the LAR values of both species were similar.
The fraction of plant biomass in foliage (FL) was also negatively related to logH and positively to NM (Table 2, r2 = 0·38, P < 0·001 for all data pooled). Again, the interaction terms were non‐significant in the separate slope model (P > 0·7 for both) and the species effect was non‐significant according to the following common slope analysis (P > 0·1).
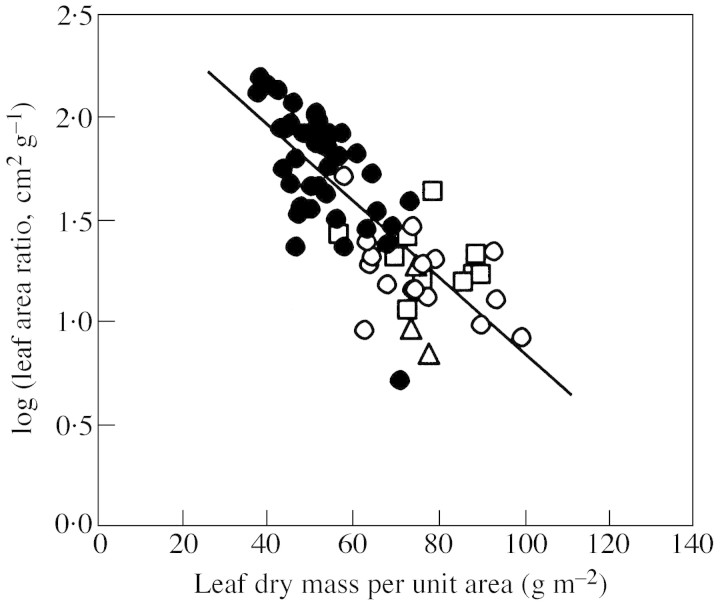
Given that LAR is dependent on both MA and FL (LAR = FL/MA; Fig. 5), the plasticity in both of its components with respect to NM and logH controls the variation of LAR along gradients of nutrient availability and with tree size. To determine the extent to which the NM‐ and logH‐related variability in LAR can be ascribed to variation in FL and MA, we calculated the expected values of FL and MA using the minimum and maximum measured values of logH and NM in interspecific regressions (Fig. 4A). For the observed range of logH (–1·50 ... 0·23), the expected value of FL varied 6·7‐fold, and that of MA only 1·9‐fold. For the observed range in NM (1·21 ... 3·36), the expected value of FL varied 1·9‐fold, and that of MA 1·3‐fold. Thus, we conclude that FL responded more plastically than foliar structure to both logH and NM, and was, accordingly, a more important determinant of LAR.
Fig. 5. Leaf area ratio in relation to leaf dry mass per unit area. The regression was fitted through all data. Symbols as in Fig. 1.
Foliar photosynthetic characteristics and construction cost vs. MA, nitrogen and tree height
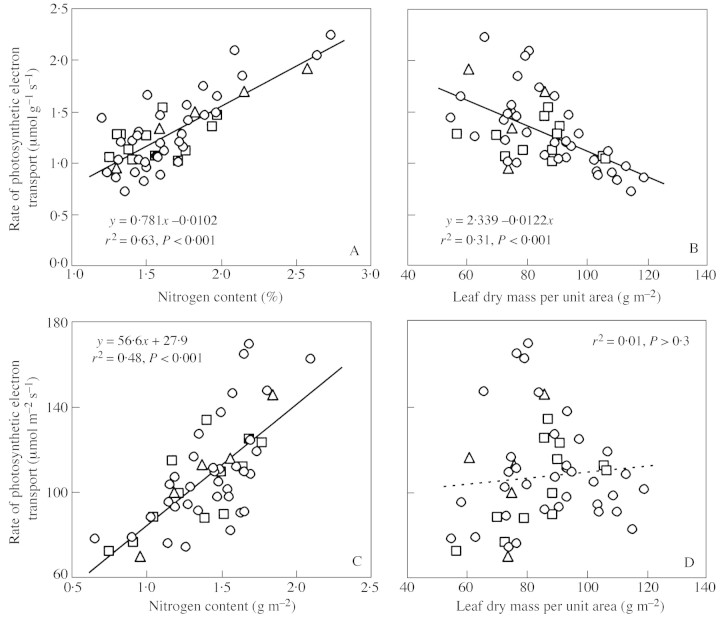
The light‐saturated rate of photosynthetic electron transport per unit dry mass (JM) was positively related to foliar nitrogen content (Fig. 6A), whereas the regressions were significant for each individual species (P < 0·05), as well as for all data pooled. However, JM scaled negatively with MA (Fig. 6B). The rate of photosynthetic electron transport per unit area (JA) correlated also positively with NA (Fig. 6C), but because of the negative correlation between JM and MA, JA (JA = JM·MA) was independent of MA (Fig. 6D). A multiple regression analysis of JM vs. MA and NM (r2 = 0·73) indicated that the negative scaling of JM did not solely result from NM effects on MA (Fig. 3A), but both MA (P < 0·001) and NM (P < 0·001) were significant determinants of JM. The ratio of JM to NM was negatively related to MA (r2 = 0·30, P < 0·001), further showing that the nitrogen use efficiency in photosynthesis declines with increasing MA.
Fig. 6. Relationships between the rate of light‐saturated photosynthetic electron transport (J) per unit leaf dry mass in dependence on leaf nitrogen content per unit dry mass (A) and MA (B); and J per unit leaf area in relation to nitrogen content per unit area (C) and MA (D). The rate of photosynthetic electron transport was determined from chlorophyll fluorescence measurements (eqn. 2). The data were fitted by linear regressions. Symbols as in Fig. 1.
Multiple linear regression of JM vs. NM and logH indicated that, at common NM, JM was lower in larger trees (r2 = 0·68, P < 0·01 for logH), possibly because of a larger MA (cf. Figs 4A and 6B). However, JA did not depend significantly on logH (P > 0·1) in the multiple regression of JA vs. NA and logH. None of the correlations highlighted differed between species (P > 0·5 according to common‐slope ANCOVA analyses).
Foliar construction cost (G) tended to be higher in sites with greater nutrient availability (Table 3) and there was a positive relationship between G and NM for all data pooled (r2 = 0·63, P < 0·001), but not with logH (P > 0·4).
DISCUSSION
Changes in leaf size with nitrogen status
Strong positive relationships between average leaf area and foliar nitrogen content were found in all species (Fig. 2, Table 2). According to laboratory studies, enhanced nitrogen availability increases both the rate of leaf expansion as well as cell division (MacAdamet al., 1989; Tayloret al., 1993; Roggatzet al., 1999) and leads to larger individual leaves (Karlsson and Nordell, 1989; Trápani and Hall, 1996; Simset al., 1998a). Whole plant experiments further indicate that, in addition to larger leaves, the number of leaves may also increase with increasing N availability (Ackerly and Bazzaz, 1995; Grubbet al., 1996). Although the same total foliar area may be formed by a fewer larger leaves or by a larger number of smaller leaves, we suggest that the plant strategy to increase the area of individual leaves with increasing nitrogen availability has distinctive ecological advantages over the strategy to increase leaf number only. First, height growth becomes an increasingly more important competitive attribute as the nutrient limitations decrease, because enhanced nutrient supply leads to augmented light competition (Tilman, 1986; Gleeson and Tilman, 1992). As the interspecific correlations suggest, the branching requirements are less for larger leaves (Givnish, 1984; King, 1991, 1998). Thus, larger leaves allow a greater fractional investment of plant biomass in height growth relative to the lateral growth (King, 1991). In addition, simulation studies demonstrate that with increasing length of leaf blades and petioles, the degree of foliar self‐shading by other leaves and stem decreases, improving the light interception efficiency of unit foliar area (Takenaka, 1994). All the species of Betula studied here possess short petioles, and increases in leaf lamina size should significantly improve the light interception efficiency in these species.
Given this ecological reasoning, interspecific differences in leaf size (Table 3) may partly explain the contrasting competitive ability of the studied species (B. nana < B. pubescens < B. pendula) in nutrient‐rich early‐successional habitats. Especially in B. nana, the extremely small leaf size and lack of petioles leads to a large fraction of foliar area that is shaded by the stem. We suggest that this, in combination with genetically constrained height growth potential, is the primary factor limiting the competitive potential of this relic of the last glaciation period in nutrient‐rich habitats.
Leaf dry mass per unit area in relation to foliar nitrogen
In agreement with the contrasting patterns of nitrogen content vs. MA relationships of previous studies, MA vs. NM relations were weaker than leaf size vs. NM dependencies (Table 2). Nevertheless, there was a statistically robust negative correlation between NM and MA in B. pubescens (Table 2). Although the MA of both B. nana and B. pendula was non‐significantly related to NM, these data fit the same regression surface with B. pubescens (Fig. 4A), indicating a functional convergence in foliar characteristics. These non‐significant relationships may be a manifestation of lower plasticity, but may have also resulted from clustering of MA data of B. nana and B. pendula at relatively low N contents (Fig. 4A).
The relationship between NM and MA was qualitatively identical when a whole‐plant average MA or MA of the uppermost leaves was used in the regressions. This consistency demonstrates that the negative effect of site nutrient conditions on MA was not solely caused by increased self‐shading within the canopy. Given that self‐shading was ruled out as the only determinant of low MA at higher NM, it is pertinent to ask by which additional mechanisms the changes in MA in B. pubescens may have occurred along the nutrient gradients. Fundamental insight into the nutrient availability effects on MA may be obtained by distinguishing between the two components of MA, leaf thickness (T) and density (D, MA = T·D), that may vary independently (Witkowski and Lamont, 1991; Niinemets, 1999). The strong positive relationship between lateral expansion growth and NM suggests that the growth in thickness may also respond to enhanced N availability in a similar manner. Such a positive relationship has been found in some investigations (Juriket al., 1982; Thompsonet al., 1992; Simset al., 1998a), and may be the consequence of larger cells in leaves produced at higher N availability (Roggatzet al., 1999). Yet, there are consistent negative effects of N on D (Witkowski and Lamont, 1991; Niinemetset al., 2001, recalculated from Juriket al., 1982; Thompsonet al., 1992) that are generally larger than the positive effects of N on T, such that the product variable, MA, decreases with increasing nutrient availability (Juriket al., 1982; Thompsonet al., 1992). Data demonstrate that the number of cells per unit area is larger (Roggatzet al., 1999), and the fraction of intercellular air space lower (Juriket al., 1982) in nitrogen‐limited leaves, providing an explanation of greater density of these leaves. Moreover, starch (Waringet al., 1985; Bowler and Press, 1996; Simset al., 1998b) and lignin (Waringet al., 1985) contents may accumulate, further increasing leaf density at a common thickness.
Modification of leaf size and MA by tree size
Average leaf area was positively related to total tree height in three Betula species (Fig. 2), as in previous studies on young trees of Betula pubescens ssp. tortuosa (Sveinbjörnsson, 1987; Sennet al., 1992). Apart from the ecological benefits of larger leaves in terms of the efficiency of height growth, shorter plants have branches and stems with lower diameter. Accordingly, both mechanical and hydraulic constraints may be responsible for the limited maximum leaf size in smaller plants. Leaf size generally scales positively with the diameter of supporting branchlet (White, 1983). Although we observed a continuous increase in leaf size with increasing plant height (Fig. 2), other investigations conducted over a larger range of plant height and age indicate that the leaf size vs. plant age or height relationship may be a curve with a maximum, with the leaf size either levelling off or decreasing after the leaves have reached the maximum size (Montfort and Müller, 1951; Steeleet al., 1989). Increasing hydraulic limitations and greater water stress in taller trees (Ryan and Yoder, 1997; Mencuccini and Magnani, 2000) may provide an explanation for this curvilinearity.
In contrast to the non‐linear relationships between leaf size and plant height, MA increases linearly with increasing tree size (Steeleet al., 1989; Niinemets 1997a; Niinemetset al., 1999), as was also observed in our study in B. nana and B. pubescens (Table 2, Fig. 4A). Similarly to MA increases in nutrient‐limited leaves, the positive scaling of MA with tree size primarily results from increases in leaf density (Niinemets, 1997a, 1999) rather than from increases in thickness (but cf. Malkina, 1983; Niinemetset al., 1999). Because increases in density are compatible with larger tissue elastic moduli that allow a greater change in leaf water potential with a lower tissue water loss (Niinemets, 2001), it has been suggested that the tree‐size related increase in MA is an acclimation response to a more severe water stress in the canopies of larger trees (Niinemets 1997a).
Biomass partitioning in relation to N and tree size
Increases in MA with increasing tree size and with decreasing nitrogen content (Fig. 4A) in B. pubescens evinced that construction of extensive foliar area in terms of foliar biomass investment in leaves may be more expensive in taller trees and at low N (Figs 4B and 5). Our study indicates that, in all species, changes in LAR (LAR = FL/MA) resulted primarily from modifications in fractional biomass allocation to leaves, FL, rather than in MA. Because the plants grow faster at a greater nutrient supply, the plants of the same age are also larger at higher nutrient availability. Given the scaling of plant size with nutrient availability and MA with tree size (Fig. 4A), we hypothesize that the plastic modifications in MA in response to nutrient availability may be constrained by tree size effects on MA.
The evidence that NM effects on MA, LAR and FL were non‐significant in B. nana but significant in B. pubescens (Table 2) confirms quantitatively the suggestion that more competitive species with larger growth rates are more responsive to nutrients (Coomes and Grubb, 2000). However, the non‐significant effect of NM on MA in B. pendula apparently contradicts this explanation. Nevertheless, although we did not study LAR and FL in B. pendula, previous investigations have demonstrated that LAR and FL are plastically modified along nutrient availability treatments in this species (McDonaldet al., 1986, 1992). Thus, the allocation patterns suggest that B. pendula fits the general hypothesis of a larger plasticity in more competitive species.
Nitrogen status and tree size vs. the foliage photosynthetic characteristics and the construction cost
As observed previously (for a review, see Field and Mooney, 1986), increased foliar N contents strongly enhanced foliar photosynthetic capacities (Fig. 6A, C). Studies dealing with the leaf structure vs. photosynthetic capacity relationships along light gradients have further found a strong positive correlation between MA and foliage photosynthetic capacity per unit area (e.g. Ellsworth and Reich, 1993; Niinemetset al., 1998a). This correlation has been interpreted as indicative of greater thickness of leaves with larger MA (Niinemetset al., 1999), and consequently, characterizing an accumulation of photosynthetic biomass per unit leaf area with increasing MA (Niinemetset al., 1998a). However, we found no relationships between the capacity of photosynthetic electron transport (J) per unit area (JA) and MA (Fig. 6D), and a negative correlation between J per unit dry mass (JM) and MA (Fig. 6B). We hypothesize that the negative scaling of JM with MA at a common foliar nitrogen content resulted from increases in leaf density with decreasing nutrient availability. Leaves with a greater density have larger intercellular gas‐ and liquid‐phase transfer resistances to CO2 (Syvertsenet al., 1995), and consistently lower photosynthesis rates at common stomatal limitations (Niinemets, 1999).
Studies have brought evidence of a negative scaling of foliar nitrogen contents per unit dry mass (Niinemets, 1997a) and leaf assimilation potentials with increasing tree size (Grulke and Miller, 1994). Although the positive effects of tree size on MA led to a greater nitrogen content per unit leaf area in our study, JA was independent of tree size, and JM decreased with increasing tree size. Greater density and diffusive limitations in leaves with greater MA may also be responsible for the negative effects of tree height on JM in the three Betula species studied.
Despite the extensive modifications in MA (Fig. 4A), foliage construction cost (G) changed little across different foliar morphologies. Although carbon‐rich lignin (63·3 %, calculated according to Nimz, 1974) and tannins may accumulate at lower nutrient availabilities (Waringet al., 1985), there is also evidence of higher contents of non‐structural carbohydrates in nutrient‐limited leaves (Waringet al., 1985). Given that the non‐structural carbohydrates have low carbon content (44·4 % for starch), simultaneous accumulation of both the expensive and cheap compounds at low nutrient availability may be a reason for lower G at low nutrient availability. Yet, we found that G was strongly related to NM, which provides an estimate of foliar protein contents. Because proteins are rich in carbon (53·5 %), the construction of leaf photosynthetic apparatus with high N content is also costly in terms of carbon investments in unit foliar biomass. Although G should not always be related to site fertility (Chapin, 1989), our finding agrees with the majority of studies (Lafitte and Loomis, 1988; Griffinet al., 1993; Griffinet al., 1996; Poorter and Villar, 1997) demonstrating a larger construction cost in leaves with higher N content.
CONCLUSIONS
The information summarized indicates that the effects of internal plant N on MA are complex in temperate deciduous woody species, and may partly be mediated through enhanced self‐shading and increased plant size at higher nutrient availability. Yet, there are also important independent effects of nitrogen on foliar structure, which seem mainly to result in denser leaves at lower N. Increases in density may provide an explanation for a stronger reduction in foliar photosynthetic capacities with decreasing nitrogen content than may be predicted from decreases in NM alone. High‐density mesophyll is generally an adaptation to water‐limited environments such as Mediterranean communities and semi‐deserts (Niinemets, 2001), and the functional significance of dense leaves in nutrient‐limited habitats remains to be discovered.
The biomass allocation responds more readily to differences in N than MA, indicating a larger plasticity in allocation than in foliar structure. However, as the trees increase in size, the allocation in foliage consistently decreases. Thus, the positive effect of high internal nutrient availability on foliar biomass investment in leaves becomes buffered by concomitant increases in plant size that reduce FL. Given the strong controls of plant size, the studies on N influences on foliar structure and plant biomass allocation should also include plant size as a covariate in statistical comparisons.
ACKNOWLEDGEMENTS
We thank Aljona Lukjanova (Institute of Ecology, Tallinn University of Educational Sciences) for help with the chlorophyll fluorescence measurements, and Kai Kimmel (Endla State Nature Reserve) for permission to perform the study in Endla. We value the comments on the manuscript by Professor David T. Bell (University of Western Australia), Dr D. R. Causton (Institute of Biological Sciences, University of Wales, Aberystwyth, UK) and an anonymous reviewer. The research was funded by the Estonian Science Foundation (grant 4584), the Estonian Minister of Education (grants 0180517s98 and 0281770Bs01), the National Research Council, National Academy of Sciences, USA, and the Bayreuther Institut für Terrestrische Ökosystemforschung (BITÖK), University of Bayreuth, Germany (BBWFT grant 0339476C).
Supplementary Material
Received: 14 May 2001; Returned for revision: 10 September 2001; Accepted: 23 October 2001.
References
- AckerlyDD, Bazzaz FA.1995. Leaf dynamics, self‐shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 101: 289–298. [DOI] [PubMed] [Google Scholar]
- AckerlyDD, Reich PB.1999. Convergence and correlations among leaf size and function in seed plants: a comparative test using independent contrasts. American Journal of Botany 86: 1272–1281. [PubMed] [Google Scholar]
- AtkinsonMD.1992. Biological flora of the British Isles No. 175. Betula pendula Roth. (B. verrucosa Ehrh.) and B. pubescens Ehrh. Journal of Ecology 80: 837–870. [Google Scholar]
- BowlerJM, Press MC.1996. Effects of elevated CO2, nitrogen form and concentration on growth and photosynthesis of a fast‐ and slow‐growing grass. The New Phytologist 132: 391–401. [DOI] [PubMed] [Google Scholar]
- ChapinFSIII.1989. The cost of tundra plant structures: evaluation of concepts and currencies. The American Naturalist 133: 1–19. [Google Scholar]
- ChapinFSIII, Schulze E‐D, Mooney HA.1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447. [Google Scholar]
- ChapinFSIII, Vitousek PM, Van Cleve K.1986. The nature of nutrient limitation in plant communities. The American Naturalist 127: 48–58. [Google Scholar]
- CoomesDA, Grubb PJ.2000. Impacts of root competition in forests and woodlands: a theoretical framework and review of experiments. Ecological Monographs 70: 171–207. [Google Scholar]
- CornelissenJHC, Castro‐Díez P, Carnelli AL.1998. Variation in relative growth rate among woody species. In: Lambers H, Poorter H, van Vuuren MMI, eds. Inherent variation in plant growth. Physiological mechanisms and ecological consequences Leiden: Backhuys Publishers, 363–392. [Google Scholar]
- CornelissenJHC, Castro‐Díez P, Hunt R.1996. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. Journal of Ecology 84: 755–765. [Google Scholar]
- DammanAWH.1986. Hydrology, development, and biogeochemistry of ombrogenous peat bogs with special reference to nutrient relocation in a western Newfoundland bog. Canadian Journal of Botany 64: 384–394. [Google Scholar]
- de GrootWJ, Thomas PA, Wein RW.1997. Biological flora of the British Isles No. 194. Betula nana L. and Betula glandulosa Michx. Journal of Ecology 85: 241–265. [Google Scholar]
- DierssenK.1977. Zur Synökologie von Betula nana in Mitteleuropa. Phytocoenologia 4: 180–205. [Google Scholar]
- EgnerH, Riehm H, Domingo W.1960. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. Annals of the Royal Agricultural College of Sweden 26: 1–199. [Google Scholar]
- EllsworthDS, Reich PB.1993. Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96: 169–178. [DOI] [PubMed] [Google Scholar]
- EvansGC.1972. The quantitative analysis of plant growth, Oxford: Blackwell Science. [Google Scholar]
- FieldC, Mooney HA.1986. The photosynthesis–nitrogen relationship in wild plants. In: Givnish TJ, ed. On the economy of plant form and function. Proceedings of the Sixth Maria Moors Cabot Symposium, ‘Evolutionary constraints on primary productivity: adaptive patterns of energy capture in plants’ Cambridge: Cambridge University Press, 25–55. [Google Scholar]
- FinérL.1992. Nutrient concentrations in Pinus sylvestris growing on an ombrotrophic pine bog, and the effects of PK and NPK fertilization. Scandinavian Journal of Forest Research 7: 205–218. [Google Scholar]
- FredeenAL, Gamon JA, Field CB.1991. Responses of photosynthesis and carbohydrate‐partitioning to limitations in nitrogen and water availability in field‐grown sunflower. Plant, Cell and Environment 14: 963–970. [Google Scholar]
- FreyT.1977. IBP research at the Vooremaa forest ecology station. In: Frey T, ed. Spruce forest ecosystem structure and ecology Vol. 1. Introductory data on the Estonian Vooremaa project. Estonian IBP Report 11. Tartu: Academy of Sciences of the Estonian S.S.R., Estonian Republican Committee for IBP, 21–36. [Google Scholar]
- GentyB, Briantais J‐M, Baker NR.1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- GivnishTJ.1984. Leaf and canopy adaptations in tropical forests. In: Medina E, Mooney HA, Vásquez‐Yánes C, eds. Physiological ecology of plants of the wet tropics. Proceedings of an international symposium held in Oxatepec and Los Tuxtlas, Mexico, June 29 to July 6, 1983 Tasks for vegetation science 12, The Hague: Dr. W. Junk Publishers, 51–84. [Google Scholar]
- GleesonSK, Tilman D.1992. Plant allocation and the multiple limitation hypothesis. The American Naturalist 139: 1322–1343. [Google Scholar]
- GriffinKL, Thomas RB, Strain BR.1993. Effects of nitrogen supply and elevated carbon dioxide on construction cost in leaves of Pinus taeda (L.) seedlings. Oecologia 95: 575–580. [DOI] [PubMed] [Google Scholar]
- GriffinKL, Winner WE, Strain BR.1996. Construction cost of loblolly and ponderosa pine leaves grown with varying carbon and nitrogen availability. Plant, Cell and Environment 19: 729–738. [Google Scholar]
- GrimshawHM, Allen SE, Parkinson JA.1989. Nutrient elements. In: Allen SE, ed. Chemical analysis of ecological materials 2nd ed. Oxford: Blackwell Science, 81–159. [Google Scholar]
- GrubbPJ, Lee WG, Kollmann J, Wilson JB.1996. Interaction of irradiance and soil nutrient supply on growth of seedlings of ten European tall‐shrub species and Fagus sylvatica Journal of Ecology 84: 827–840. [Google Scholar]
- GrulkeNE, Miller PR.1994. Changes in gas exchange characteristics during the life span of giant sequoia: implications for response to current and future concentrations of atmospheric ozone. Tree Physiology 14: 659–668. [DOI] [PubMed] [Google Scholar]
- HilbertDW.1990. Optimization of plant root:shoot ratios and internal nitrogen concentration. Annals of Botany 66: 91–99. [Google Scholar]
- HiroseT.1988. Modelling the relative growth rate as a function of plant nitrogen concentration. Physiologia Plantarum 72: 185–189. [Google Scholar]
- HobbieEA, Macko SA, Williams M.2000. Correlations between foliar δ15N and nitrogen concentrations may indicate plant–mycorrhizal interactions. Oecologia 122: 273–283. [DOI] [PubMed] [Google Scholar]
- IngestadT, Lund A‐B.1979. Nitrogen stress in birch seedlings. I. Growth technique and growth. Physiologia Plantarum 45: 137–148. [Google Scholar]
- IngestadT, McDonald AJS.1989. Interaction between nitrogen and photon flux density in birch seedlings at steady‐state nutrition. Physiologia Plantarum 77: 1–11. [Google Scholar]
- JonassonS.1981. Plant communities and species distribution of low alpine Betula nana heaths in northernmost Sweden. Vegetation 44: 51–64. [Google Scholar]
- JurikTW, Chabot JF, Chabot BF.1982. Effects of light and nutrients on leaf size, CO2 exchange, and anatomy in wild strawberry (Fragaria virginiana). Plant Physiology 70: 1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KarlssonPS, Nordell KO.1987. Growth of Betula pubescens and Pinus sylvestris seedlings in a subarctic environment. Functional Ecology 1: 37–44. [Google Scholar]
- KarlssonPS, Nordell KO.1989. Effects of leaf duration, nutrient supply, and temperature on the seasonal pattern of growth and nitrogen uptake in tree seedlings in a subarctic environment. Canadian Journal of Botany 67: 211–217. [Google Scholar]
- KingDA.1991. Tree allometry, leaf size and adult tree size in old‐growth forests of western Oregon. Tree Physiology 9: 369–381. [DOI] [PubMed] [Google Scholar]
- KingDA.1998. Influence of leaf size on tree architecture: first branch height and crown dimensions in tropical rain forest trees. Trees: Structure and Function 12: 438–445. [Google Scholar]
- KörnerC.1991. Some often overlooked plant characteristics as determinants of plant growth: a reconsideration. Functional Ecology 5: 162–173. [Google Scholar]
- KolbTE, Steiner KC, McCormick LH, Bowersox TW.1990. Growth response of northern red oak and yellow poplar seedlings to light, soil moisture and nutrients in relation to ecological strategy. Forest Ecology and Management 38: 65–78. [Google Scholar]
- KõlliR, Kährik R.1970. Phytomass and its ash content in spruce and pine forests of the wood sorrel site type. In: Reimets E, Reintam L, Rooma I, Sepp R, Tarandi K, Turbas E, eds. Soil regimes and processes Transactions of Estonian Agricultural Academy 65, Tartu: Estonian Agricultural Academy, 233–261. (In Russian). [Google Scholar]
- KüppersM, Koch G, Mooney HA.1988. Compensating effects to growth of changes in dry matter allocation in response to variation in photosynthetic characteristics induced by photoperiod, light and nitrogen. In: Evans JR, von Caemmerer S, Adams WW III, eds. Ecology of photosynthesis in sun and shade Australia: CSIRO, 287–298. [Google Scholar]
- KullO, Niinemets Ü.1993. Variation in leaf morphometry and nitrogen concentration in Betula pendula Roth., Corylus avellana L. and Lonicera xylosteum L. Tree Physiology 12: 311–318. [DOI] [PubMed] [Google Scholar]
- LafitteHR, Loomis RS.1988. Calculation of growth yield, growth respiration and heat content of grain sorghum from elemental and proximal analyses. Annals of Botany 62: 353–361. [Google Scholar]
- LathamRE.1992. Co‐occurring tree species change rank in seedling performance with resources varied experimentally. Ecology 73: 2129–2144. [Google Scholar]
- LevinSA, Mooney HA, Field C.1989. The dependence of plant root:shoot ratios on internal nitrogen concentration. Annals of Botany 64: 71–75. [Google Scholar]
- MacAdamJW, Volenec JJ, Nelson CJ.1989. Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiology 89: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MalkinaIS.1983. Relationships between photosynthesis of oak leaves and their structure and age of trees. Lesovedeniye 4: 68–71. (In Russian). [Google Scholar]
- MasingV.1990. The champ trees of Estonia. Eesti Loodus 33: 806–807. (In Estonian). [Google Scholar]
- McDonaldAJS, Lohammar T, Ericsson A.1986. Uptake of carbon and nitrogen at decreased nutrient availability in small birch (Betula pendula Roth.) plants. Tree Physiology 2: 61–71. [DOI] [PubMed] [Google Scholar]
- McDonaldAJS, Lohammar T, Ingestad T.1992. Net assimilation rate and shoot area development in birch (Betula pendula Roth.) at different steady‐state values of nutrition and photon flux density. Trees: Structure and Function 6: 1–6. [Google Scholar]
- MencucciniM, Magnani F.2000. Comment on ‘Hydraulic limitation of tree height: a critique’ by Becker, Meinzer and Wullschleger. Functional Ecology 14: 135–137. [Google Scholar]
- MezianeD, Shipley B.1999. Interacting determinants of specific leaf area in 22 herbaceous species: effects of irradiance and nutrient availability. Plant, Cell and Environment 22: 447–459. [Google Scholar]
- MontfortC, Müller L.1951. Grundsätzliches zur Lebensrhythmik der Mistel (Viscum album L.) im jährlichen Längenzuwachs und in der Blattgestaltung. Berichte Deutscher Botanischer Gesellschaft 64: 297–303. [Google Scholar]
- NiinemetsÜ.1997a Distribution patterns of foliar carbon and nitrogen as affected by tree dimensions and relative light conditions in the canopy of Picea abies Trees: Structure and Function 11: 144–154. [Google Scholar]
- NiinemetsÜ.1997b Energy requirement for foliage construction depends on tree size in young Picea abies trees. Trees: Structure and Function 11: 420–431. [Google Scholar]
- NiinemetsÜ.1998. Growth of young trees of Acer platanoides and Quercus robur along a gap–understory continuum: interrelationships between allometry, biomass partitioning, nitrogen and shade‐tolerance. International Journal of Plant Sciences 159: 318–330. [Google Scholar]
- NiinemetsÜ.1999. Research review. Components of leaf dry mass per area—thickness and density—alter leaf photosynthetic capacity in reverse directions in woody plants. The New Phytologist 144: 35–57. [Google Scholar]
- NiinemetsÜ.2001. Climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs at the global scale. Ecology 82: 453–469. [Google Scholar]
- NiinemetsÜ, Kull K.1994. Leaf weight per area and leaf size of 85 Estonian woody species in relation to shade tolerance and light availability. Forest Ecology and Management 70: 1–10. [Google Scholar]
- NiinemetsÜ, Kull O.1998. Stoichiometry of foliar carbon constituents varies along light gradients in temperate woody canopies: implications for foliage morphological plasticity. Tree Physiology 18: 467–479. [DOI] [PubMed] [Google Scholar]
- NiinemetsÜ, Kull O, Tenhunen JD.1998a An analysis of light effects on foliar morphology, physiology and light interception in temperate deciduous woody species of contrasting shade tolerance. Tree Physiology 18: 681–696. [DOI] [PubMed] [Google Scholar]
- NiinemetsÜ, Kull O, Tenhunen JD.1999. Variability in leaf morphology and chemical composition as a function of canopy light environment in co‐existing trees. International Journal of Plant Sciences 160: 837–848. [DOI] [PubMed] [Google Scholar]
- NiinemetsÜ, Bilger W, Kull O, Tenhunen JD.1998b Acclimation to high irradiance in temperate deciduous trees in the field: changes in xanthophyll cycle pool size and in photosynthetic capacity along a canopy light gradient. Plant, Cell and Environment 21: 1205–1218. [Google Scholar]
- NiinemetsÜ, Ellsworth DS, Lukjanova A, Tobias M.2001. Site fertility and the morphological and photosynthetic acclimation of Pinus sylvestris needles to light. Tree Physiology 21: 1231–1244. [DOI] [PubMed] [Google Scholar]
- NimzH.1974. Das Lignin der Buche—Entwurf eines Konstitutionsschemas. [Beech lignin—proposal of a constitutional scheme]. Angewandte Chemie 86: 336–344. [Google Scholar]
- OrenR, Sheriff DW.1995. Water and nutrient acquisition by roots and canopies. In: Smith WK, Hinckley TM, eds. Resource physiology of conifers. Acquisition, allocation, and utilization San Diego: Academic Press, 39–74. [Google Scholar]
- PaavilainenE.1980. Effect of fertilization on plant biomass and nutrient cycle on a drained dwarf shrub pine swamp. Communicationes Instituti Forestalis Fenniae 98: 1–71. [Google Scholar]
- PoorterH.1990. Interspecific variation in relative growth rate: on ecological causes and physiological consequences. In: Lambers H, Cambridge ML, Konings H, Pons TL, eds. Causes and consequences of variation in growth rate and productivity of higher plants The Hague: SPB Academic Publishing, 45–68. [Google Scholar]
- PoorterH.1994. Construction costs and payback time of biomass: a whole plant perspective. In: Roy J, Garnier E, eds. A whole plant perspective on carbon–nitrogen interactions The Hague: SPB Academic Publishing, 111–127. [Google Scholar]
- PoorterH, Nagel O.2000. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology 27: 595–607. [Google Scholar]
- PoorterH, Remkes C.1990. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83: 553–559. [DOI] [PubMed] [Google Scholar]
- PoorterH, Villar R.1997. The fate of acquired carbon in plants: chemical composition and construction costs. In: Bazzaz FA, Grace J, eds. Plant resource allocation San Diego: Academic Press, 39–72. [Google Scholar]
- ReichPB, Walters MB.1994. Photosynthesis–nitrogen relations in Amazonian tree species. II. Variation in nitrogen vis‐à‐vis specific leaf area influences mass‐ and area‐based expressions. Oecologia 97: 73–81. [DOI] [PubMed] [Google Scholar]
- ReichPB, Kloeppel BD, Ellsworth DS, Walters MB.1995. Different photosynthesis‐nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia 104: 24–30. [DOI] [PubMed] [Google Scholar]
- ReintamL.1970. Characteristics of some soils formed on red‐brown moraine and problems of the delimitation of sod‐podzolic, pseudopodzolic and brown forest types. In: Reimets E, Reintam L, Rooma I, Sepp R, Tarandi K, Turbas E, eds. Soil regimes and processes Transactions of Estonian Agricultural Academy 65, Tartu: Estonian Agricultural Academy, 195–232. (In Russian). [Google Scholar]
- RoggatzU, McDonald AJS, Stadenberg I, Schurr U.1999. Effects of nitrogen deprivation on cell division and expansion in leaves of Ricinus communis L. Plant, Cell and Environment 22: 81–89. [Google Scholar]
- RosatiA, Day KR, DeJong TM.2000. Distribution of leaf mass per unit area and leaf nitrogen concentration determine partitioning of leaf nitrogen within tree canopies. Tree Physiology 20: 271–276. [DOI] [PubMed] [Google Scholar]
- RyanMG, Yoder BJ.1997. Hydraulic limits to tree height and tree growth. What keeps trees from growing beyond a certain height? BioScience 47: 235–242. [Google Scholar]
- SchreiberU, Bilger W, Neubauer C.1994. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E‐D, Caldwell MM, eds. Ecophysiology of photosynthesis Ecological studies 100, Berlin: Springer Verlag, 49–70. [Google Scholar]
- SennJ, Hanhimäki S, Haukioja E.1992. Among‐tree variation in leaf phenology and morphology and its correlation with insect performance in the mountain birch. Oikos 63: 215–222. [Google Scholar]
- SimsDA, Seemann JR, Luo Y.1998a Elevated CO2 concentration has independent effects on expansion rates and thickness of soybean leaves across light and nitrogen gradients. Journal of Experimental Botany 49: 583–591. [Google Scholar]
- SimsDA, Seemann JR, Luo Y.1998b The significance of differences in the mechanisms of photosynthetic acclimation to light, nitrogen and CO2 for return on investment in leaves. Functional Ecology 12: 185–194. [Google Scholar]
- SteeleMJ, Coutts MP, Yeoman MM.1989. Developmental changes in Sitka spruce as indices of physiological age. I. Changes in needle morphology. The New Phytologist 113: 367–375. [DOI] [PubMed] [Google Scholar]
- SveinbjörnssonB.1987. Biomass proportioning as related to plant size in juvenile mountain birch near Abisko, Swedish Lapland. Reports of the Kevo Subarctic Research Station 20: 1–8. [Google Scholar]
- SyvertsenJP, Lloyd J, McConchie C, Kriedemann PE, Farquhar GD.1995. On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant, Cell and Environment 18: 149–157. [Google Scholar]
- TakenakaA.1994. Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot. Ecological Research 9: 109–114. [Google Scholar]
- TanW, Hogan GD.1998. Dry weight and N partitioning in relation to substrate N supply, internal N status and developmental stage in jack pine (Pinus banksiana Lamb.) seedlings: implications for modelling. Annals of Botany 81: 195–201. [Google Scholar]
- TaylorG, McDonald AJS, Stadenberg I, Freer‐Smith PH.1993. Nitrate supply and the biophysics of leaf growth in Salix viminalis Journal of Experimental Botany 44: 155–164. [Google Scholar]
- ThompsonWA, Kriedemann PE, Craig IE.1992. Photosynthetic response to light and nutrients in sun‐tolerant and shade‐tolerant rainforest trees. I. Growth, leaf anatomy and nutrient content. Australian Journal of Plant Physiology 19: 1–18. [Google Scholar]
- ThompsonWA, Stocker GC, Kriedemann PE.1988. Growth and photosynthetic response to light and nutrients of Flindersia brayleyana F. Muell, a rainforest tree with broad tolerance to sun and shade. Australian Journal of Plant Physiology 15: 299–315. [Google Scholar]
- TilmanD.1986. Resources, competition and the dynamics of plant communities. In: Crawley MJ, ed. Plant ecology Oxford: Blackwell Science, 51–75. [Google Scholar]
- TitusJE, Wagner DJ.1984. Carbon balance for two Sphagnum mosses: water balance resolves a physiological paradox. Ecology 65: 1765–1774. [Google Scholar]
- TrápaniN, Hall AJ.1996. Effects of leaf position and nitrogen supply on the expansion of leaves of field grown sunflower (Helianthus annuus L.). Plant and Soil 184: 331–340. [Google Scholar]
- TurnerIM.1994. A quantitative analysis of leaf form in woody plants from the world’s major broadleaved forest types. Journal of Biogeography 21: 413–419. [Google Scholar]
- VeberK.1974. Vegetation history of the Endla mire system. In: Kumari E, ed. Estonian wetlands and their life Estonian contributions to the IBP 7, Tallinn: Valgus, 160–182. [Google Scholar]
- VertregtN, Penning de Vries FWT.1987. A rapid method for determining the efficiency of biosynthesis of plant biomass. Journal of Theoretical Biology 128: 109–119. [Google Scholar]
- WangJR, Hawkins CDB, Letchford T.1998. Relative growth rate and biomass allocation of paper birch (Betula papyrifera) populations under different soil moisture and nutrient regimes. Canadian Journal of Forest Research 28: 44–55. [Google Scholar]
- WaringRH, McDonald AJS, Larsson S, Ericsson T, Wiren A, Arwidsson E, Ericsson A, Lohammar T.1985. Differences in chemical composition of plants grown at constant relative growth rates with stable mineral nutrition. Oecologia 66: 157–160. [DOI] [PubMed] [Google Scholar]
- WassenMJ, Olde Venterink HGM, de Swart EOAM.1995. Nutrient concentrations in mire vegetation as a measure of nutrient limitation in mire ecosystems. Journal of Vegetation Science 6: 5–16. [Google Scholar]
- WhitePS.1983. Corner’s rules in eastern deciduous trees: allometry and its implications for the adaptive architecture of trees. Bulletin of the Torrey Botanical Club 110: 203–212. [Google Scholar]
- WitkowskiETF, Lamont BB.1991. Leaf specific mass confounds leaf density and thickness. Oecologia 88: 486–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.