Abstract
Stomatal closure can explain the inhibition of net CO2 uptake by a leaf subjected to a mild drought: the photosynthetic apparatus appears resistant to lack of water. Changes in both the water content of leaves maintained in a constant environment and the ambient CO2 molar fraction during measurements on well‐hydrated leaves lead to similar effects on net CO2 uptake and whole chain electron transport as estimated by leaf chlorophyll fluorescence measurements. In particular, it is shown that photosystem II (PSII) functioning and its regulation are not qualitatively changed during desiccation and that the variations in PSII photochemistry can simply be understood by changes in substrate availability in this condition. Moreover, an analysis of the literature shows that when inhibition of net CO2 uptake by C3 leaves under drought (Phaseolus vulgaris L., Helianthus annus L. and Solanum tuberosum L.) was lower than 80 %, elevated CO2 completely restored the photosynthetic capacity. The CO2 molar fraction in the chloroplasts declines as stomata close in drying leaves. As a consequence, in C3 plants, ribulose‐1,5‐bisphosphate oxygenation increases and becomes the main sink for photosynthetic electrons. Depending on the prevailing photon flux density, the O2 uptake through photorespiratory activity can entirely replace carbon dioxide as an electron acceptor, or not. The rate of the Mehler reaction remains low and unchanged during desiccation. However, drought could also involve CO2‐sensitive modification of the photosynthetic metabolism depending on plant growth conditions and possibly also on plant species.
Key words: Chloroplastic carbon dioxide molar ratio, drought stress, high CO2 effect, Lavatera trimestris L. var. splendens, Pisum sativum L., photorespiration, photosynthesis, stomatal effect
INTRODUCTION
Reductants produced by linear electron transfer in the thylakoid membrane are mainly used to reduce carbon dioxide and dioxygen in the chloroplasts. In C3 plants this occurs mainly through the activity of ribulose 1,5‐bisphosphate carboxylase/oxygenase (Rubisco) (Ruuska et al., 2000), which is the first enzyme of both the photosynthetic carbon reduction (PCR) and carbon oxidation (PCO) cycles. For most C4 plants at ambient CO2 molar ratio (Ca), as Rubisco oxygenase is inhibited by the high CO2 molar ratio in the bundle sheath cells, oxygenation of ribulose bisphosphate (RuBP) (if any) is low, and dioxygen reduction occurs mainly through both the Mehler reaction and the activity of Cytochrome a3 and the alternative oxidase in the mitochondrion. CO2 and O2 are competitors at the catalytic sites of Rubisco and the nature of this competition is well documented and modelled (Von Caemmerer, 2000). The reactions that are responsible for direct reduction of O2 have been reviewed recently (Asada, 1999; Foyer and Noctor, 2000). They mainly include mechanisms at the photosystem I (PSI) level, either reduced ferredoxin or FeS‐X centres, although plastoquinone and P680 in its triplet state can also reduce O2.
The electrons produced by photosystem II (PSII) activity are also used to reduce acceptors other than CO2 and O2. In particular, nitrogen assimilation by photosynthetic cells may require 10–20 % of the total electron flow (Champigny, 1995). Oxaloacetate (OAA) can also be reduced to malate by NADPH when the activation state of NADP+‐malate dehydrogenase is high enough. As this activation state is driven by the reduction of disulfur bonds on the enzyme, this reaction is considered to be a valve which allows a flow of reducing equivalents to the cytoplasm and the mitochondrion through the OAA/malate shuttle, and thus is a redox state control of the stroma (Scheibe, 1987). Some of the electrons reducing ferredoxin can also be recycled to the plastoquinone pool (Arnon and Chain, 1975). Direct O2 reduction through the Mehler reaction, cyclic electron flow and functioning of the malate valve are only linked with the build‐up of a low pH in the lumen and thus contribute to maintaining both ATP/NADPH at a value required for net CO2 assimilation and a trans‐thylakoidal ΔpH large enough to regulate the photosynthetic photochemistry and electron transfer in a fluctuating environment.
Fluxes of electrons in the photosynthetic membrane are highly regulated and depend strongly on both substrate availability and the redox state of the transfer paths. In C3 plants particularly, decreasing the ambient CO2 molar fraction under saturating light (Rubisco‐limited situation) causes a decline in the whole chain electron transport rate, while under limiting light (electron‐limited situation) whole chain electron transport remains unchanged even at very low Ca (Badger, 1985; Cornic and Briantais, 1991).
The extent to which drought affects the rate of electron transfer in the thylakoid membranes and the partitioning of the electrons produced by PSII activity between the potential acceptors in the chloroplasts has been a matter of debate in the past decade. A discussion of electron transfer during drought must first include both an assessment of the resistance of the photosynthetic system to drought, and an understanding of the substrates available to the chloroplasts in this condition. Here we summarize evidence showing that the photosynthetic apparatus is resistant to drought, and that when stomata close in dehydrating plants, the electrons produced by PSII are mainly used to drive the oxygenation of RuBP. We also show that CO2‐sensitive photosynthetic reactions are inhibited in pea plants growing at low temperature and subjected to drought.
THE PHOTOSYNTHETIC APPARATUS IS RESISTANT TO DROUGHT
It has often been shown that the maximum quantum yield of PSII photochemistry measured by the ratio Fv/Fm (the variable to the maximum chlorophyll fluorescence emissions) after a long period of dark adaptation is not changed even by a severe drought (relative water content of 50 to 40 %; e.g. Cornic et al., 1987; Genty et al., 1987). Likewise the maximum quantum yield of PSI measured at the temperature of liquid nitrogen is not affected in this condition (Cornic et al., 1987). As a whole, linear electron transfer is very resistant to drought ( Kaiser et al., 1981; Sharkey and Badger, 1982; Genty et al., 1987) and can remain unchanged in isolated Xanthium strumarium mesophyll cells (Sharkey and Badger, 1982) down to a water potential of –4·0 MPa, showing an inhibition of only about 20 % at –6·4 MPa.
Figure 1 shows variation in ΦPSII (the operating quantum yield of PSII photochemistry), qp (a relative estimation of open PSII centres) and Fv′/Fm′ (the quantum yield of open PSII centres) as a function of ΦCO2 (the quantum yield of gross CO2 uptake) measured on Zea mays L. leaves in normal air (21 % O2 + N2). As shown by Genty et al. (1989) these three parameters are related as followed:
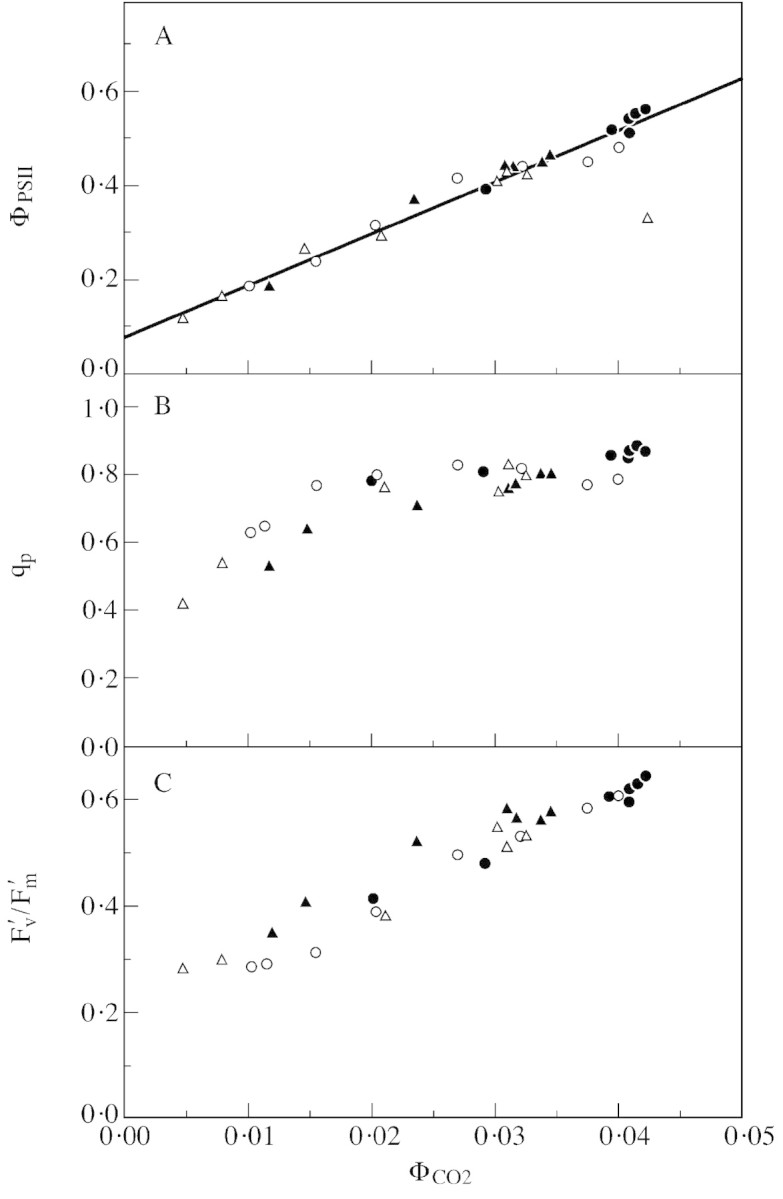
Fig. 1. ΦPSII (A), qp (B) and Fv′/Fm′ (C) as a function of ΦCO2. Measurements were made on Zea mays L. plants either at different ambient CO2 molar ratios (Ca) on well‐hydrated leaves (closed circles) or after water was withheld, on dehydrating leaves (open circles) at normal Ca (about 360 ppm). Gas exchange chlorophyll fluorescence emission measurements were coupled as described in Cornic and Briantais (1991). Conditions during measurements were as follows: leaf temperature, 23·5 ± 0·6 °C; O2 molar ratio, 21 % in nitrogen; vapour pressure deficit (VPD), 0·7 ± 0·1 kPa; photon flux density (PFD), 450 µmol m–2 s–1 (K. Saccardy and G. Cornic, unpubl. res.).
ΦPSII = qp(Fv′/Fm′)
Measurements were made on leaves of plants growing in a growth cabinet and either watered regularly or allowed to dehydrate slowly. In both cases the relationships were the same, showing clearly that drought did not change PSII functioning or the regulation of its functioning. In agreement with Cornic (1994), measurements also showed that drought did not induce the build‐up of an extra electron sink.
In Z. mays leaves, the rate of electron transfer to sinks other than CO2 was low (see the intercept with the vertical axis of the relation between ΦPSII and ΦCO2). Similar results were obtained with C3 plants in nitrogen with 1 % O2 to inhibit photorespiration (see Cornic, 1994). Provided the relationship between ΦPSII and ΦCO2 (as in Fig. 1) is the same for well‐watered and drying plants, it is possible to use ΦPSII to calculate the rate of whole chain electron transport during drought, assuming that the way the electrons are used does not change the quantum yield of PSII photochemistry. In this case ΦPSII can also be used directly as an indicator of the relative variation of whole chain electron transfer if the drying plants are under constant light.
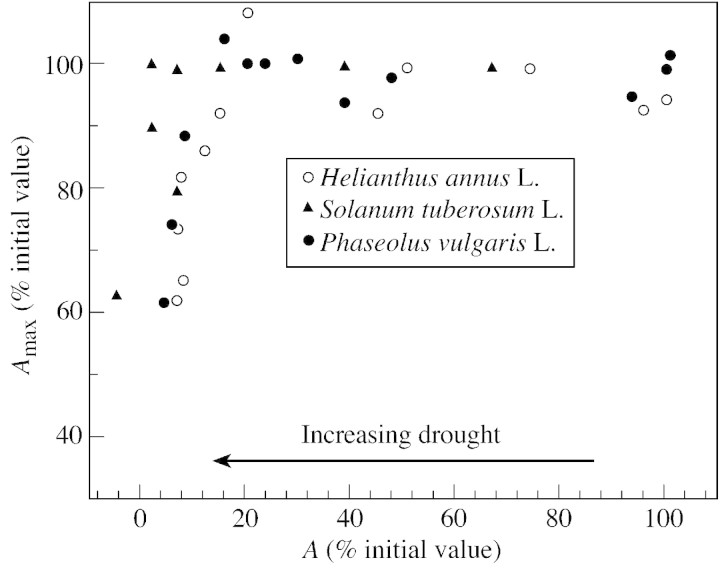
In addition, measurements performed at very high Ca show that the mechanisms that are responsible for the maximum rate of photosynthesis, including ATP synthesis, are resistant to drought (Fig. 2). However, data from the literature are not easy to compare as the variations A (net CO2 assimilation by leaves) measured at normal Ca (about 350 ppm) and Amax measured at elevated Ca (up to 10 %) during drought are plotted either as a function of leaf relative water deficit or leaf water potential. A comparison between these two types of data becomes possible if A itself is taken as an indicator of the desiccation state of the leaves: a decrease in A indicating an increase in desiccation. Figure 2 does indeed show that elevated Ca could restore the full capacity of photosynthesis (Amax) of P. vulgaris (Cornic et al., 1989) and of H. annus (Graan and Boyer, 1990) until A measured at normal Ca was inhibited by 80 % by desiccation. An inhibition of A by 80 % corresponds to a relative leaf water deficit of about 25 % and to a leaf water potential of –1·2 MPa for P. vulgaris and H. annuus, respectively. The range of leaf water deficit over which Amax remains changed, or changes only little, although A declines, could characterize what is termed in this paper ‘a mild drought’. Results obtained by Tourneux and Peltier (1995) on Solanum tuberosum are also plotted (Fig. 2). They show a very similar relationship although they were obtained by measuring 16O2 and 18O2 using mass spectrometry. It is worth noting that in the three experiments described in Fig. 2, drought was induced either by withholding the watering of whole plants (desiccation occurring in a couple of weeks in P. vulgaris or in 2–3 d in H. annuus) or by drying in the dark (S. tuberosum leaf discs). Kaiser (1987) also obtained very similar data.
Fig. 2. Relationship between Amax, the net photosynthesis (net CO2 or net O2 evolution) measured at elevated Ca (up to 10 %) and A, the net CO2 uptake by leaves maintained at normal Ca. Both Amax and A were measured during a drought. A is taken as an indicator of the water status in the leaf: it decreases as desiccation increases. Data are taken from Cornic et al. (1989, Figs 1 and 2A), Graan and Boyer (1990, Fig. 5A) and Tourneux and Peltier (1995, Figs 4C and 5).
IN C3 PLANTS, ELECTRONS PRODUCED BY PSII ACTIVITY ARE MAINLY USED TO RUN THE PCR AND PCO CYCLE
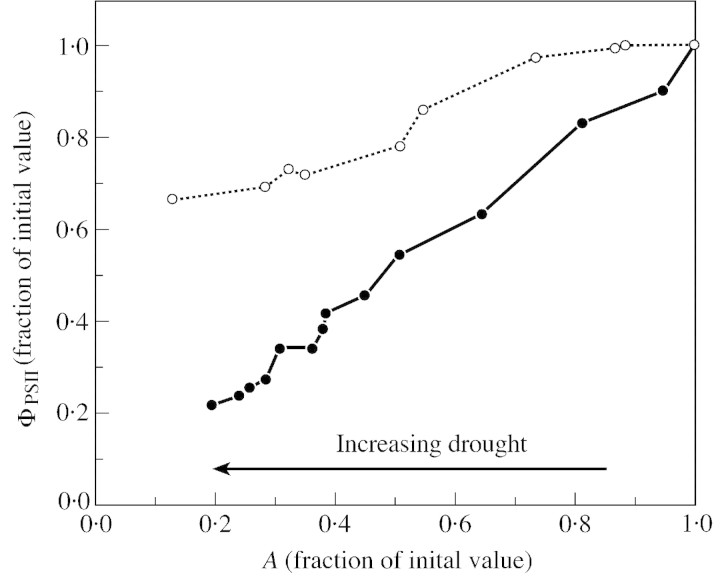
Estimation of whole chain electron transfer on intact leaves can be done either by using chlorophyll fluorescence emission measurements as explained above, or by measuring the net O2 CO2‐dependent evolution together with 18O2 uptake using a mass spectrometer. In this case, addition of the two values gives gross photosynthesis which is proportional to the whole chain electron transfer rate. Measurements performed to date by either of these methods on C3 leaves subjected to drought indicate that electrons are produced in excess of the amount needed for CO2 assimilation (e.g. Cornic and Briantais, 1991; Tourneux and Peltier, 1995; Biehler and Fock, 1996). Thomas and André (1982) were the first to note that there was a relative increase in O2 uptake while A was declining in soybean subjected to drought stress. This was largely confirmed by others. As an example, Fig. 3 shows the relative variation in ΦPSII as a function of the relative values of A measured in air containing either 21 or 1 % O2 and normal Ca (350 ppm) on dehydrating cut bean leaves. When A has decreased to 20 % of its initial value, ΦPSII is inhibited by more than 80 % in 1 % O2, while it has declined only by about 35 % in 21 % O2. This shows that oxygen can indeed efficiently replace carbon dioxide as an electron acceptor. The small decline in ΦPSII during the desiccation of the leaf in 21 % O2 could be due to a limitation of ribulose 5‐phosphate regeneration in this condition (see discussion of Fig. 6).
Fig. 3. Relationships between relative value of ΦPSII and A during desiccation of detached leaves from P. vulgaris. The O2 molar fraction during measurements was either 21 % (closed circles) or 1 % (open circles) in nitrogen. Other conditions were as follows: leaf temperature, 23·5 °C initially, increasing to 24·9 °C at the end of the measurements; PFD, 450 µmol m–2 s–1; and Ca, about 360 ppm. Relative water content of the leaves at the end of the experiments was 76·2 and 72·8 % in 21 and 1 % O2, respectively (G. Cornic, unpubl. res.).
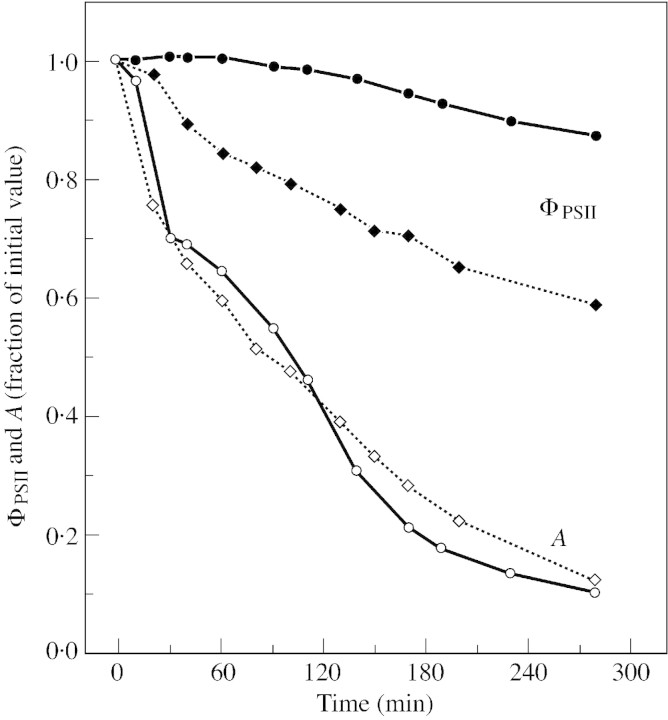
Fig. 6. Variation in relative values of ΦPSII and A during desiccation of cut leaves of L. trimestris. Chlorophyll fluorescence emission was done in parallel with net CO2 uptake during the experiments (see Figs 4 and 5). Closed symbols, ΦPSII measurements; open symbols, A measurements. Light conditions 450 (circles) or 990 (squares) µmol m–2 s–1. These measurements were performed simultaneously with the measurements presented in Fig. 4A and B.
Given the in vitro specificity factor of Rubisco, and assuming that 18O2 uptake was mainly due to photorespiratory activity, Tourneux and Peltier (1995) were able to use gas exchange data to calculate values for chloroplast CO2 molar fraction (Cc) in dehydrating potato leaves that agreed closely with published values obtained by 13C/12C isotope discrimination. This suggests that RuBP oxygenation is indeed the main path for O2 uptake. Moreover, it is possible to calculate a specificity factor for Rubisco using gas exchange and chlorophyll fluorescence emission data for P. vulgaris leaves that agrees well with published values (Cornic and Briantais, 1991; Ghashghaie and Cornic, 1994), showing that alternative electron fluxes, including the Mehler reaction, are not quantitatively important. This is also consistent with Fig. 1, which shows that drought did not induce increased alternative electron fluxes on slowly dehydrating Z. mays leaves. Using transgenic tobacco plants with reduced Rubisco activity and a large electron transport capacity relative to Rubisco oxygenase, Ruuska et al. (2000) came to the same conclusion, both at high Ca and at the CO2 compensation point which is supposed to mimic a drought‐stressed condition. Consistently, Badger et al. (2000) could not see any increase in the Mehler reaction in dehydrating Madrone (Azbutus menziesii) leaves.
As noted by Noctor and Foyer (1998), in the absence of ATP consumption, the Mehler reaction might not occur because of an efficient photosynthetic control in this condition. In a leaf under water stress and having a low photosynthetic rate, ATP consumption is likely to be low.
NET CO2 UPTAKE BY LEAVES SUBJECTED TO DESICCATION DECLINES BECAUSE STOMATAL CLOSURE REDUCES CO2 AVAILABILITY TO THE CHLOROPLASTS
The above discussion provides a model that can be tested: if the proportion of photosynthetic electrons allocated to the reduction of O2 increases in the chloroplasts during drought because of photorespiratory activity, then we expect the CO2 molar fraction in the leaf to decrease at the same time (Fig. 3).
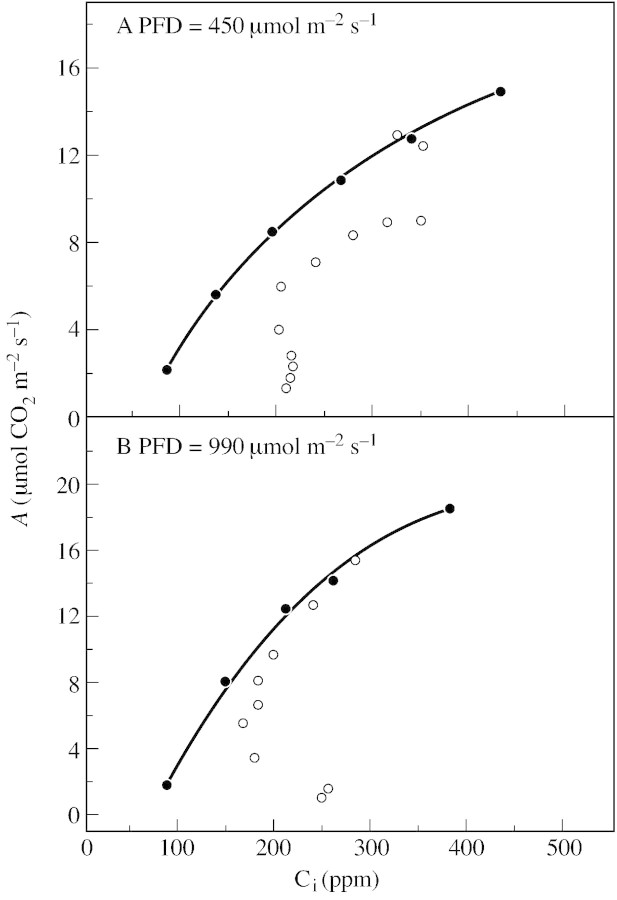
Figure 4 shows the relationship between A and Ci (the CO2 molar fraction at the evaporating sites within the leaf) obtained first by changing Ca around a Lavatera trimestris L. (var. splendens) leaf and secondly by dehydrating the same leaf rapidly (within 4 h) at normal Ca. The experiment was done both under limiting and nearly saturating light. Although there was a clear decline in Ci when the leaves began to wilt, the A/Ci curves during dehydration were not the same as the A/Ci curves measured on well‐hydrated leaves. The differences between the two were particularly obvious for measurements done under low light (Fig. 4A) and clearly evident when Ci was lower than 200 ppm for measurements done under high light (Fig. 4B). The differences can be attributed to both patchy stomatal closure (which might have been important because of the rapid dehydration of the leaves) and, when the fluxes are low, to the increasing importance of cuticular transpiration (Boyer et al., 1997). However, what it is really important to know is the CO2 molar fraction at the chloroplast level, Cc, or else a relative value of Cc.
Fig. 4. Relationship between A and the leaf internal CO2 molar ratio (Ci) measured on detached Lavatera trimestris L. leaves either before (well‐hydrated, closed circles) or after (open circles) a rapid desiccation. Plants were grown in winter, in a peat soil, in a temperature‐controlled glasshouse in the University of Illinois at Urbana‐Champaign, USA (temperature from about 20 °C during the day to 17 °C during the night). Measurements were made with a LICOR 6400 under either a PFD of 450 (A) or 990 (B) µmol m–2 s–1. Other conditions were as follows: leaf temperature 25 ± 0·3 °C; Ca, 350 ppm; and VPD, 1·5 ± 0·5 kPa. Relative water content of the leaves at the end of the experiments was 70·4 and 67·7 % for A and B, respectively (G. Cornic and S. Long, unpubl. res.).
Knowing the relation between ΦPSII and ΦCO2 in a non‐photorespiratory condition for L trimestris leaves, and using parallel measurements of ΦPSII during the two sets of measurements described in Fig. 4, it is possible to calculate the rate of carboxylation (Jc) and the rate of oxygenation (Jo) by the leaves (Epron and Cornic, 1993). As the oxygen uptake in this situation is mainly due to oxygenase activity (see the discussion in the preceding section), Jc/Jo is related to Cc as follows:
Jc/Jo = S(Cc/O)
where O is the concentration of oxygen dissolved in the chloroplasts and S the O2/CO2 specificity factor of Rubisco.
As the O concentration is not expected to change much during these measurements, this relation shows that plotting A as a function of Jc/Jo is equivalent to plotting A as a function of Cc if S remains constant.
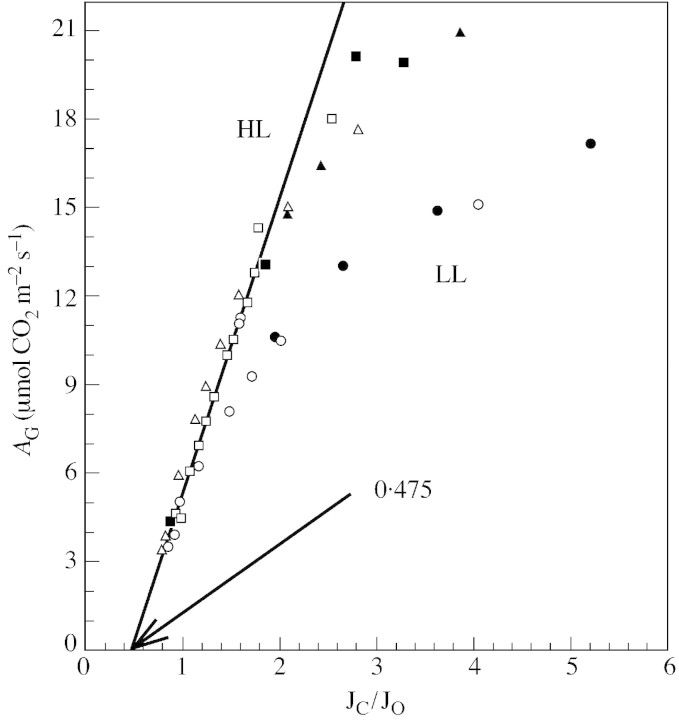
Figure 5 shows that the relationship between AG (estimation of gross photosynthesis: = A + dark respiration) and Jc/Jo is the same when A is measured at different Ca or at the same Ca when the leaf is dehydrating (data from Fig. 4 and additional measurements). This observation strongly suggests that: (1) net CO2 uptake during dehydration declines only because the CO2 molar fraction is declining in the chloroplasts; and (2) S is not changed during desiccation. It is worth noting that the value of Jc/Jo shown in Fig. 5 is what we expect at the different Ci assuming a specificity factor of 80, which is commonly measured in C3 plants, and that the Jc/Jo ratio at Γ* (when AG = 0) is very close to the theoretical value of 0·5.
Fig. 5. Relation between AG (estimation of gross photosynthesis = A + dark respiration) and Jc/Jo where Jc is the rate of RuBP carboxylation and Jo the rate of RuBP oxygenation calculated as in Epron and Cornic (1993) by measuring chlorophyll fluorescence emission (with an FMS2, Hansatech fluorometer) in parallel with net CO2 uptake during the experiments presented in Fig. 4. As explained in the text, the ratio Jc/Jo is proportional to Cc, the carbon dioxide molar fraction in the chloroplasts. Measurements were performed at a PFD of 450 µmol m–2 s–1 (LL, circles) or under a PFD of 990 µmol m–2 s–1 (HL, triangles and squares), on well‐hydrated leaves (closed symbols) at different Ca, or during dehydration of the cut leaves at constant Ca (open symbols). Data were taken from Fig. 4A and B (circles and triangles). Other data from measurements taken under a PFD of 990 µmol m–2 s–1 were added (squares). Experimental conditions as in Fig. 4. The correlation line was calculated for all data corresponding to Jc/Jo < 1·4 (G. Cornic and S. Long, unpubl. res.).
Using these data, it is also possible to calculate the actual Cc values assuming that Γ* = 42·5 ppm at a leaf temperature of 25 °C (Brooks and Farquhar, 1985; Bernacchi et al., 2001) and considering the relationship:
Cc = 2Γ* (Jc/Jo)
Cc values for leaves maintained under high light at normal Ca are about 50 ppm lower than Ci, and the ratio of Cc/Ca is 0·47 and 0·57 when Ca = 340 ppm, depending on the assumption about dark respiration when calculating Jc and Jo. These values are in good agreement with published values (for a discussion see Tourneux and Peltier, 1995).
During dehydration of L. trimestris leaves (Figs 4 and 5), A declines in a similar fashion in high and low light (Fig. 6), and is less than 20 % of its initial value after 5 h of dehydration. However, ΦPSII decreased by only 10 and 40 % under low and high light, respectively. It is worth noting that under low light after 2 h of dehydration, ΦPSII had hardly changed whereas A was already inhibited by about 60 %. This is to be expected if A is limited by Cc during desiccation: under limiting light (electron‐limiting situation) the electron transfer rate does not decline, but it does under high light (Rubisco‐limiting situation) (Badger, 1985). A similar observation was made by Biehler and Fock (1996) who measured whole chain electron transport (measurement of 16O2 and 18O2 by mass spectrometry) both under saturating and limiting light, together with net CO2 uptake, by slowly dehydrating leaves of Triticum plants. Thus we expect an absolute increase in photorespiration rate in C3 leaves dehydrating under limiting light and only a relative (compared with net CO2 uptake) increase when C3 leaves are dehydrating under saturating light.
DROUGHT CAN ALSO INDUCE INHIBITION OF PHOTOSYNTHETIC METABOLISM WHICH IS SENSITIVE TO CO2
Figure 7 shows the relationship between ΦPSII and A measured at a leaf temperature of 23·5 ± 0·6 °C during slow dehydration (10–15 d after water was withheld) of Pisum sativum L. plants grown either at normal (25/20 °C, day/night) or low temperature (8–9/6 °C, day/night). ΦPSII of plants grown at normal temperature remained constant while net CO2 uptake by dehydrating leaves decreased by about 35 %. In contrast, ΦPSII declined as soon as A was inhibited by drought in plants grown at low temperature. In both growth conditions the relation between qp and A during drought was similar to the relationship between ΦPSII and A. In plants grown at normal temperature, there was clearly another electron acceptor during the early stage of the drought (presumably O2; see Fig. 3) which entirely replaced CO2.
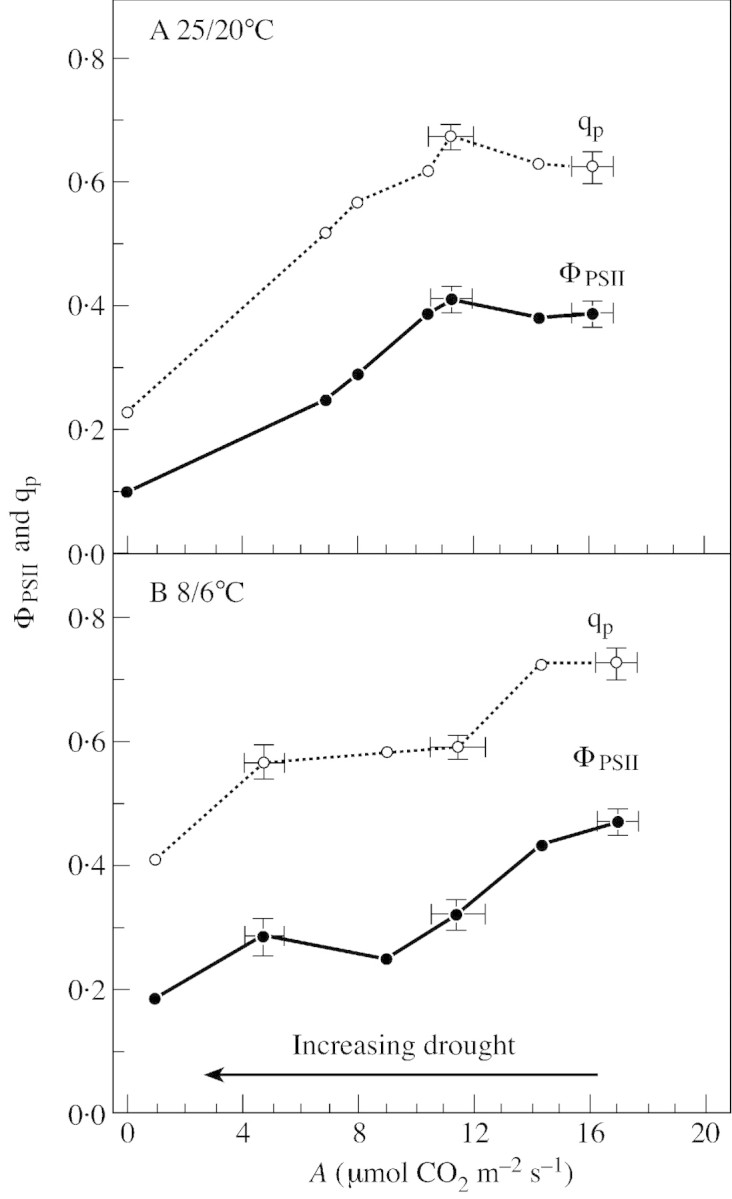
Fig. 7. Variation in ΦPSII and qp as a function of A during dehydration of Pisum sativum L. plants obtained by withholding water. Plants were grown on a peat soil in a growth cabinet and watered every 2 d. A, Plants grown at 25/20 °C (day/night), 16 h light period (normal temperature); relative water content of the leaves was about 62 % at the end of the experiment. B, Plants grown at 8–9/6 °C (day/night), 16 h light period (low temperature); relative water content of the leaves was about 69 % at the end of the experiment. In both conditions, PFD at the top of the plants during growth was 350 µmol m–2 s–1. Measurements were made as explained in Fig. 1. Conditions during measurements were: leaf temperature, 23·5 ± 0·5 °C; Ca, 360 ± 5 ppm; VPD, 0·6 ± 0.2 kPa; and PFD, 450 µmol m–2 s–1. The standard deviation of the mean is shown when it was possible to calculate the mean of three to five close measurements (N. Manuel and G. Cornic, unpubl. res.).
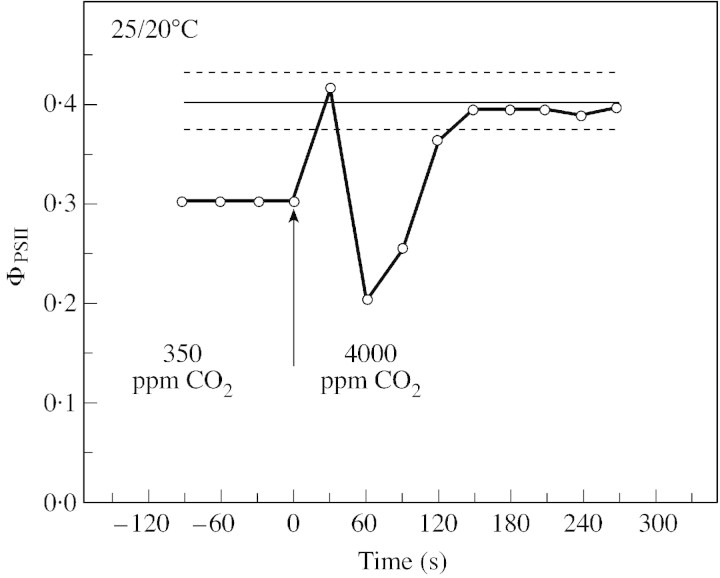
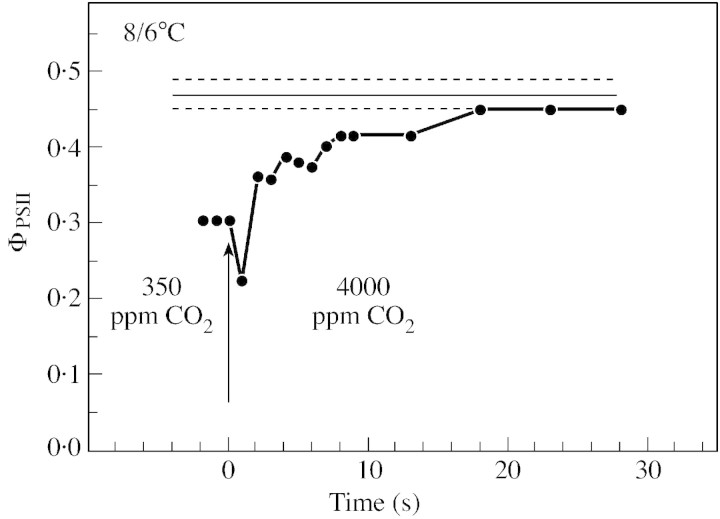
To check whether the decline in ΦPSII observed during drought was due to an inhibition of whole chain electron transport by low Cc or by damage to photosynthetic processes caused by the decreasing water content of the leaves, we abruptly increased Ca from 350 to 4000 ppm around wilted leaves. For plants grown at normal temperature (Fig. 8), such a shift in Ca values caused an immediate increase in ΦPSII, in an oscillating mode, from 0·3 to the control value, showing that whole chain electron transfer was limited only by the availability of carbon dioxide. The effect of drought on ΦPSII of leaves of plants grown at low temperature was also removed when Ca was increased to 4000 ppm. However, the increase from 0·3 to the control value was much slower and, as shown in Fig. 9, was only complete after about 20 min. In plants grown at low temperature, it is likely that an inhibition of CO2‐sensitive photosynthetic reaction(s) was induced during drought. Comparing ABA‐treated with drought‐stressed leaves, Graan and Boyer (1990) noted that ‘changes’ other than stomatal closure occur ‘at low leaf water potential that are not present in ABA‐treated leaves’. These authors concluded ‘the nature of these changes is not known but appears to involve an altered but CO2‐sensitive photosynthetic metabolism’. Inactivation of Rubisco by low Ci in wilted leaves might be part of these CO2‐sensitive processes, as suggested by Meyer and Genty (2000). However, because ABA‐treated leaves, in which Ci is expected to be low, did not behave like wilted leaves (Graan and Boyer, 1990) and because high CO2 slowly removed the inhibition of ΦPSII in dehydrated leaves of pea plants grown at low temperature (Fig. 9), all the CO2‐sensitive inhibition cannot be due to desiccation. Sucrose Phosphate Synthase (SPS) activity is generally inhibited during drought (Vassey and Sharkey, 1989; Vassey et al., 1991; Castrillo, 1992), due presumably to the low carbon dioxide molar fraction prevailing in the leaf when stomata close. Moreover this inhibition can be largely removed at elevated Ca (Vassey et al., 1991). Such a decline in SPS activity in dehydrating leaves could lead to a Pi limitation of photosynthesis. We can thus hypothesize that exportation of triose phosphates is involved in the CO2‐sensitive process inhibited by drought. Presumably, this effect could depend both on growth conditions, as shown here by the comparison between P. sativum plants grown at normal and low temperature, and on the plant species.
Fig. 8. Typical variation over time of ΦPSII when Ca was changed abruptly from 350 to 4000 ppm around dehydrated leaves of P. sativum plants grown at normal temperature (relative water content of the leaf = 68·2 %). Other experimental conditions were as in Fig. 7. The line parallel to the x‐axis is the mean ΦPSII value measured on well‐hydrated leaves ± s.e. (dotted lines, n = 4).
Fig. 9. Typical variation over time of ΦPSII when Ca was changed abruptly from 350 to 4000 ppm around dehydrated leaves of P. sativum plants grown at low temperature (relative water content of the leaf = 74·5 %). For details see Fig. 8 legend.
CONCLUSIONS
Up to a leaf relative water content of about 70 %, it is likely that stomatal closure plays the main role in the decline in leaf photosynthesis and that the photosynthetic machinery remains intact. This allows the leaf to respond rapidly to changes in the water status of the environment. As shown here in Fig. 2, the lower limit of this ‘safe’ range might be given by the leaf relative water content (or the leaf water potential) causing about 80 % of inhibition of net CO2 uptake by the leaf under drought. Under such circumstances, oxygenation of RuBP in C3 plants can efficiently replace carboxylation of RuBP when the stomata close. Nevertheless, in this situation the activity of whole chain electron transport can be down‐regulated if the electron flow is sink‐limited, as is likely to occur when the leaf is exposed to saturating or nearly saturating light conditions. This will of course cause a concomitant increase in non‐radiative dissipation of the energy trapped at the PSII level as discussed by Weis et al. (1987) and Genty et al. (1989). As shown here in Fig. 1 and detailed in Cornic (1994), drought does not qualitatively change the regulation of PSII activity. However, depending on growth conditions and on the plant species, drought could inhibit some parts of the photosynthetic metabolism. As shown in Fig. 9, this inhibition is removed by high CO2. This type of response to stomatal closure (Cornic, 1994) implies that some reactions of photosynthetic metabolism are down‐regulated, presumably due to low CO2.
ACKNOWLEDGEMENTS
Thanks to L. Ainsworth, C. Bernacchi and Dr S. Naidu for critical reading and language corrections.
Supplementary Material
Received: 20 May 2001; Returned for revision: 17 October 2001; Accepted: 3 December 2001.
References
- ArnonDI, Chain RK.1975. Regulation of ferredoxin‐catalysed photosynthetic phosphorylation. Proceedings of the National Academy of Sciences of the USA 72: 4961–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AsadaK.1999. The water to water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50: 601–639. [DOI] [PubMed] [Google Scholar]
- BadgerMR.1985. Photosynthetic oxygen‐exchange. Annual Review of Plant Physiology 36: 27–53. [Google Scholar]
- BadgerMR, von Caemmerer S, Ruuska S, Nakano H.2000. Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philosophical Transactions of the Royal Society of London B 355: 1433–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BernacchiCL, Singsaas EL, Pimentel C, Portis Jr AR, Long SP.2001. Improved temperature response functions for models of Rubisco‐limited photosynthesis. Plant Cell and Environment 24: 253–259. [Google Scholar]
- BiehlerK, Fock H.1996. Evidence for the contribution of the Mehler peroxidase reaction in dissipating excess electrons in drought‐stressed wheat. Plant Physiology 112: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BoyerJS, Wong SC, Farquhar GD.1997. Gas exchange across leaf cuticule at various water potentials. Plant Physiology 114: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BrooksA, Farquhar GD.1985. Effect of temperature on the CO2/O2 specificity of ribulose‐1, 5‐bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165: 397–406. [DOI] [PubMed] [Google Scholar]
- CastrilloM.1992. Sucrose metabolism in bean plants under water deficit. Journal of Experimental Botany 43: 1557–1561. [Google Scholar]
- ChampignyML.1995. Integration of photosynthetic carbon and nitrogen metabolism in higher plants. Photosynthesis Research 46: 117–127. [DOI] [PubMed] [Google Scholar]
- CornicG.1994. Drought stress and high light effects on leaf photosynthesis. In: Baker N, Bowyer JS, ed. Photoinhibition of photosynthesis. Oxford: Bios. [Google Scholar]
- CornicG, Briantais JM.1991. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 183: 178–184. [DOI] [PubMed] [Google Scholar]
- CornicG, Papageorgiou I, Louason G.1987. Effects of a rapid and a slow drought cycle followed by rehydration on stomatal and non‐stomatal components of leaf photosynthesis in Phaseolus vulgaris L. Journal of Plant Physiology 126: 309–318. [Google Scholar]
- CornicG, le Gouallec JL, Briantais JM, Hodges M. 1989 Effect of a high light treatment during a drought stress on photosynthetic capacities of two C3 plants: Phaseolus vulgaris and Elatostema repens Planta 177: 84–90. [DOI] [PubMed] [Google Scholar]
- EpronD, Cornic G.1993. The use of chlorophyll fluorescence to investigate the effects of dehydration on leaf photosynthesis. Current Topics in Plant Physiology 1: 177–183. [Google Scholar]
- FoyerCH, Noctor G.2000. Oxygen processing in photosynthesis: regulation and signalling. New Phytologist 146: 359–388. [Google Scholar]
- GentyB, Briantais JM, Baker NR.1989. The relationships between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- GentyB, Briantais JM, Viera da Silva JB.1987. Effects of drought on primary photosynthetic processes of cotton leaves. Plant Physiology 83: 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GhashghaieJ, Cornic G.1994. Effect of temperature on partitioning of photosynthetic electron flow between CO2 and O2 reduction and on the CO2/O2 specificity of rubisco. Journal of Plant Physiology 143: 642–650. [Google Scholar]
- GraanT, Boyer JS.1990. Very high CO2 partially restores photosynthesis in sunflower at low water potentials. Planta 181: 378–384. [DOI] [PubMed] [Google Scholar]
- KaiserWH.1987. Effect of water deficit on photosynthetic capacity. Physilogia Plantarum 71: 142–149. [Google Scholar]
- KaiserWM, Kaiser G, Prachuab PK, Wildman SG, Heber U. 1981. Photosynthesis under osmotic stress. Inhibition of photosynthesis of intact chloroplasts, protoplasts and leaf slices at high osmotic potentials. Planta 167: 416–422. [DOI] [PubMed] [Google Scholar]
- MeyerS, Genty B.2000. Heterogeneous inhibition of photosynthesis over the leaf surface of Rosa rubiginosa L. during water stress and abscisic acid treatment: induction of a metabolic component by limitation of CO2 diffusion. Planta 210: 126–131. [DOI] [PubMed] [Google Scholar]
- NoctorG, Foyer CH 1998. A re‐evaluation of the ATP:NADPH budget during C3 photosynthesis: a contribution from nitrate assimilation and its associated respiratory activity? Journal of Experimental Botany 49: 1895–1908. [Google Scholar]
- RuuskaSA, Badger MR, Andrews TJ, von Caemmerer S.2000. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. Journal of Experimental Botany 51: 357–368. [DOI] [PubMed] [Google Scholar]
- ScheibeR.1987. NADP+‐malate dehydrogenase in C3‐plants: regulation and role of a light‐activated enzyme. Physiologia Plantarum 71: 393–400. [Google Scholar]
- SharkeyTD, Badger MR.1982. Effects of water stress on photosynthetic electron transport, photophosphorylation, and metabolite levels of Xanthium strumarium mesophyll cells. Planta 156: 199–206. [DOI] [PubMed] [Google Scholar]
- ThomasDA, André M 1982. The response of oxygen and carbon dioxide exchanges and root activity to short term water stress in soybean. Journal of Experimental Botany 33: 393–405. [Google Scholar]
- TourneuxC, Peltier G.1995. Effect of water deficit on photosynthetic oxygen exchange measured using 18O2 and mass spectrometry in Solanum tuberosum L. leaf discs. Planta 195: 570–577. [Google Scholar]
- VasseyTL, Sharkey TD.1989. Mild water stress of Phaseolus vulgaris plants leads to reduced starch synthesis and extractable surcrose phosphate synthase activity. Plant Physiology 89: 1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VasseyTL, Quick PW, Sharkey TD, Stitt M.1991. Water stress, carbon dioxide, and light effects on sucrose‐phosphate synthase activity in Phaseolus vulgaris Physiologia Plantarum 81: 37–44. [Google Scholar]
- VonCaemmererS.2000. Biochemical models of leaf photosynthesis. Techniques in plant sciences. No. 2. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- WeisE, Berry JA.1987. Quantum efficiency of photosystem‐II in relation to energy‐dependent quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 894: 198–208. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.