Abstract
Drought‐affected plants experience more than just desiccation of their organs due to water deficit. Plants transpire 1000 times more molecules of water than of CO2 fixed by photosynthesis in full sunlight. One effect of transpiration is to cool the leaves. Accordingly, drought brings about such multi‐stresses as high temperatures, excess photoradiation and other factors that affect plant viability. Wild watermelon serves as a suitable model system to study drought responses of C3 plants, since this plant survives drought by maintaining its water content without any wilting of leaves or desiccation even under severe drought conditions. Under drought conditions in the presence of strong light, wild watermelon accumulates high concentrations of citrulline, glutamate and arginine in its leaves. The accumulation of citrulline and arginine may be related to the induction of DRIP‐1, a homologue of ArgE in Escherichia coli, where it functions to incorporate the carbon skeleton of glutamate into the urea cycle. Immunogold electron microscopy reveals the enzyme to be confined exclusively to the cytosol. DRIP‐1 is also induced by treating wild watermelon with 150 mm NaCl, but is not induced following treatment with 100 µm abscisic acid. The salt treatment causes the accumulation of γ‐aminobutyrate, glutamine and alanine, in addition to a smaller amount of citrulline. Citrulline may function as a potent hydroxyl radical scavenger.
Key words: Wild watermelon (Citrullus lanatus L.); drought; photosynthesis; electron sink; active oxygen; ascorbate peroxidase; citrulline; arginine; γ‐aminobutyrate, ArgE
HOW DOES DROUGHT AFFECT PLANTS?
The total photon flux density of visible light from the sun is 2200 µmol m–2 s–1 on the surface of the ground. Ten to twenty per cent of photons are reflected from the leaf surface and transmitted through leaves (Geller and Nobel, 1984). The remaining photons cause physical, chemical or biological events in leaves, to a greater or lesser extent, corresponding to a photon flux density of 120 cal m–2 s–1. A small fraction of this energy may be dissipated as fluorescent light. Plant leaves use yet another portion of energy from incident light for fixing CO2. The rate of net carbon assimilation of C3 plants is usually 20–30 µmol m–2 s–1 in the ordinary atmosphere (Miyake and Yokota, 2000). Carbon fixation and reduction utilizes 4·4–6·8 cal m–2 s–1 (Fig. 1). Thus plant leaves use only 3·7–5·7 % of total photon energy received by chlorophyll. The remaining 114–116 cal m–2 s–1 of the sun’s energy is dissipated as heat, either through physical or biochemical events in the leaves. Some of the heat may be evolved through the dissipation of photon energy by reducing oxygen in photosystem I (PSI) and the subsequent decomposition of active oxygens formed there (Smirnoff, 1993; Asada, 1999). The flux of electrons in PSI to oxygen increases with increasing photon flux densities, and may exceed the flux of electrons to the carbon reduction cycle under full sunlight (Miyake and Yokota, 2000). Evaporation of water molecules through the stomata can cool plant leaves to an adequate temperature (Hashimoto et al., 1984). The rate of transpiration reaches 6–10 mmol H2O m–2 s–1 (Larcher, 1995), which is enough to dissipate the heat released in full sunlight at a rate of 80–130 cal m–2 s–1. Thus, the loss of water through transpiration is not simply an inevitable consequence of incorporating CO2 from the atmosphere into the leaves, but also has a positive function: that of dissipating heat safely (Lange, 1959; Hashimoto et al., 1984; Larcher, 1995). However, what happens when the amount of available water is decreased or severely limited? The first option is for plants to close their stomata (Cornic and Massacci, 1996). This decreases the inflow of CO2 into the leaves and directs more electrons to the formation of active oxygen species. As the rate of transpiration decreases, so too does the amount of heat that can be dissipated safely by this method. Plants suffer from multiconstraints, including injury of cell components by active oxygen and increasing temperature, under these conditions. Thus, drought is stressful and dangerous to plants, particularly those exposed to direct sunlight, and gives rise to many physiological responses in such plants.
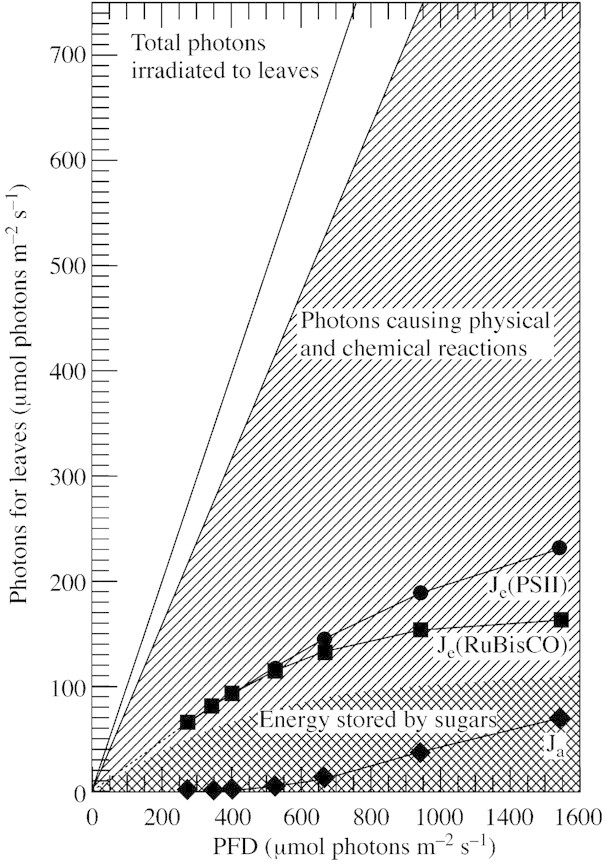
Fig. 1. Efficiency of light energy utilization by plant leaves. The diagonally hatched area represents total photons captured by leaves. Je(PSII) is the flux of electrons passing through photosystem II to the electron transport chain. Je(RubiscO) is the flux of electrons to photosynthetic carbon reduction and oxidation cycles. The difference between Je(PSII) and Je(Rubisco), [Je(PSII) – Je(Rubisco)] corresponds to Ja, the electron flux in alternative electron transport, composed of the flux to molecular oxygen in photosystem I and the flux in the photosystem II cyclic electron transport. The net carbon assimilation rate is assumed to correspond to the amount of energy stored in sugars (cross‐hatched shading). Note that photon energy corresponding to the area depicted by the diagonal lines must be discarded as heat.
IDEAL MODEL PLANTS FOR LEARNING ABOUT DROUGHT
The critical factor in determining whether or not a plant is a suitable model for studying drought response lies in its ability to survive exposure to such conditions. In analysing the responses of such plants, one may learn how the plant react to drought. Domesticated plants, which have been exploited for thousands of years, are selected for their productivity and the quality of their crops. Many genes that were functional in wild habitats may have been silenced, but the genes for productivity and quality are strongly expressed in crop plants. Crop plants show many responses when they are subjected to drought. However, it should be noted that many of these responses are death‐directed responses.
There are many species that can survive drought in the presence of full sunlight (Larcher, 1995). Succulent (CAM) plants are an extreme example. These plants close their stomata in the daytime and can survive drought without transpiration. Ice plants can induce the CAM system and other protective measures to survive drought and saline environments (Bohnert et al., 1999; Cushman and Bohnert, 1999). These plants differ from crop plants in their photosynthetic mechanism and morphological appearance.
In the Botswana desert, annual precipitation is about 200 mm and rain is restricted to the spring and summer (Gibson, 1996). In the desert, annual plants grow up during these wetter seasons but can be injured by severe drought after the rains. After C4 grasses have turned yellow because of a deficit of available water, one C3 species still remains green and survives for a longer period. This plant is wild watermelon. Ecologically, wild watermelon is known to be a xerophyte and can tolerate arid environments by maintaining its water status (Larcher, 1995). This is accomplished by extending its developed, geophyte‐type root system into deep ground water. Interestingly, this plant is still drought resistant despite having an underdeveloped root system when grown in a small pot in a laboratory growth chamber (Fig. 2). When 3‐week‐old wild watermelon plants are subjected to drought conditions by withholding water and maintaining the plants at 35 °C under 1000 µmol m–2 s–1 illumination, the stomata gradually close with decreasing soil water content (Kawasaki et al., 2000). Transpiration and net CO2 assimilation stop within 2 d. However, the plants survive these severe drought conditions for at least an additional 5 d without any detectable transpiration and net CO2 assimilation, and without any loss of leaf water. How the plants manage the light energy captured by leaves under these conditions was the initial focus of this study. The strategy adopted by wild watermelon plants to survive drought is discussed with reference to previously published and new data.

Fig. 2. Drought‐tolerant and susceptible plants under drought in the light. Three‐week‐old wild watermelon, cucumber and radish plants in the same pot were subjected to stress for 5 d by withholding watering. Note that the radish plants were already stressed by high temperature and high light intensity before the drought stress experiments.
RESPONSES OF WILD WATERMELON TO DROUGHT
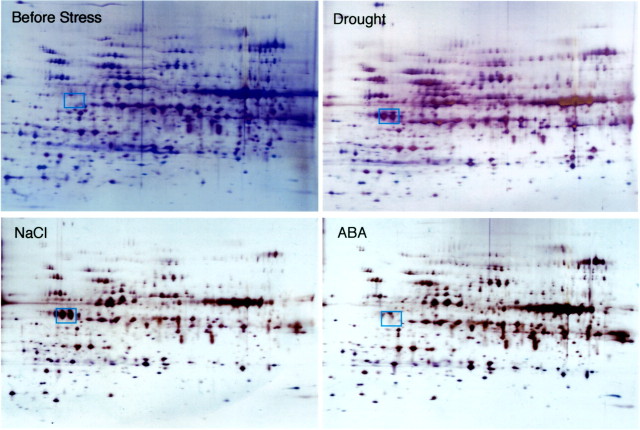
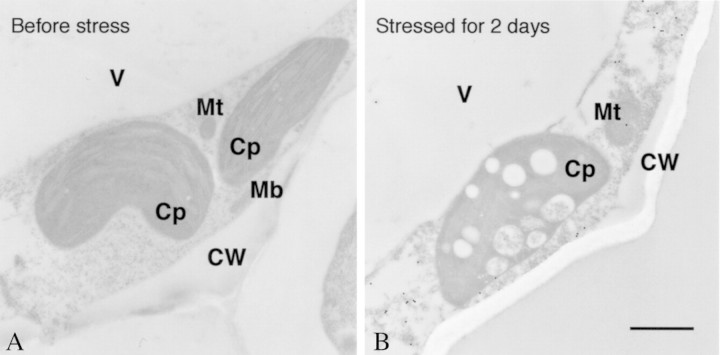
Resolving total soluble proteins by two‐dimensional electrophoresis is a powerful tool by which to analyse changes in the protein composition of the leaves of stressed plants. As drought progresses, the concentration of several peptides increases in wild watermelon leaves (Fig. 3). One of these drought‐induced peptides (DRIP), DRIP‐1, is produced in abundance. The DRIP‐1 cDNA has been cloned based on its N‐terminal sequence (Kawasaki et al., 2000), and is a homologue of the ArgE/DapE/Acy1/CPG2/YscS family (Biagini and Puigserver, 2001). ArgE is N‐acetylornithine deacetylase, and catalyses hydrolysis of the N‐acetyl group of N‐acetylornithine to give ornithine and acetate in Escherichia coli (Meinnel et al., 1992). This reaction is the final step of the acetyl cycle for arginine synthesis, which serves to incorporate the carbon skeleton of glutamate into the urea cycle (Fig. 4). The whole sequence of the cDNA has no extra sequence for translocation of the translated peptide into an organelle (Kawasaki et al., 2000). In fact, immunogold electron microscopy with the antibody raised against a synthetic peptide revealed gold particles exclusively in the cytosol of the mesophyll cells of wild watermelon leaves (Fig. 5). No particles were seen in chloroplasts or mitochondria. This observation is consistent with the evidence from the deduced amino acid sequence for the cDNA for DRIP‐1.
Fig. 3. Two‐dimensional electrophoresis of the soluble fraction of the third leaves of wild watermelon plants stressed by drought, 100 µm ABA and 150 mm NaCl for 2 d. Experiments were performed as reported previously (Kawasaki et al., 2000). DRIP‐1 is marked with squares.
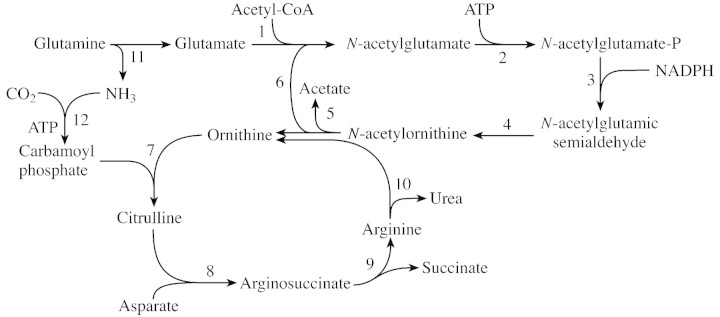
Fig. 4. Pathway for citrulline and arginine synthesis from glutamate. The names of enzymes functioning in the metabolic steps from 1 to 12 are as follows: 1, N‐acetylglutamate synthase; 2, N‐acetylglutamate kinase; 3, N‐acetylglutamate phosphate reductase; 4, N‐acetylornithine aminotransferase; 5, N‐acetylornithine deacetylase (DRIP‐1 may function for this step); 6, glutamate N‐acetyltransferase; 7, ornithine transcarbamylase; 8, arginosuccinate synthase; 9, arginine synthase; 10, arginase; 11, glutaminase and glutamate dehydrogenase; and 12, carbamoyl phosphate synthetase.
Fig. 5. Immunoelectron microscopy of wild watermelon leaves before and after drought stress for 2 d with the anti‐DRIP‐1 antibody. Samples were prepared for immunoelectron microscopy as described by Seo et al. (2000). Sections were incubated with anti‐DRIP‐1 antibody raised in rabbits to the synthetic peptide (Tyr‐248 to Glu‐263 of DRIP‐1) and then reacted with anti‐rabbit IgG conjugated with 20 nm diameter particles (Biocell Research Laboratories, Cardiff, UK). After immunolabelling, the sample was stained with uranyl acetate. Bar = 1 µm. CW, Cell wall; Cp, chloroplast; Mb, microbody; Mt, mitochondrion; V, vacuole.
The operation of the urea cycle depends on the presence of the carbamoyl group from ammonia and CO2, as well as carbamoyl phosphate synthase and ornithine carbamoyl transferase (the citrulline‐synthesizing enzyme), which are localized in chloroplasts in plants (Shibata et al., 1986; Ludwig, 1993). The urea cycle functions not only to remobilize nitrogen atoms from arginine (the nitrogen‐storage amino acid) (Ludwig, 1993), but also to synthesize ornithine and arginine for producing polyamines and some alkaloids in plants (Hashimoto and Yamada, 1994). Arginine is also a constituent amino acid of proteins. The acetyl cycle is highly regulated by the end product of the cycle, arginine, by feedback inhibition (Shargool et al., 1988). In this context, it is of interest to note that N‐acetylglutamate is essential for the reaction of carbamoylphosphate synthetase in rat liver (Alonso and Rubio, 1983). Since N‐acetylornithine‐glutamate acetyltransferase is found in the chloroplasts of pea leaves (Taylor and Stewart, 1981), DRIP‐1 in the cytosol may be involved in additional sequestration of the glutamate skeleton into the urea cycle. The pathway from glutamate to N‐acetylornithine and the subcellular localization of the enzymes involved in the pathway from glutamate to N‐acetylornithine remains unclear in plant cells.
REGULATION OF EXPRESSION OF THE DRIP‐1 GENE
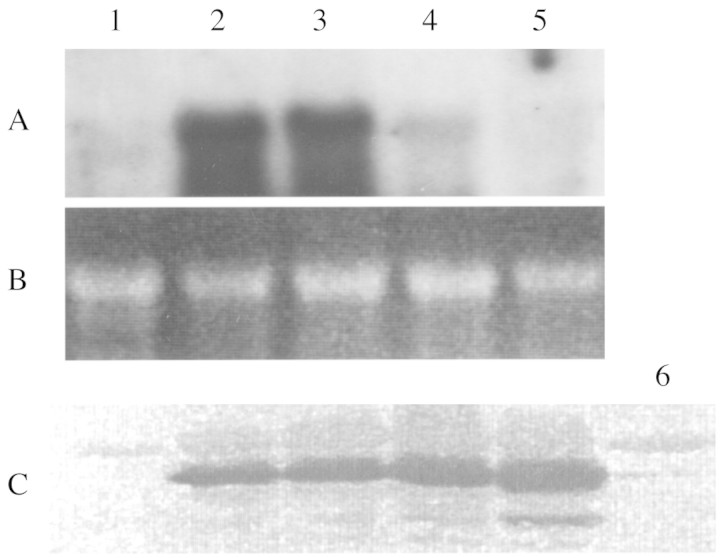
When irrigation is halted, the leaves of wild watermelon maintain their substantial rates of transpiration and CO2 assimilation for the first 24 h (Kawasaki et al., 2000). Figure 6 shows that the DRIP‐1 transcript was induced on the first day and that the level was maintained for a further day before the transcript disappeared. Western blot analysis reveals that the DRIP‐1 protein was preserved for another 2 d, in contrast to the DRIP‐1 transcript. This indicates that once DRIP‐1 is induced in the cytosol, its catalytic action can continue for an extended time. Re‐watering plants that have been droughted for 2 d results in recovery of transpiration and net assimilation rates to almost original levels within 2 d (Kawasaki et al., 2000). DRIP‐1 was still preserved after the recovery of transpiration and assimilation (Fig. 6). The complete degradation of the protein may require a longer period.
Fig. 6. Northern and Western blot analyses of the expression of the gene for DRIP‐1 in the leaves of wild watermelon plants subjected to drought and treated with 100 µm ABA for 2 d. For the Northern analysis in panel A, 10 µg of total RNA was used for gel electrophoresis and blotted onto nylon membranes, which were then probed with 32P‐DRIP‐1 cDNA. B, Ethidium bromide staining of the ribosomal RNA for confirmation of equal loading of RNA samples. For the Western analysis (C), 10 µg of total proteins was electrophoresed and then transferred to PVDF membranes. The DRIP‐1 polypeptide was probed with anti‐DRIP‐1 antibody used in Fig. 4. Lane 1, Before stress; lane 2, droughted for 1 d; lane 3, droughted for 2 d; lane 4, droughted for 3 d; lane 5, 4 d after rewatering of the plants droughted for 2 d; lane 6, treated with 100 µm ABA for 2 d.
These results indicate that expression of the gene for DRIP‐1 is transcriptionally regulated. Since the stomata of the leaves of wild watermelon are still functionally open and transpiration is in progress on the first day, it seems unlikely that abscisic acid (ABA) is involved in the induction of DRIP‐1. To determine whether ABA is a hormonal signal in the drought response of DRIP‐1, water supplemented with 100 µm ABA was applied daily to plants growing at 28 °C under 500 µmol photons m–2 s–1 illumination in the daytime. Net assimilation had decreased to one‐half of its initial rate 4 h after the first ABA treatment, to one‐third after 28 h and to less than 10 % after 52 h. In the ABA experiments, the DRIP‐1 protein was induced at a very low level 52 h after the start of treatment (Fig. 6). ABA responsive genes seem to be defined as those inducible by exogenous ABA treatment within 24 h (Pla et al., 1993; Shen and Ho, 1995; Ingram and Bartels, 1996). The quite different responses observed between stomatal behaviour and DRIP‐1 induction following drought and ABA treatments may rule out the involvement of ABA in the induction of DRIP‐1 during drought, as in the case of thioredoxin‐like protein in potato and wild tomato plants (Rey et al., 1998). The soil water content decreased to half of the original level 24 h after watering stopped. However, the leaf water content showed no decrease at all. This may imply that the root system possesses a mechanism to sense drought and releases a signalling messenger to the leaves to induce DRIP‐1 at a transcriptional level.
A NEW COMPATIBLE SOLUTE: CITRULLINE
Many physiological studies of drought and salt responses in plants have indicated that the accumulation of compatible solutes, or osmolytes, which can protect cells and cell components from damage, is one of the important ways in which plants maintain homeostasis (Hanson and Hitz, 1982). High concentrations of osmolytes may also function to lower the water potential, to retain cell water and to take up more water from the soil. Reported osmolytes include free amino acids, polyols, quaternary amines and sulfate esters, and are highly soluble in water (Hanson and Hitz, 1982; Yancey et al., 1982; Kempf et al., 1998). Large amounts of proline accumulate in many plants (Delauney and Verma, 1993; Yoshiba et al., 1995; Liu and Zhu, 1997). Although the exact biochemical function of proline as a compatible solute has not been studied well, physiological and transgenic studies have revealed that it plays an effective role (Nanjo et al., 1999). Glycinebetaine is accumulated in some salt‐tolerant plants in the Chenopodiaceae and Gramineae (Hanson and Hitz, 1982; Takabe et al., 1998). This compatible solute can protect membranes and enzymes from high concentrations of salt (Hayashi et al., 1997; Alia et al., 1998a, b).
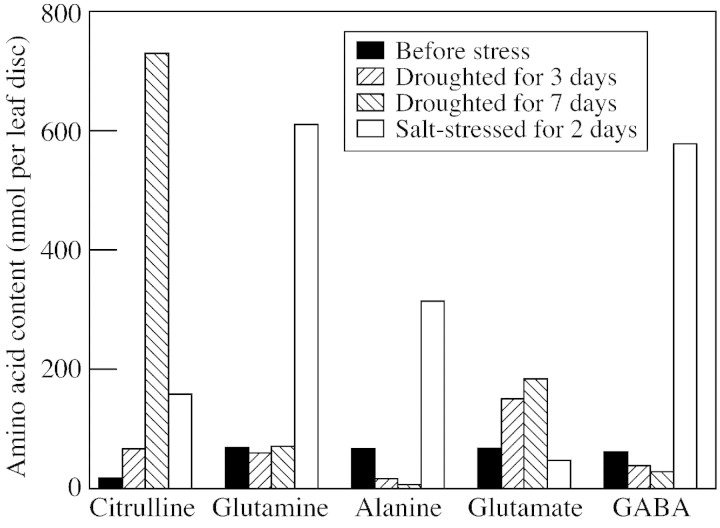
Wild watermelon primarily accumulates citrulline, and then glutamate and arginine, in place of proline and glycinebetaine (Kawasaki et al., 2000). The amount of citrulline accumulated reached 24 µmol g–1 f. wt, or a concentration of 0·6 m assuming that citrulline is located only in the cytosol which constitutes 5 % of the total volume of developed mesophyll cells (Fig. 8). Since chloroplasts are the subcellular site for synthesis of citrulline in plants, and low molecular weight metabolites can pass freely through the nuclear envelope, citrulline may be dispersed among these subcellular compartments. In this case, the concentration of citrulline would be 0·2–0·3 m. The concentrations of glutamate and arginine are each one‐quarter of that of citrulline.
Fig. 8. Changes in the free‐amino acid composition in the leaves of wild watermelon droughted for 3 and 7 d and treated with 150 mm NaCl for 2 d. Experimental details are described in Kawasaki et al. (2000).
In drought, electrons that have no productive destination in the electron transport chain are directed to the reduction of molecular oxygen to form superoxide and hydrogen peroxide (Miyake and Yokota, 2000). The flux of electron transfer to oxygen in PSI forms about 50 % of the flux of the alternative electron transport (Ja in Fig. 1). The remaining 50 % is the flux in cyclic electron transfer in PSII (Miyake and Yokota, 2001). Ja increases with increasing light intensity and with decreasing intercellular CO2 concentrations. In other words, Ja is a major electron sink in drought conditions (Miyake and Yokota, 2000, 2001).
Plants have an efficient system for decomposing active oxygen species, using the enzymes superoxide dismutase (SOD) and ascorbate peroxidase (APX) in chloroplasts (Asada, 1999). These enzymes are inactivated by hydrogen peroxide in vitro, although they are important active oxygen scavenging enzymes (Miyake and Asada, 1996; Casano et al., 1997; Jewett et al., 1999). The response to drought and high‐light stress has been examined in tobacco plants expressing catalase from E. coli (Shikanai et al., 1998). Wild‐type tobacco plants show strong bleaching of leaves after 72 h of drought in the light, a symptom of drought stress, whilst transgenic tobacco plants with the extra catalase retain their normal appearance under these conditions. Chloroplast APX is completely inactivated 48 h after the start of drought experiments in both wild‐type and transgenic plants. These results suggest that the introduced catalase partially protects transgenic leaves from the stress, but is not enough to protect APX from attack by hydrogen peroxide. Interestingly, leaves of wild watermelon plants induce chloroplast APX during drought stress (C. Miyake and A. Yokota, unpubl. res.). Therefore, there must be a mechanism(s) to enable APX to continue functioning without a loss in activity under drought conditions.
In addition to these enzymes, plant chloroplasts contain thioredoxin peroxidase for degrading hydrogen peroxide and lipid peroxides (Baier and Dietz, 1999). This enzyme may be coupled to the electron transport chain and can use electrons from PSI to decompose the peroxides (Yamamoto et al., 1999). The prosthetic group of the enzyme is not heme but the enzyme has two cysteine residues in a CXXC motif. The three‐dimensional structure of the rat homologue of thioredoxin peroxidase has been resolved (Hirotsu et al., 1999). Not being susceptible to hydrogen peroxide, this enzyme may be involved in the degradation of hydrogen peroxide in addition to lipid peroxides.
Thus, hydrogen peroxide and superoxide can be decomposed by target enzymes. Transition metal ions such as cuprous and ferrous ions may be released from enzymes and electron carriers in membranes during drought stress. Hydrogen peroxide and ferrous ions form hydroxyl radicals, the most reactive of the active oxygen species, in the Fenton reaction (Hauptmann and Cadenas, 1997). Hydroxyl radicals react with many cell components non‐specifically leading to decomposition. Branching groups of amino acid residues of proteins, protein peptide bonds, diphosphoester bonds, base groups of DNA and RNA, and unsaturated bonds of fatty acids are all susceptible to attack from hydroxyl radicals. There is no known enzyme for the specific decomposition of hydroxyl radicals. Passive defense mechanisms may operate: ‘(1) to prevent hydroxyl radical generation by the chelation of divalent metal ions; and (2) to repair or eliminate molecules damaged by hydroxyl radicals’ (Acworth et al., 1997). Alternatively, the hydroxyl radicals may be scavenged by reaction with various compounds.
Animals may decompose hydroxyl radicals with metallothionein. Ascorbate and α‐tocopherol react with hydroxyl radicals in vitro but whether these compounds participate in active scavenging of the radicals in vivo is unclear. Arginine can also be decomposed by hydroxyl radicals (Stadtman, 1993). The most reactive moiety in the arginine molecule under air‐equilibrated conditions is the α‐amino group of the amino acid. The α‐carbon of the amino acid is oxidatively converted to aldehyde after releasing the amino and carboxyl groups of arginine. Arginine accumulated in the leaves of wild watermelon up to 0·1 m and so may function as a hydroxyl radical scavenger.
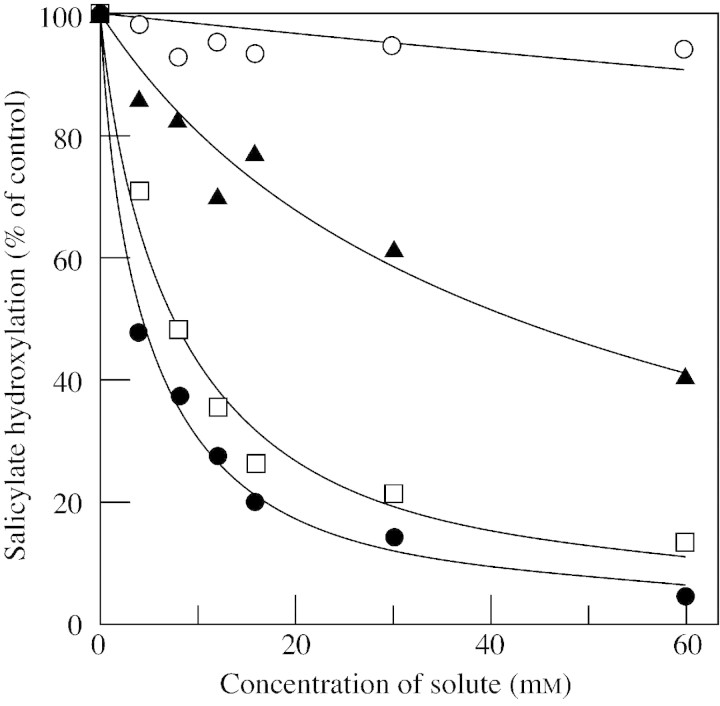
Citrulline has a similar chemical structure to arginine and is a good candidate as a radical scavenger. The second‐order rate constant for the reaction between citrulline and hydroxyl radicals is 3·9 × 109 m–1 s–1 (Akashi et al., 2001). Citrulline is one of the most efficient scavengers among compatible solutes, as shown in Fig. 7. Citrulline effectively protects DNA and metabolic enzymes from oxidative injuries. Interestingly, activities of metabolic enzymes are not inhibited by up to 600 mm citrulline. We reason that citrulline contributes to oxidative stress‐tolerance under drought conditions as a novel hydroxyl radical‐scavenger.
Fig. 7. Hydroxyl radical‐scavenging activities of citrulline and various compatible solutes. Citrulline (closed circles), mannitol (squares), proline (triangles) and glycinebetaine (open circles). Results are from Akashi et al. (2001).
One may pose the question of how wild watermelon becomes heat tolerant. DnaK is induced in this plant as drought conditions progress (Kawasaki et al., 2000). However, we do not know how the amino acids accumulated in the leaves under drought imbue heat tolerance, as does glycinebetaine (Alia et al., 1998a, b).
DROUGHT AND SALT: SIMILAR EFFECTS AND SIMILAR RESPONSES?
As the water content in the soil decreases the salt content increases. In other words, plants often encounter salt stress in addition to desiccation during drought stress. The interrelation between desiccation and salt stress in the drought responses of wild watermelon plants was investigated. Plants were irrigated with water containing 150 mm NaCl every day under illumination of 500 µmol photons m–2 s–1. After 2 d, DRIP‐1 was induced by this NaCl treatment to a level similar to that observed under drought (Fig. 3). However, another response of the watermelon plants to the salt treatment was quite different from that of drought. DRIP‐1 was induced on the first day after the start of the drought stress (Fig. 3), but citrulline accumulated much later (Fig. 8). Conversely, changes in the amino acid content and composition were very rapid under salt stress. In addition to this difference between the two stresses, the amino acids synthesized in salt‐stressed plants were quite different from those detected in droughted plants. Citrulline was accumulated to a lesser extent than under drought, although a large amount of DRIP‐1 had been induced. Instead, glutamine and γ‐aminobutyrate (GABA) accumulated. The amount of GABA was 15–20 µmol g–1 f. wt, the largest amount reported to date (Shelp et al., 1999). GABA can function as a scavenger of hydroxyl radicals (Smirnoff and Cumbes, 1989). It is not clear why citrulline was not synthesized. Presumably, glutamate was decarboxylated to GABA by glutamate decarboxylase (Shelp et al., 1999) rather than being N‐acetylated to bring the carbon skeleton of glutamate to ornithine. Alanine was also accumulated during the salt stress, but to a lesser extent. The same amino acids are accumulated under salt stress in other plants (Stewart and Larcher, 1980; Hanson and Hitz, 1982; Rhodes et al., 1986).
Irrespective of the mechanism involved, accumulation of different amino acids during drought compared with salt stress indicates that the response of wild watermelon to the two stresses is not the same. During drought stress, the soil loses water, and the soil water potential decreases as drought progresses. Wild watermelon has been known to forgo its branching thin roots and root hairs to protect the roots from massive leakage of water (Larcher, 1995). In this case salts cannot enter the roots. In salt stress (Fig. 8), NaCl was administered with sufficient water. Although transpiration through the stomata is decreased with salt stress, some salt may be transported to the leaves with water. Glutamate decarboxylase may be activated by salt through binding of a Ca2+/calmodulin complex to the enzyme (Shelp et al., 1999), or acetyl transfer to glutamate from acetyl‐CoA may be inhibited by salt. Thus, drought and salt stresses are quite different environmental stimuli for the plant.
DROUGHT RESPONSES OF OTHER WILD PLANTS
Wild watermelon is not the only C3 plant to show severe drought tolerance in nature. A desert C3 plant, Retama raetam, is an interesting example (Wittler et al., 2001). This plant is a stem‐assimilating, evergreen plant. The canopy of the whole plant body encounters quite different light conditions in the dry season in the Negev desert of Israel. The upper stems of the plant canopy receive high light, but the lower stems can maintain their photosynthetic activity under the shade of the upper canopy. Some proteins, including APX and Rubisco, disappear under stress but mRNAs for these proteins persist; cytosol‐located transcripts in association with polysomes and chloroplast‐located transcripts are present in their free forms. With rain, stems in the upper canopy use these mRNAs to synthesize cellular proteins in several hours.
Wild tomato can preserve its water content under drought (Tabaeizadeh, 1998). Several genes have been identified that are expressed under this stress.
Thus, some wild C3 plants have quite unique and interesting responses to drought and high light intensities. The responses of these wild plants are different from those encountered in domesticated plants and those adapted to low light intensity. Precise analyses of wild C3 plants may reveal other strategies used by C3 plants to survive in hostile environments.
ACKNOWLEDGEMENT
This study was supported by the ‘Research for the Future’ programme (JSPS‐RFTF96R16001 and JSPS‐RFTF97R 16001) of the Japan Society for the Promotion of Science.
Supplementary Material
Received: 6 June 2001; Returned for revision: 17 October 2001; Accepted: 11 January 2002.
References
- AcworthIN, McCabe DR, Maher TJ.1997. The analysis of free radicals, their reaction products, and antioxidants. In: Baskin SI, Salem H, eds. Oxidants, antioxidants, and free radicals Washington, DC: Taylor & Francis, 23–77. [Google Scholar]
- AkashiK, Miyake C, Yokota A.2001. Citrulline, a novel compatible solute in drought‐tolerant wild watermelon leaves, is an efficient hydroxyl radical‐scavenger. FEBS Letters 508: 438–442. [DOI] [PubMed] [Google Scholar]
- Alia,Hayashi H, Sakamoto A, Murata N.1998a Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant Journal 16: 155–161. [DOI] [PubMed] [Google Scholar]
- Alia,Kondo Y, Sakamoto A, Nonaka H, Hayashi H, Saradhi PP, Chen TH, Murata N.1998b Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Molecular Biology 38: 1011–1019. [DOI] [PubMed] [Google Scholar]
- AlonsoE, Rubio V.1983. Binding of N‐acetyl‐l‐glutamate to rat liver carbamoyl phosphate synthetase (ammonia). European Journal of Biochemistry 135: 331–337. [DOI] [PubMed] [Google Scholar]
- AsadaK.1999. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50: 601–639. [DOI] [PubMed] [Google Scholar]
- BaierM, Dietz KJ.1999. Protective function of chloroplast 2‐cysteine peroxiredoxin in photosynthesis. Evidence from transgenic Arabidopsis. Plant Physiology 119: 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BiaginiA, Puigserver A.2001. Sequence analysis of the aminoacylase‐1 family. A new proposed signature for metalloexopeptidases. Comparative Biochemistry and Physiology, Series B. Biochemistry and Molecular Biology 128: 469–481. [DOI] [PubMed] [Google Scholar]
- BohnertHJ, Su H, Shen B.1999. Molecular mechanism of salinity tolerance. In: Shinozaki K, Yamaguchi‐Shinozaki K, eds. Molecular responses to cold, drought, heat and salt stress in higher plants Austin: R.G. Landes, 29–60. [Google Scholar]
- CasanoLM, Gomez LD, Lascano HR, Gonzalez CA, Trippi VS.1997. Inactivation and degradation of CuZn‐SOD by active oxygen species in wheat chloroplasts exposed to photooxidative stress. Plant and Cell Physiology 38: 433–440. [DOI] [PubMed] [Google Scholar]
- CornicG, Massacci A.1996. Leaf photosynthesis under drought stress. In: Baker NR, ed. Photosynthesis and the environment Dordrecht, The Netherlands: Kluwer, 347–366. [Google Scholar]
- CushmanJ, Bohnert H.1999. Crassulacean acid metabolism: molecular genetics. Annual Review of Plant Physiology and Plant Molecular Biology 50: 305–332. [DOI] [PubMed] [Google Scholar]
- DelauneyA, Verma DPS.1993. Proline biosynthesis and osmoregulation in plants. Plant Journal 4: 215–223. [Google Scholar]
- GellerGN, Nobel PS 1984. Cactus ribs – Influence On PAR interception and CO2 uptake. Photosynthetica 18: 482–494. [Google Scholar]
- GibsonAC.1996. Structure–function relations of warm desert plants. Berlin: Springer‐Verlag. [Google Scholar]
- HansonAD, Hitz WD.1982. Metabolic responses of mesophytes to plant water deficits. Annual Review of Plant Physiology and Plant Molecular Biology 33: 163–203. [Google Scholar]
- HashimotoT, Yamada Y.1994. Alkaloids biosynthesis: molecular aspects. Annual Review of Plant Physiology and Plant Molecular Biology 45: 257–285. [Google Scholar]
- HashimotoY, Ino T, Kramer PJ, Naylor AW, Strain BR.1984. Dynamic analysis of water stress of sunflower leaves by means of a thermal image processing system. Plant Physiology 76: 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HauptmannN, Cadenas E.1997. The oxygen paradox: biochemistry of active oxygen. In: Scandalios JG, ed. Oxidative stress and the molecular biology of antioxidant defenses Plainview: CHSL Press, 1–20. [Google Scholar]
- HayashiH, Alia, Mustardy L, Deshnium P, Ida M, Murata N.1997. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant Journal 12: 133–142. [DOI] [PubMed] [Google Scholar]
- HirotsuS, Abe Y, Okada K, Nagahara N, Hori H, Nishino T, Hakoshima T.1999. Crystal structure of a multifunctional 2‐cys peroxiredoxin heme‐binding protein 23 kDa/proliferation‐associated gene product. Proceedings of the National Academy of Sciences of the USA 96: 12333–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IngramJ, Bartels D.1996. The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 377–403. [DOI] [PubMed] [Google Scholar]
- JewettSL, Rocklin AM, Ghanevati M, Abel JM, Marach JA.1999. A new look at a time‐worn system: Oxidation of CuZn‐SOD by H2O2 Free Radical Biology and Medicine 26: 905–918. [DOI] [PubMed] [Google Scholar]
- KawasakiS, Miyake C, Kouchi T, Yokota A.2000. Responses of wild watermelon to drought stress: accumulation of an ArgE homologue and citrulline in leaves during water deficit. Plant and Cell Phyiology 41: 864–873. [DOI] [PubMed] [Google Scholar]
- KempfB, Bremer E.1998. Uptake and synthesis of compatible solutes as microbial stress responses to high‐osmolality environments. Archives of Microbiology 170: 319–330. [DOI] [PubMed] [Google Scholar]
- LangeOL.1959. Untersuchungen über den Wärmehaushalt und Hitzeresistenz mauretanischer Wüsten‐ und Savannenpflanzen. Flora 147: 595–651. [Google Scholar]
- LarcherW.1995. Physiological plant ecology. 3rd edn. Berlin: Springer. [Google Scholar]
- LiuJ, Zhu JK.1997. Proline accumulation and salt‐stress‐induced gene expression in a salt‐hypersensitive mutant of arabidopsis. Plant Physiology 114: 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LudwigRA.1993. Arabidopsis chloroplasts dissimilate l‐arginine and l‐citrulline for use as N source. Plant Physiology 101: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MeinnelT, Schmitt E, Mechulam Y, Blanquet S.1992. Structural and biochemical characterization of the Escherichia coli argE gene product. Journal of Bacteriology 174: 2323–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MiyakeC, Asada K.1996. Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate; hydrogen peroxide decomposes Compound I of ascorbate peroxidase. Plant and Cell Physiology 37: 423–430. [Google Scholar]
- MiyakeC, Yokota A.2000. Determination of the rate of photoreduction of O2 in the water‐water cycle in watermelon leaves and enhancement of the rate by limitation of photosynthesis. Plant and Cell Physiology 41: 335–343. [DOI] [PubMed] [Google Scholar]
- MiyakeC, Yokota A.2001. Cyclic flow of electrons within PSII in thylakoid membranes. Plant and Cell Physiology 42: 508–515. [DOI] [PubMed] [Google Scholar]
- NanjoT, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi‐Shinozaki K, Shinozaki K.1999. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana FEBS Letters 19: 205–10. [DOI] [PubMed] [Google Scholar]
- PlaM, Vilardell J, Guiltinan MJ, Marcotte WR, Niogret MF, Quatrano RS, Pages M.1993. The cis‐regulatory element CCACGTGG is involved in ABA and water‐stress responses of the maize gene rab28. Plant Molecular Biology 21: 259–66. [DOI] [PubMed] [Google Scholar]
- ReyP, Pruvot G, Becuwe N, Eymery F, Rumeau D, Peltier G.1998. A novel thioredoxin‐like protein located in the chloroplast is induced by water deficit in Solanum tuberosum L. plants. Plant Journal 13: 97–107. [DOI] [PubMed] [Google Scholar]
- RhodesD, Handa S, Bressan R.1986. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiology 82: 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SeoS, Okamoto M, Iwai T, Iwano M, Fukui K. Isogai A, Nakajima N, Ohashi Y.2000. Reduced levels of chloroplast FtsH protein in tobacco mosaic virus‐infected tobacco leaves accelerate the hypersensitive reaction. Plant Cell 12: 917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShargoolPD, Jain JC, McKay G.1988. Ornithine biosynthesis and degradation in plant cells. Phytochemistry 27: 1571–1574. [Google Scholar]
- ShelpBJ, Bown AW, McLean MD.1999. Metabolism and functions of gamma‐aminobutyric acid. Trends in Plant Science 4: 446–452. [DOI] [PubMed] [Google Scholar]
- ShenQ, Ho TD.1995. Functional Dissection of an abscisic acid (ABA)‐inducible gene reveals two independent ABA‐responsive complexes each containing a G‐box and a novel cis‐acting element. Plant Cell 7: 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShibataH, Ochiai H, Sawa Y, Miyoshi S.1986. Localization of carbamoylphosphate synthetase and aspartate carbamoyltransferase in chloroplasts. Plant Physiology 80: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShikanaiT, Takeda T, Yamauchi H, Nano S, Tomizawa K, Yokota A, Shigeoka S.1998. Inhibition of ascorbate peroxidase under oxidative stress in tobacco having bacterial catalase in chloroplasts. FEBS Letters 428: 47–51. [DOI] [PubMed] [Google Scholar]
- SmirnoffN.1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist 125: 27–58. [DOI] [PubMed] [Google Scholar]
- SmirnoffN, Cumbes QJ.1989. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28: 1057–1060. [Google Scholar]
- StadtmanER.1993. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal‐catalyzed reactions. Annual Reviews of Biochemistry 62: 797–821. [DOI] [PubMed] [Google Scholar]
- StewartGR, Larher F.1980. Accumulation of amino acids and related compounds in relation to environmental stress. In: Midlin BJ, ed. The biochemistry of plants: a comprehensive treatise, vol. 5 New York: Academic Press, 609–635. [Google Scholar]
- TabaeizadehZ.1998. Drought‐induced responses in plant cells. International Review of Cytology 182: 193–247. [DOI] [PubMed] [Google Scholar]
- TakabeT, Nakamura T, Nomura M, Hayashi Y, Ishitani M, Muramoto Y, Tanaka A, Takabe T.1998. Glycinebetaine and the genetic engineering of salinity tolerance in plants. In: Satoh K, Murata N, eds. Stress responses of photosynthetic organisms Amsterdam: Elsevier, 115–131. [Google Scholar]
- TaylorAA, Syewart GR.1981. Tissue and subcellular localization of enzymes of arginine metabolism in Pisum sativum Biochemical and Biophysical Research Communications 101: 1281–1289. [DOI] [PubMed] [Google Scholar]
- WittlerR, Merquiol E, Halla‐Herr E, Rachmilevitch S, Kaplan A, Cohen M.2001. Living under a ‘dormant’ canopy: a molecular acclimation mechanism of the desert plant Retama raetam Plant Journal 25: 407–416. [DOI] [PubMed] [Google Scholar]
- YamamotoH, Miyake C, Dietz KJ, Tomizawa K, Murata N, Yokota A.1999. Thioredoxin peroxidase in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Letters 447: 269–273. [DOI] [PubMed] [Google Scholar]
- YanceyPH, Clark ME, Hand SC, Bowlus RD, Samero GN.1982. Living with water stress: Evolution of osmolyte systems. Science 217: 1214–1222. [DOI] [PubMed] [Google Scholar]
- YoshibaY, Kiyosue T, Katagiri T, Ueda H, Moriguchi T, Yamaguchi‐Shinozaki K, Wada K, Harada Y, Shinozaki K.1995. Correlation between the induction of a gene for Δ‐pyrrole‐5‐carboxylate synthetase and the accumulation of proline in Arabodopsis thaliana under osmotic stress. Plant Journal 7: 751–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.