Abstract
We review the photosynthetic responses to drought in field‐grown grapevines and other species. As in other plant species, the relationship between photosynthesis and leaf water potential and/or relative water content in field‐grown grapevines depends on conditions during plant growth and measurements. However, when light‐saturated stomatal conductance was used as the reference parameter to reflect drought intensity, a common response pattern was observed that was much less dependent on the species and conditions. Many photosynthetic parameters (e.g. electron transport rate, carboxylation efficiency, intrinsic water‐use efficiency, respiration rate in the light, etc.) were also more strongly correlated with stomatal conductance than with water status itself. Moreover, steady‐state chlorophyll fluorescence also showed a high dependency on stomatal conductance. This is discussed in terms of an integrated down‐regulation of the whole photosynthetic process by CO2 availability in the mesophyll. A study with six Mediterranean shrubs revealed that, in spite of some marked interspecific differences, all followed the same pattern of dependence of photosynthetic processes on stomatal conductance, and this pattern was quite similar to that of grapevines. Further analysis of the available literature suggests that the above‐mentioned pattern is general for C3 plants. Even though the patterns described do not necessarily imply a cause and effect relationship, they can help our understanding of the apparent contradictions concerning stomatal vs. non‐stomatal limitations to photosynthesis under drought. The significance of these findings for the improvement of water‐use efficiency of crops is discussed.
Key words: Vitis vinifera L., grapevine, Mediterranean sclerophylls, C3 plants, photosynthesis, stomatal conductance, photochemistry, carboxylation, drought, gas exchange, chlorophyll fluorescence
INTRODUCTION
The debate as to whether drought mainly limits photosynthesis through stomatal closure or through metabolic impairment has been running since the earliest reports on the effects of drought on photosynthesis (Jones, 1973; Boyer, 1976; Quick et al., 1992; Lawlor and Uprety, 1993; Cornic, 1994; Lawlor, 1995; Tezara et al., 1999; Cornic, 2000; Flexas and Medrano, 2002a, b). During the last decade, stomatal closure was generally accepted to be the main determinant for decreased photosynthesis under mild to moderate drought (Sharkey, 1990; Chaves, 1991; Ort et al., 1994; Cornic and Massacci, 1996). Previously described non‐stomatal effects were mostly attributed to the presence of non‐homogeneous stomatal closure during drought (Downton et al., 1988; Terashima et al., 1988). However, evidence has been accumulating that shows that photophosphorylation (Havaux et al., 1987; Meyer and de Kouchkovsky, 1992), RuBP regeneration (Giménez et al., 1992; Gunasekera and Berkowitz, 1993) and Rubisco activity (Castrillo and Calcagno, 1989; Medrano et al., 1997) are impaired under drought. More recently, Lawlor and co‐workers (Tezara et al., 1999) pointed out that impaired photophosphorylation and ATP synthesis was the main factor limiting photosynthesis in sunflower, even under mild drought. Thus, the old controversy has surfaced again (Cornic, 2000; Flexas and Medrano, 2002a, b), and was discussed at the SEB Meeting in Canterbury, UK, in April 2001 (Cornic and Fresneau, 2002; Lawlor, 2002; Tang et al., 2002).
Comparing results from different authors is complex due to interspecific differences in the response of stomatal conductance and photosynthesis to leaf water potential and/or relative water content, the parameters most often used to assess the degree of drought (Lawlor, 1995; Cornic and Massacci, 1996). It is clear that stomata close progressively as drought progresses, followed by parallel decreases of net photosynthesis. However, stomatal conductance is not controlled by soil water availability alone, but by a complex interaction of factors internal and external to the leaf.
It is certainly recognized that leaf water status interacts with stomatal conductance and transpiration and, under water stress, a good correlation is often observed between leaf water potential and stomatal conductance. However, the precise relationship is dependent, among other factors, on the species studied, the drought history of the individuals studied, the size of pots in which the plants are rooted or the environmental conditions during drought (Schulze and Hall, 1982; Tardieu and Simonneau, 1998; Flexas et al., 1999a; Tyree, 1999). Even within a given species, comparing results from different studies may be difficult. For instance, we have observed that the photosynthetic response to pre‐dawn leaf water potential differs among grapevines, and depends on conditions during plant growth and measurements, as well as on the cultivar examined (Flexas et al., 1998, 1999a, b; Escalona et al., 1999; Bota et al., 2001).
Moreover, stomata often close in response to drought before any change in leaf water potential and/or leaf water content is detectable (Gollan et al., 1985; Socías et al., 1997). It is now well established that there is a drought‐induced root‐to‐leaf signalling, promoted by soil drying and reaching the leaves through the transpiration stream, which induces closure of stomata. This chemical signal has been shown to be abscisic acid (ABA), which is synthesized in the roots in response to soil drying (Davies and Zhang, 1991). However, its role is not simple, and a direct correlation between xylem ABA content and stomatal conductance has been shown only in some cases (Correia et al., 1995; Socías et al., 1997). Leaf water potential (Tardieu and Davies, 1992; Socías et al., 1997; Tardieu and Simmoneau, 1998), plant nutritional status (Schurr et al., 1992), xylem sap pH (Davies, 2002), farnesyltransferase activity (Pei et al., 1998) and other factors seem to modulate stomatal sensitivity to ABA. Xylem hydraulic conductivity, which is sometimes decreased under drought, has been shown to modulate stomatal closure directly (Salleo et al., 2000; Hubbard et al., 2001). Finally, stomata also close as leaf‐to‐air vapour pressure deficit (VPD) increases (Raschke, 1979; Dai et al., 1992; Oren et al., 1999), irrespective of soil water availability.
In summary, this complex regulation of stomatal conductance is related to important differences among species and genotypes in the response of stomata to leaf water potential, relative water content, ABA and other parameters, making it difficult to define a pattern of photosynthetic responses to drought. An interesting case is represented by species like grapevine that show isohydric behaviour (Choné et al., 2001). These species can show substantial photosynthetic limitations without any detectable change in their leaf water potential or relative water content (Tardieu and Simmoneau, 1998), thus raising questions as to the suitability of these parameters as a basis for comparison when studying the effects of drought on photosynthesis.
Nevertheless, it must be emphasized that a high degree of co‐regulation of stomatal conductance (gs) and photosynthesis is usually found (Wong et al., 1979; Farquhar et al., 2001). Since gs is responsive to all the external (soil water availability, VPD) and internal (ABA, xylem conductivity, leaf water status) factors related to drought, it represents a more integrative basis for the overall effects of drought than leaf water potential and relative water content. Therefore, in searching for a common pattern of photosynthetic response to drought, we have used gs as an integrative parameter reflecting the water stress experienced by the plant. However, stomatal movements are very dynamic due to complex regulation by multiple factors. For this reason, mid‐morning, light‐saturated stomatal conductance (which is usually correlated with the average daily mean conductance) was taken as a representative value of gs. This was preferred to midday gs because, as drought becomes progressively more intense, the daily peak conductance drops and is displaced from around midday towards the early morning hours (Vadell et al., 1995; Flexas et al., 1999a).
The present report reviews a series of studies of the response of grapevines and other species to progressive drought. In these studies we relate every photo synthetic parameter (measured at steady state and light saturation) to the maximum light‐saturated stomatal conductance observed for that plant at the moment of measuring.
RELATING THE ELECTRON TRANSPORT RATE TO LIGHT‐SATURATED STOMATAL CONDUCTANCE GENERALIZES ITS RESPONSES TO DROUGHT IN GRAPEVINES
Early studies of chlorophyll fluorescence in irrigated and non‐irrigated grapevines growing in the field during summer (Flexas et al., 1998) showed that permanent photoinhibition, as determined by pre‐dawn photochemical efficiency (Fv/Fm), was rare even under severe drought. The rate of light‐saturated electron transport (ETR), measured at midday, sometimes decreased in non‐irrigated plants, but decreased to a lesser extent than net CO2 assimilation (An). This was understood as indicative of a relative increase in photorespiration, which has been known to occur under drought since the early studies by Lawlor and co‐workers (Lawlor and Fock, 1975, 1977a, b; Lawlor, 1976a, b; Lawlor and Pearlman, 1981) and is now well accepted (Wingler et al., 1999, 2000). We have recently demonstrated that O2 uptake increases significantly in water‐stressed grapevines, presumably due mainly to photorespiration and only due in minor part to an increase in the Mehler reaction (Flexas et al., 1999b, 2002a). At the time of the first study (Flexas et al., 1998), we assumed that photorespiration might be an important photoprotective mechanism in field‐grown grapevines, as suggested for other species (Heber et al., 1996; Kozaki and Takeba, 1996), since ETR remained relatively high even under severe stress. Moreover, although there was a certain tendency for ETR to decrease with decreasing pre‐dawn leaf water potential (Ψ), a non‐significant relationship was observed between these two parameters (Fig. 1A). These results contrasted with the highly significant linear relationship that was observed recently between ETR and Ψ in 2‐year‐old grapevines of the same cultivar (Tempranillo), maintained in large pots and grown under field conditions (Flexas et al., 1999a, see Fig. 1A). Figure 1B shows that the response of stomatal conductance to Ψ was also different in field‐grown and potted grapevines, possibly due to differences in the root system, osmotic adjustment and/or stomatal sensitivity to drought. Interestingly, when ETR was plotted against gs, a single hyperbolic function satisfactorily fitted data from both field‐grown and potted plants (Flexas et al., 2002a) (Fig. 1C). From gs values of 400 down to about 150 mmol H2O m–2 s–1, ETR is little affected. Lower gs values lead to steep reductions of ETR. A study with 22 different grapevine cultivars, rooted in pots and grown under field conditions (Bota et al., 2001), also revealed that ETR correlated better with gs than with either leaf relative water content (RWC) or Ψ (Flexas et al., 2002a).
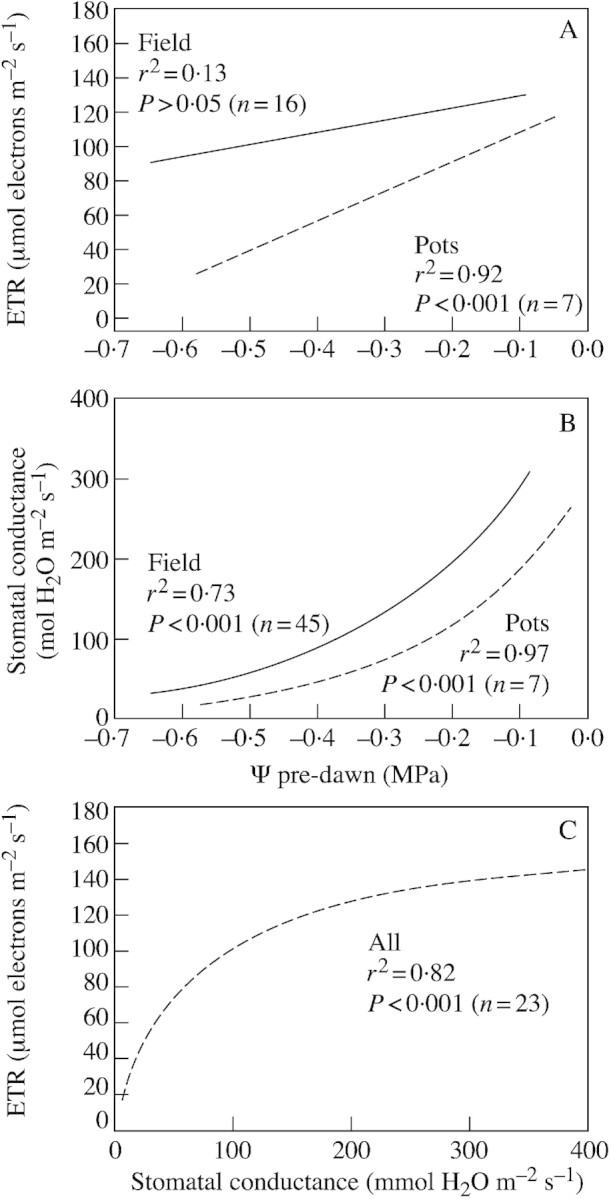
Fig. 1. Relationships between photosynthetic electron transport rate (ETR) and pre‐dawn leaf water potential (Ψ)(A), and stomatal conductance and pre‐dawn leaf water potential (B) in field‐grown (solid lines) and potted (broken lines) grapevines. Only the regression fits are shown, indicating their correlation coefficient and significance (data from Flexas et al., 1999a). When ETR was plotted against stomatal conductance (C), a single hyperbolic correlation was observed including field and potted plants (data from Flexas et al., 2002a).
The primary correlation between An and gs was already known to exist for both field‐ and pot‐grown plants (Escalona et al., 1999; Flexas et al., 2002a). The most surprising of these new results was that the use of gs also generalized the response of a parameter that, in principle, was not directly related to stomatal closure. That is, a secondary strong relationship was also found between ETR and gs.
LIGHT‐SATURATED STOMATAL CONDUCTANCE GENERALIZES THE RESPONSES OF MANY PHOTOSYNTHETIC PARAMETERS TO DROUGHT IN GRAPEVINES
Apart from ETR, pre‐dawn Fv/Fm and the sub‐stomatal CO2 concentration (Ci) have also been shown in previous studies to be more dependent on gs than on Ψ (Flexas et al., 1998; Escalona et al., 1999). On the basis of these observations, as well as on theoretical considerations given in the Introduction, we hypothesized that the use of gs as an integrative parameter reflecting the water stress condition of the plant would help to generalize a pattern of response of different photosynthetic processes to drought. To test this hypothesis, we related different photosynthetic parameters, studied in both field‐ and pot‐grown grapevines between 1994 and 2000, to the corresponding light‐saturated gs (Flexas et al., 2002a). These parameters included An, Ci, the estimated gross photosynthesis (Ag), ETR, the ratios ETR/An and ETR/Ag, leaf dark respiration (RD), pre‐dawn Fv/Fm, non‐photochemical quenching of chlorophyll fluorescence at midday (NPQ) and parameters derived from analyses of An–Ci curves, such as the apparent carboxylation efficiency (ϵ), leaf light respiration (RL), CO2 compensation point (Γ) and the CO2‐saturated rate of photosynthesis (Asat). All parameters were found to be highly significantly correlated to gs, and accurately fitted data from both field‐grown and potted plants, as well as data from 23 different cultivars (Flexas et al., 2002a).
Drought usually leads to erroneous calculation of Ci due to patchy stomatal closure (Downton et al., 1988; Terashima et al., 1988) and different cuticular conductance to water vapour and CO2 (Boyer et al., 1997). These limitations were taken into account and estimated, and the true Ci was re‐calculated accordingly (Osmond et al., 1997a; Escalona et al., 1999; Flexas et al., 2002a). Therefore, the Ci data used in the present paper should be free of errors, except for the low accuracy of gas‐exchange determinations at very low gs.
Irrespective of the origin of the data (year, season, irrigation treatment, field‐ or pot‐grown plants), significant regression patterns were observed between each parameter and gs. Three regions could be differentiated on these regressions along a gradient of gs during the development of drought. Decreases in gs from 0·4 to 0·15 mol H2O m–2 s–1 (corresponding to a mild water stress) were paralleled by a decline in An and a progressive decline in the sub‐stomatal CO2 concentration. This suggested that stomatal limitations to photosynthesis were dominant. The ratio ETR/An increased, mirroring the decline in Ci, which suggested an increased rate of photorespiration. At lower values of gs (0·15–0·05 mol H2O m–2 s–1), Ci still decreased, but the electron transport rate and the carboxylation efficiency started to decline. At this stage, both stomatal and non‐stomatal limitations were therefore important. Further reductions of gs (< 0·05 mol H2O m–2 s–1) led to steeper reductions of An, ETR and ϵ, and to steep increases in Ci, indicating that non‐stomatal limitations to photosynthesis became dominant. Under these conditions pre‐dawn Fv/Fm occasionally decreased. Although the ratio ETR/An and Γ increased exponentially with decreases in gs, the ratio ETR/Ag remained almost constant through the entire range of gs, suggesting that the Mehler reaction did not increase substantially as stress progressed.
In summary, these results show that in addition to An and ETR, other important photosynthetic parameters were correlated to gs in a simple manner, whereas their correlation to Ψ and RWC was dependent on experimental conditions.
DO THESE RELATIONSHIPS GIVE INSIGHTS INTO THE PROCESSES LIMITING PHOTOSYNTHESIS UNDER DROUGHT IN GRAPEVINES?
The curves of best fit between four parameters (An, ETR, Asat and ϵ) and gs are shown in Fig. 2. These parameters were selected because they represent very important components of photosynthesis: An is the actual rate of photosynthesis, ETR reflects the capacity for energy and reductant synthesis, Asat may be related to the potential photosynthetic capacity and ϵ reflects, to some extent, the activity and activation state of Rubisco.
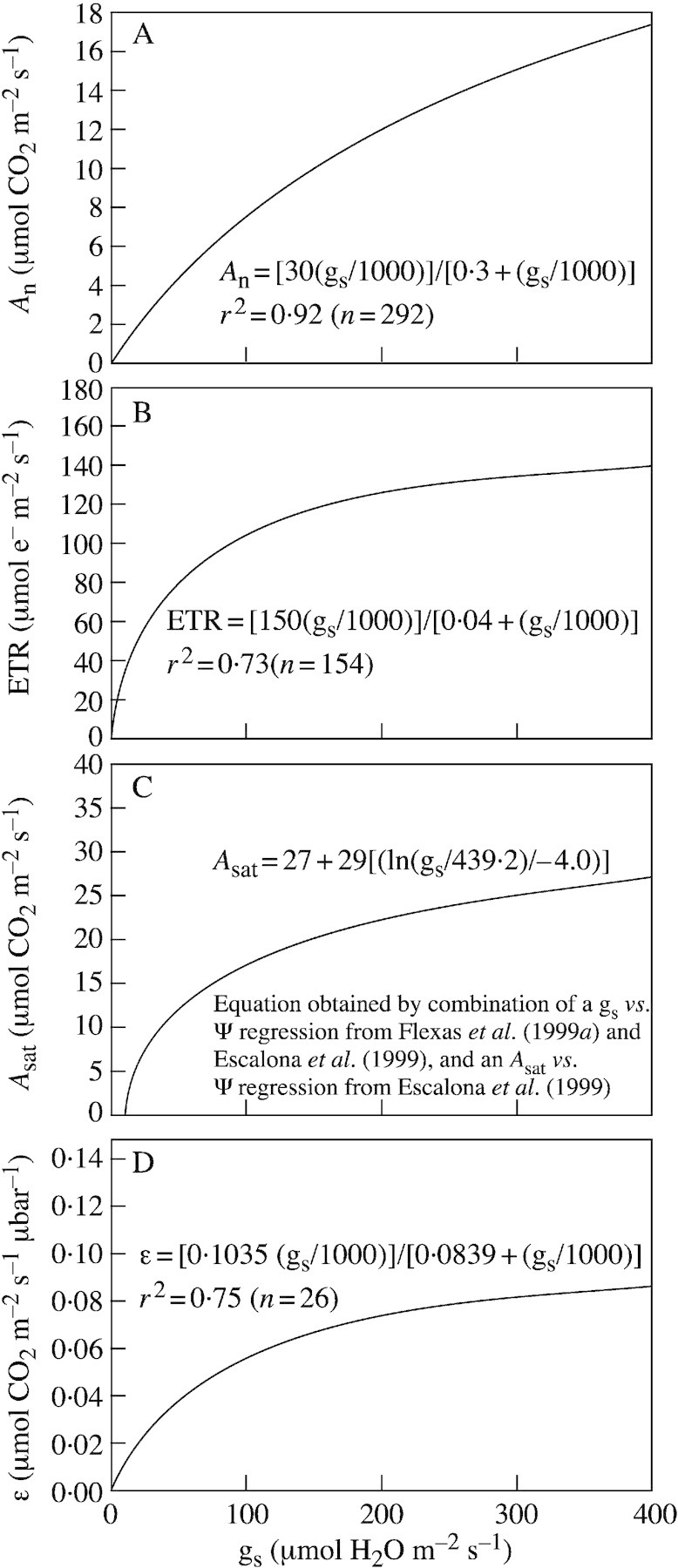
Fig. 2. Relationships between stomatal conductance (gs) and: net CO2 assimilation (An) (A), photosynthetic electron transport rate (ETR) (B), light‐ and CO2‐saturated net CO2 assimilation (Asat) (C), and apparent carboxylation efficiency, estimated as the initial slope of An–Ci curves (ϵ) (D). Data correspond to field‐grown grapevines, and only the best‐fitting correlation curves are shown, all of them being hyperbolic and highly significant. Leaf temperatures ranged from 28·5 to 39·3 °C (data from Flexas, 2000).
Once these general relationships are established, one can evaluate the relative importance of each process in photosynthetic limitation at any given degree of water stress, represented by a value of gs (Fig. 3). As drought progresses, the proportional decrease in the parameters studied was much less than the decline in stomatal conductance for any given interval of the latter. For instance, when gs was halved, An decreased by only 30 %. Therefore, during that interval, Ci decreased, whereas the intrinsic water use efficiency (An/gs) and the rate of photorespiration increased (not shown). At the same time, Asat decreased by 20 % and ETR and ϵ decreased by less than 10 %. Therefore, over that range of gs (i.e. mild drought), stomatal closure seems to be the main cause of decreased photosynthesis. This does not mean that non‐stomatal limitations are absent, but simply that they are not the dominant factor limiting photosynthesis. For instance, decreasing Asat suggests that the capacity for RuBP regeneration is adjusted progressively since early stomata closure.
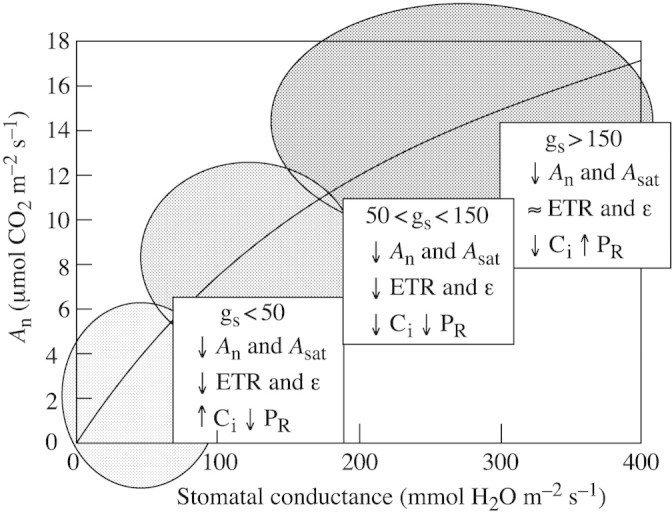
Fig. 3. Schematic pattern of response of photosynthesis in grapevines to drought, using gs as a reference parameter. Three main regions are distinguished, and the down‐regulation of different photosynthetic parameters is indicated for every region.
Further reduction of gs leads to more important reductions of all the parameters studied. When gs is 100 mmol H2O m–2 s–1, An decreases by 50 %, Asat by 35 %, and ETR and ϵ by 25–30 %. When gs equals 50 mmol H2O m–2 s–1, An decreases by 70 %, Asat and ϵ by 50 %, and ETR by 40 %. Below this threshold of gs, Ci increases (not shown), suggesting the predominance of non‐stomatal limitations to photosynthesis.
These results in field‐grown grapevines reveal a pattern of gradual response of photosynthesis to water stress, similar to that proposed by Lawlor (1995). After an early effect of drought resulting in partial stomatal closure, a metabolic adjustment takes place through limited RuBP‐regeneration (possibly due to impaired ATP synthesis, see below). Further reductions of gs as drought progresses lead to reduced photochemistry and carboxylation efficiency. Photoinhibition eventually occurs under conditions of very severe drought and almost complete stomatal closure.
WHAT ABOUT OTHER SPECIES?
To further test the generality of the relationships between different photosynthetic parameters and light‐saturated gs, six Mediterranean sclerophyllous trees and shrubs were subjected to progressive soil drying (Gulías et al., 2002). We had previously shown that in one of these species, Pistacia lentiscus L., drought induced a cascade of photosynthetic regulations qualitatively similar to that of grapevines, first involving stomatal closure and, later, non‐stomatal regulation (Gulías et al., 2002). Three of these species (Quercus ilex, Rhamnus alaternus and R. ludovici‐salvatoris) showed proportional decreases of gs and RWC in response to soil drying. In contrast, the other three (Quercus pubescens, Pistacia lentiscus and P. terebinthus) showed similar decreases of gs but their RWC remained almost constant (Flexas, Gulías, Abadía and Medrano, unpubl. res.). In spite of this distinct behaviour, all six species showed a similar pattern of dependency of different photosynthetic parameters on gs. We have superimposed results obtained for these six species over the relationships obtained for grapevines (Fig. 4), and also added to the figure results from other authors to increase the genetic and environmental variability. All the data points added are similar to the relationship for grapevine in respect to An, ETR and ϵ. This was surprising given that the species studied represent a substantial variety of life forms and photosynthetic characteristics. The data that fitted least well were those for Asat for the six sclerophyllous species (Fig. 4C). The fact that these species share with grapevines a common relationship between An and gs, while displaying such a divergence in their relationship between Asat and gs, could reflect a higher mesophyll resistance in the sclerophyllous species. It has been shown that sclerophyllous and woody species generally have a substantially higher mesophyll resistance than more mesophytic species (Lloyd et al., 1992; Epron et al., 1995; Evans and von Caemmerer, 1996). Additionally, the data from other authors were not dissimilar to the relationships found in grapevines. The non‐origin intercept of the data from Martin and Ruiz‐Torres (1992) was probably due to the fact that the relationships were not obtained from the original data, but rather from a combination of the best‐fit relationships given by the authors for the plots of gs, Asat and ϵ vs. Ψ.
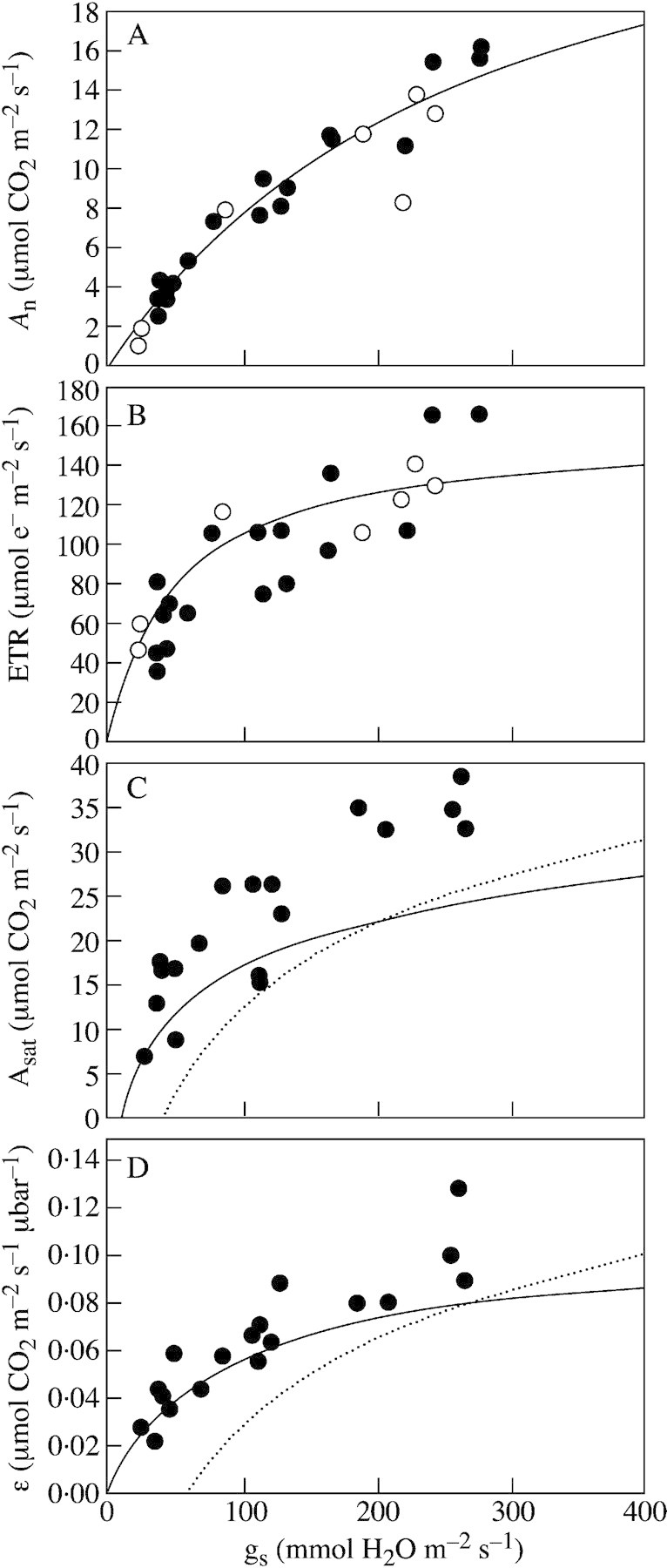
Fig. 4. This is the same as Fig. 2, after adding some data from the literature on other species to achieve a broad range of species and plant types. In all plots (A–D), data from Gulías et al. (2002) on six different Mediterranean sclerophyllous shrubs (filled circles) have been added. In A and B, data from other authors and species have been added (open circles). These include data on three different tropical understorey plants (from Ishida et al., 1999), on the evergreen sclerophyllous shrub, Pistacia lentiscus (from Flexas et al., 2001), and on alfalfa (Medicago sativa) plants (Antolín and Sánchez‐Díaz, 1993). In C and D, curves of best fit (dotted lines) between the plotted parameters obtained by Martin and Ruiz‐Torres (1992) in wheat (Triticum aestivum) are shown.
From the present data it is concluded that, although there is wide variability among species and genotypes in the maximum values of photosynthesis and stomatal conductance, as well as in the variations of leaf Ψ and RWC (Schulze and Hall, 1982; Vadell and Medrano, 1992; Bota et al., 2001), the photosynthesis to conductance ratio is largely maintained (see also Farquhar et al., 1987; Lloyd et al., 1992; Bota et al., 2001). Even when relationships between different photosynthetic parameters and gs are influenced by the species (Fig. 4; see Schulze and Hall, 1982; Farquhar et al., 1987), the species‐effect seems to be much less than that on photosynthesis and RWC or Ψ.
DO GAS‐EXCHANGE DATA MATCH THE BIOCHEMICAL EVIDENCE?
The present results support a quite generalized pattern of down‐regulation of different photosynthetic parameters in response to drought when using light‐saturated gs as a reference parameter. Such a pattern can be used to analyse the relative importance of every process at any given degree of stress. Nevertheless, all the evidence presented to date derives from in vivo measurements of gas exchange and chlorophyll fluorescence, and the interpretation of the results ultimately lies in the model of Farquhar and co‐workers (Farquhar, 1980; von Caemmerer and Farquhar, 1981) and its derivatives. The validation of this model still needs to be extended, especially in respect to long‐term responses (Farquhar et al., 2001).
To test the validity of this gas‐exchange model for the estimation of drought‐depressed rates of certain biochemical reactions, the results presented here are compared with those of other authors in which destructive, biochemical determinations were made in control and water‐stressed plants at the same time as gas‐exchange measurements. In particular, two important assumptions of the gas‐exchange model require validation. First, in the model, control of RuBP regeneration is ascribed to ETR but, as recognized by Farquhar et al. (2001), it could also be limited by other components of the photosynthetic carbon reduction cycle. Secondly, the apparent carboxylation efficiency (ϵ) was thought to be controlled by Rubisco activity, but other mesophyll limitations to photosynthesis may also exert control over ϵ. It is important to address both aspects for the study of photosynthetic responses to drought.
Decreased capacity for RuBP regeneration should come from decreased ATP synthesis under moderate water stress
Decreased capacity for RuBP regeneration, as determined by the CO2‐saturated rate of photosynthesis, has been shown many times to be an early response to drought, decreasing much earlier than ϵ (von Caemmerer and Farquhar, 1984; Martin and Ruiz‐Torres, 1992; Escalona et al., 1999; see Figs 3 and 4). Determination of RuBP content of leaves from water‐stressed plants seems to confirm that decreased capacity for RuBP regeneration is an early response to drought (Giménez et al., 1992; Gunasekera and Berkowitz, 1993). Farquhar’s model of photosynthesis assumes that this may be due to decreased ETR. However, the introduction of chlorophyll fluorescence techniques has shown that under mild drought Asat is usually reduced to a much greater extent than ETR (Figs 3 and 4). Tezara et al. (1999) have suggested that decreased ATP synthesis through ATPase impairment would lead to reduced RuBP regeneration. Whether impaired ATPase would also affect ETR or not depends on the precise mechanism of impairment, which is still not well understood, and other possible unknown metabolic adjustments. In spite of these uncertainties, there seems to be an agreement between gas exchange and biochemical literature. Clearly, both limited RuBP regeneration and impaired ATP synthesis still occur at high light‐saturated gs (over 150 mmol H2O m–2 s–1), i.e. in early phases of drought development (Younis et al., 1979; Turner et al., 1985; Havaux et al., 1987; Meyer and de Kouchkovsky, 1992; Tezara et al., 1999). To our knowledge, there is only one report (Ortiz‐López et al., 1991) of no inhibition of ATPase even at lower gs. The causes for reduced ATP synthesis under mild drought remain to be determined.
Decreased carboxylation capacity does not reflect only decreased Rubisco activity
The wide use of An–Ci curves has led to several reports showing a decrease in the apparent carboxylation efficiency (and thus, presumably, Rubisco activity) even at mild to moderate water stress in a number of species (Figs 3 and 4; see Martin and Ruiz‐Torres, 1992; Antolín and Sánchez‐Díaz, 1993; Faver et al., 1996; Escalona et al., 1999). However, assays of Rubisco activity from water‐stressed leaves have generally led to the conclusion that both its activity and activation state remain unaffected until the stress is severe (Jones, 1973; Beadle and Jarvis, 1977; Sharkey and Seemann, 1989; Plaut and Federman, 1991; Parry et al., 1993; Lal et al., 1996; Tezara et al., 1999; Wingler et al., 1999; Parry et al., 2002). Inhibition of Rubisco activity at mild to moderate water deficits has been reported only occasionally (Castrillo and Calcagno, 1989; Holaday et al., 1992; Medrano et al., 1997).
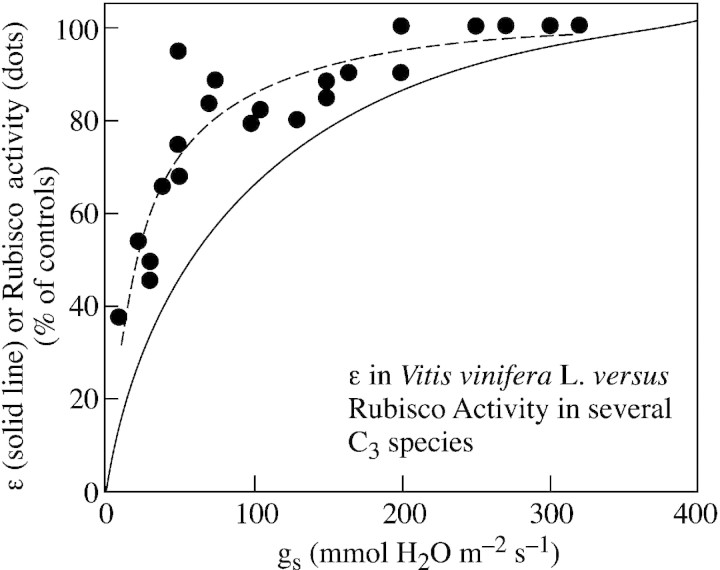
Therefore, for the particular case of Rubisco activity, it seems that the photosynthetic model of Farquhar et al. (1980) does not match the biochemical determinations. This is illustrated in Fig. 5, which shows the relationship between gs and both ϵ measured in grapevines and Rubisco activity determined in vitro for different species, including grapevines, by different authors. The results are expressed as a percentage of the control (unstressed) values to facilitate comparison of different units used by different authors, as well as to compare ϵ with Rubisco activity. Again, gs proves to be a solid reference parameter, since it generalizes the response of Rubisco activity to drought among a wide range of species and conditions. It is clear that two different relationships are obtained, the differences initially increasing with decreasing gs. When gs is between 50 and 150 mmol H2O m–2 s–1, ϵ is about 20–30 % lower than the measured Rubisco activity.
Fig. 5. Relationship between apparent carboxylation efficiency (ϵ) and stomatal conductance (gs) in field‐grown grapevines (solid line). Only the curve of best fit is plotted. ϵ is expressed as a % of the maximum observed values. Data of initial and/or total Rubisco activity have been added to the figure (filled circles), expressed as a % of maximum values for comparison. We selected data on Rubisco activity from the available literature in which gs was given. These data are from the following species and references: Helianthus annus (Pancovic et al., 1999; Tezara et al., 1999), Hordeum vulgare (Lal et al., 1996; Wingler et al., 1999, 2000), Medicago sativa (Antolín and Sánchez‐Díaz, 1993), Trifolium subterraneum (Medrano et al., 1997), Triticum aestivum (Holaday et al., 1992), Vicia faba (Lal et al., 1996) and Vitis vinifera (Bota, unpubl. res.). Broken line shows the curve of best fit for Rubisco activity data.
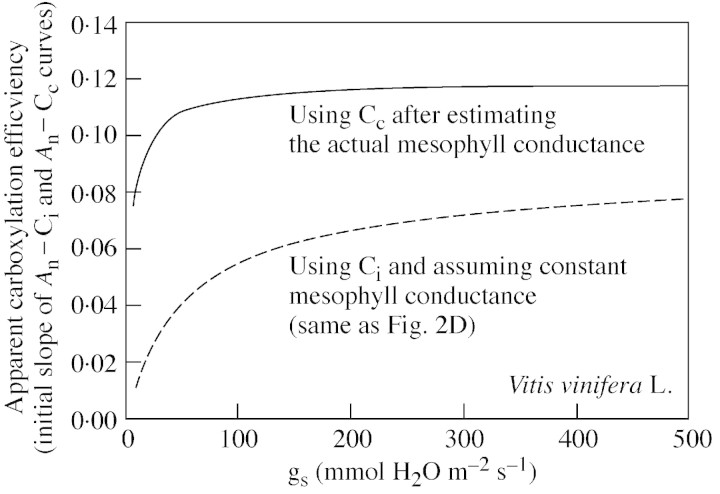
A possible explanation arises given that ϵ is underestimated whenever Ci is proportionally overestimated. Although we took into account patchy stomatal closure and cuticular conductance when calculating Ci (see previous sections), a different problem, namely varying mesophyll resistance, would lead to large and variable differences between Ci and the actual CO2 concentration at the carboxylation site (Cc), so ϵ would no longer be representative of the actual carboxylation efficiency. Increased mesophyll resistance, due either to changes in leaf internal anatomy or impaired carbonic anhydrase, has been suggested to occur under water stress (Beadle and Jarvis, 1977; Cornic et al., 1989; Renou et al., 1990; Lal et al., 1996; Roupsard et al., 1996). To test this possibility, we estimated Cc from combined gas‐exchange and chlorophyll fluorescence measurements, according to a current model (Epron et al., 1995; Valentini et al., 1995), and assuming that all the reducing power generated by the electron transport chain is used for photosynthesis and photorespiration, with only a negligible proportion being consumed by the Mehler reaction and other processes. The data obtained suggested that mesophyll conductance was decreasing as gs declined (Flexas et al., 2002a). Thereafter, we converted An–Ci curves to An–Cc curves, and recalculated ϵ on this new basis. Figure 6 shows the relationship obtained between ϵ and light‐saturated gs using this new approach and should be compared with Fig. 2D showing the relationship based on the typical An–Ci approach. Clearly, the new relationship is much more similar to that between Rubisco activity and light‐saturated gs obtained from the literature (Fig. 5).
Fig. 6. Effect of changing mesophyll conductance in the calculation of the apparent carboxylation (ϵ). The graph shows ϵ as a function of stomatal conductance in field‐grown, drought‐stressed grapevines, calculated directly from An–Ci curves (broken line) or after calculating Cc, from An–Cc curves (solid line). Data are from Flexas et al., 2002a).
These findings seem to confirm an early study by Beadle and Jarvis (1977), who showed a decreased mesophyll conductance in Picea sitchensis as drought progressed without any inactivation of Rubisco as determined in vitro. It is suggested that drought‐induced down‐regulation of mesophyll conductance to CO2 is much more important than previously thought. Nevertheless, these results are simply based on a model that requires many assumptions to be made, so they are not conclusive. A more extensive analysis of the effects of drought on mesophyll resistance is therefore needed.
IMPLICATIONS OF THE PRESENT RESULTS AND PRACTICAL APPLICATIONS
We have shown that in general the drought‐regulation of a wide range of parameters related to photosynthesis seems more dependent on stomatal conductance than on typical parameters reflecting leaf water status. As these relationships are similar for different plant species and different circumstances, one inherent implication could be that under drought, down‐regulation of different photosynthetic processes depends more on CO2 availability in the mesophyll (i.e. on stomatal closure) than on leaf water potential or leaf water content, as suggested previously (Sharkey, 1990). This could be understood as a direct adjustment of photosynthetic metabolism to CO2 availability, which is well known to act as a regulator of Rubisco (Perchorowicz and Jensen, 1983; Meyer and Genty, 1999), nitrate reductase (Kaiser and Förster, 1989) and sucrose phosphate synthase (Vassey et al., 1991). Low CO2 also promotes increased trans‐thylakoid ΔpH, which induces increased NPQ. Nevertheless, these suggestions are merely based on statistical correlative evidence, and further studies are required to prove them. In particular, it remains to be determined if low CO2 availability, or the pH changes resulting from it, are capable of promoting down‐regulation of other important photosynthetic steps such as ATP synthesis.
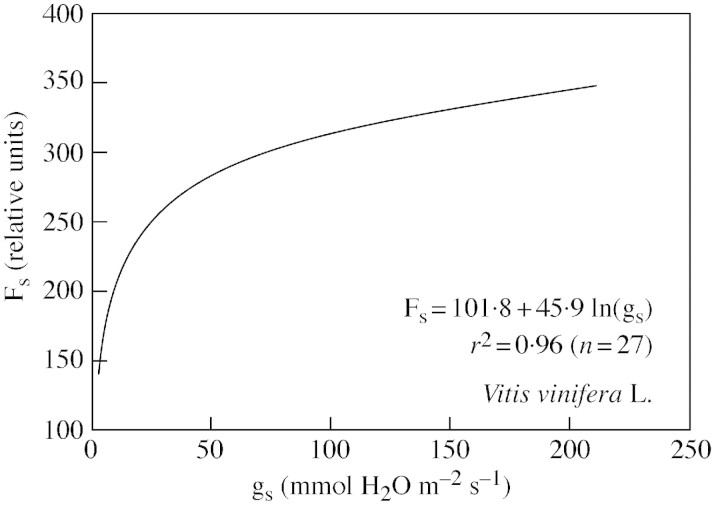
Irrespective of the uncertainties raised about the mechanistic reasons for the strong dependence of any photosynthetic parameter on gs, it reveals an integrated down‐regulation of the whole photosynthetic process as drought progresses, in accordance with theories of integrated ‘photosynthetic control’ (Foyer et al., 1990). This integrated regulation of photosynthesis is reinforced by this analysis since a direct correlation was described between gs, determined at a given light intensity, and a fluorescence parameter which, in principle, may have little dependence on stomatal conductance, the steady‐state chlorophyll fluorescence (Fs) (Fig. 7; see Ounis et al., 2001; Flexas et al., 2002b). Figure 8 shows a tentative scheme of such a photosynthetic control under drought, which can be summarized as follows. Under drought, stomata close in proportion to the degree of stress, progressively limiting CO2 availability in the chloroplast. CO2 assimilation is reduced and the CO2 : O2 ratio drops, thereby increasing photorespiration and/or the Mehler reaction. Since these processes consume relatively less ATP than does photosynthesis, they should lead to a certain increase of trans‐thylakoid ΔpH (Schreiber and Neubauer, 1990; Osmond et al., 1997b). Impaired ATPase and/or reduced ETR may also interfere with the build‐up of trans‐thylakoid ΔpH. The xanthophyll de‐epoxidation that follows increased ΔpH should lead to increased NPQ. Thermal dissipation in the antenna becomes progressively more important and Fs is consequently lowered.
Fig. 7. Relationship between steady‐state chlorophyll fluorescence (Fs) and stomatal conductance in leaves of grapevine, measured at a PPFD of 500 µmol photons m–2 s–1 (from Ounis et al., 2001).
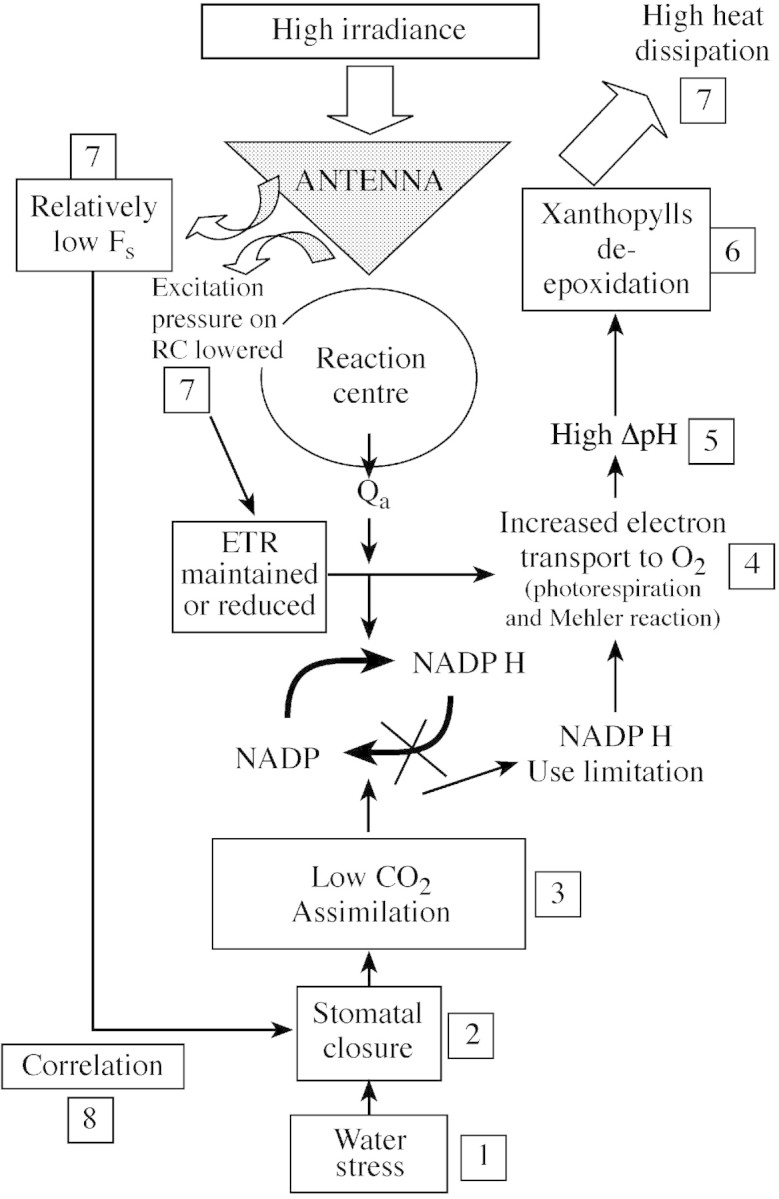
Fig. 8. Proposed mechanism of leaf response to water stress, explaining the observed interrelationships among different photosynthetic processes. Numbers indicate a proposed order for reading the scheme. See text for more details.
The relationship between Fs and gs provides a method for remote sensing of water stress. In grapevines, Fs/Fo declines steeply when non‐stomatal limitations become important (when gs drops below 100–150 mmol H2O m–2 s–1, see Figs 3, 4 and 7). Under moderate water deficit, i.e. when photosynthesis is mainly limited by stomatal conductance, a complete recovery of the maximum An occurred just one night after irrigation (Flexas et al., 1999a). However, if gs reaches values as low as 50 mmol H2O m–2 s–1, photosynthesis does not reverse after irrigation (Quick et al., 1992). Thus, proper monitoring of Fs would be a useful tool for deciding when irrigation must be applied to maintain plants at a limit between severe water stress and luxurious water consumption, thus rationalizing use of irrigation water. This method is especially useful because it does not depend on measuring fluorescence during saturating flashes, even during remote sensing (Moya et al., 1998; Flexas et al., 2000, 2002b; Ounis et al., 2001).
ACKNOWLEDGEMENT
We thank David Lawlor for introducing us to research on drought, for helpful comments and advice, and for his friendship.
Supplementary Material
Received: 1 May 2001; Returned for revision: 2 August 2001; Accepted: 7 September 2001.
References
- AntolínMC, Sánchez‐Díaz M.1993. Effects of temporary droughts on photosynthesis of alfalfa plants. Journal of Experimental Botany 44: 1341–1349. [Google Scholar]
- BeadleCL, Jarvis PG.1977. Effects of shoot water status on some photosynthetic partial processes in Sitka spruce. Physiologia Plantarum 41: 7–13. [Google Scholar]
- BotaJ, Flexas J, Medrano H.2001. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Annals of Applied Biology 138: 353–361. [Google Scholar]
- BoyerJS.1976. Photosynthesis at low water potentials. Philosophical Transactions of the Royal Society B 273: 501–512. [Google Scholar]
- BoyerJS, Wong SC, Farquhar GD.1997. CO2 and water vapour exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiology 114: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CastrilloM, Calcagno AM.1989. Effects of water stress and rewatering on ribulose 1,5‐bisphosphate carboxylase activity, chlorophyll and protein contents in two cultivars of tomato. Journal of Horticultural Science 64: 717–724. [Google Scholar]
- ChavesMM.1991. Effects of water deficits on carbon assimilation. Journal of Experimental Botany 42: 1–16. [Google Scholar]
- ChonéX, van Leeuwen C, Dubourdieu D, Gaudillère JP.2001. Stem water potential is a sensitive indicator of grapevine water status. Annals of Botany 87: 477–484. [Google Scholar]
- CornicG.1994. Drought stress and high light effects on leaf photosynthesis. In: Baker NR, Bowyer JR, eds. Photoinhibition of photosynthesis: from molecular mechanisms to the field. Oxford: BIOS Scientific Publishers. [Google Scholar]
- CornicG.2000. Drought stress inhibits photosynthesis by decreasing stomatal aperture – not by affecting ATP synthesis. Trends in Plant Science 5: 187–188. [Google Scholar]
- CornicG, Massacci A.1996. Leaf photosynthesis under drought stress. In: Baker NR, ed. Photosynthesis and the environment. The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- CornicG, Le Gouallec J‐L, Briantais JM, Hodges M.1989. Effect of dehydration and high light on photosynthesis of two C3 plants (Phaseolus vulgaris L. and Elatostema repens (Lour.) Hall f.). Planta 177: 84–90. [DOI] [PubMed] [Google Scholar]
- CornicG, Fresneau C.2002. Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Annals of Botany 89: 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CorreiaMJ, Pereira JS, Chaves MM, Rodrigues ML, Pacheco C.A.1995. ABA xylem concentrations determine maximum daily leaf conductance of field‐grown Vitis vinifera L. plants. Plant Cell and Environment 18: 511–521. [Google Scholar]
- DaiZ, Edwards GE, Ku MSB.1992. Control of photosynthesis and stomatal conductance in Ricinus communis L. (castor bean) by leaf to air vapor pressure deficit. Plant Physiology 99: 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesWJ, Zhang J.1991. Root signals and the regulation of growth and development of plants in drying soil. Annual Review of Plant Physiology and Plant Molecular Biology 42: 55–76. [Google Scholar]
- DaviesWJ, Gowing DJG.1999. Plant responses to small perturbations in soil water status. In: Press MC, Scholes JD, Barker MG. Physiological Plant Ecology Oxford: Blackwell Sciences, 67–89. [Google Scholar]
- DowntonWJS, Loveys BR, Grant WJR.1988. Non‐uniform stomatal closure induced by water stress causes putative non‐stomatal inhibition of photosynthesis. New Phytologist 110: 503–509. [DOI] [PubMed] [Google Scholar]
- EpronD, Godard D, Cornic G, Genty B.1995. Limitation of net CO2 assimilation rate by internal resistances to CO2 transfer in the leaves of two tree species (Fagus sylvatica L. and Castanea sativa Mill.). Plant Cell and Environment 18: 43–51. [Google Scholar]
- EscalonaJM, Flexas J, Medrano H.1999. Stomatal and non‐stomatal limitations of photosynthesis under water stress in field‐grown grapevines. Australian Journal of Plant Physiology 26: 421–433. [Google Scholar]
- EvansJR, von Caemmerer S.1996. Carbon dioxide diffusion inside leaves. Plant Physiology 110: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FarquharGD, von Caemmerer S, Berry JA.1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- FarquharGD, von Caemmerer S, Berry JA.2001. Models of photosynthesis. Plant Physiology 125: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FarquharGD, Hubick KT, Terashima I, Condon AG, Richards RA.1987. Genetic variation in the relationship between photosynthetic CO2 assimilation rate and stomatal conductance to water loss. In: Biggens J, ed. Progress in photosynthesis research Vol IV. Dordrecht: Martinus Nijhoff Publishers. [Google Scholar]
- FaverKL, Gerik TJ, Thaxton PM, El‐Zik KM.1996. Late season water stress in cotton: II. Leaf gas exchange and assimilation capacity. Crop Science 36: 922–928. [Google Scholar]
- FlexasJ, Medrano H.2002a Drought‐inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Annals of Botany 89: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FlexasJ, Medrano H.2002b Photosynthetic responses of C3 plants to drought. In: Hemantaranjan A, ed. Advances in Plant Physiology IV. Jodhpur: Scientific Publishers. [Google Scholar]
- FlexasJ, Escalona JM, Medrano H.1998. Down‐regulation of photosynthesis by drought under field conditions in grapevine leaves. Australian Journal of Plant Physiology 25: 893–900. [Google Scholar]
- FlexasJ, Escalona JM, Medrano H.1999a Water stress induces different levels of photosynthesis and electron transport rate regulations in grapevines. Plant, Cell and Environment 22: 39–48. [Google Scholar]
- FlexasJ, Badger M, Chow WS, Medrano H, Osmond CB.1999b Analysis of the relative increase in photosynthetic O2 uptake when photosynthesis in grapevine leaves is inhibited following low night temperatures and/or water stress. Plant Physiology 121: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FlexasJ, Briantais J‐M, Cerovic Z, Medrano H, Moya I.2000. Steady‐state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: a new remote sensing system. Remote Sensing of the Environment 73: 283–297. [Google Scholar]
- FlexasJ, Gulías J, Jonasson S, Medrano H, Mus M.2001. Seasonal patterns and control of gas exchange in local populations of the Mediterranean evergreen shrub Pistacia lentiscus L. Acta Oecologica 22: 33–43. [Google Scholar]
- FlexasJ, Bota J, Escalona JM, Sampol B, Medrano H.2002a Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology (in press). [DOI] [PubMed] [Google Scholar]
- FlexasJ, Escalona JM, Evain S, Gulías J, Moya I, Osmond CB, Medrano H.2002b Steady state chlorophyll fluorescence (Fs) measurements as a tool to follow variations of net CO2 assimilation and stomatal conductance during water‐stress in C3 plants. Physiologia Plantarum 114: 231–240. [DOI] [PubMed] [Google Scholar]
- FoyerCH, Furbank R, Harbinson J, Horton P.1990. The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynthesis Research 25: 83–100. [DOI] [PubMed] [Google Scholar]
- GiménezC, Mitchell VJ, Lawlor DW.1992. Regulation of photosynthesis rate of two sunflower hybrids under water stress. Plant Physiology 98: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GollanT, Turner NC, Schulze E‐D.1985. The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content. III. In the sclerophyllous woody species Nerium oleander Oecologia 65: 356–362. [DOI] [PubMed] [Google Scholar]
- GulíasJ, Flexas J, Abadía A, Medrano H.2002. Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: possible factors explaining the declining distribution of an endemic Balearic species (Rhamus ludovici‐salvatoris). Tree Physiology (in press). [DOI] [PubMed] [Google Scholar]
- GunasekeraD, Berkowitz GA.1993. Use of transgenic plants with Rubisco antisense DNA to evaluate the rate limitation of photosynthesis under water stress. Plant Physiology 103: 629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HarauxF, de Kouchkovsky Y.1998. Energy coupling and ATP synthase. Photosynthesis Research 57: 231–251. [Google Scholar]
- HavauxM, Canaani O, Malkin S.1987. Inhibition of photosynthetic activities under slow water stress measured in vivo by the photoacoustic method. Physiologia Plantarum 70: 503–510. [Google Scholar]
- HeberU, Bligny R, Streb P, Douce R.1996. Photorespiration is essential for the protection of the photosynthetic apparatus of C3 plants against photoinactivation under sunlight. Botanica Acta 109: 307–315. [Google Scholar]
- HoladayAS, Ritchie SW, Nguyen HT.1992. Effects of water deficit on gas‐exchange parameters and ribulose 1,5‐bisphosphate carboxylase activation in wheat. Environmental and Experimental Botany 32: 403–410. [Google Scholar]
- HubbardRM, Ryan MG, Stiller V, Sperry JS.2001. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant, Cell and Environment 24: 113–121. [Google Scholar]
- IshidaA, Nakano T, Matsumoto Y, Sakoda M, Hoe Ang L.1999. Diurnal changes in leaf gas exchange and chlorophyll fluorescence in tropical tree species with contrasting light requirements. Ecological Research 14: 77–88. [Google Scholar]
- JonesHG.1973. Moderate‐term water stresses and associated changes in some photosynthetic parameters in cotton. New Phytologist 72: 1095–1105. [Google Scholar]
- KaiserWM, Förster J.1989. Low CO2 prevents nitrate reduction in leaves. Plant Physiology 91: 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KozakiA, Takeba G.1996. Photorespiration protects C3 plants from photooxidation. Nature 384: 557–560. [Google Scholar]
- LalA, Ku MSB, Edwards GE.1996. Analysis of inhibition of photosynthesis due to water stress in the C3 species Hordeum vulgare and Vicia faba: electron transport, CO2 fixation and carboxylation capacity. Photosynthesis Research 49: 57–69. [DOI] [PubMed] [Google Scholar]
- LawlorDW.1976a Water stress induced changes in photosynthesis, photorespiration, respiration and CO2 compensation concentration of wheat. Photosynthetica 10: 378–387. [Google Scholar]
- LawlorDW.1976b Assimilation of carbon into photosynthetic intermediates of water‐stressed wheat. Photosynthetica 10: 431–439. [Google Scholar]
- LawlorDW.1983. Integration of biochemical processes in the physiology of water stressed plants. In: Marcelle R, Clijters H, von Puche M, eds. Effects of stress on photosynthesis. The Hague‐Boston‐London: Martinus Nijhoff/Dr W. Junk Publishers. [Google Scholar]
- LawlorDW.1995. The effects of water deficit on photosynthesis. In: Smirnoff N, ed. Environment and plant metabolism. Flexibility and acclimation. Oxford: BIOS Scientific Publisher. [Google Scholar]
- LawlorDW.2002. Limitation to photosynthesis in water‐stressed leaves: stomal vs. metabolism and the role of ATP. Annals of Botany 89: 871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LawlorDW, Fock H.1975. Photosynthesis and photorespiratory CO2 evolution of water‐stressed sunflower leaves. Planta 126: 247–258. [DOI] [PubMed] [Google Scholar]
- LawlorDW, Fock H.1977a Photosynthetic assimilation of 14CO2 by water‐stressed sunflower leaves in two oxygen concentrations and the specific activity of products. Journal of Experimental Botany 28: 320–328. [Google Scholar]
- LawlorDW, Fock H.1977b Water stress induced changes in the amounts of some photosynthetic assimilation products and respiratory metabolites of sunflower leaves. Journal of Experimental Botany 28: 329–337. [Google Scholar]
- LawlorDW, Khanna‐Chopra R.1984. Regulation of photosynthesis during water stress. In: Sybesma C, ed. Advances in photosynthesis research Vol IV. The Hague‐Boston‐Lancaster: Martinus Nijhoff/Dr W. Junk Publishers. [Google Scholar]
- LawlorDW, Pearlman JG.1981. Compartmental modelling of photorespiration and carbon metabolism of water stressed leaves. Plant, Cell and Environment 4: 37–52. [Google Scholar]
- LawlorDW, Uprety DC.1993. Effects of water stress on photosynthesis of crops and the biochemical mechanism. In: Abrol YP, Mohanty P, Govinjee, eds. Photosynthesis: photoreactions to plant productivity. New Dehli: Oxford and IBH Publishing Co. PVT. Ltd. [Google Scholar]
- LloydJ, Syversten JP, Kriedemann PE, Farquhar GD.1992. Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant, Cell and Environment 15: 873–899. [Google Scholar]
- MartinB, Ruiz‐Torres NA.1992. Effects of water‐deficit stress on photosynthesis, its components and component limitations, and on water use efficiency in wheat (Triticum aestivum L.). Plant Physiology 100: 733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MedranoH, Parry MA, Socias X, Lawlor DW.1997. Long term water stress inactivates Rubisco in subterranean clover. Annals of Applied Biology 131: 491–501. [Google Scholar]
- MeyerS, de Kouchkovsky Y.1992. ATPase state and activity in thylakoids from normal and water‐stressed lupin. FEBS Letters 303: 233–236. [DOI] [PubMed] [Google Scholar]
- MeyerS, Genty B.1999. Heterogeneous inhibition of photosynthesis over the leaf surface of Rosa rubiginosa L. during water stress and abscisic acid treatment: induction of a metabolic component by limitation of CO2 diffusion. Planta 210: 126–131. [DOI] [PubMed] [Google Scholar]
- MoyaI, Camenen L, Latouche G, Mauxion C, Evain S, Cerovic Z.1998. An instrument for the measurement of sunlight excited plant fluorescence. In: Garab G, ed. Photosynthesis: mechanisms and effects, Vol. V. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- OrenR, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schäfer KVR.1999. Survey and synthesis of intra‐ and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant, Cell and Environment 22: 1515–1526. [Google Scholar]
- OrtDR, Oxborough K, Wise RR.1994. Depressions of photosynthesis in crops with water deficits. In: Baker NR, Bowyer JR, eds. Photoinhibition of photosynthesis: from molecular mechanisms to the field. Oxford: BIOS Scientific Publishers. [Google Scholar]
- Ortiz‐LópezA, Ort DR, Boyer JS.1991. Photophosphorylation in attached leaves of Helianthus annuus at low water potentials. Plant Physiology 96: 1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OsmondCB, Flexas J, Neff R.1997a Imaging chlorophyll fluorescence in vivo with an almost portable system. In: Ball M, Clark‐Walker GD, Farquhar GD, Gunning BES, Morgan IG, Osmond CB, eds. Research School of Biological Sciences. Annual Report 1997. Canberra: RSBS. [Google Scholar]
- OsmondCB, Maxwell K, Björkman O, Badger M, Leegood R.1997b Too many photons: photorespiration, photoinhibition and photo oxidation. Trends in Plant Science 4: 119–121. [Google Scholar]
- OunisA, Evain S, Flexas J, Tosti S, Moya I.2001. Adaptation of the PAM‐fluorometer for remote sensing of chlorophyll fluorescence. Photosynthesis Research 68: 113–120. [DOI] [PubMed] [Google Scholar]
- PankovicD, Sakac, Z, Kevresan, S, Plesnicar, M.1999. Acclimation to long‐term water deficit in the leaves of two sunflower hybrids: photosynthesis, electron transport and carbon metabolism. Journal of Experimental Botany 50: 127–138. [Google Scholar]
- ParryMAJ, Delgado E, Vadell J, Keys AJ, Lawlor DW, Medrano H.1993. Water stress and the diurnal activity of ribulose‐1,5‐bisphosphate carboxylase in field grown Nicotiana tabacum genotypes selected for survival at low CO2 Plant Physiology and Biochemistry 31: 113–120. [Google Scholar]
- ParryMAJ, Andralojc PJ, Khan S, Lea PJ, Keys AJ.2002. Rubisco activity: effects of drought stress. Annals of Botany 89: 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PeiZ‐M, Ghassemian M, Kwak CM, McCourt P, Schroeder JI.1998. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282: 287–290. [DOI] [PubMed] [Google Scholar]
- PerchorowiczJT, Jensen RG.1983. Photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Regulation by CO2 and O2 Plant Physiology 71: 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PlautZ, Federman E.1991. Acclimation of CO2 assimilation in cotton leaves to water stress and salinity. Plant Physiology 97: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QuickWP, Chaves MM, Wendler R, David M, Rodrigues ML, Passaharinho JA, Pereira JS, Adcock MD, Leegood RC, Stitt M.1992. The effect of water stress on photosynthetic carbon metabolism in four species grown under field conditions. Plant, Cell and Environment 15: 25–35. [Google Scholar]
- RaschkeK.1979. Movements of stomata. In: Haupt W, Feinleib ME, eds. Encyclopedia of plant physiology New Series vol. 7. Physiology of movements. Berlin: Springer‐Verlag. [Google Scholar]
- RenouJ‐L, Gerbaud A, Just D, André M.1990. Differing substomatal and chloroplastic CO2 concentrations in water‐stressed wheat. Planta 182: 415–419. [DOI] [PubMed] [Google Scholar]
- RoupsardO, Gross P, Dreyer E.1996. Limitation of photosynthetic activity by CO2 availability in the chloroplasts of oak leaves from different species and during drought. Annales des Sciences Forestieres 53: 243–54. [Google Scholar]
- SalleoS, Nardini A, Pitt F, Lo Gullo MA.2000. Xylem cavitation and hydraulic control of stomatal conductance in Laurels (Laurus nobilis L.). Plant, Cell and Environment 23: 71–79. [Google Scholar]
- SchreiberU, Neubauer C.1990. O2‐dependent electron flow, membrane energisation and the mechanism of non‐photochemical quenching of chlorophyll fluorescence. Photosynthesis Research 25: 279–293. [DOI] [PubMed] [Google Scholar]
- SchulzeE‐D, Hall AE.1982. Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In: Lange OL, Nobel PS, Osmond CB, eds. Encyclopedia of plant physiology Vol. 12 B. Physiological plant ecology II. Berlin‐Heidelberg‐New York: Springer‐Verlag. [Google Scholar]
- SchurrU, Gollan T, Schulze E‐D.1992. Stomatal responses to drying soil in relation to changes in the xylem sap composition of Helianthus annuus II. Stomatal sensitivity to abscisic acid imported from the xylem sap. Plant, Cell and Environment 15: 561–567. [Google Scholar]
- SharkeyTD.1990. Water stress effects on photosynthesis. Photosynthetica 24: 651. [Google Scholar]
- SharkeyTD, Seeman JR.1989. Mild water stress effects on carbon‐reduction cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiology 89: 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SocíasFX, Correia MJ, Chaves MM, Medrano H.1997. The role of abscisic acid and water relations in drought responses of subterranean clover. Journal of Experimental Botany 48: 1281–1288. [Google Scholar]
- TangA‐C, Kawamitsu Y, Kanechi M, Boyer JS.2002. Photosynthetic oxygen evolution at low water potential in leaf discs lacking an epidermis. Annals of Botany 89: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TardieuF, Davies WJ.1992. Stomatal response to ABA is a function of current plant water status. Plant Physiology 98: 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TardieuF, Simmonneau T.1998. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49: 419–432. [Google Scholar]
- TerashimaI, Wong SC, Osmond CB, Farquhar GD.1988. Characterization of non‐uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant and Cell Physiology 29: 385–394. [Google Scholar]
- TezaraW, Mitchell VJ, Driscoll SD, Lawlor DW.1999. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401: 914–917. [Google Scholar]
- TurnerLB, Wellburn AR.1985. Changes in adenylate nucleotide levels in the leaves of Capsicum annuum during water stress. Journal of Plant Physiology 120: 111–122. [Google Scholar]
- TyreeMT.1999. Water relations and hydraulic architecture. In: Pugnaire FI, Valladares F, eds. Handbook of functional plant ecology. New York: Marcel Dekker Inc. [Google Scholar]
- VadellJ, Medrano H.1992. Effect of drought on subterranean clover. 2. Genetic variability of photosynthesis, transpiration and stomatal conductance. Photosynthetica 27: 433–440. [Google Scholar]
- VadellJ, Cabot C, Medrano H.1995. Diurnal time course of leaf gas exchange rates and related characters in drought‐acclimated and irrigated Trifolium subterraneum Australian Journal of Plant Physiology 22: 461–469. [Google Scholar]
- ValentiniR, Epron D, De Angelis P, Matteucci G, Dreyer E.1995. In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Quercus cerris L.) Leaves: diurnal cycles under different levels of water supply. Plant, Cell and Environment 18: 631–640. [Google Scholar]
- VasseyTL, Quick WP, Sharkey TD, Stitt M.1991. Water stress, carbon dioxide and light effects on sucrose phosphate synthase activity in Phaseolus vulgaris Physiologia Plantarum 81: 37–44. [Google Scholar]
- von CaemmererS, Farquhar GD.1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387. [DOI] [PubMed] [Google Scholar]
- von CaemmererS, Farquhar GD.1984. Effects of partial defoliation, changes of irradiance during growth, short‐term water stress and growth at enhanced p(CO2) on photosynthetic capacity of leaves of Phaseolus vulgaris L. Planta 160: 320–329. [DOI] [PubMed] [Google Scholar]
- WinglerA, Lea PJ, Quick WP, Leegood RC.2000. Photorespiration: metabolic pathways and their role in stress protection. Philosophical Transactions of the Royal Society B 355: 1517–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WinglerA, Quick WP, Bungard RA, Bailey KJ, Lea PJ, Leegood RC.1999. The role of photorespiration during drought stress: an analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant, Cell and Environment 22: 361–373. [Google Scholar]
- WongSC, Cowan IR, Farquhar GD.1979. Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426. [Google Scholar]
- YounisHM, Boyer JS, Govindjee.1979. Conformation and activity of chloroplast coupling factor exposed to low chemical potential of water in cells. Biochimica et Biophysica Acta 548: 328–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.