Abstract
Insufficient soil moisture during summer months is now the major cause of sugar beet yield losses in the UK. However, selection for increased drought tolerance has not been a breeding priority until recently. Genetic variation for drought tolerance is an essential prerequisite for the development of more stress‐tolerant varieties, but commercial sugar beet varieties seem to have similar yield responses to drought. The objective of this study was to assess the degree of genotypic variation for drought tolerance within a wide range of sugar beet germplasm and genebank accessions within Beta. Thirty sugar beet genotypes were screened under field drought conditions, and putative drought tolerant and sensitive lines (in terms of yield reduction in polythene‐covered vs. irrigated plots) were identified. Significant genotype × water treatment interactions were found for dry matter yield and relative leaf expansion rate. Genotypic differences for drought susceptibility index were also significant. Differential sensitivity of seedling shoot growth to water deficit was examined by comparing 350 genebank accessions in a simple growth chamber screen. Methods of data management were devised to highlight lines for entry into subsequent field tests. The results of the field and seedling screens indicate that there is variation for tolerance to water deficits within sugar beet and related types, and that there are lines that show greater drought tolerance than selected commercial varieties. Divergent lines showing contrasting behaviour should aid in the identification of key morpho‐physiological traits that confer drought tolerance.
Key words: Beta vulgaris, sugar beet, drought tolerance, genetic variation, leaf expansion, relative growth rate
INTRODUCTION
Drought is a major limitation to crop productivity worldwide (Boyer, 1982). For most major food crops, improvement in drought tolerance is an important breeding objective, and significant advances have been made over the past 10–20 years (Boyer, 1996). Only recently, however, has the impact of drought been recognized as a major cause of yield losses in sugar beet (Jaggard et al., 1998; Pidgeon et al., 2001). In dry regions, such as California or southern Europe, the crop is grown under irrigation. However, in other beet production areas where irrigation is not normally applied, such as the UK and parts of Germany, summer rainfall amounts are unpredictable and are usually insufficient to meet the crop’s water requirements. In the east of England, for example, average precipitation receipts for June to September are 150 mm, whereas evapotranspiration accounts for nearly 350 mm during the same period (Jaggard et al., 1998). Furthermore, many of the soils in this region are sandy with low water holding capacity. Averaged nationally for the UK, annual drought‐induced yield losses were estimated to be 10 % of potential production, and rose to 30 % in drier years (Jaggard et al., 1998). Only a small fraction of the beet crop is irrigated in the UK and there is little opportunity for new water abstraction licences. Therefore, the best avenue for improvement of beet production in drought‐prone areas is the development of varieties with increased drought tolerance.
Two essential building blocks of any crop improvement programme are genetic variation and sources of germplasm containing enhanced expression of desired traits. Studies on the yield performance of sugar beet varieties under dry conditions, on soils of low water holding capacity or in response to irrigation have not found any significant genotypic differences (van der Beek et al., 1993; Fisher and Kerr, 1998; Kerr, 2000). This may reflect the narrow genetic base of commercial varieties (Bosemark, 1979; McGrath et al., 1999). In this case, introgression of germplasm from wild populations would be the only way to increase the genetic variation for characters that could contribute to drought tolerance in sugar beet. However, introgression of exotic material into breeding lines requires additional time and expense to sort out ‘weedy’ characters while preserving desired agronomic traits (Lewellen, 1992). Therefore, the objective of this study was to assess the degree of genotypic variation for drought tolerance within a wide range of sugar beet germplasm and related Beta types. Results from these initial experiments are important in determining the direction of research and in designing strategies for breeding programmes.
Two approaches were used. First, yield and growth responses of a wide range of sugar beet genotypes were compared under drought conditions in the field. Secondly, a large number of Beta genebank accessions were tested in a growth chamber screen for differential sensitivity of seedling shoot growth to water deficits. Field trials of genotypes are time‐consuming and expensive. Furthermore, space is limited, so only a restricted number of genotypes can be tested in 1 year. Deciding which genotypes to include in field trials is an important decision, and each entry should fulfil the purposes of the test. There are at least 10 000 accessions of Beta types available from genebanks such as the BAZ genebank in Braunschweig, Germany. However, there is little information on individual phenotypes, and no indication of the degree of potential drought tolerance. Hence, one of the objectives of an EU‐sponsored project was to screen some of these accessions in a simple growth chamber test for drought tolerance. The same accessions were also screened in other tests for disease resistance (Luterbacher et al., 2000). The aim of the drought‐tolerance screen was to highlight accessions worthy of further investigation for promising traits or as possible entries in field trials.
MATERIALS AND METHODS
Field screening
Thirty‐two sugar beet genotypes (Table 1) were planted in the field in a factorial, randomized complete block design. Two genotypes showed poor establishment and were eliminated from further analysis. Seeds were drilled in 1·5 m × 3 m plots on 12 May 1999 on loamy sand near Bury St Edmunds, Suffolk, UK. Plots were replicated four times with two treatments, either full irrigation or no irrigation. Irrigation was applied by drip tape placed between plant rows spaced 50 cm apart. The tape was buried 2–8 cm below the surface of the soil. Plots were irrigated with approx. 18 mm water per week, split into two applications. The irrigation regime commenced on 9 July and ended 1 d before harvest (6 Oct. 1999). The entire experiment was covered using semi‐permanent polythene tunnels (Haygrove Tunnels Ltd, Redbank, UK) on 15 Jun. 1999. The tunnels were approx. 138 m long, 7·3 m wide and 3·9 m high at the highest point. The tunnels were open at the ends and at the sides where the polythene reached to within 1·5 m of the ground. Four such tunnels adjacent and connected to each other covered the experimental area. Rainfall runoff from the polythene was carried away from the area by drainage furrows extending the length of the tunnels and within a 2 m wide gap between the plots in adjacent tunnels.
Table 1.
List of genotypes tested in the field in 1999
| ID | Variety name | Donor/source | Country of origin | Ploidy |
| 1802 | Janacscz Iii | Ford‐Lloyda | Poland | |
| 2122 | Villanyi | ATPb | Hungary | |
| 2123 | Nagykalloi | ATP | Hungary | |
| 3360 | Dieckmann’s Maxima | BAZc | Germany | |
| 3362 | Kaweaa | BAZ | Germany | |
| 3787 | Pedigree | BAZ | The Netherlands | |
| 4106 | Dobrovicka A | BAZ | Czech Republic | |
| 5385 | Lablabu | USDAd | Afganistan | |
| 6071 | Alba | BAZ | Italy | |
| 6177 | BAZ | Georgia | ||
| 7066 | Kleinwanzlebener Zz | BAZ | Germany | |
| 7829 | Uladovskaya | VIRe | Russia | |
| 7912 | L’Govskaya | VIR | Russia | |
| 8035 | Moldavskaya | VIR | Russia | |
| KWS1 | KWSf | Diploid | ||
| KWS2 | KWS | Diploid | ||
| KWS3 | KWS | Diploid | ||
| KWS4 | KWS | Diploid | ||
| KWS5 | KWS | Diploid | ||
| KWS6 | KWS | Diploid | ||
| KWS7 | KWS | Triploid | ||
| KWS8 | KWS | Diploid | ||
| KWS9 | KWS | Triploid | ||
| Nicola | Nicola | British Sugar | Triploid | |
| NOV1 | Syngentag | Diploid | ||
| NOV6 | Syngenta | Diploid | ||
| NOV7 | Syngenta | Diploid | ||
| NOV8 | Syngenta | Diploid | ||
| Oberon | Oberon | British Sugar | Diploid | |
| Roberta | Roberta | British Sugar | Diploid | |
| SBSI24367 | BP Mashad | SBSIh | Iran | |
| SBSI24369 | BP Karadj | SBSI | Iran |
Ploidy and geographical locations are listed for genotypes where information is available. For some of the lines, the four‐digit ID number is the BAZ genebank accession number.
a Dr B. Ford‐Lloyd, University of Birmingham.
b Institute for Agrobotany, Hungary.
c Federal Centre for Breeding Research on Cultivated Plants (BAZ) Genebank, Germany.
d National Plant Germplasm System, United States Department of Agriculture.
e N.I. Vavilov All‐Russian Scientific Research Institute of Plant Industry, Russia.
f KWS SAAT AG, Germany.
g Novartis Seeds International AB – HILLESHÖG, Landskrona, Sweden.
h Sugar Beet Seed Institute, Iran.
The effect of the tunnels on crop microclimate was significant, but did not create combinations of weather factors that are unlikely to occur in practice. The polythene was 100 µm thick, which transmitted over 90 % of the incident PAR when new, decreasing to approx. 80 % transmission by the end of the experiment. Windrun was also decreased by 50–60 % under the tunnels, thus during the period that the crop was covered by polythene estimated cumulative evapotranspiration was 260 mm; this was 24 % less than that of the crop outside the tunnels. The amount of soil water available to the crop at this site was approx. 167 mm, derived from soil survey data and assuming a functional rooting depth of 120 mm (Hodge et al., 1984).
Standard cultural practices for the beet crop were followed. Briefly, the field was fertilized before cultivation with 600 kg ha–1 kainit (K : Na : Mg, 12 : 18 : 3), 200 kg ha–1 0 : 24 : 24 (N : P : K) and 100 kg ha–1 N (as NH4NO3). Pre‐ and post‐emergence herbicides were applied, followed by hand hoeing where necessary to control weeds. Plots were sprayed with flusilazole/carbendazim fungicide to control powdery mildew on 8 August.
Relative leaf expansion rate (RLER) was measured between 2 and 9 September when older leaves of droughted plants were visibly wilted. RLER was calculated by measuring the length and width of the fourth newly formed lamina. This leaf was marked and measured again 7 d later. Preliminary experiments showed that by applying a correction factor these dimensions accurately estimated the actual leaf area measured with a leaf area meter (DeltaT, Cambridge, UK). The correction factor did not change significantly with leaf age or variety. Another preliminary study showed that the relative expansion rate of the fourth leaf remained constant for approx. 21 d, after which the rate decreased. Therefore, small differences between plants in the age or shape of the selected leaf did not significantly bias comparisons based on the calculation of RLER. RLER was calculated according to the equation:
RLER = (lnA2 – lnA1)/(t2 – t1)
where A is estimated leaf area and t is time. Since all genotypes were measured on the same days, chronological rather than thermal time was deemed acceptable in this case.
A central area of 1·5 × 2 m within each plot was harvested by hand on 13 October. Roots were washed and weighed, then subsampled for measurement of tissue water content and dry weight. Plant tops (including the crown, petiole and laminae) were weighed in the field using an electronic load cell, then subsampled for determination of tissue water content and dry weight. Drought tolerance was defined using the susceptibility index (SI; Fischer and Maurer, 1978), which is the relative yield loss divided by the mean yield loss of all tested genotypes in the experiment. The degree of yield loss due to water deficit compared with irrigated controls is:
YL = (1 – [Yd/Yi])
where YL is the relative yield loss, Yd is the yield under drought and Yi is the irrigated yield. Then,
SI = YL/(1 – [Xd/Xi])
where Xd is the mean yield under drought conditions of all tested genotypes, and Xi is the mean irrigated yield of all genotypes.
Growth chamber screen
Accessions to be tested in the growth chamber screen were chosen from a ‘core’ collection in the BAZ Beta genebank (Braunschweig, Germany). The aim was to select accessions that represented diverse genetic backgrounds and geographical locations. Approx. 50 seeds from each accession were steeped in thiram fungicide for 24 h prior to sowing in potting compost [Levington F2S containing N : P : K (5 : 8 : 3) and microelements]. Once seedlings emerged (approx. 1 week after sowing), they were transplanted into 130 ml pots containing the same medium and allowed to grow for a further 3 weeks. A subset of ten seedlings was harvested to assess the shoot dry matter at the start of the experiment. The remaining plants were randomly assigned to either drought or irrigated treatments and transferred to a growth chamber (16 h light period, 23/18 °C day/night temperatures). The irrigated plants were watered to pot capacity once each day, and the drought regime consisted of a similar irrigation only once every 3–4 d. Preliminary tests showed that by 13 d after transplanting, pre‐dawn leaf water potentials in the water‐limited treatment decreased to –1·5 MPa prior to irrigation, while irrigated leaf water potentials remained near –0·5 MPa (Mylonopoulos, 2000). After 4 weeks in the growth chamber, plants were harvested. From each genotype, ten plants were harvested in the controls and 15 plants in the stressed treatment. Shoots were excised at the soil surface, oven‐dried at 80 °C for 48 h, then weighed. Approx. 18 genotypes were tested in each batch, which always included a benchmark sugar beet variety (‘Saxon’).
Dry weights were transformed and relative growth rate (RGR) was calculated in a similar way to RLER. Drought tolerance of individual lines was expressed in terms of SI (as above, based on RGR instead of yield), or relative to the growth reduction of Saxon within each test batch according to the formula:
reduction in RGR relative to Saxon = (1 – [YLx/YLSaxon])
where YLx is the drought loss of a test genotype (RGR basis), and YLSaxon is the drought loss of Saxon. Test batches that showed no significant difference (P < 0·05) in RGR of Saxon between irrigated and droughted treatments were discarded. In a few cases this was necessary because the stressed plants were over‐watered or the controls were under‐watered.
RESULTS
Field screen
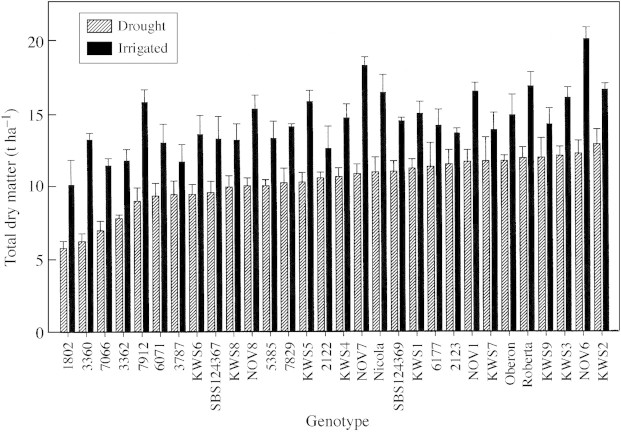
Under irrigated conditions, total dry matter yields varied substantially among the 30 sugar beet genotypes tested in the field (Fig. 1; Table 2). This was not surprising since the collection included improved varieties selected for the UK environment, while others were older varieties, landrace or open pollinated varieties adapted to quite different local conditions (e.g. line 5385 from Afghanistan, collected originally in 1954). Similarly, yields under droughted conditions were also significantly different among genotypes (P < 0·001). The drought treatment was effective in decreasing yields substantially (Table 2), but all lines managed to continue growing throughout the season using only stored soil moisture.
Fig. 1. Total dry matter (harvestable root plus shoot) yield of sugar beet genotypes grown with or without irrigation in the field. Bars = s.e. (n = 4).
Table 2.
Analysis of variance for the effects of water treatment and genotype on total dry matter yield (t ha–1), root yield (fresh root biomass, t ha–1), sugar yield (t ha–1) and relative leaf expansion rate (RLER, d–1)
| DM yield | Root yield | Sugar yield | RLER | SI | |||||||
| Source | d.f. | F | P | F | P | F | P | F | P | F | P |
| Genotype (G) | 29 | 8·35 | <0·001 | 18·92 | <0·001 | 15·46 | <0·001 | 1·44 | 0·081 | 1·84 | 0·018 |
| Treatment (T) | 1 | 349·8 | <0·001 | 630·8 | <0·001 | 289·0 | <0·001 | 128·5 | <0·001 | – | – |
| G × T | 29 | 1·61 | 0·032 | 2·00 | 0·003 | 2·13 | 0·001 | 1·97 | 0·004 | – | – |
Drought susceptibility index (SI) computed from droughted and irrigated total dry matter yields (see text).
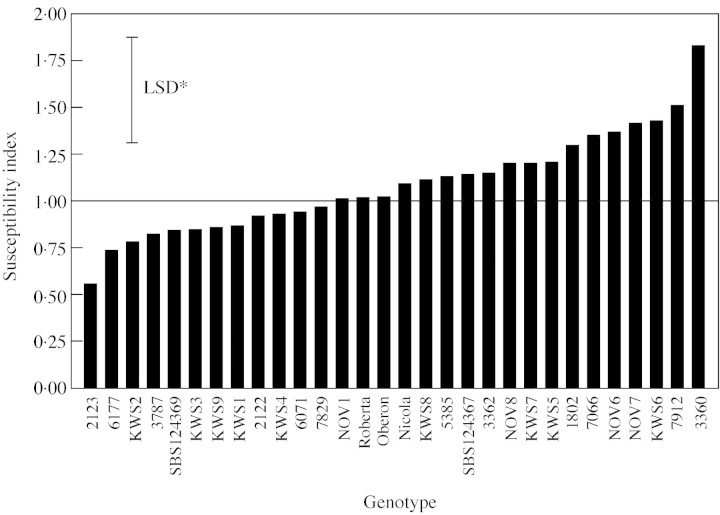
Importantly, there was a significant genotype × water treatment interaction for dry matter yield, indicating that the genotypes responded to drought differentially (Table 2). Similar results were obtained with related yield variables (root fresh weight and sugar yield). Values for drought‐induced yield losses, calculated as SI, also varied by more than three‐fold (P < 0·05; Fig. 2). It is interesting to note that the SI values of the three commercial varieties (Roberta, Nicola and Oberon) were clustered together, which corroborates other comparisons limited to UK varieties (Fisher and Kerr, 1998).
Fig. 2. Drought susceptibility index (SI) based on total dry matter yield losses. Genotypes were grown in the field with or without irrigation. Bar indicates LSD for genotype effect (significant at P < 0·05). Horizontal line at SI = 1 indicates the mean response: genotypes with SI less than 1 were more drought tolerant than average.
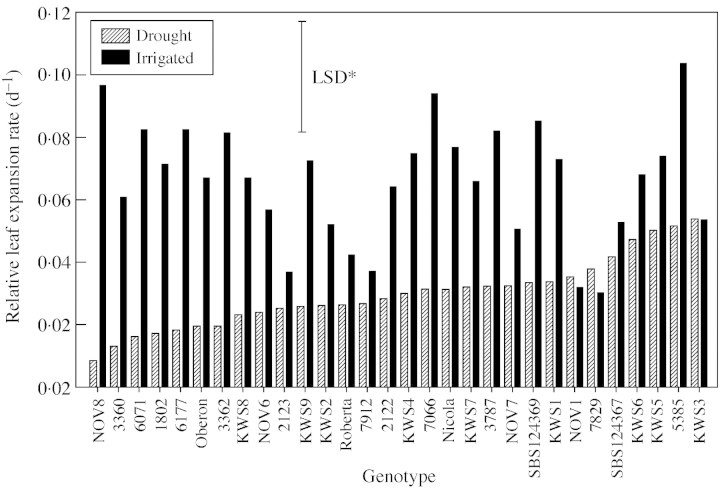
If there is genetic variation for drought tolerance, which is a complex trait, then it follows that there is also variation in the component morpho‐physiological traits that confer tolerance. One of the plant processes most sensitive to drought is leaf expansion (Hsiao, 1973). Thus, it seemed relevant to examine variation for sensitivity of leaf expansion rates to drought. There was extensive variation between genotypes for RLER under irrigated conditions, but differences were not statistically different due to the variation in growth rates between individual leaves within a genotype (Fig. 3). More replications are required to minimize this source of error. However, the interaction between genotypes and the water treatment was highly significant (P < 0·01). Line KWS3 showed intermediate RLER under irrigated conditions, but was little affected by drought; line NOV8, which had high irrigated RLER, was severely inhibited by drought. Regression analysis of irrigated RLER vs. the percentage loss in RLER due to drought showed that genotypes showing high irrigated RLER had the greatest drought‐induced decreases in RLER (r2 = 0·64, P < 0·01). Perhaps not surprisingly, differences in RLER between irrigated and droughted plots measured on one occasion late in the season did not correlate with SI. However, RLER of droughted plants was associated with the percentage of green crop cover on droughted plots measured 20 d later (r = 0·44, P < 0·05, 28 d.f.).
Fig. 3. Relative leaf expansion rates of the fourth newly formed lamina on genotypes grown in the field with or without irrigation. Bar indicates the LSD for genotype × water treatment interaction (significant at P < 0·01).
Growth chamber screen
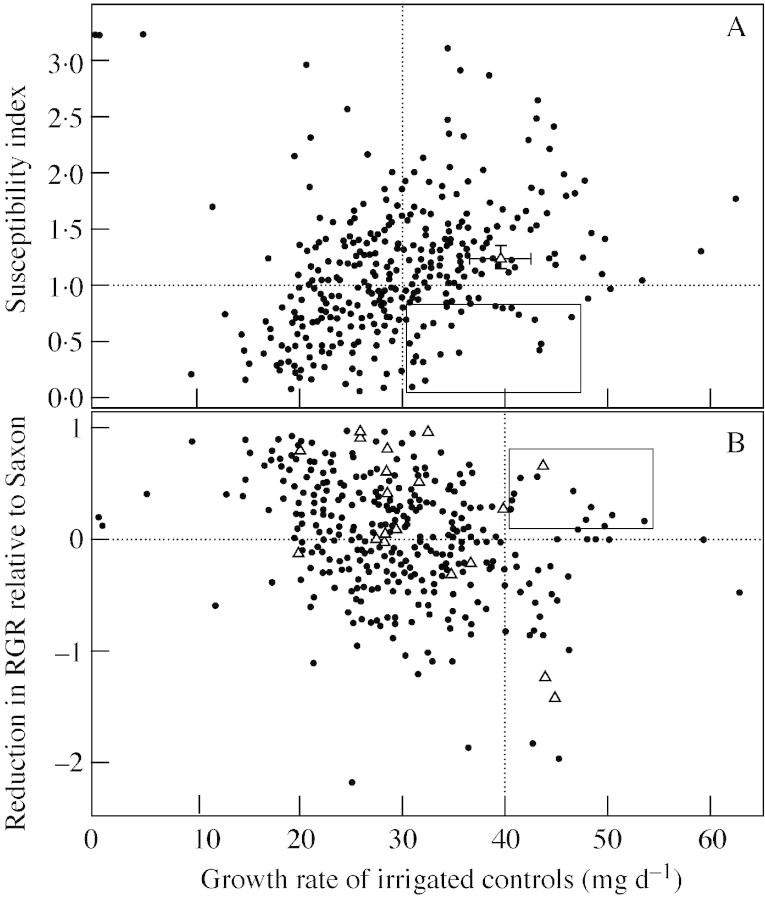
The results of screening 350 Beta genebank accessions are shown in Fig. 4. These accessions comprised a wide range of Beta types: sugar beet, leaf beet, fodder beet, garden beet and various populations of the subspecies maritima, all within the species vulgaris; 10 % of the entries were related species, such as adanensis and macrocarpa. Differences due to plant size and growth habit were minimized by using RGR instead of absolute growth rates. In addition, to make fair comparisons between genotypes and to combine data from batches tested at different times, the RGR data were normalized by computing the drought susceptibility index (SI). SI compares the drought performance of each line according to its growth potential under well‐watered conditions, and is adjusted for the severity of the stress imposed within a particular test batch. In this way drought tolerance per se was compared with few confounding influences.
Fig. 4. Response of shoot growth to irrigation and water deficit in the growth chamber screen. Each symbol represents a different genebank accession. A, Susceptibility index to water deficit based on relative growth rate (RGR) of shoots. The mean response for the benchmark variety, Saxon, is shown (triangle); bars are ± s.e. Inset box indicates accessions that show control growth rates greater than the mean rate of all genotypes (vertical line) and a susceptibility index less than 0·9. Horizontal line at SI = 1 indicates the mean susceptibility of all combined genotypes within a test batch. B, Reduction in RGR for each accession compared with the reduction in RGR of Saxon, computed for each test batch. Accessions that are sugar beet types are shown separately (triangles). The inset box indicates accessions that show control growth rates greater than the mean growth rate for Saxon, and less growth inhibition than the mean response of Saxon. The horizontal line indicates a reduction in RGR equivalent to that for the mean response of Saxon.
There was a general trend for rapidly growing plants to show more relative growth inhibition, in part because large plants consumed more of the water in the limited pot volume. Separating the entries by growth rate under well‐watered conditions minimizes the chances of identifying one entry as a ‘drought‐tolerant’ genotype only because it has a low growth rate under all conditions. An ideal entry would be one that has a high well‐watered growth rate in combination with a low SI. A high growth rate avoids the problem noted above, and would be a desirable character. Entries of this sort were highlighted by applying a two‐way screen, by plotting SI vs. control growth rate (Fig. 4A). Only a few entries satisfied both demands of having a faster than average growth rate and an SI value less than 1 (Fig. 4A, box).
The influence of batch‐to‐batch variation was minimized by expressing the reduction in RGR relative to that of Saxon within each batch (Fig. 4B). Test batches differed slightly, in part because plants were grown in the glasshouse before transfer to the growth chamber and therefore seasonal effects on early seedling growth may have altered the responses between batches. In the SI calculation, the reduction in RGR is adjusted, based on the drought intensity index of the entire batch (see Materials and Methods), whereas in Fig. 4B the reduction in RGR is adjusted to the performance of Saxon. As shown in Fig. 4A, the response of Saxon was reasonably consistent, but the drought intensity index can be swayed by a few extreme entries within a batch. This does not happen in the assessment illustrated in Fig. 4B, and it is perhaps the more robust method of the two. A small number of the entries were sugar beet types (Fig. 4B, triangles). These also showed a wide range of responses and were not clustered near to the result for Saxon, which earlier field studies (Amaducci et al., 1976; van der Beek et al., 1993; Fisher and Kerr, 1998) would have predicted.
Although the basis of SI and the reduction in RGR relative to Saxon differs slightly, eight entries appeared within the ‘elite’ box in both methods. Four were maritima accessions, and the other entries were a fodder beet, sugar beet, an unclassified vulgaris type and one Beta adanensis accession. Inclusion in the ‘elite’ box means that these accessions grew faster than the mean rate for Saxon under well‐watered conditions, but shoot growth was less inhibited under water deficit than the mean response of all the genotypes, and also less inhibited than that of Saxon. Current work involves re‐testing these selected accessions; those that repeatedly fall within the ‘elite’ box merit consideration for testing in field trials.
DISCUSSION
Genetic variation is an essential prerequisite for any crop improvement programme. In sugar beet, it did not appear that sufficient genetic variation existed for drought tolerance, and that improvement would be obtained ‘neither easily or quickly’ (van der Beek, 1993). As with most crops, selection for agronomic characters over time tends to narrow the genetic base (McGrath et al., 1999) and studies that compared commercial varieties seemed to confirm this by finding no significant genotypic differences for drought tolerance (e.g. Amaducci et al., 1976). However, these studies examined only limited numbers of locally adapted varieties that may have had related genetic backgrounds. There is now evidence that there is as much genetic variation within a wide range of sugar beet germplasm as there is within pools of wild subspecies and related Beta species (Hjerdin et al., 1994; Desplanque et al., 1999; McGrath et al., 1999). This genetic variation is reflected in the genotypic differences in drought tolerance we obtained from field and growth chamber comparisons. Further work is needed to rigorously test the genetic basis of these genotypic differences. For instance, estimates of heritability and combining ability within a defined population could be used to quantify the degree of genetic variability for some of the measured traits.
An ideal variety combines high yield potential with yield stability. Drought tolerance will contribute to better yield stability since water supply is the largest variable affecting yields (Jaggard et al., 1998; Pidgeon et al., 2001). Genotypes that show consistent and contrasting drought responses are useful tools to determine the morpho‐physiological traits that confer drought tolerance. For instance, the three‐fold difference in SI make lines 2123 and 3360 (Fig. 2) potentially useful materials for experiments comparing mechanisms of drought tolerance.
From a production point of view, absolute yield is also vitally important. Therefore, any improvement in drought tolerance must not be detrimental to yield potential (the yield obtained under optimum, irrigated conditions), or at least any decrease in yield potential must be offset by an increase in yield stability. There was a positive association between droughted and irrigated yields (r = 0·69, P < 0·01, 28 d.f.), and certain lines (e.g. NOV6) showed high yield potential under both conditions. Thus, conventional selection for high yield potential may be used to realise yield gains under drought, but caution is needed as there were exceptions: NOV7 and 7912 had high irrigated yields but were not in the upper quartile for yields under drought. Furthermore, the data indicate that selecting for high yield potential does not guarantee drought tolerance per se. Among the 30 beet genotypes tested there was no relationship between growth potential and sensitivity to drought determined by dry matter yield loss (r2 = 0·06). The three commercial hybrids that were tested (Roberta, Nicola and Oberon) had large irrigated yields but were not the most drought‐tolerant lines; nor were the poorest yielding lines the most drought sensitive (Fig. 2). NOV6 had high yields, but was also one of the most drought‐sensitive lines. A comparison of wheat landraces and cultivars also showed no association between SI and yield potential (Ehdaie et al., 1988). In sugar beet, the lack of association between SI and yield potential indicates that yield potential and SI are not mutually exclusive, and drought tolerance does not necessarily entail a reduction in yield. An encouraging example was KWS2, which had the highest droughted yield and one of the lowest SI values.
The challenge is to identify specific traits conferring drought tolerance that can be incorporated into lines of high yield potential. Unlike maize and wheat, in which improvements can be realised by directly selecting for yield in dry environments, selection gains in sugar beet will probably depend on the linkage of molecular markers with QTLs controlling these traits and then ‘pyramiding’ the most useful markers into one or more breeding lines while performing selections in a conventional breeding environment.
One physiological trait that could be important is RLER, since final yield in sugar beet is directly related to the interception of solar radiation, determined largely by the rate of development of the crop canopy and maintenance of functional leaf area (Scott and Jaggard, 1993). Evidence for genotypic variation in drought tolerance was reinforced by measurements of RLER that showed significant genotype × water treatment interaction. Unfortunately, the lack of correlation with SI within this data set questions the efficacy of RLER as an indirect selection tool. However, before RLER is ruled out altogether, more tests under different levels of stress and at different stages of crop development are needed.
The objective of the seedling screen was to test a large number of genotypes and, because of time and space limitations, each line was tested only once. However, a benchmark sugar beet genotype (Saxon) was included in each test batch. The variation in SI and growth rate of controls for Saxon, shown in Fig. 4A, indicates the consistency of the response between test batches. Assuming that the variation in response of Saxon is typical of most entries, the wide range of growth rates and SI values in Fig. 4A is not solely a reflection of random error or environmental variation but is probably due mostly to genetic variation for drought responses.
Accessions falling within the ‘elite’ box in successive growth chamber tests would suggest that these plants have mechanisms that protect and permit shoot growth under water‐limited conditions. These repeated tests are currently being conducted, along with comparisons of field‐tested genotypes in the growth chamber screen. The growth chamber screen was not designed to provide any information about root growth or biomass partitioning between roots and shoots, which are important in the field. The confined root environment of these pot experiments would not reflect accurately the phenotype of root growth under field conditions. Nevertheless, if genes can be identified that control the expression of traits that enhance the performance of lines in this test environment, then it might be possible to incorporate these traits into other genetic backgrounds for crop improvement.
The objective of current work is to use genotypes that show divergent responses to drought to explore in greater detail characters that influence drought performance. Field tests have been repeated to assess the consistency of the ranking of tolerant and susceptible lines by selecting out of the 30 genotypes tested in this study eight genotypes representing extreme responses. Results will be described in a future paper.
ACKNOWLEDGEMENTS
Special thanks to Chris Clark and Mich LeBloa for assistance with field trials, to Jo Smith, Kathy Bean and Kevin Sawford for assistance with the seedling screen, and to John Pidgeon and Keith Jaggard for commenting on the manuscript. The field experiment was sponsored by the British Beet Research Organisation (BBRO), with additional support from KWS, Syngenta, the Perry Foundation and the Chadacre Trust. Thanks to Dr Seyed Sadeghian of the Sugar Beet Institute of Iran for the kind donation of seed. Acquisition of many accessions for the field tests was mediated by Dr Lothar Frese of the BAZ genebank, Braunschweig, Germany. The growth chamber screen was also conducted in collaboration with Dr Frese, which was co‐funded by the BBRO and a CEC grant for project GENRES CT95 42 under council regulation 1467/94.
Supplementary Material
Received: 25 June 2001; Returned for revision: 16 October 2001; Accepted: 11 January 2002.
References
- AmaducciMT, Caliandro A, Cavazza L, de Caro A, Venturi G.1976. Effects of irrigation on different sugar beet varieties in different locations and years. Proceedings of the 39th Winter Congress of the International Institute for Sugar Beet Research, 423–448. [Google Scholar]
- BosemarkNO.1979. Genetic poverty of the sugar beet in Europe. Proceedings of the Conference on Broadening the Genetic Base of Crops, Wageningen, 1978, 29–35. [Google Scholar]
- BoyerJS.1982. Plant productivity and environment. Science 218: 443–448. [DOI] [PubMed] [Google Scholar]
- BoyerJS.1996. Advances in drought tolerance in plants. Advances in Agronomy 56: 187–218. [Google Scholar]
- DesplanqueB, Boudry P, Broomberg K, Saumitou‐Laprade P, Cuguen J, Van Dijk H.1999. Genetic diversity and gene flow between wild, cultivated and weedy forms of Beta vulgaris L. (Chenopodiaceae), assessed by RFLP and microsatellite markers. Theoretical and Applied Genetics 98: 1194–1201. [Google Scholar]
- EhdaieB, Waines JG, Hall AE.1988. Differential responses of landrace and improved spring wheat genotypes to stress environments. Crop Science 28: 838–842. [Google Scholar]
- FischerRA, Maurer R.1978. Drought resistance in spring wheat cultivars. I. Grain yield responses. Australian Journal of Agricultural Research 29: 897–912. [Google Scholar]
- FisherSJ, Kerr SP.1998. Sugar beet varietal responses to irrigation and autumn growth. Aspects of Applied Biology 52: 173–178. [Google Scholar]
- HjerdinA, Säll T, Nilsson NO, Bornman CH, Halldén C.1994. Genetic variation among wild and cultivated beets of the section Beta as revealed by RFLP analysis. Journal of Sugar Beet Research 31: 59–67. [Google Scholar]
- HodgeCAH, Burton RGO, Corbett WM, Evans R, Seale RS.1984. Soils and their use in Eastern England. Soil Survey of England and Wales, Bulletin Number 13. [Google Scholar]
- HsiaoTC.1973. Plant responses to water stress. Annual Review of Plant Physiology 24: 519–570. [Google Scholar]
- JaggardKW, Dewar AM, Pidgeon JD.1998. The relative effects of drought stress and virus yellows on the yield of sugarbeet in the UK, 1980–1995. Journal of Agricultural Science 130: 337–343. [Google Scholar]
- KerrS.2000. Variety interactions with sowing, soils and harvest. British Sugar Beet Review 68: 18–22. [Google Scholar]
- LewellenRT.1992. Use of plant introductions to improve populations and hybrids of sugarbeet. In: Shands HL, Weisner LE, eds. Use of plant introductions in cultivar development. CSSA Special Publication Number 20. Madison: Crop Science Society of America. [Google Scholar]
- LuterbacherMC, Smith JM, Asher MJC, Frese L.2000. Disease resistance in collections of Beta species. Journal of Sugar Beet Research 37: 39–47. [Google Scholar]
- McGrathJM, Derrico CA, Yu Y.1999. Genetic diversity in selected, historical US sugarbeet germplasm and Beta vulgaris ssp. maritima Theoretical and Applied Genetics 98: 968–76. [Google Scholar]
- MylonopoulosI.2000. Evaluation of sugar beet (Beta vulgaris L.) genotypes under water deficit conditions in a controlled environment. MSc Thesis, University of Bristol, UK. [Google Scholar]
- PidgeonJD, Werker AR, Jaggard KW, Richter GM, Lister DH, Jones PD.2001. Climatic impact on the productivity of sugar beet (Beta vulgaris L.) in Europe 1961 – 1995. Agricultural and Forest Meteorology 109: 27–37. [Google Scholar]
- ScottRK, Jaggard KW.1993. Crop physiology and agronomy. In: Cooke DA, Scott RK, eds. The sugar beet crop. London: Chapman and Hall. [Google Scholar]
- van der BeekMA, Houtman HJ.1993. Does interaction between varieties and drought stress exist? Proceedings of the 56th Congress of the International Institute for Sugar Beet Research, 151–169. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.