Abstract
Although active oxygen species are produced at high rates in both the chloroplasts and peroxisomes of the leaves of C3 plants, most attention has focused on the potentially damaging consequences of enhanced chloroplastic production in stress conditions such as drought. This article attempts to provide quantitative estimates of the relative contributions of the chloroplast electron transport chain and the glycolate oxidase reaction to the oxidative load placed on the photosynthetic leaf cell. Rates of photorespiratory H2O2 production were obtained from photosynthetic and photorespiratory flux rates, derived from steady‐state leaf gas exchange measurements at varying irradiance and ambient CO2. Assuming a 10 % allocation of photosynthetic electron flow to the Mehler reaction, photorespiratory H2O2 production would account for about 70 % of total H2O2 formed at all irradiances measured. When chloroplastic CO2 concentration rates are decreased, photorespiration becomes even more predominant in H2O2 generation. At the increased flux through photorespiration observed at lower ambient CO2, the Mehler reaction would have to account for more than 35 % of the total photosynthetic electron flow in order to match the rate of peroxisomal H2O2 production. The potential signalling role of H2O2 produced in the peroxisomes is emphasized, and it is demonstrated that photorespiratory H2O2 can perturb the redox states of leaf antioxidant pools. We discuss the interactions between oxidants, antioxidants and redox changes leading to modified gene expression, particularly in relation to drought, and call attention to the potential significance of photorespiratory H2O2 in signalling and acclimation.
Key words: Oxidative load, photorespiration, H2O2, Mehler–peroxidase, wheat (Triticum aestivum), barley (Hordeum vulgare), catalase, glutathione, antioxidant, modelling
INTRODUCTION: OXIDATIVE LOAD AND THE ANTIOXIDANT SYSTEM
It is generally accepted that stress‐induced deregulation of plant metabolism leads to the enhanced production of active oxygen species (AOS), the cellular titre of which is policed by the antioxidant system (Noctor and Foyer, 1998). Both AOS and soluble antioxidants are involved in signalling processes in plants: the picture that is emerging suggests that relatively stable oxidants (H2O2) and antioxidants (ascorbate, glutathione) act as sensors of the ‘oxidative load’ on the cell (Noctor et al., 2000). According to this view, mild increases in oxidative load trigger events that lead to acclimation and enhanced resistance, while more severely increased and sustained loads tip the developmental balance towards senescence and death.
A modified balance between AOS generation and the antioxidant system affects the expression of antioxidative enzymes, and is also involved in processes such as the hypersensitive response to pathogen attack (Smirnoff, 1993; Levine et al., 1994; Noctor and Foyer, 1998; Veljovic‐Jovanovic et al., 2001). Both hydrogen peroxide (H2O2) and glutathione have been implicated in signalling cascades and in the control of gene expression (Levine et al., 1994; Foyer et al., 1997; Willekens et al., 1997). Drought is an example of an abiotic stress in which components of the antioxidative system are perturbed or up‐regulated (Smirnoff, 1993). It can therefore be inferred that enhanced production of AOS, a compromised capacity to remove AOS or both, elicits acclimatory events during drought (Smirnoff, 1993). Most discussion has centred on accelerated AOS production through side‐reactions in the chloroplast, in particular the formation of superoxide and H2O2 linked to auto‐oxidation of components associated with photosystem I (PSI; Mehler reaction). Less attention has been paid to the influence of photorespiration.
PHOTORESPIRATION AND OXIDATIVE LOAD
Photorespiration occurs at high rates in the leaves of C3 plants (Foyer and Noctor, 2000). Probably the best accepted ‘function’ of this pathway is that of an alternative electron sink. The considerable energy used in photorespiratory C and N recycling lowers the quantum yield of photosynthesis, thereby making light utilization in CO2 fixation less efficient. This effect could be physiologically advantageous in conditions such as drought stress, where stomatal closure may decrease the availability of CO2 to the photosynthetic apparatus. Labelling studies by Lawlor and colleagues were among the earliest indications that as water becomes scarce the photorespiratory pathway accelerates relative to net photosynthesis (Lawlor, 1976; Lawlor and Pearlman, 1981). Increased allocation of energy to photorespiration could mitigate deleterious effects such as photoinhibition by allowing metabolism to continue using the products of photosynthetic electron transport (Osmond and Grace, 1995). However, while the operation of photorespiration may decrease the probability of photoinhibition and attenuate AOS production in the chloroplast, the photorespiratory C recycling pathway involves the obligatory production of H2O2 in the peroxisomes through the action of glycolate oxidase. Increased photorespiratory flux during drought could, therefore, significantly exacerbate the oxidative load on the photosynthetic cell. While considerable attention has been paid to the significance of photorespiration as an alternative sink for light energy, little or none has focused on the potential importance of the attendant high rates of H2O2 generation.
ESTIMATION OF PHOTORESPIRATORY RATES BY DERIVATION OF THE RATE OF RUBP OXYGENATION (VO) FROM GAS EXCHANGE MEASUREMENTS
Photorespiration is masked by photosynthetic O2 evolution and CO2 assimilation. Even where labelling experiments enable O2 consumption or CO2 evolution to be monitored in the light, accurate quantification of photorespiration is complicated by the concurrent operation of other processes that also consume O2 and/or release CO2. One convenient method that is largely able to overcome these difficulties is derivation of the rate of photorespiration from measured rates of CO2 uptake through modelling (Sharkey, 1988; von Caemmerer, 2000), which has the advantage that photorespiratory flux can be derived solely from measurements of leaf gas exchange. This approach requires estimation of chloroplastic CO2 and O2 concentrations (Cc and Oc, respectively), as well as the rate of non‐photorespiratory CO2 release. The first two parameters can be calculated (Cc) or assumed (Oc) with reasonable accuracy (see legend to Fig. 1). To calculate Cc from the intercellular CO2 con centration, Ci, it is necessary to use a value for the mesophyll transfer conductance, gi. This value has been estimated at around 0·3–0·6 mol m–2 s–1 for species with photosynthetic rates similar to those of wheat and potato (Loreto et al., 1992; von Caemmerer et al., 1994). We used a value of 0·3 since at ambient CO2 this gave Cc/Ci close to 0·7 (von Caemmerer and Evans, 1991). To estimate non‐photorespiratory CO2 release in the light it was assumed for the data of Figs 1–5 that this value was equal to 50 % of the rate measured in the dark. The assumption concerning non‐photorespiratory CO2 release only significantly affects the derived values of rate of ribulose‐1,5‐bisphosphate (RuBP) oxygenation (vo) and rate of RuBP utilization (vRuBP) at very low ambient CO2 (Table 1).
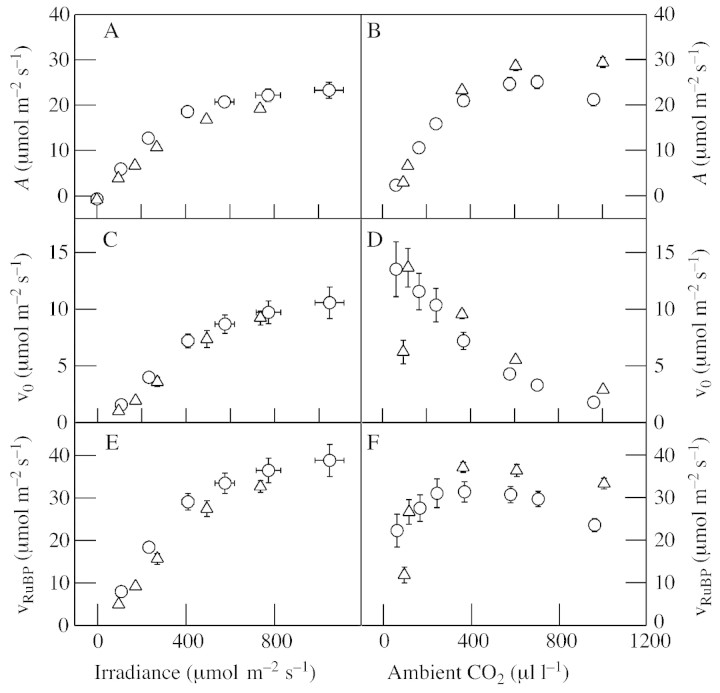
Fig. 1. Rate of net photosynthesis and derived parameters plotted against irradiance (A, C, E) and ambient CO2 concentration (B, D, F) for attached leaves of wheat (circles) and potato (triangles). A and B, Net CO2 uptake; C and D, rate of RuBP oxygenation (vo); E and F, rate of total RuBP utilization (vRuBP). Wheat (Triticum aestivum ‘Cannon’) and potato (Solanum tuberosum ‘désirée’) were grown in soil and slow‐release fertilizer to the age of 4–5 weeks (wheat) or 6–9 weeks (potato) in controlled‐environment glasshouses (day length 14 h, day/night temperature 22/18 °C, irradiance 250 µmol quanta m–2 s–1). Plants were transferred to the laboratory for measurements of steady‐state photosynthesis. CO2 and H2O exchange were measured in a multi‐chamber system designed and developed at Rothamsted by Lawlor and colleagues (Paul et al., 1990). The measuring systems consisted of four or six temperature‐ and humidity‐controlled chambers, illuminated from above by floodlamps and connected to a gas‐mixer and infra‐red gas analyser (IRGA). All experiments were conducted at 20 °C, 50 % relative humidity and 21 % O2. CO2 composition was controlled by a gas mixer and irradiance by neutral density sheets. For wheat, middle sections of the fourth leaf were used; for potato, the half of a fully expanded leaf distal from the petiole was introduced into the chamber. For irradiance curves, CO2 was 360 µl l–1; for CO2 curves, irradiance was 650–750 µmol m–2 s–1 at the leaf surface. Leaves were incubated for 30 min in darkness, before illumination at each condition until a steady‐state rate of photosynthesis was reached (30–40 min). To calculate vo and vRuBP, the Rubisco specificity factor (Srel) was taken to be 110 (Keys, 2000) and the chloroplastic oxygen concentration (Oc) was assumed to be that of water in equilibrium with air at 20 °C (276 µm). The chloroplastic CO2 concentration (Cc) was derived from Ci by taking a CO2 transfer conductance through the mesophyll (gi) of 0·3 mol m–2 s–1 (von Caemmerer et al., 1994) and assuming that the rate of CO2 uptake affects Cc relative to Ci as in Ruuska et al. (2000): Cc = Ci – A/gi. Cc was converted to a molar concentration by applying a CO2 solubility constant at 20 °C of 0·0392 mol l–1 (von Caemmerer, 2000). The ratio of oxygenation to carboxylation was calculated as O : C = (1/Srel)(Oc/Cc) and vo was derived according to Sharkey (1988): vo = (A – R)/(1/O : C – 0·5), where R is non‐photorespiratory CO2 release in the light (negative value). The ‘real’ or gross rate of carboxylation at Rubisco was derived as vc = A + 0·5 vo – R, and the total rate of RuBP utilization as vRuBP = vo + vc.
Table 1.
Effect of the rate of non‐photorespiratory CO2 evolution on parameters derived from measurements of net CO2 uptake (A) by attached wheat leaves at different Ca
| vo | vc | vRuBP | |||||
| Ca (µl l–1) | A | 1 | 2 | 1 | 2 | 1 | 2 |
| 61 | 2.3 | 15.5 | 12.0 | 9.37 | 8.29 | 24.9 | 20.3 |
| 166 | 10.5 | 12.0 | 11.2 | 15.8 | 16.1 | 27.8 | 27.4 |
| 243 | 15.8 | 10.6 | 10.2 | 20.4 | 20.9 | 31.0 | 31.0 |
| 368 | 20.9 | 7.32 | 7.09 | 23.9 | 24.5 | 31.2 | 31.5 |
| 578 | 24.6 | 4.35 | 4.23 | 26.1 | 26.7 | 30.4 | 30.9 |
| 704 | 25.0 | 3.34 | 3.25 | 26.0 | 26.7 | 29.4 | 29.9 |
| 960 | 21.1 | 1.81 | 1.76 | 21.4 | 22.0 | 23.2 | 23.8 |
n = four leaves on different plants, standard errors 15 % of value or less. Values in column 1 are calculated assuming that non‐photorespiratory CO2 evolution in the light is equal to the rate of dark respiration [after 30 min darkness, mean rate (n = 4) was –0·68 ± 0·05 µmol CO2 m–2 s–1]. Values in column 2 are calculated assuming that non‐photorespiratory CO2 evolution is zero in the light. At each Ca value, CO2 uptake was monitored for 30 min at an irradiance of 650–750 µmol m–2 s–1. All rates are expressed in µmol m–2 s–1.
Figure 1 shows typical irradiance and CO2 response curves of wheat and potato leaves. For both plants, net CO2 uptake by leaves responded similarly to increasing irradiance and CO2 (Fig. 1A and B). Values of vo increased with irradiance in a similar fashion to net CO2 uptake (Fig. 1, compare A and C). When photosynthesis was manipulated by CO2 concentration, vo changed antagonistically to net CO2 uptake in wheat, with the highest vo occurring at the lowest net CO2 uptake and vice versa (Fig. 1, compare B and D). The response was similar in potato except that vo decreased at the lowest CO2 concentration (approx. 60 µl l–1). We have also observed this effect in some experiments with wheat (see Fig. 5) and the effect of changing CO2 availability on absolute photorespiratory flux is discussed below. While the light response of vRuBP was similar to that of net CO2 uptake and vo (Fig. 1E), vRuBP was generally much less responsive to CO2 than these processes (Fig. 1F). The broad optimum illustrates the capacity of oxygenation to substitute for carboxylation in the utilization of RuBP and hence photosynthetic energy. This is reflected by the increase in the ratio of RuBP oxygenation to RuBP carboxylation (O : C) as the ambient CO2 concentration (Ca) falls (Fig. 2).
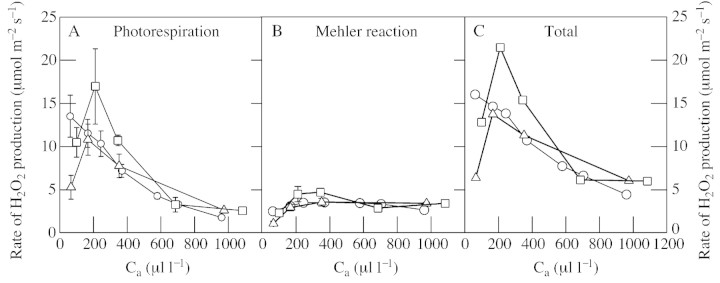
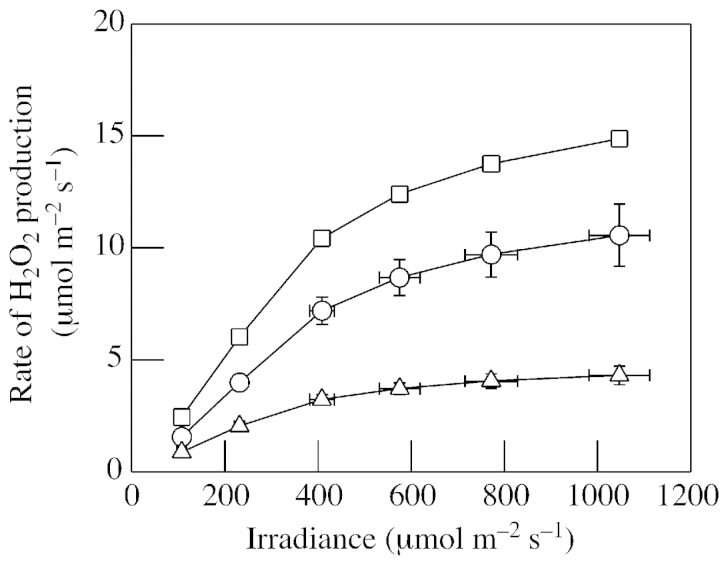
Fig. 5. Changes in H2O2 production with ambient CO2 concentration in attached wheat leaves. The production in photorespiration (A), O2 reduction in the chloroplast (B) and the total amount generated (C) were calculated as described for Fig. 4. Different symbols show three independent experiments. Circles show values that are the means ± s.e. of three different plants, the same set of plants being measured at each CO2 concentration. Squares and triangles show means ± s.e. of four different plants, with a different set of plants being measured at each CO2 concentration.
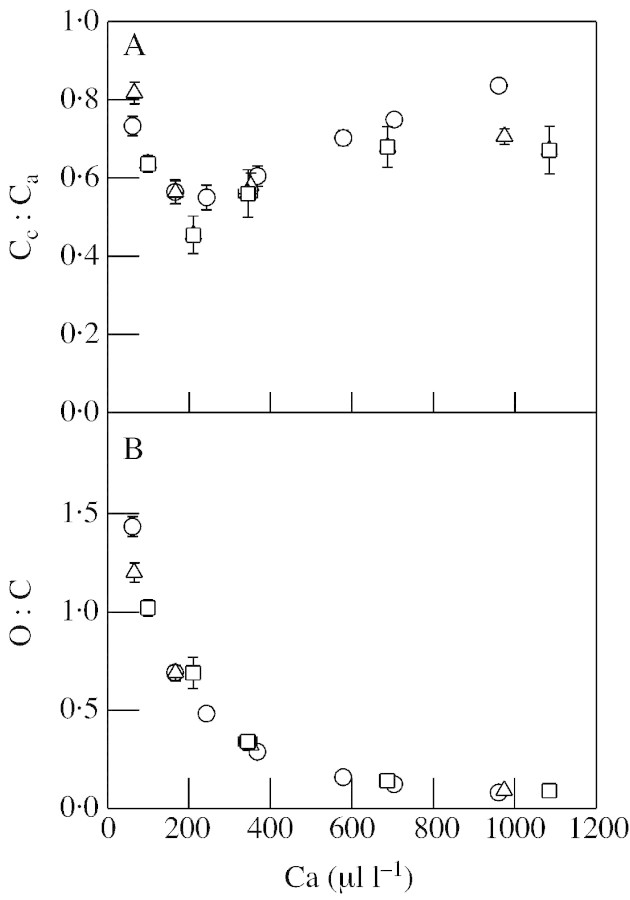
Fig. 2. Modelled data derived from measurements of net CO2 uptake and intercellular CO2 concentration (Ci) in attached wheat leaves at different CO2 concentrations. Ca and Cc, ambient and chloroplastic CO2 concentration, respectively; O : C, ratio of RuBP oxygenation to RuBP carboxylation. Data are shown from three independent experiments (methods as in legend to Fig. 1). For each experiment, values are means ± s.e. of three (circles) or four (triangles, squares) leaves. Circles, Experiment in which four leaves, each attached to a different plant, were measured in the steady‐state (30–40 min illumination at the Ca values indicated). Triangles and squares, Experiments in which each measurement at each Ca value was carried out with different leaves, each attached to a different plant. Irradiance was 675–838 µmol m–2 s–1, temperature was 20 °C and gas composition was 21 % O2, CO2 concentration (Ca) as indicated and balance N2.
DO THE MODELLED DATA PROVIDE AN ACCURATE MEASURE OF PHOTORESPIRATION?
During drought stress, the calculated value of Cc was predicted to decline sharply at relatively mild relative water deficits (Tourneux and Peltier, 1995), and a good estimate of this parameter is key to accurate modelling of the rate of photorespiration (see legend to Fig. 1). In the data shown in Fig. 2, Cc/Ca values were highest at low CO2, where net CO2 utilization is relatively low, and at supra‐atmospheric CO2, where external CO2 is high relative to net CO2 uptake (Fig. 2A). Intermediate values of Ca gave somewhat lower values (Fig. 2A). Using data from 18O2 uptake measurements, Tourneux and Peltier (1995) calculated a Cc/Ca value of 0·56 at atmospheric CO2, assuming that non‐photorespiratory CO2 evolution is not inhibited by light. In agreement with these data, analysis of the gas exchange data obtained in the three experiments of Fig. 2 gave Cc/Ca as 0·61 ± 0·03 (circles), 0·58 ± 0·03 (triangles) and 0·56 ± 0·06 (squares) at Ca = 345–368 µl l–1 (Fig. 2A).
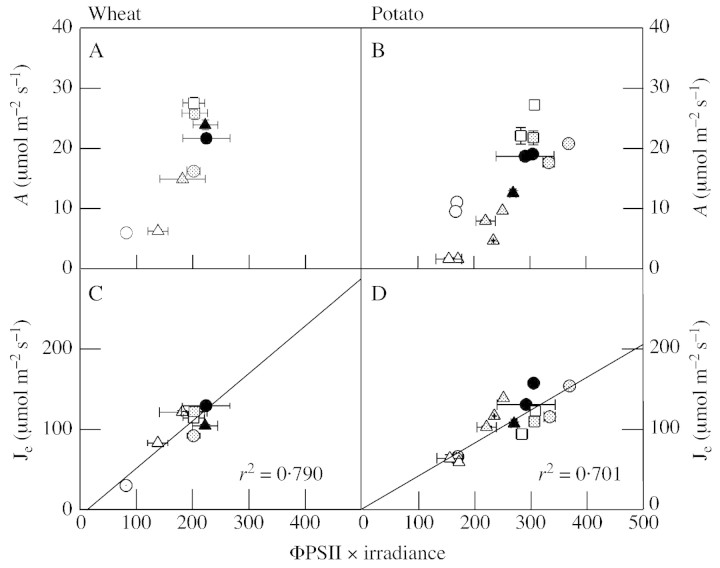
Oxygenation and carboxylation are often considered the major sinks for photosynthetic reducing power, and this notion is supported by comparisons of gas exchange analysis with chlorophyll fluorescence analysis (Ruuska et al., 2000). If this is so, the relative rate of linear electron transport [Φphotosystem II (PSII) × irradiance] should correlate with the total demand of oxygenation and carboxylation for electrons, denoted Je. We calculated Je from values of net CO2 uptake measured under widely varying conditions of irradiance, Ca, or ambient O2 concentration, and compared the values obtained to the relative rate of linear electron transport calculated from chlorophyll fluorescence measurements (for details, see legend to Fig. 3). The data show that the two parameters are related by a straight line that passes close to the origin and that has a reasonable correlation coefficient, both for wheat and potato (Fig. 3C and D). As well as the linear relationship observed, the calculations of Je gave values close to those predicted by chlorophyll fluorescence analysis, even where the measured rates of net CO2 uptake were very different. For example, wheat leaves at 230 µl l–1 CO2 gave a measured rate of net CO2 uptake of approx. 15 µmol m–2 s–1; in wheat leaves at 2 % O2 the measured rate was 28 (Fig. 3A). However, fluorescence analysis predicted almost identical rates of electron transport and this was borne out by the Je values calculated from gas exchange (Fig. 3C). These data suggest that the modelled data provide a reasonably good estimate of the rate of photorespiration.
Fig. 3. Relationship between net CO2 uptake (A and B), the derived rate of electron flow associated with carboxylation and oxygenation (C and D), and the relative rate of linear electron transport, calculated from chlorophyll fluorescence quenching. A and C, Attached leaves of wheat; B and D, attached leaves of potato. Chlorophyll fluorescence was measured simultaneously with gas exchange using an oxy‐blot fluorometer. The fluorescence excitation beam and emission signal, as well as saturating light flashes, were passed down a fibre‐optic held in fixed position for each chamber by mountings built at Rothamsted. The optic was held at 45° to the leaf surface and monitored that part of the leaf which corresponded to the central third of the leaf portion in the chamber. All leaves used gave Fv/Fm > 0·8 after 30 min dark incubation. Photosynthesis was then induced by illumination under conditions of varying light (circles), CO2 (triangles) or O2 (squares). On attainment of the steady‐state rate of net CO2 uptake (30–40 min illumination), the fluorescence of leaves in each chamber was monitored sequentially for 3 min, during which time Fm′ was measured by applying two saturating flashes (4000 µmol quanta m–2 s–1, 2 s duration, 2 min between flashes). The photochemical yield of PSII (ΦPSII) was calculated according to Genty et al. (1989) and Je was calculated as 4 (vo + vc), assuming that each carboxylation event gives rise to 2 PGA and that each oxygenation event produces 1·5 PGA + 0·5 NH3, whose reassimilation in the chloroplast involves ferredoxin‐dependent glutamate synthase (2 e– per NH3). All points represent the means of two or four different plants (standard errors are shown where values are means of four). For wheat, black circles show data at ‘control’ conditions (irradiance = 750 µmol m–2 s–1, CO2 = 382 µl l–1, O2 = 21 %). White and grey circles, Gas composition as for ‘controls’ but irradiance = 112 and 340 µmol m–2 s–1, respectively. Triangles show data at the same light and O2 tension except CO2 = 123 (white), 232 (grey) and 1055 (black) µl l–1. Squares show data at control light and CO2 but O2 = 2 % (white) and 7 % (grey). For potato, ‘control’ conditions were as for wheat and are denoted by grey squares. White and black circles, Control gas composition but irradiance = 235 and 1250 µmol m–2 s–1, respectively. Triangles show data at control light and O2 but CO2 = 60 (white), 96 (grey with cross), 150 (grey) and 220 (black) µl l–1. Squares, Conditions as for squares in wheat data.
DOES PHOTORESPIRATORY FLUX INCREASE IN ABSOLUTE TERMS WHEN CO2 AVAILABILITY FALLS?
The above data show the increased allocation of energy to photorespiration as CO2 availability decreases. Similar effects are known to occur during drought. The combined use of chlorophyll fluorescence and gas exchange showed that the total electron transport rate decreased much less than net CO2 uptake with decreasing Ci or during drought stress (Cornic and Briantais, 1991). Mass spectrometric measurements of 18O2 uptake and net O2 evolution demonstrated that during drought, gross O2 release at PSII was maintained much higher than net O2 evolution in potato (Tourneux and Peltier, 1995) or net CO2 uptake in wheat (Biehler and Fock, 1996). Lastly, electron transport rates derived from chlorophyll fluorescence analysis decreased less than net CO2 uptake in droughted barley leaves (Wingler et al., 1999).
Although it is clear that photorespiration increases relative to net CO2 uptake during drought, it is less evident whether flux through the photorespiratory pathway increases in absolute terms. This question is relevant to the oxidative effect mediated by photorespiration during drought. Neither chlorophyll fluorescence nor mass spectrometric analysis using 18O2 uptake can provide an unequivocal answer to this question because of possible concomitant changes in the rate of the Mehler reaction. Biehler and Fock (1996) approached the problem by estimations of 14C‐glycolate labelling, which was reported to decrease during drought. A recent study by the same group quantified photorespiration in tomato leaves by measuring 12CO2 evolution in the first few seconds after switching to supplying 13CO2 only, and subtracting estimated rates of non‐photorespiratory CO2 evolution (Haupt‐Herting et al., 2001). It was reported that although photorespiration increased markedly compared with net CO2 uptake, and remained high in droughted leaves, decreased water potential led to decreased photorespiratory flux (Haupt‐Herting et al., 2001). On the other hand, studies of several barley mutants with decreased amounts of enzymes involved in the photorespiratory pathway suggest that flux does increase to some extent during mild drought stress (Wingler et al., 1999).
The modelled data shown in Fig. 1 suggest that photo respiration will increase in absolute terms as chloroplastic CO2 concentration becomes restricted. In most of our experiments, we have observed an optimum for calculated rates of vo at Ca equal to 100–200 µl l–1. Whether or not vo declines at very low CO2 probably depends on factors such as irradiance (Cornic and Briantais, 1991), since one of the factors that could restrict photorespiratory flux at low CO2 is a limiting rate of RuBP regeneration (von Caemmerer, 2000). Additional evidence for absolute increases in photo respiratory flux at CO2 concentrations below atmospheric comes from other experiments in which we measured leaf amino acids by freeze‐clamping leaves during steady‐state photosynthesis (Novitskaya et al., 2002). In these experiments, the Gly/Ser ratio correlated with the calculated value of vo, whether this was manipulated by CO2, irradiance or O2. In wheat leaves, Gly/Ser was 4·8 ± 1·1, 4·8 ± 1·3, 8·0 ± 1·2, 8·3 ± 0·8 and 7·3 ± 0·9 at Ca values (µl l–1) of 350, 210, 167, 99 and 66, respectively. In potato leaves, the ratios were 4·3 ± 1·1, 5·7 ± 1·0 and 7·5 ± 0·3 at Ca = 300, 218 and 105 µl l–1, respectively (all values means ± s.e. of four different plants; Novitskaya et al., 2002).
HOW MUCH H2O2 IS PRODUCED THROUGH PHOTORESPIRATION AND THE MEHLER REACTION?
The stoichiometry of the glycolate pathway dictates that H2O2 generation in photorespiration equals vo. The rate of H2O2 production in the chloroplast is difficult to estimate, but a value of 10 % is often cited for the proportion of electrons flowing to O2 at PSI (Foyer and Noctor, 2000). Some authors have estimated higher values (e.g. Miyake and Yokota, 2000), while studies at very high concentrations of CO2 and on plants with low Rubisco suggest that the proportion is probably considerably less than 10 % (Ruuska et al., 2000). If the ATP yields of linear electron transport are sufficient to satisfy almost all the requirements of stromal metabolism, then it remains as yet unclear how the photosynthetic system could allow a high proportion of electron flow to O2 in the Mehler reaction (Noctor and Foyer, 2000). In view of these considerations and the incisive data of Ruuska et al. (2000), we consider here the value of 10 % as a likely maximum value for the proportion of electrons allocated to the Mehler–peroxidase reaction.
Assuming this constant value, then the rate of H2O2 formation via both the Mehler reaction and photorespiration increases more or less in proportion with net CO2 uptake, when the latter is manipulated by changes in irradiance (Fig.). At all irradiances, photorespiratory H2O2 production accounts for about 70 % of total H2O2 formed (Fig. 4). When chloroplastic CO2 concentration falls, as is likely during drought, photorespiration becomes even more dominant in the production of H2O2 (Fig. 5). The data represented by circles in Fig. 5 show that the calculated rate of H2O2 generation increases gradually as CO2 is decreased, so that maximum rates are observed at the lowest Ca value (60 µl l–1). The other two curves suggest an optimum CO2 concentration for photorespiratory H2O2 generation, below which photorespiration and the attendant H2O2 production decline (Fig. 5; compare data for potato leaves in Fig. 1D). It should be noted that the measurements at very low CO2 are those on which the largest error is to be expected: first, it is under these conditions that the assumption concerning the rate of non‐photorespiratory CO2 release is most influential (Table 1); and secondly, small changes or inaccuracies in the measurement of net CO2 uptake and/or Ci can produce large changes in the calculated value of vo. Whatever the response of photorespiratory flux at very low CO2, all three curves suggest that photorespiratory H2O2 production will increase (by 1·5‐ to 2‐fold) at Cc concentrations below those maintained in well‐watered plants in air (Fig. 5). Our Ca curves were carried out at 650–750 µmol m–2 s–1, which at air CO2 concentrations drives a rate of photorespiratory H2O2 generation that is about 85 % of the value predicted at the near‐saturating irradiance of 1100 µmol m–2 s–1 (Fig. 4). The data of Fig. 4 predict that at an irradiance of 650–750 µmol m–2 s–1, mild drought may drive photorespiratory H2O2 generation faster than saturating irradiance in non‐drought conditions (compare Figs 4 and 5).
Fig. 4. Effect of irradiance on modelled rates of H2O2 production in photorespiration and following superoxide production at PSI. Triangles, H2O2 production in the Mehler reaction; cicles, H2O2 production in photorespiration; squares, total H2O2 production. Steady‐state photo synthesis was measured in attached leaves of wheat at Ca = 350 µl l–1, 212 and irradiance as indicated. Photorespiratory H2O2 production is equal to vo, derived as in Fig. 1. H2O2 production in the chloroplast assumes that 10 % of total electron flow through the photosynthetic electron transport chain is linked to the Mehler–peroxidase reaction. Calculation of total electron flow assumes no sinks other than the Mehler–peroxidase reaction, photorespiration and CO2 fixation. The utilization of electrons associated with photorespiration and CO2 fixation was calculated as described for Fig. 3. If the Mehler reaction accounts for 10 % of photosynthetic electrons, then total electron flow, JII, is 40/9 (vo + vc), and steady‐state H2O2 production in the chloroplast = 0·025 JII (O2 + 2e– + 2H+ = H2O2, plus two electrons required in the peroxidatic conversion of H2O2 to H2O = four electrons per H2O2 generated).
Low Ca values drive up the total oxidative load on the photosynthetic cell (Fig. 5A). It is clear that under most conditions, and particularly when CO2 is scarce, most of the H2O2 produced is formed by glycolate oxidation. The only condition in which H2O2 is produced faster in the chloroplast than in the peroxisome is at artificially high Ca (Fig. 5B). It is possible that in contrast to our assumption of a constant electron allocation to the Mehler reaction, the allocation increases as Cc drops during drought, although the data shown in Fig. 3, particularly for wheat, provide little evidence of large changes in sinks other than CO2 fixation and photorespiration. Although the Mehler reaction could increase during drought, we can calculate from our data that this reaction would have to account for approx. 38–30 % of total electron flow in order to equal the rate of photorespiratory H2O2 production at Ca = 61–166 µl l–1. In a recent study of watermelon leaves at different CO2 concentrations, a considerable residual electron flow, which could not be accounted for by electron flow linked to CO2 fixation and photorespiration, was present at all ambient CO2 concentrations (Miyake and Yokota, 2000). This residual flow included a component that required O2 higher than 1·7 % and which the authors attributed to a physiological Mehler reaction linked to oxidation of fer redoxin and/or monodehydroascorbate reductase (Miyake and Yokota, 2000). Expressed as a fraction of total electron flow, this component increased about two‐fold as Ci dropped to around 50 µl l–1 though the increase in absolute rate was less marked (Miyake and Yokota, 2000). Using a combined approach of 18O2 measurements and estimations of photorespiratory flux through glycolate labelling, Biehler and Fock (1996) concluded that the Mehler reaction accounted for less than 15 % of photosynthetic electrons in unstressed wheat and increased to approx. 29 % in wheat subjected to drought. It therefore remains possible that the Mehler reaction increases during drought, but exactly how this could happen remains unclear. Our current understanding suggests that the two factors which most strongly influence the rate of the Mehler reaction in vivo will be irradiance (Fig. 4) and ATP demand (Foyer and Noctor, 2000). While photorespiration has a slightly higher ATP : reductant demand than CO2 fixation, fairly small increases in the Mehler reaction would be sufficient to support the rise in O : C during drought, even assuming the complete absence of other ATP‐generating reactions (e.g. cyclic electron transport through PSI, nitrite reduction). Drought‐induced uncoupling of the thylakoid electron transport chain from photophosphorylation cannot be completely excluded, though this idea receives little support from the well known increases in non‐photochemical quenching observed in droughted leaves (Demmig et al., 1988; Cornic and Briantais, 1991; Biehler and Fock, 1996; Wingler et al., 1999).
ANTIOXIDANT DEFENCES AND H2O2: POTENTIAL INFLUENCE IN DROUGHT‐ASSOCIATED SIGNALLING
Earlier concepts that active oxygen species (AOS) exert their effects through physicochemical damage have been replaced by one that recognizes the active role of AOS in signalling. In particular, drought‐induced increases in superoxide and H2O2 by the thylakoid membranes are well documented (e.g. Bartoli et al., 1999). The extent of accumulation of these oxidants is determined by the capacity of the major redox buffers of the plant cell, ascorbate and glutathione, and the associated enzymes of antioxidant defence. Moreover, many studies have documented the responses of these antioxidants to drought and the general enhancement of antioxidant defence that accompanies prolonged exposure to water deficits (Smirnoff, 1993; Iturbe‐Ormaetxe et al., 1998). Several of these have used transformed plants with increased expression of antioxidant enzymes to increase drought tolerance. In particular, overexpression of either superoxide dismutase (SOD) or ascorbate peroxidase in the chloroplast (McKersie et al., 1996; Yan et al., 2002) has been shown to confer a degree of extra protection against water deficits. Similarly, overexpression of Fe‐SOD in poplar chloroplasts was found to protect the photosynthetic electron transport system from over‐reduction at low CO2 partial pressures (Arisi et al., 1998). This is a particularly interesting observation as SOD is already present at high activities in chloroplasts. Moreover, SOD catalyses one of the fastest reactions known to biology and the enzyme‐catalysed reaction is limited only by the rate of diffusion. It is therefore unlikely that enhanced protection of PSII activity is due simply to increased enzyme capacity. An alternative explanation is that Fe‐SOD acts as a metabolite channel at PSI, not only catalysing superoxide dismutation, but also channelling H2O2 along the thylakoid membrane surface, directly to ascorbate peroxidase (APX), which then reduces H2O2 to H2O. It has previously been suggested that SOD overexpression facilitates increased stress tolerance via enhancement of tissue H2O2 contents, which signal changes in gene expression leading to general increases in defence responses. Although this has never been demonstrated, the systemic accumulation of H2O2 is associated with the expression of defence genes in the hypersensitive response and with wounding or mechanical stimulation (e.g. Alvarez et al., 1998). H2O2 can act as a local signal, for example, as a second messenger of hormone action (Orozco‐Cardenas et al., 2001) or, in the extreme case, as a signal for hypersensitive cell death.
It was initially suggested that an oxidative burst is involved in the triggering of drought stress responses (Shinozaki and Yamaguchi‐Shinozaki, 1997). Emerging evidence is confirming this notion. H2O2 and ABA interact in controlling the stomatal aperture, and both are involved in the regulation of Ca2+ channel function (Pei et al., 2000). The ATMPK3 gene, encoding a mitogen‐activated protein kinase (MAPK), is involved in responses to changes in osmolarity, and is activated by H2O2. The subsequent H2O2‐induced MAPK cascade acts to repress auxin responses (Kovtun et al., 2000). ABA synthesis is regulated by redox factors: antioxidants block drought‐induced ABA accumulation (Jia and Zhang, 2000). The pools of the endogenous antioxidants ascorbate and glutathione could therefore be regulators of ABA‐linked signalling in vivo. It is important to note that the apoplast contains large amounts of ascorbate, but little or no glutathione. In addition, there is little capacity for ascorbate regeneration in the apoplast, and monodehydroascorbate and dehydroascorbate have either to be re‐reduced at the membrane surface or returned to the cytosol for re‐reduction. This means that large redox changes resulting from hormone or stress‐induced H2O2 production are more likely to occur in the apoplast than in the cytoplasm because redox buffering capacity is relatively low in the first compartment. There is therefore potential for multiple interactions between H2O2, antioxidants and drought‐induced hormones. Mutants are likely to be invaluable in uncovering such interactions, as they enable the effects of perturbations in compartment‐specific components to be elucidated.
PERTURBATION OF ANTIOXIDANT POOLS BY PHOTORESPIRATION
Extra‐chloroplastic antioxidant capacity in C3 plants is dominated by peroxisomal catalase. This is undoubtedly because of the need to cope with the abundant production of photorespiratory H2O2 at high light and/or low CO2 (Figs 4 and 5). Catalase is energy‐efficient, since it catalyses a dismutation reaction that does not consume reductant. But the enzyme has a low affinity for H2O2, and this may explain the presence of other antioxidative enzymes in the leaf peroxisome (Foyer and Noctor, 2000). However, the isolation of a barley mutant deficient in catalase via a ‘photorespiratory’ screen underlined the indispensability of the enzyme (Kendall et al., 1983). This mutant can grow and set seed under conditions where photorespiration is suppressed, but transfer to air in the light causes characteristic lesions, loss of vigour and eventually leaf death. Smith et al. (1984) reported that prior to the appearance of these symptoms, the leaf glutathione pool became both very oxidized and increased in size by about six‐fold (within 4 d). These effects were confirmed in transformed tobacco into which a construct containing an antisense catalase coding sequence had been introduced (Willekens et al., 1997). In contrast, maize catalase mutants show no marked phenotype (Scandalios, 1994), reinforcing the tight link between the major leaf peroxisomal form of this enzyme and photorespiration.
We have reinvestigated the kinetics of the perturbation of the leaf glutathione pool on transfer of the mutant from 0·6 % CO2 to air (Fig. 6). In addition, we developed a reliable method to assay leaf H2O2 (Veljovic‐Jovanovic et al., 2002) and also measured the leaf ascorbate redox state, to ascertain whether changes in glutathione redox state reflect a generalized cellular oxidation. In both the mutant and the wild‐type, H2O2 was increased by transfer to air. It should be noted that although photorespiration was increased after transfer from 0·6 % CO2, the irradiance in the chamber (approx. 250 µmol m–2 s–1) was not sufficient to drive very high rates of photorespiratory H2O2 production. A rate of about 5 µmol H2O2 m–2 s–1 can be predicted at an irradiance of 250 µmol m–2 s–1. Assuming a leaf chlorophyll content of 100 mg m–2, this rate would entail a total production of photorespiratory H2O2 of around 5 mmol mg–1 chl in 48 h (2 d of 14 h light). Even though transfer to air caused a measurable increase in H2O2 in both types of plant, the increase was no more than 50 nmol mg–1chl within this time (Fig. 6). It is therefore clear that even in the mutant, 99·999 % of the H2O2 produced was metabolized. Although the mutant showed a rate of photosynthesis that was 30 % lower than that of the wild‐type, rates were maintained in both types of plants for the duration of the experiment (data not shown). Therefore, within the time period of the experiment H2O2 metabolism did not occur through routes that caused appreciable cellular damage.
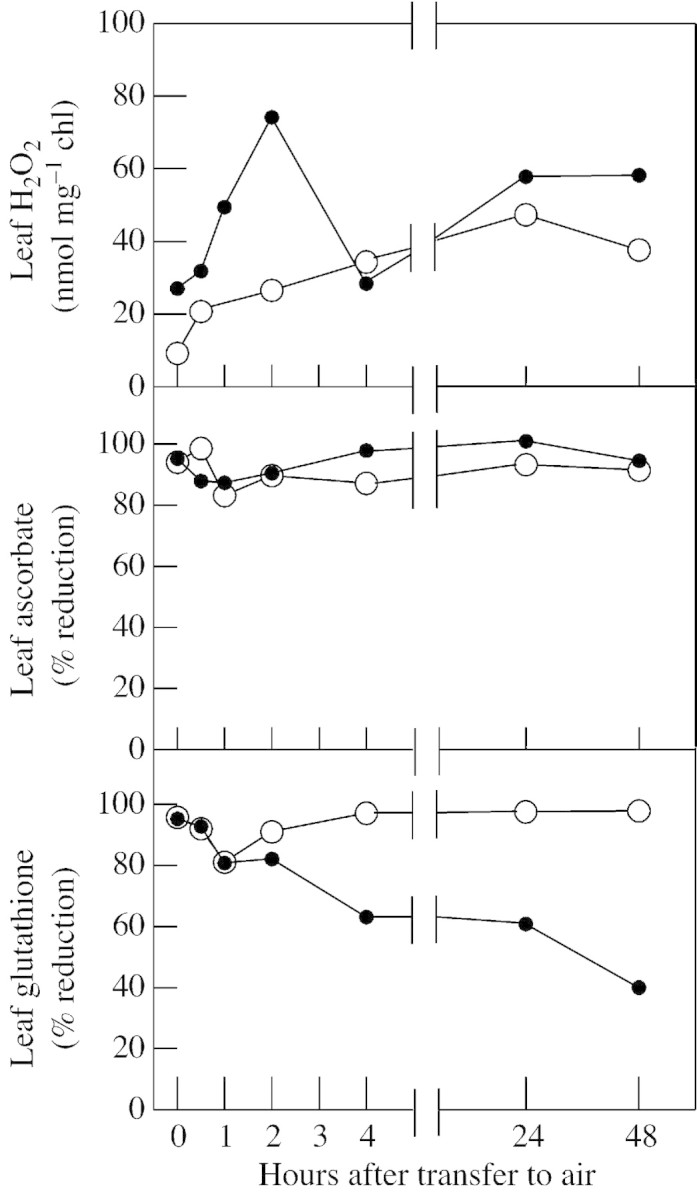
Fig. 6. Changes in leaf H2O2 and the redox states of the principal soluble leaf antioxidants, ascorbate and glutathione, in wild‐type barley (open circles) and a mutant deficient in catalase (closed circles). Barley (Hordeum vulgare, var. Maris Mink) and the catalase‐deficient mutant (RPr 79/4; Kendall et al., 1983) were grown under the same conditions as wheat (Fig. 1), except that ambient CO2 was artificially maintained at 0·6 %. Suppression of photorespiration is necessary for healthy growth of the mutant (Kendall et al., 1983). Six weeks after seeds were sown, plants were transferred to a chamber in which conditions were identical to that in which they had been grown, except that the ambient CO2 concentration was 400 µl l–1. Leaf samples were taken at the indicated times after transfer, for determination of H2O2, glutathione and ascorbic acid. H2O2 was extracted and determined by a modified peroxidase‐coupled assay (Veljovic‐Jovanovic et al., 2002). Reduced and total ascorbate, and total and oxidized glutathione, were assayed as described in Foyer et al. (1995).
The ability of photorespiration to perturb leaf antioxidant redox states is evidenced by the transient oxidation of ascorbate and glutathione in both mutant and wild‐type (Fig. 6). Only in the case of the glutathione pool in the mutant is the oxidation sustained, however, and this occurs despite no appreciable increase in leaf H2O2 or ongoing oxidation of the ascorbate pool. The oxidation of glutathione is akin to observations in barley leaves subjected to severe drought, where the oxidized form of glutathione increases from a typical value of 5 % or less to around 40 % of the total pool (Smirnoff, 1993). Several differences exist, however, between the changes in the glutathione pool in the catalase mutants and those observed during drought. First, unlike the changes in glutathione in the catalase mutants, drought‐induced perturbation of the glutathione redox state does not always increase the total pool of glutathione. Secondly, several reports suggest that leaf ascorbate pools become smaller and also, sometimes, more oxidized during drought (Smirnoff, 1993). In plants with low catalase activity, however, both the redox state (Fig. 6) and total content (data not shown) of ascorbate were unaffected by transfer to photorespiratory conditions. In tobacco with low catalase, an increased ascorbate pool was associated with faster photorespiration, but this may well have been an irradiance effect since, in this study, accelerated photorespiration was achieved by transfer from low to high light (Willekens et al., 1997). We are currently analysing the effects of drought on leaf ascorbate and glutathione in wheat.
Can it be assumed that in wild‐type plants catalase capacity is so high that drought‐induced increases in photorespiratory H2O2 production will never be perceived as an oxidative stress? The existence of peroxisomal isoforms of ascorbate peroxidase may be rationalized by the need to prevent leakage of H2O2 into the cytosol (Foyer and Noctor, 2000). It cannot be discounted that the operation of peroxisomal APX, together with other enzymes of the ascorbate–glutathione cycle, could be an important route through which high rates of photorespiration could impact on the redox state of glutathione. The experiment of Fig. 6 might be considered of doubtful physiological relevance: the transient perturbation of the glutathione pool in wild‐type plants could be attributable to a lag period before catalase becomes fully synthesized or active after transfer from high CO2. Nevertheless, the data do underline the potential of high rates of photorespiratory H2O2 production to produce specific perturbations in important components of leaf redox homeostasis. Moreover, while catalase is present in the leaf peroxisomes of C3 plants in very high amounts, the enzyme turns over rapidly and must be continually resynthesized (Hertwig et al., 1992). In his review of the relationship between drought and AOS production, Smirnoff (1993) noted that ‘it would be instructive to know if turnover of catalase is influenced by water deficit’. This remains an important question. Salt stress engendered decreases in catalase activity in rye leaf pieces, as well as net oxidation of both ascorbate and glutathione and loss of chlorophyll (Streb and Feierabend, 1996). In these experiments, however, leaf pools of glutathione and ascorbate were not significantly increased. In contrast, inhibition of catalase with aminotriazole led to a net accumulation of both reduced and oxidized forms of glutathione, whereas ascorbate oxidation did not occur and chlorophyll loss was less significant (Streb and Feierabend, 1996). There were therefore clear differences between salt stress and catalase inhibition in these experiments. As in our experiments with catalase‐deficient barley, appreciable increases in leaf H2O2 were not observed in either salt stress or as a result of catalase inhibition (Streb and Feierabend, 1996). Using wheat, we are currently investigating the response to drought of transcripts encoding catalase and APX.
CHLOROPLASTIC AND PEROXISOMAL AOS PRODUCTION: ROLES IN DAMAGE AND SIGNALLING
Most studies suggest that drought‐induced decreases in net CO2 uptake are due to stomatal limitations, and the data we present are consistent with this notion. Modelling photorespiratory rates as a function of Ca suggests that photorespiratory flux will increase during drought. Because of the difficulties of relating Ci to leaf water status (Sharkey and Seemann, 1989), it is difficult to know at what point during the progression of drought increased photorespiratory flux will occur. The analysis of Wingler et al. (1999) in barley suggests that absolute flux began to increase at leaf water potentials as high as –1 MPa (which were reached after 8–10 d of drought in their experiments) and continued to increase until a water potential of –1·5 MPa was reached. This may well correspond to relatively mild decreases in Ci, perhaps equivalent to those observed here at Ca values in the range 100–200 µl l–1, where photorespiratory flux was maximal. Thereafter, further decreases in Ci may diminish absolute rates of RuBP oxygenation. Our data suggest that when RuBP oxygenation is maximal, the total oxidative load posed on the cell will owe more to photorespiratory H2O2 production than to chloroplastic events. From the point of view of physicochemical damage, however, AOS produced in the thylakoids might be locally very dangerous. Although the chloroplast has robust antioxidant defences, production of the hydroxyl radical through iron‐catalysed reduction of H2O2 by superoxide or ascorbate may pose a serious threat. Another dangerous species for photosynthetic function could be singlet oxygen produced in the thylakoid membrane. It is clear, however, that the increased oxidative load that accompanies most, if not all, stresses exerts its effect as much through modifications in gene expression as through physicochemical damage. Particular attention has been paid to oxidative events occurring at the apoplast, but we also call particular attention to the significance of photorespiratory H2O2 in signalling and acclimation. Photosynthetic events can modify nuclear gene expression (Karpinski et al., 1997; Pfannschmidt et al., 2001) but the intermediaries are ill‐defined. In C3 plants at least, the photorespiratory pathway enables the light‐dependent deposition of H2O2 outside the chloroplast where this oxidant, or antioxidants with which it interacts, may be involved in triggering acclimatory events in abiotic stresses such as drought.
ACKNOWLEDGEMENTS
The authors thank BBSRC for financial support. S.V.J. was the recipient of two Royal Society fellowships. L.N. was the recipient of a Rothamsted International fellowship.
Supplementary Material
Received: 18 July 2001; Returned for revision: 1 November 2001; Accepted: 12 November 2001.
References
- AlvarezME, Pennell RI, Meijer P‐J, Ishikawa Am, Dixon RA, Lamb C.1998. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92: 773–784. [DOI] [PubMed] [Google Scholar]
- ArisiACM, Cornic G, Jouanin L, Foyer CH.1998. Overexpression of iron superoxide dismutase in transformed poplar modifies the regulation of photosynthesis at low CO2 partial pressures or following exposure to the prooxidant herbicide methyl viologen. Plant Physiology 117: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BartoliCG, Simontacchi M, Tambussi E, Beltrano J, Montaldi E, Puntarulo S.1999. Drought and watering‐dependent oxidative stress: effect on antioxidant content in Triticum aestivum L. leaves. Journal of Experimental Botany 50: 375–383. [Google Scholar]
- BiehlerK, Fock H.1996. Evidence for the contribution of the Mehler‐peroxidase reaction in dissipating excess electrons in drought‐stressed wheat. Plant Physiology 112: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CornicG, Briantais J‐M.1991. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 183: 178–184. [DOI] [PubMed] [Google Scholar]
- DemmigB, Winter K, Kruger A, Czygan FC.1988. Zeaxanthin and the heat dissipation of excess light energy in Nerium oleander exposed to a combination of high light and water stress. Plant Physiology 87: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FoyerCH, Noctor G.2000. Oxygen processing in photosynthesis: regulation and signalling. New Phytologist 146: 359–388. [Google Scholar]
- FoyerCH, Lopez‐Delgado H, Dat JF, Scott IM.1997. Hydrogen peroxide‐ and glutathione‐associated mechanisms of acclimatory stress tolerance and signalling. Physiologia Plantarum 100: 241–254. [Google Scholar]
- FoyerCH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L.1995. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiology 109: 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GentyB, Briantais J‐M, Baker NR.1989. The relationship between the quantum yield of photosynthetic electron transport and the quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- Haupt‐HertingS, Klug K, Fock HP.2001. A new approach to measure gross CO2 fluxes in leaves. Gross CO2 assimilation, photorespiration, and mitochondrial respiration in the light in tomato under drought stress. Plant Physiology 126: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HertwigB, Streb P, Feierabend J.1992. Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiology 100: 1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe‐OrmaetxeI, Escuredo PR, Arrese‐Igor C, Becana M.1998. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiology 116: 173–181. [Google Scholar]
- JiaW, Zhang J.2000. Water stress‐induced abscisic acid accumulation in relation to reducing agents and sulfhydryl modifiers in maize plant. Plant, Cell and Environment 23: 1389–1395. [Google Scholar]
- KarpinskiS, Escobar C, Karpinska B, Creissen G, Mullineaux PM.1997. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KendallAC, Keys AJ, Turner JC, Lea PJ, Miflin BJ.1983. The isolation and characterisation of a catalase‐deficient mutant of barley (Hordeum vulgare L.). Planta 159: 505–511. [DOI] [PubMed] [Google Scholar]
- KeysAJ.1999. Biochemistry of photorespiration and the consequences for plant performance. In: Bryant JA, Burrell MM, Kruger NJ, eds. Plant carbohydrate biochemistry Oxford, UK: BIOS Scientific, 147–161. [Google Scholar]
- KovtunY, Chiu WL, Tena G, Sheen J.2000. Functional analysis of oxidative stress‐activated mitogen‐activated protein kinase cascade in plants. Proceedings of the National Academy of Sciences of the USA 97: 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LawlorDW.1976. Assimilation of carbon into photosynthetic inter mediates of water‐stressed wheat. Photosynthetica 10: 431–439. [Google Scholar]
- LawlorDW, Pearlman JG.1981. Compartmental modelling of photo respiration and carbon metabolism of water‐stressed leaves. Plant, Cell and Environment 4: 37–52. [Google Scholar]
- LevineA, Tenhaken R, Dixon R, Lamb C.1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593. [DOI] [PubMed] [Google Scholar]
- LoretoF, Harley PC, Di Marco G, Sharkey TD.1992. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology 98: 1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersieBD, Bowley SR, Harjanto E, Leprince O.1996. Water‐deficit tolerance and field performance of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiology 111: 1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MiyakeC, Yokota A.2000. Determination of the rate of photoreduction of O2 in the water‐water cycle in watermelon leaves and enhancement of the rate by limitation of photosynthesis. Plant Cell Physiology 41: 335–343. [DOI] [PubMed] [Google Scholar]
- NoctorG, Foyer CH.1998. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49: 249–279. [DOI] [PubMed] [Google Scholar]
- NoctorG, Foyer CH.2000. Homeostasis of adenylate status during photosynthesis in a fluctuating environment. Journal of Experimental Botany 51: 347–356. [DOI] [PubMed] [Google Scholar]
- NoctorG, Veljovic‐Jovanovic S, Foyer CH.2000. Peroxide processing in photosynthesis: antixoidant coupling and redox signalling. Proceedings of the Royal Society of London, B 355: 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NovitskayaL, Trevanion S, Driscoll D, Foyer CH, Noctor G.2002. How does photorespiration modulate leaf amino acid contents? A dual approach through modelling and metabolite analysis. Plant, Cell and Environment (in press). [Google Scholar]
- Orozco‐CárdenasML, Narváez‐Vásquez J, Ryan CA.2001. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin and methyl jasmonate. Plant Cell 13: 179–191. [PMC free article] [PubMed] [Google Scholar]
- OsmondCB, Grace SC.1995. Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? Journal of Experimental Botany 46: 1351–1362. [Google Scholar]
- PaulMJ, Lawlor DW, Driscoll SP.1990. The effect of temperature on photosynthesis and carbon fluxes in sunflower and rape. Journal of Experimental Botany 41: 547–555. [Google Scholar]
- PeiZM, Murata Y, Benning G, Thomine S, Klüsener Allen GJ, Grill E, Schroeder JI.2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- PfannschmidtT, Allen JF, Oelmüller R.2001. Principles of redox control in photosynthesis gene expression. Physiologia Plantarum 112: 1–9. [Google Scholar]
- RuuskaSA, Badger MR, Andrews TJ, von Caemmerer S.2000. Photosynthetic electron sinks with reduced amounts of Rubisco: little evidence for significant Mehler reaction. Journal of Experimental Botany 51: 357–368. [DOI] [PubMed] [Google Scholar]
- ScandaliosJG.1994. Regulation and properties of plant catalases. In: Foyer CH, Mullineaux PM, eds. Causes of photooxidative stress and amelioration of defense systems in plants Boca Raton, Florida: CRC Press, 275–315. [Google Scholar]
- SharkeyTD.1988. Estimating the rate of photorespiration in leaves. Physiologia Plantarum 73: 147–152. [Google Scholar]
- SharkeyTD, Seemann JR.1989. Mild water stress effects on carbon‐reduction‐cycle intermediates, ribulose bisphosphate carboxylase activity and spatial homogeneity of photosynthesis in intact leaves. Plant Physiology 89: 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShinozakiK, Yamaguchi‐Shinozaki K.1997. Gene expression and signal transduction in water‐stress response. Plant Physiology 115: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SmirnoffN.1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist 125: 27–58. [DOI] [PubMed] [Google Scholar]
- SmithIK, Kendall AC, Keys AJ, Turner JC, Lea PJ.1984. Increased levels of glutathione in a catalase‐deficient mutant of barley (Hordeum vulgare L.). Plant Science Letters 37: 29–33. [Google Scholar]
- StrebP, Feierabend J.1996. Oxidative stress responses accompanying photoinactivation of catalase in NaCl‐treated rye leaves. Botanica Acta 109: 125–132. [Google Scholar]
- TourneuxC, Peltier G.1995. Effect of water deficit on photosynthetic oxygen exchange measured using 18O2 and mass spectrometry in Solanum tuberosum Planta 195: 570–577. [Google Scholar]
- Veljovic‐JovanovicS, Noctor G, Foyer CH.2002. Artefactual effects during hydrogen peroxide determination and consequences for the estimation of leaf contents. Plant Biochemistry and Physiology (in press). [Google Scholar]
- Veljovic‐JovanovicS, Pignocchi C, Noctor G, Foyer CH.2001. Low ascorbic acid in the vtc 1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiology 127: 426–435. [PMC free article] [PubMed] [Google Scholar]
- von CaemmererS.2000. Biochemical models of leaf photosynthesis. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- von CaemmererS, Evans JR.1991. Determination of the CO2 pressure in chloroplasts from leaves of several C3 plants. Australian Journal of Plant Physiology 18: 287–305. [Google Scholar]
- von CaemmererS, Evans JR, Hudson GS, Andrews TJ.1994. The kinetics of ribulose‐1,5‐bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195: 88–97 [Google Scholar]
- WillekensH, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W.1997. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO Journal 16: 4806–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WinglerA, Quick WP, Bungard RA, Bailey KJ, Lea PJ, Leegood RC.1999. The role of photorespiration during drought stress: an analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant, Cell and Environment 22: 361–373. [Google Scholar]
- YanJ, Wang Y, Allen R, Holaday AS, Tissue D, Zhang H.2002. Protection of photosynthesis and seed production under water‐deficit conditions in transgenic tobacco plans that over‐express Arabidopsis ascorbate peroxidase. Plant Physiology (in press). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.