Abstract
Many bacteria regulate diverse physiological processes in concert with their population size. Bacterial cell‐to‐cell communication utilizes small diffusible signal molecules, which the bacteria both produce and perceive. The bacteria couple gene expression to cell density by eliciting a response only when the signalling molecules reach a critical threshold (a point at which the population is said to be ‘quorate’). The population as a whole is thus able to modify its behaviour as a single unit. Amongst Gram‐negative bacteria, the quorum sensing signals most commonly used are N‐acylhomoserine lactones (AHLs). It is now apparent that AHLs are used for regulating diverse behaviours in epiphytic, rhizosphere‐inhabiting and plant pathogenic bacteria and that plants may produce their own metabolites that interfere with this signalling. Transgenic plants that produce high levels of AHLs or which can degrade bacterial‐produced AHLs have been made. These plants have dramatically altered susceptibilities to infection by pathogenic Erwinia species. In addition, such plants will prove useful tools in determining the roles of AHL‐regulated density‐dependent behaviour in growth promoting, biological control and pathogenic plant‐associated bacterial species.
Key words: N‐Acylhomoserine lactones, AHLs, Erwinia, potato, rhizosphere
INTRODUCTION
Microbial success critically depends on the ability to perceive and respond rapidly to changes in the local environment. For any individual bacterium, one of the most important of these environmental factors will be the number and growth status of its fellows within its immediate vicinity. Such information may allow the bacterium to anticipate future availability of nutrients or build up of toxins. More importantly, it enables the individuals within the population to coordinate ecological strategies that would not be successful if attempted by a small number of bacteria acting independently. The ability to monitor the local population density is dependent on a cell‐to‐cell communication system that employs small diffusible signalling molecules. This phenomenon has been termed ‘quorum sensing’ since initiation of a concerted population response depends on the population reaching a minimal population unit or ‘quorum’ [reviewed in Withers et al. (2001) and Whitehead et al. (2001)]. Examples of such density‐dependent multicellular behaviour in prokaryotes include diverse processes such as bioluminescence, sporulation, swarming, antibiotic biosynthesis, plasmid conjugal transfer and the production of virulence determinants in animal, fish and plant pathogens (Swift et al., 1996). A number of quorum signalling molecules have been identified in both Gram‐negative and Gram‐positive species. In Gram‐negative bacteria, one of the most widespread and best understood families of signal molecules is the N‐acylhomoserine lactones (AHLs) which vary predominantly in the presence or absence of an acyl chain C3 substituent (oxo‐ or hydroxy‐) and length of the N‐acyl side chain (four to 14 carbons). Examples of some of the known AHLs together with density‐dependent behaviour that they have been shown to regulate are shown in Table 1.
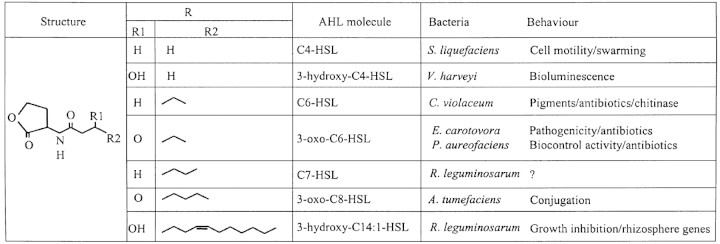
Table 1. Examples of a number of AHLs and some of the density‐dependent behaviours that they are known to regulate
AHL BIOSYNTHESIS AND PERCEPTION
Several bacterial species produce the same AHL, though in each it may be used to regulate different biological processes. Thus LuxI of the marine bacterium Vibrio fischeri, synthesizes N‐(3‐oxohexanoyl)‐l‐homoserine lactone (3‐oxo‐C6‐HSL), which regulates bioluminescence in a cell‐density‐dependent manner, while CarI of Erwinia carotovora also produces 3‐oxo‐C6‐HSL, which, in this bacterium, is responsible for the induction of the secreted plant cell wall‐degrading exoenzymes and of the antibiotic carbapenem (Bainton et al., 1992; Jones et al., 1993). The cviI gene of the soil bacterium Chromobacterium violaceum encodes the enzyme for N‐hexanoyl‐l‐homoserine lactone (C6‐HSL) synthesis, which is structurally very similar to 3‐oxo‐C6‐HSL and which induces production of the purple pigment violacein as well as antifungal chitinases (McClean et al., 1997; Chernin et al., 1998). Inactivation or deletion of luxI, carI or cviI results in loss of density‐dependent bioluminescence, virulence or violacein production, respectively. The relevant operons can, however, be induced by the addition of an exogenous supply of an appropriate AHL to the mutant bacteria. Utilizing such operons, reporter strains of Escherichia coli and C. violaceum have been developed that indicate the presence of particular AHLs through light or pigment production (McClean et al., 1997; Winson et al., 1998).
AHL biosynthetic enzymes fall into three groups. The LuxI type was the first to be discovered and appears to be the most common. These enzymes generate the homoserine lactone moiety from S‐adenosyl methionine and derive the acyl side chain from the appropriately charged acyl‐acyl carrier protein (acyl‐ACP) or acyl‐coenzyme A (acyl‐CoA). Many of these enzymes can produce more than one AHL by utilizing alternative acyl‐ACP or acyl‐CoA side chain precursors. A second group of AHL biosynthetic enzymes, the LuxM type, has no significant homology to the LuxI type but appears to catalyse AHL synthesis from the same substrates (Hanzelka et al., 1999). A third class of AHL synthase, HdtS, has been identified from the biocontrol strain Pseudomonas fluorescens F113 (Laue et al., 2000). HdtS has no homology to either LuxI or LuxM. Many bacteria utilize more than one AHL biosynthetic enzyme and in some cases the synthases used may belong to different classes (Hanzelka et al., 1999). It is not clear why three different classes of enzymes should have evolved to perform the same role; perhaps possession of different AHL synthases may afford some protection against competitor or host species developing inhibitory molecules that target the synthase.
AHLS AND ERWINIA CAROTOVORA INFECTIONS
AHLs were initially identified over two decades ago as ‘autoinducers’ of bioluminescence in certain marine bacteria (Eberhard et al., 1981; Cao and Meighen, 1989). The finding 10 years later that production of carbapenem antibiotic by the terrestrial plant pathogen Erwinia carotovora was also regulated by identical self‐produced AHLs was the first indication of how widespread this form of signalling would prove to be (Bainton et al., 1992).
As well as antibiotic production, AHLs were found to be responsible for the induction of a glycine‐rich protein (Harpin) that strongly elicits the plant hypersensitive reaction (Mukherjee et al., 1997). Even more importantly, AHLs provide global control of exoenzyme production by E. carotovora. E. carotovora carI mutants appear to be completely avirulent in a tobacco test system; they can neither macerate plant tissue nor multiply in planta because they lack pectin lyase, pectate lyase, polygalacturonase, cellulase and protease (Jones et al., 1993; Pirhonen et al., 1993). It is pertinent to ask how the expression of these exoenzymes only at high cell density in wild‐type cells may contribute to the success of Erwinia species as plant pathogens. An explanation has been proposed by Pirhonen et al. (1993): under aerobic conditions, a successful E. carotovora infection requires a relatively high inoculum and the progression of the disease is then a competition between bacterial multiplication and the development of plant resistance (Perombelon and Kelman, 1980). Thus, production of macerating enzymes at low cell densities would not give rise to a successful infection but would result in induction of the local and systemic plant defence response, which in turn would hamper subsequent infections. Such resistance to E. carotovora infection is seen when the plant defence response is artificially induced by application of salicylic acid (Palva et al., 1994).
If an infecting bacterium were to encounter AHL levels that indicated that it was part of a far larger population than it actually was, it might be induced to mount a pathogenic attack prematurely. The course of the ensuing infection might be drastically altered, with the plant being able to mount a successful defence to a weak attack. To test this theory, plants were engineered to express the yenI gene of Yersinia enterocolitica (Fray et al., 1999). In Y. enterocolitica, and in E. coli expressing the yenI gene product, C6‐HSL and 3‐oxo‐C6‐HSL are produced in a 1 : 1 ratio; both AHLs are naturally produced by a number of E. carotovora species (Nasser et al., 1998; Fig. 1). Initially, transgenic tobacco plants were made to test the feasibility of synthesizing bacterial AHL signalling molecules in planta. The predicted AHLs were made at biologically active levels in these plants, but only when YenI was targeted to the chloroplasts (Fray et al., 1999). This requirement for plastid location is probably due to the availability in this organelle of the immediate precursors for AHL synthesis. The presence of suitable precursors probably reflects the prokaryotic origin of plastids. By using HPLC‐mass spectrometry, the AHLs synthesized were confirmed as being 3‐oxo‐C6‐HSL and C6‐HSL and these were present at levels of 0·41 µg and 0·35 µg g–1 f. wt, respectively, approximating to the 1 : 1 ratio produced by YenI in Y. enterocolitica. In bacteria, AHLs with an acyl side chain of six carbons or less have been shown to diffuse freely across the bacterial membrane (though this is not the case for AHLs with a 12 carbon side chain; Pearson et al., 1999). Consistent with this, the 3‐oxo‐C6‐HSL and C6‐HSL produced in the chloroplasts appear to diffuse freely across the plastid and plasma membranes and can be detected at the plant cell surface by bacterial AHL biosensors.
Fig. 1. Thin layer chromatogram showing the range of AHLs produced by various Erwinia carotovora species. After chromatography, AHLs were located by overlaying the chromatogram with agar seeded with the Chromobacterium violaceum strain mutant for cviI (the gene required for AHL synthesis). The presence of AHLs is indicated by complementation of the mutation and restoration of the production of the purple pigment, violacein. A, 3‐oxo‐C6‐HSL standard; B, C6‐HSL standard; C–F, spent bacterial culture supernatants of Erwinia carotovora subsp. carotovora SCRI 193 (C); Erwinia carotovora subsp. atroseptica SCRI 1043 (D); Erwinia carotovora subsp. atroseptica SCRI 1039 (E); and Erwinia chrysanthemi SCRI 1043 (F). Most strains produce both 3‐oxo‐C6‐HSL and C6‐HSL as well as additional AHLs in some cases. The sensitivity of the C. violaceum reporter strain varies according to the AHL being detected, thus the intensity of pigment is not a direct indicator of the relative abundance of each AHL.
AHL‐producing tobacco plants were able to restore pathogenicity to the avirulent E. carotovora CarI mutant (Fig. 2). However, tobacco is not a normal host for E. carotovora and, in order for an infection to occur, the inoculum level used was already in excess of the quorum‐sensing threshold. To test whether small inocula of wild‐type E. carotovora could be induced to attempt a pathogenic attack prematurely on AHL‐producing plants, and whether such an infection would result in increased resistance, YenI‐expressing potato lines were made. These plants were infected using stem stab inoculations with a range of titres of wild‐type E. carotovora atroseptica. Surprisingly, the plants proved to be susceptible at inoculum levels as low as 102, levels that in an untransformed plant did not cause disease symptoms (Fray et al., unpubl. res.). This raises the question of why Erwinia does not normally attempt an infection at these lower cell densities if such an infection is likely to be successful. There are two possible explanations: it might be a reflection of the strategies employed by either the bacteria or the plant.

Fig. 2. AHL‐producing transgenic tobacco plants restore pathogenicity to an avirulent AHL‐deficient Erwinia carotovora subsp. carotovora mutant (PNP22). The photograph shows the leaves 4 d after infection. 1, Wild‐type tobacco inoculated with wild‐type Erwinia carotovora; 2, Wild‐type tobacco inoculated with AHL‐negative Erwinia carotovora mutant PNP22; 3, AHL‐producing tobacco line inoculated with Erwinia carotovora PNP22.
The bacterial strategy
The quorum sensing threshold might be set by the bacteria at a level that is above that needed for a successful infection in potato, but this threshold might still represent the level that maximizes the bacteria’s chances of success. E. carotovora atrosceptica infects plant species other than potato: in these species an inoculum of 102 may not be sufficient to overcome the plant’s defence responses. Without a mechanism for identifying each potential host plant and modifying its behaviour according to host plant species, setting a higher threshold for the expression of pathogenicity‐associated genes may represent the best evolutionary strategy. Furthermore, other bacterial species will also be present in the rhizosphere or on the plant surface, some of which are likely to be making identical or similar AHLs. This could result in cross‐talk between species and an over‐estimation by Erwinia of its own population size. Indeed, such cross‐talk between species in the rhizosphere has been demonstrated by Wood et al. (1997). Setting a higher threshold for activation might be one mechanism to compensate for this.
The plant strategy
Plants may have evolved strategies to interfere with the bacteria’s AHL signalling system to prevent them from initiating a pathogenic attack. Such interference could include the production of signal mimics, signal blockers or signal‐degrading enzymes or the production of compounds that block the activity of the AHL‐producing enzymes. Examples of compounds with signal inhibiting properties are known and there is evidence for the production of AHL inhibitory molecules by bacteria, algae and plants. In the simplest case, an AHL produced by one bacterial species may be antagonistic to the activity of an AHL used by a second species. This is seen in the case of Chromobacterium violaceum where the cognate AHL contains an acyl side chain of six carbons. The presence of this molecule or closely related analogues induces the production of the purple pigment violacein. However, related molecules with acyl side chains of ten or more carbons do not activate violacein production and actually inhibit the normal response to the cognate molecule (McClean et al., 1997; Fig. 3). More complex blocking molecules are produced by the Australian marine alga, Delisea pulchra. This macroalga produces halogenated furanones which have some structural similarity to AHLs (Table 2). It appears that D. pulchra uses these AHL blockers in vivo to inhibit bacterial cell swarming and attachment responses, thus preventing the build‐up of bacterial biofilms on the algal surfaces (Givskov et al., 1996; Gram et al., 1996). Teplitski et al. (2000) reported AHL inhibitory activities in exudates from pea seedlings. The compounds responsible have not been identified, but they preferentially partition into polar solvents (unlike the AHL molecules themselves). We have also found compounds in a number of plant extracts that have similar partitioning characteristics in aqueous solvents: inhibitory activities were particularly pronounced in extracts from a number of fruit, including grape and strawberry (Fig. 3). Bacterial phenotypes controlled through quorum sensing are frequently regulated by additional environmental cues. In some cases, population‐density signals can be modulated or overridden by factors such as oxygen tension, nutrient starvation, iron limitation or catabolite repression. It is possible that the plant‐produced compounds are indirectly altering the bacterial AHL response rather than targeting it directly, but even if this were the case such compounds could prove to be important in determining the outcome of interactions between higher plants and a diversity of pathogenic and symbiotic bacteria. The evidence for AHL‐inhibitory molecules in potato plants is not as clear as that for certain fruit or for pea seedling exudates. However, if these plants have adopted a strategy of limiting pathogenic attacks by blocking quorum sensing molecules, then engineering them to make AHLs would run counter to the existing defence mechanism and might render such plants more susceptible to infection.

Fig. 3. Inhibition of quorum sensing responses. A, In a reverse assay, 3‐oxo‐C6‐HSL was added to a top agar containing the Chromobacterium violaceum CviI– biosensor strain. Inhibition of quorum sensing by the long chain 3‐oxo‐C12‐HSL, or by dilutions of a crude grape extract (60–7·5 µl made up to 60 µl with H20), results in inhibition of violacein production but not bacterial growth. B and C, Other plant extracts also inhibit AHL responses. Antibiotic production by wild‐type Erwinia carotovora is revealed by inhibition of growth in a lawn of an Escherchia coli strain sensitive to carbabenem. In B, the well contains water whilst in C it contains a crude strawberry extract. Inhibition of antibiotic production (an AHL response) is revealed by growth of the E. coli lawn adjacent to the well containing the fruit extract.
Table 2. Quorum sensing‐related signal molecules
A, The basic N‐acyl homoserine lactone structure (see also Table 1). B, Halogenated furanones produced by a marine alga block bacterial AHL responses. C and D, Some cyclic dipeptides produced by both bacteria and fungi can activate or repress the bacterial AHL receptor. E, The volatile quorum‐sensing molecule produced during infections by Ralastonia solanacearum. F, Proposed structure for one of the quorum‐sensing molecules used by the plant pathogen Xanthomonas campestris. G and H, Examples of butyrolactone signal molecules produced by some soil inhabiting Streptomyces species.
ALTERNATIVE APPROACHES FOR INTERFERING WITH AHL SIGNALLING IN PLANT‐ASSOCIATED MICROBES
Supplying transgenic plants with the ability to block or degrade AHL signals may provide an alternative approach for engineering resistance to E. carotovora species. There is no evidence that the long chain AHLs function as inhibitors of shorter chain AHL‐induced responses in systems other than Chromobacterium violaceum. Even if long chain AHLs could suppress exoenzyme production by E. carotovora species, problems would remain with their use as some have been shown to have immune modulatory effects in mice and human leukocyte immunoassays (Telford et al., 1998). Engineering plants to produce inhibitory halogenated furanones might be possible, although the genes directing their biosynthesis have yet to be cloned from Delisea pulchra. Enhancing and extending the natural inhibitory activity apparent in some plant tissues may prove to be a more attractive approach. This is currently hampered by the fact that the nature of the inhibitory molecule(s) is unknown.
A bacterial isolate, Bacillus sp. 240B1, from soil was identified as having AHL degrading activity. The gene (aiiA) for this AHL degrading activity was cloned and shown to contain motifs conserved in several groups of metallohydrolases (Dong et al., 2000). When expressed in a heterologous E. carotovora system, AiiA inactivated the endogenous AHLs and reduced bacterial virulence. Recently, transgenic tobacco and potato plants expressing the aiiA gene have been made (Dong et al., 2001). These plants show considerable resistance to E. carotovora pv. carotovora infections even at very high bacterial inocula. Even where local disease symptoms were exhibited, the plants appeared to mount a defence response and recover. This appears to be a very promising approach to preventing AHL signalling in plant‐associated bacteria. The same authors showed that AiiA is a lactonase, destroying AHL activity by opening the heterocyclic ring. An AHL‐degrading activity has also been found in the soil bacterium Varivorax paradoxus (Leadbetter and Greenberg, 2000). In this case the enzyme is an AHL‐acylase, cleaving the acyl side chain off the homoserine lactone moiety. The gene encoding this enzyme has yet to be cloned but, if it is identified, a transgenic plant approach might also prove effective.
As an alternative to the GM plant approach, a bacterial strain that efficiently degrades AHLs and that is capable of colonizing the rhizosphere might prove an effective biological control agent against certain plant pathogenic bacteria.
AHLS AND OTHER PLANT‐ASSOCIATED BACTERIA—DO WE WANT TO BLOCK QUORUM SENSING?
Many Gram‐negative bacteria found in association with plants have been shown to produce AHL signal molecules (Cha et al., 1998). The list includes epiphytic, pathogenic, rhizosphere‐inhabiting and nitrogen‐fixing symbionts. For many of these species, the genes under AHL regulation and the ecological roles of quorum sensing have not been studied in detail but, in the case of Pseudomonas spp., AHL production was found to be more common among plant‐associated species than among soil‐borne species (Elasri et al., 2001).
Some plant pathogenic bacteria have AHL sensing systems where the link with pathogenicity is indirect. In the plant pathogen Agrobacterium tumefaciens, conjugal transfer of the Ti plasmid between bacteria is induced by AHLs. However, AHLs form part of a more complex regulatory circuit that is triggered by opines produced by the transformed host plant tissue (reviewed in Farrand, 1998). For octopine‐type Ti plasmids, a luxR homologue (TraR) forms part of an octopine inducible operon that includes enzymes required for octopine catabolism. TraR responds to the AHL 3‐oxo‐C8‐HSL by inducing the genes required for plasmid transfer; in addition, it also up‐regulates the synthesis of 3‐oxo‐C8‐HSL itself. The situation is similar for nopaline‐type Ti plasmids, but, in this case, agrocinipines act on a repressor to relieve the repression of TraR and to allow AHL‐induced plasmid transfer when an appropriate cell density has been reached. Thus, A. tumefaciens genetically modifies plants to produce opines which have a direct effect upon AHL production and perception by A. tumefaciens itself. However, whilst AHLs contribute to the maintenance (or spread) of the Ti plasmid within the A. tumefaciens population, they do not appear to be directly required for plant infection.
For other plant pathogens, a link between AHLs and pathogenicity is not apparent. Pseudomonas syringae B728a makes AHLs, but disruption of the genes responsible had no effect on pathogenicity or swarming (Kinscherf and Willis, 1999). In contrast, it is apparent that AHL responses play a direct role in the colonization and behaviour of many bacteria beneficial to plants.
Rhizobium leguminosarum produces a number of different AHL molecules (Lithgow et al., 2000) using four different AHL biosynthetic genes (two on the symbiotic plasmid, one on a second large plasmid and one on the chromosome). The largest AHL contains an acyl side chain of 14 carbons, which is unusual both for the length of the acyl side chain and for the fact that it is not fully saturated. This AHL was originally identified as a ‘small bacteriocin’ because of its growth inhibitory effects on several strains of R. leguminosarum (Hirsch, 1979; Wijffelman et al., 1983), though Gray et al. (1996) subsequently concluded that it induced exponentially growing cells to go into stationary phase rather than causing cell death. As well as the effect on growth, this large AHL was also found to induce expression of the rhizosphere‐expressed rhiABC operon and to promote plasmid transfer. A complex regulatory circuit operates in this species, with the three other AHL synthesizing loci being regulated by this long chain AHL (Lithgow et al., 2000). A role for AHL signalling in nodulation by R. leguminosarum is implied by the finding that mutating an AHL receptor decreased nodulation (Cubo et al., 1992). The related species R. etli CNPAF512 produces at least seven AHLs; disruption of a gene responsible for the production of two of these resulted in a doubling in the number of nitrogen‐fixing nodules (Rosemeyer et al., 1998). This latter finding is intriguing in the light of the discovery that some legumes appear to secrete AHL antagonists from their roots (Teplitski et al., 2000).
Pseudomonas aureofaciens 30–84 (a soil‐borne bacterium that colonizes the wheat rhizosphere) inhibits the fungus Gaeumannomyces graminis var. tritici, the causative agent of take‐all disease of wheat (Wood and Pierson, 1996), and has thus been used as a biological control agent. P. aureofaciens 30–84 synthesizes three phenazine antibiotics which are responsible (at least in part) for this antifungal activity. Expression of the phenazine biosynthetic operon is controlled by C6‐HSL, which is synthesized by the phzI gene product (Wood and Pierson, 1996). Disruption of phzI abolishes bio‐control activity, but this can be restored in situ by co‐inoculation with a C6‐HSL producing strain that supplies the signalling molecule in trans (Wood et al., 1997). Restoration of biocontrol activity to phzI–strains can also be conferred by AHL‐synthesizing plant tissues (Fray et al., 1999). AHLs are likely to have a role in promoting active secondary metabolite production in many bio‐control strains of pseudomonads (Whitehead et al., 2001), as has also been shown for Pseudomonas chlororaphis PCL1391 (which inhibits Fusarium oxysporum infections of tomato; Chin‐A‐Woeng et al., 2001).
It is possible that gross disruption of AHL‐based communication in the rhizosphere may adversely affect the colonization or behaviour of a number of important growth‐promoting or biocontrol species (Zhang and Pierson, 2001). The disadvantages of this disruption may outweigh any potential gains from reduced pathogenicity of other bacteria.
OTHER BACTERIAL QUORUM SENSING SYSTEMS OF PLANT‐ AND RHIZOSPHERE‐ASSOCIATED BACTERIA
Apart from AHLs, a number of other molecules that effect AHL quorum sensing or that act as quorum sensing molecules in their own right are produced by plant‐ and rhizosphere‐associated bacteria.
Several species of Pseudomonas, including biological control strains of P. fluorescens, produce cyclic dipeptides (diketopiperazines; Table 2). Though there is no obvious similarity between these molecules and AHLs, they appear to be capable of directly activating or antagonizing the receptors normally used by bacteria for AHL perception (Holden et al., 1999). Such compounds are not confined to bacteria; several of these active cyclic dipeptides are also produced by the fungal plant pathogen Alteneria alternata and two, cyclo(l‐Pro‐l‐Tyr) and cyclo(l‐Pro‐l‐Phe), act as host‐specific phytotoxins against spotted knapweed (Centaurea maculosa; Stierle et al., 1988). The physiological/ecological role of these molecules in relation to quorum sensing (if any) remains to be established.
The plant pathogen Ralstonia solanacearum causes wilting and death in several hundred plant species including economically important crops such as potato, tomato and banana. During infection, R. solanacearum integrates a number of self‐ and plant‐derived signals. The first intercellular signal produced by the bacteria is 3‐hydroxy‐palmitic acid‐methyl ester (Table 2), which is volatile and acts as a quorum sensing molecule. This acts through a phosphorelay to derepress a transcriptional activator, PhcA, which is itself induced by a plant‐derived signal. PhcA then acts to induce the production of extracellular polysaccharides and various other virulence factors and AHLs, though the role of these AHLs in signalling during the infection process is unclear at the present time (Denny, 1999).
Xanthomonas campestris pv. campestris, the causative agent of black rot in crucifers, also regulates extracellular polysaccharide production via diffusible intercellular signal molecules. These molecules are not fully characterized, but the lactone structure shown in Table 2 was proposed for one of these (Chun et al., 1997).
Gram‐negative bacteria are not alone in exhibiting density‐dependent behaviour. Among the Streptomyces, small diffusible butyrolactone‐containing (BL) autoinducers direct the late log phase‐dependent production of antibiotics and novel pigments and may also induce differentiation and spore production (Table 2; Horinouchi and Beppu, 1992). As with AHL synthesis, BL production is inhibited by cerulenin (which irreversibly inhibits β‐ketoacyl‐acyl carrier protein synthetase) suggesting that, like AHLs, one of the precursors is derived from fatty acid biosynthesis; the other precursor of BLs is probably a glycerol derivative.
Becker et al. (1997) presented evidence that BL‐mediated communication between Streptomyces species contributed to pathogen inhibition in a soil naturally suppressive to S. scabies, the causative agent of potato scab disease. However, they concluded that there was no cross‐talk between the AHL and BL quorum sensing systems.
CONCLUSIONS
Quorum sensing is an example of multicellular behaviour in prokaryotes and regulates diverse physiological processes in beneficial and pathogenic bacteria. AHLs are one of the most widely used quorum sensing molecules among Gram‐negative bacteria and the ability now exists to engineer plants to give false information to bacteria that use this form of signalling. This can be achieved either through AHL production in planta or by engineering plants with the ability to degrade AHL molecules. The latter approach is particularly promising as a mechanism to confer disease resistance to Erwinia species. However, the role of AHL signalling in a number of important plant‐associated bacteria remains to be clarified. The degree of communication within and between mixed bacterial communities and the role that naturally produced plant metabolites may have upon this communication are likely to be fruitful areas for research in the coming years.
ACKNOWLEDGEMENTS
Work in the author’s laboratory is supported by a Biotechnology and Biological Sciences Research Council Sir David Phillips Fellowship awarded to R.G.F. and by European Framework Five grant QLK3‐CT‐2000–31759, which are both gratefully acknowledged. I thank James Newton for reviewing the manuscript.
Supplementary Material
Received: 31 July 2001; Returned for revision: 10 October 2001; Accepted: 26 November 2001.
References
- BaintonNJ, Stead P, Chhabra SR, Bycroft BW, Salmond GPC, Stewart GSAB, Williams P.1992. N‐(3‐oxohexanoyl)‐l‐homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora Biochemical Journal 288: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BeckerDM, Kinkel LL, Schottel JL.1997. Evidence for interspecies communication and its potential role in pathogen suppression in a naturally occurring disease suppressive soil. Canadian Journal of Microbiology 43: 985–990. [Google Scholar]
- CaoJG, Swartzman E, Miyamoto C, Meighen E.1989. Regulatory mutants of the Vibrio‐harveyi‐Lux system. Journal of Bioluminescence and Chemiluminescence 3: 207–212. [Google Scholar]
- ChaC, Gao P, Chen YC, Shaw PD, Farrand SK.1998. Production of acyl‐homoserine lactone quorum‐sensing signals by gram‐negative plant‐associated bacteria. Molecular Plant‐Microbe Interactions 11: 1119–1129. [DOI] [PubMed] [Google Scholar]
- CherninLS, Winson MK, Thompson JM, Haran S, Bycroft BW, Chet I, Williams P, Stewart GSAB. 1998. Chitinolytic activity in Chromobacterium violaceum: Substrate analysis and regulation by quorum sensing. Journal of Bacteriology 180: 4435–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin‐A‐WoengTFC, van den Broek D, de Voer G, van der Drift KMGM, Tuinman S, Thomas‐Oates JE, Lugtenberg BJJ, Bloemberg GV.2001. Phenazine‐1‐carboxamide production in the biocontrol strain Pseudomonas chlororaphis PCL1391 is regulated by multiple factors secreted into the growth medium. Molecular Plant–Microbe Interactions 14: 969–979. [DOI] [PubMed] [Google Scholar]
- ChunW, Cui J, Poplawsky A.1997. Purification, characterization and biological role of a pheromone produced by Xanthamonas campestris pv. campestris Physiological and Molecular Plant Pathology 51: 1–14. [Google Scholar]
- CuboMT, Economou A, Murphy G, Johnston AWB, Downie JA. 1992. Molecular characterization and regulation of the rhizosphere‐expressed genes rhiABCR that can influence nodulation by Rhizobium‐leguminosarum biovar viviae. Journal of Bacteriology 174: 4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DennyTP.1999. Autoregulator‐dependent control of extracellular polysaccharide production in phytopathogenic bacteria. European Journal of Plant Pathology 105: 417–430. [Google Scholar]
- DongYH, Xu JL, Li XZ, Zhang LH.2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum‐sensing signal and attenuates the virulence of Erwinia carotovora Proceedings of the National Academy of Sciences of the USA 97: 3526–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DongYH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH.2001. Quenching quorum‐sensing‐dependent bacterial infection by an N‐acyl homoserine lactonase. Nature 411: 813–817. [DOI] [PubMed] [Google Scholar]
- EberhardA, Burlingame AL, Eberhard C, Kenyon GL, Nealson KH, Oppenheimer NJ.1981. Structural identification of autoinducer of Photobacterium‐fischeri luciferase. Biochemistry 20: 2444–2449. [DOI] [PubMed] [Google Scholar]
- ElasriM, Delorme S, Lemanceau P, Stewart G, Laue B, Glickmann E, Oger PM, Dessaux Y.2001. Acyl‐homoserine lactone production is more common among plant‐associated Pseudomonas spp. than among soilborne Pseudomonas spp. Applied Environmental Microbiology 67: 1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FarrandSK.1998. Conjugal plasmids and their transfer. In: Spaink HP, Kondorosi A, Hookyaas PJJ, eds. The Rhizobiaceae: molecular biology of model plant‐associated bacteria Dordrecht: Kluwer Academic Press, 199–233. [Google Scholar]
- FrayRG, Throup JP, Wallace A, Daykin M, Williams P, Stewart GSAB, Grierson D.1999. Plants genetically modified to produce N‐acylhomoserine lactones communicate with bacteria. Nature Biotechnology 17: 1017–1020. [DOI] [PubMed] [Google Scholar]
- GivskovM, DeNys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg PD, Kjelleberg S.1996. Eukaryotic interference with homoserine lactone‐mediated prokaryotic signalling. Journal of Bacteriology 178: 6618–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GramL, deNys R, Maximilien R, Givskov M, Steinberg P, Kjelleberg S. 1996. Inhibitory effects of secondary metabolites from the red alga Delisea pulchra on swarming motility of Proteus mirabilis Applied Environmental Microbiology 62: 4284–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrayKM, Pearson JP, Downie JA, Boboye BEA, Greenberg EP.1996. Cell‐to‐cell signaling in the symbiotic nitrogen‐fixing bacterium Rhizobium leguminosarum: autoinduction of a stationary phase and rhizosphere‐expressed genes. Journal of Bacteriology 178: 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HanzelkaBL, Parsek MR, Val DL, Dunlap PV, Cronan JE Jr, Greenberg EP.1999. Acylhomoserine lactone synthase activity of the Vibrio fischeri AinS protein. Journal of Bacteriology 9: 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HirschPR.1979. Plasmid‐determined bacteriocin production by Rhizobium leguminosarum Journal of General Microbiology 113: 219–228. [Google Scholar]
- HoldenMTG, Chhabra SR, de Nys R, Stead P, Bainton NJ, Hill PJ, Manefield M, Kumar N, Labatte M, England D, Rice S, Givskov M, Salmond GPC, Stewart GSAB, Bycroft BW, Kjelleberg SA, Williams P.1999. Quorum‐sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram‐negative bacteria. Molecular Microbiology 33: 1254–1266. [DOI] [PubMed] [Google Scholar]
- HorinouchiS, Beppu T. 1992. Autoregulatory factors and communication in actinomycetes. Annual Review of Microbiology 46: 377–398. [DOI] [PubMed] [Google Scholar]
- JonesS, Yu B, Bainton NJ, Birdsall M, Bycroft BW, Chhabra SR, Cox AJR, Golby P, Reeves PJ, Stephens S, Winson MK, Salmond GPC, Stewart GSAB, Williams P.1993. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa EMBO Journal 12: 2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KinscherfTG, Willis DK.1999. Swarming by Pseudomonas syringae B728a requires gacS (lemA) and gacA but not the acyl‐homoserine lactone biosynthetic gene ahlI Journal of Bacteriology 181: 4133–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaueRE, Jiang Y, Chhabra SR, Jacob S, Stewart GSAB, Hardman A, Downie JA, O’Gara F, Williams P.2000. The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N‐(3‐hydroxy‐7‐cis‐tetradecenoyl)homoserine lactone, via HdtS, a putative novel N‐acylhomoserine lactone synthase. Microbiology‐UK 146: 2469–2480. [DOI] [PubMed] [Google Scholar]
- LeadbetterJR, Greenberg EP.2000. Metabolism of acyl‐homoserine lactone quorum‐sensing signals by Variovorax paradoxus Journal of Bacteriology 182: 6921–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LithgowJK, Wilkinson A, Hardman A, Rodelas B, Wisniewski‐Dye F, Williams P, Downie JA.2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum‐sensing loci. Molecular Microbiology 37: 81–97. [DOI] [PubMed] [Google Scholar]
- McCleanKH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GSAB, Williams P.1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N‐acylhomoserine lactones. Microbiology‐UK 143: 3703–3711. [DOI] [PubMed] [Google Scholar]
- MukherjeeA, Cui Y, Liu Y Chatterjee AK.1997. Molecular characterization and expression of the Erwinia carotovora hrpNEcc gene, which encodes an elicitor of the hypersensitive reaction. Molecular Plant‐Microbe Interactions 10: 462–471. [DOI] [PubMed] [Google Scholar]
- NasserW, Bouillant ML, Salmond G, Reverchon S.1998. Characterization of the Erwinia chrysanthemi expl‐expR locus directing the synthesis of two N‐acyl‐homoserine lactone signal molecules. Molecular Microbiology 29: 1391–1405. [DOI] [PubMed] [Google Scholar]
- PalvaTK, Hurtig M, Saindrenan P, Palva ET.1994. Salicylic acid induced resistance to Erwinia carotovora subsp. carotovora in tobacco. Molecular Plant‐Microbe Interactions 7: 356–363. [Google Scholar]
- PearsonJP, Van Delden C, Iglewski BH.1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell‐to‐cell signals. Journal of Bacteriology 181: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PerombelonMCM, Kelman A.1980. Ecology of the soft rot erwinias. Annual Review of Phytopathology 18: 361–387. [Google Scholar]
- PirhonenM, Flego D, Heikineimo R, Palva ET. 1993. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora EMBO Journal 12: 2467–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RosemeyerV, Michiels J, Verreth C, Vanderleyden J.1998. luxI‐ and luxR‐homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris Journal of Bacteriology 180: 815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StierleAC, Cardellina JH, Strobel GA.1988. Maculosin, a host‐specific phytotoxin for spotted knapweed from Alternaria‐alternata Proceedings of the National Academy of Sciences of the USA 85: 8008–8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SwiftS, Throup JP, Williams P, Salmond GPC, Stewart GSAB.1996. Quorum sensing: a population‐density component in the determination of bacterial phenotype. Trends in Biochemical Sciences 21: 214–219. [PubMed] [Google Scholar]
- TelfordG, Wheeler D, Williams P, Tomkins PT, Appleby P, Sewell H, Stewart GSAB, Bycroft BW, Pritchard DI.1998. The Pseudomonas aeruginosa quorum‐sensing signal molecule N‐(3‐oxododecanoyl)‐1‐homoserine lactone has immunomodulatory activity. Infection and Immunity 66: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeplitskiM, Robinson JB, Bauer WD.2000. Plants secrete substances that mimic bacterial N‐acyl homoserine lactone signal activities and affect population density‐dependent behaviours in associated bacteria. Molecular Plant‐Microbe Interactions 13: 637–648. [DOI] [PubMed] [Google Scholar]
- WhiteheadNA, Barnard AML, Slater H, Simpson NJL, Salmond GPC. 2001. Quorum‐sensing in Gram‐negative bacteria. FEMS Microbiology Reviews 25: 365–404. [DOI] [PubMed] [Google Scholar]
- WijffelmanCA, Pees E, VanBrussel AAN, Hooykaas PJJ.1983. Repression of small bacteriocin excretion in Rhizobium‐Leguminosarum and Rhizobium‐Trifolii by transmissible plasmids. Molecular and General Genetics 192: 171–176. [Google Scholar]
- WinsonMK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GSAB.1998. Construction and analysis of luxCDABE‐based plasmid sensors for investigating N‐acyl homoserine lactone‐mediated quorum sensing. FEMS Microbiology Letters 163: 185–192. [DOI] [PubMed] [Google Scholar]
- WithersH, Swift S, Williams P.2001. Quorum sensing as an integral component of gene regulatory networks in Gram‐negative bacteria. Current Opinion in Microbiology 4: 186–193. [DOI] [PubMed] [Google Scholar]
- WoodDW, Pierson LS. 1996. The phzI gene of Pseudomonas aureofaciens 30–84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene 168: 49–53. [DOI] [PubMed] [Google Scholar]
- WoodDW, Gong FC, Daykin MM, Williams P, Pierson LS.1997. N‐acyl‐homoserine lactone‐mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30–84 in the wheat rhizosphere. Journal of Bacteriology 179: 7663–7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhangZG, Pierson LS. 2001. A second quorum‐sensing system regulates cell surface properties but not phenazine antibiotic production in Pseudomonas aureofaciens Applied and Environmental Microbiology 67: 4305–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




