Abstract
Plant root mucilage is known to enhance soil quality by contributing towards the soil carbon pool, soil aggregation, detoxification of heavy metal ions and interactions with rhizospheric microflora. Mucilage consists of many monosaccharide units, including fucose which can be used as an indicator for plant root based polysaccharides. This is the first report of an immunological technique developed to use anti‐fucose antibodies as markers for probing and localizing fucosyl residues in mucilage polysaccharide and, in turn, for localization of plant root mucilage. Fucose was complexed with bovine serum albumin to raise antibodies against fucose. A fucose‐directed antibody was shown to cross‐react with root cap mucilages from grasses. This antibody was used to localize root mucilage polysaccharide in maize and wheat root caps using immunogold electron microscopy. Abundant labelling could be localized on the cell wall, and in the intercellular matrix and vesicles of the peripheral root cap cells. Labelling was less intense in cells towards the centre of the root cap tissue. Control experiments confirmed that immunogold localization of fucose was specific and reliable.
Key words: Root mucilage, fucose, immunogold labelling, Zea mays, Triticum aestivum
INTRODUCTION
Plant roots release organic components into the rhizosphere. A distinction is usually made between high molecular weight compounds, termed mucilage or insoluble exudates, and smaller compounds termed soluble exudates (Traore et al., 2000). Plant root exudates are known to contribute to soil carbon by as much as 30 % of the above‐ground biomass (Buyanovski and Wagner, 1987). Root mucilages are also known to modify the rhizosphere (Osborn et al., 1999) and perform various functions at the soil–root interface including soil aggregation, providing a carbon source for microbial flora, facilitating movement of the root in the soil and ameliorating toxic effects of elements like Al3+ and Cd2+ (Rougier and Chaboud, 1985; McCully, 1999).
Plant root mucilage is a complex polysaccharide mainly composed of six sugar residues, namely glucose, galactose, mannose, fucose, xylose and arabinose, present in varying ratios in different taxa (Bacic et al., 1986). Of these six sugar residues, fucose has been used as a marker for root mucilage synthesis in root cap cells (Bowles and Northcote, 1972). In maize (Zea mays L.), fucose accounts for 20 % of root cap mucilage (Chaboud, 1983; Bacic et al., 1986), whereas in rice (Oryza sativa L.) fucose is less than 8 % (Chaboud and Rougier, 1984). According to Wright and Northcote (1974), fucose is only a minor component of wheat (Triticum aestivum L.) root mucilage.
Most studies of root mucilage production and transport have used radiolabelled monosaccharides (Harris and Northcote, 1970; Kirby and Roberts, 1971; Bowles and Northcote, 1972; Paull and Jones, 1975; Wright, 1975; Wright and Northcote, 1975; Rougier, 1976; Green and Northcote, 1979). Other researchers have used sugar‐specific lectins to localize monosaccharide components in whole roots (Rougier et al., 1979; Vermeer and McCully, 1981; Chaboud and Rougier, 1984). Roy and Vian (1991) employed a multistep detection procedure using fucose‐directed UeA lectin and subsequent localization with anti‐UeA antibody, and secondary antibody–gold particle complex to detect mucilage by electron microscopy. Other workers have used antibody probes to study plant cell walls (Willats et al., 2000). However, these cell wall‐directed antibodies are not able to discriminate between the wall and the mucilage polysaccharides that are transported across the cells in the root tip.
Using antibodies against specific saccharide residues can provide information on the location of that particular saccharide in the cell. Antibodies against various monosaccharides can be obtained by ligating sugars with bovine serum albumin (BSA) (Pazur, 1998). Here we report the subcellular localization of root mucilage polysaccharides using a BSA‐ligated fucose‐directed antibody, using an immunogold labelling technique.
MATERIALS AND METHODS
Preparation of anti‐fucose antiserum
Antibodies against fucose were raised in rabbits. BSA–fucose [ligation of BSA and α‐l‐fucose, prepared as described by Walzel et al. (1991)] was used as an antigen following the procedure described by Pazur et al. (1994). The immune serum obtained was used directly as a polyclonal antibody without further purification.
Enzyme‐linked immunosorption assay (ELISA)
ELISA was performed following the method of Haines and Patel (1997). Protein A‐alkaline phosphatase was used as secondary probe and plates were incubated at 37 °C for 1 h. Plates were then developed using p‐nitrophenyl phosphate (pNPP) as substrate. After incubation at room temperature, 100 µl 3 n NaOH stopping solution was added to each well and the plate was read at 405 nm on an ELISA reader (BioRad, model 550 microplate reader).
Immunoblot assay
The method of Willats and Knox (1999) was followed for immunoblot assays. Root mucilages from various grasses along with purified mucilage from Plantago ovata seed husk (isabgol), gum acacia, gum karraya and potato starch (2 µl equivalent to 10 µg polysaccharide), BSA and BSA–fucose, both 2 µg in 2 µl, were spotted on nitrocellulose membrane (0·45 µm; BIORAD Trans‐Blot® Transfer Medium). The membrane was blocked with phosphate‐buffered saline (PBS), pH 7·2, containing 5 % skimmed milk powder [MPBS, ‘Anikspray’, Nutricia (India) Pvt. Ltd, New Delhi, India] for 1 h followed by incubation in primary antibody at a dilution of 1 : 50 in PBST [PBS containing 0·1 % (v/v) Tween 20], for 1 h. The membrane was washed thoroughly with PBST and incubated in goat–anti‐rabbit alkaline phosphatase (Sigma, St Louis, MO, USA) at 1 : 10 000 dilution in PBST for 1 h. After thorough washing, staining was developed using NBT/BCIP (Nitro Blue Tetrazolium/5‐bromo‐4‐chloro‐3‐indolyl phosphate) (Sigma).
Plant material
Seeds of maize (Z. mays ‘Deccan 103’) and wheat (T. aestivum ‘Kundan’) were procured from the Indian Agricultural Research Institute, New Delhi. Seeds were first washed with 10 % detergent (Teepol® B‐300) for 30 min with constant shaking and then in running water for 30 min. They were surface sterilized with ethyl alcohol for 5 min, followed by five washes in sterile distilled water, and then treated with 4 % sodium hypochlorite for 5 min and washed five times with sterile distilled water for 5 min each. Seeds were germinated in a sterile moisture chamber (15 cm diameter Petri dishes lined with moist filter paper). Seedlings (48 h old) were placed in a sterile hydroponic growth chamber with their roots in sterile distilled water and incubated at 27 °C for 24 h with constant shaking at 100 r.p.m.
Tissue preparation for microscopy
After incubation for 24 h, root tips (0·5 cm from the tip) were aseptically excised with a scalpel and fixed in 0·5 m glutaraldehyde and 2·0 m paraformaldehyde in 0·1 m phosphate buffer (Na+ salt), pH 7·2 ± 0·2, for 12 h at 4 °C. The tissue was then further processed for making blocks: dehydrated in an ethanol series (30, 50, 70, 90 and 100 %) at 4 °C and infiltrated with LR white resin (London Resin Company Ltd, Berkshire, England) at the same temperature. The resin was then polymerized at 55 °C in an oven for 24 h. The blocks obtained were first sectioned for bright field light microscopy. Transverse sections (1 µm) from the root tip end were stained with 0·5 % toluidine blue O and observed. These 1 µm sections were also processed for immunolocalization of fucose epitopes by light microscopy. For electron microscopy, ultrathin sections (60–90 nm) were cut on UCT ultramicrotome (Leica Mikrosysteme Gmbh, Vienna, Austria) and collected on circular 400 mesh nickel grids.
Immunolabelling procedure for light microscopy
The procedure given by Kerr and Thorpe (1994) was followed with minor modifications. Slides with 1 µm sections were washed in PBS and blocked with 3 % skimmed milk for 30 min followed by hydrogen peroxide [20 ml H2O2 (30 %) in 80 ml methanol] for endogenous peroxidase reaction for 30 min. Slides were washed in PBS and incubated in anti‐fucose antibody (the rabbit antiserum was used in a 1 : 50 dilution in PBS) for 1·5 h at room temperature, washed thoroughly in PBST and incubated in swine anti‐rabbit antiserum (Dakopatts a/s, Glostrup, Denmark; used in 1 : 100 dilution in PBS) as a bridging antibody. Slides were then washed thoroughly in PBST and incubated in rabbit‐PAP (peroxidase‐anti‐peroxidase raised in rabbit; Sigma) for 1 h. Following another wash in PBST, slides were developed using DAB (3 3′ diaminobenzidine; Sigma) as substrate and observed with an Olympus CX 40 light microscope attached to an Olympus SC35 camera. Necessary control sections were maintained.
Immunolabelling procedure for electron microscopy
Grids were processed for immunolabelling as described by Dasgupta et al. (2000), with minor modifications. The sections were first blocked with 2 % skimmed milk in phosphate buffer (pH 7·2), followed by incubation in anti‐fucose antibody (the rabbit antiserum was used at a 1 : 50 dilution in phosphate buffer, pH 7·2, with 1 % fish gelatine) for 18 h at 4 °C, then washed thoroughly with the washing buffer (1 % fish gelatine in phosphate buffer) and incubated in goat‐anti‐rabbit–gold conjugate (British Biocell Institute, Cardiff, UK) at 1 : 50 dilution (particle size 15 nm) for 2 h. The grids were washed thoroughly and stained with 3 % aqueous uranylacetate for 1 h, washed and dried. Grids were viewed at 60 kV acceleration voltage under a Phillips CM‐10 transmission electron microscope (TEM). Images were recorded on a 35 mm Phillips CM‐10 camera. To test the specificity of immunogold localization, two controls were used. One was stained with pre‐immune rabbit serum instead of anti‐fucose antibodies and the other was stained only with goat–anti‐rabbit–gold conjugate. Transmission electron micrographs were evaluated for the number of gold particles per µm2 in the photograph. Means (± s. e.) were calculated and compared.
RESULTS AND DISCUSSION
Specificity of the anti‐fucose antibodies for root mucilage
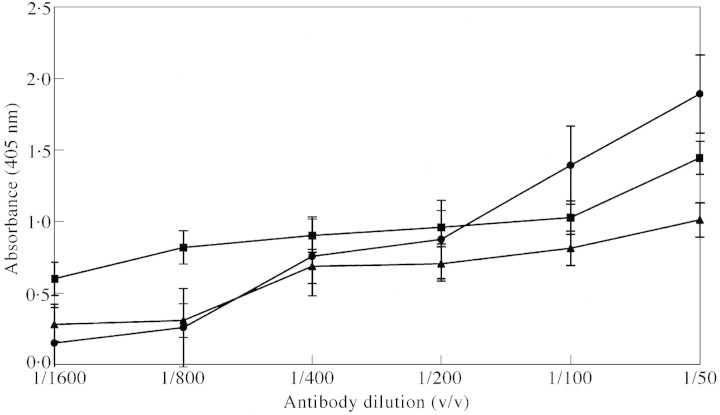
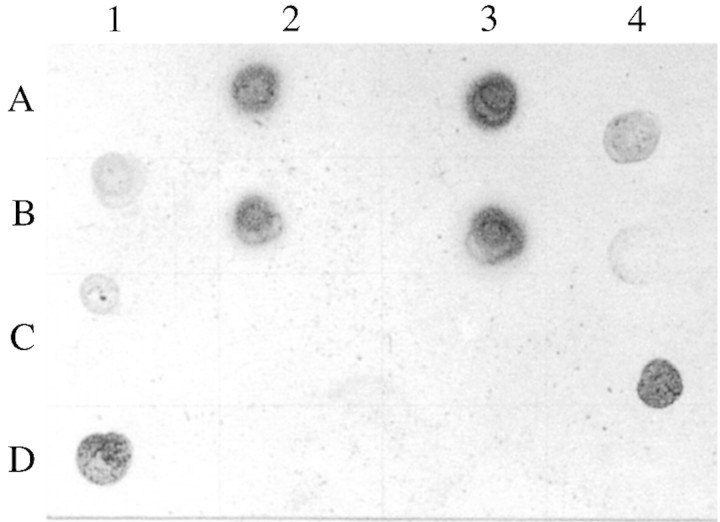
Two types of antibodies can be expected in the antiserum: anti‐fucose and anti‐BSA (Pazur et al., 1994; Pazur, 1998). We used the total antiserum against plant material and hence the anti‐BSA antiserum was not separated from anti‐fucose antiserum. ELISA (Fig. 1) gave the titre value of the anti‐fucose antiserum against maize root mucilage to be 1 : 50. Immunoblot assay (Fig. 2) showed that the anti‐fucose antiserum cross‐reacted with root mucilages from all the grasses tested. It also confirmed that anti‐fucose antiserum is specific for root mucilage and does not show any non‐specific reaction with other related polysaccharides of plant origin.
Fig. 1. ELISA (enzyme‐linked immunosorbent assay) for fucose in sequential dilution of anti‐fucose antibody. BSA–fucose (circles), BSA (triangles) and maize (squares) root mucilage. Concentration of antigens is 100 µg ml–1.
Fig. 2. Immunoblot assay of root mucilages from various grasses and some plant‐derived polysaccharides, using antiserum raised against BSA‐fucose. Blots were developed using NBT/BCIP. A1, Control blank; A2, Zea mays; A3, Triticum aestivum; A4, Hordeum vulgare; B1, Echinochloa crusgalli; B2, Oryza sativa; B3, Sorghum halepense; B4, Eleusine coracana; C1, Panicum antidotale; C2, starch; C3, gum acacia; C4, BSA‐Fucose; D1, BSA; D2, gum karraya; D3, isabgol; D4, control blank.
The layer of exuded mucilage with root border cells was clearly visible by light microscopy. Immunolocalization of the fucose epitopes by light microscopy showed that these are concentrated in the outer peripheral cells, around the sloughed root border cells, and in the exuded mucilage (Fig. 3).
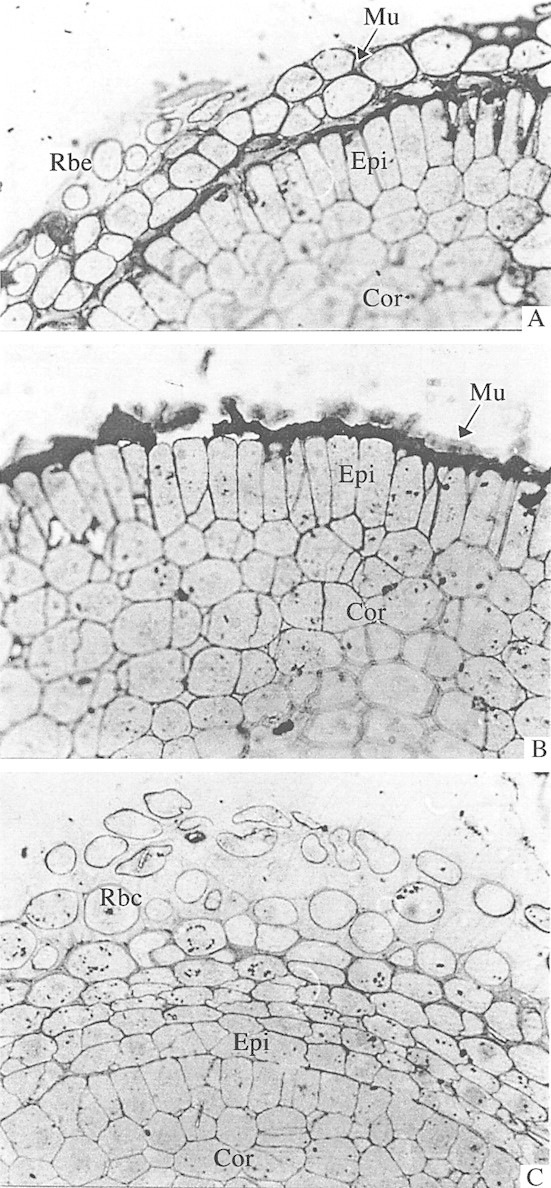
Fig. 3. Light micrographs of root tip sections of maize and wheat seedlings labelled with antiserum raised against BSA‐fucose and detected with rabbit‐PAP (peroxidase‐antiperoxidase raised in rabbit) and developed using DAB (3 3′ diaminobenzidine). A and B, Detection of fucose in exuded root mucilage and outer cell walls of the epidermis in maize (A) and wheat (B) root tips. ×400. C, Control section of maize root tip in which anti‐BSA‐fucose antiserum was replaced with pre‐immune rabbit serum. ×400. Rbc, Root border cell; Mu, exuded root mucilage; Epi, epidermis; Cor, cortex.
Immunogold localization of fucose in root tip sections
Although both the outer layer of exuded mucilage and root border cells were washed away during immunolabelling, a distinct layer on the peripheries of all sections showed heavy labelling (Fig. 4A) because remnants of the mucilage layer were stuck to the epidermal cells. The labelling was localized in the cytoplasm, cell walls and intercellular matrices of the peripheral cells (Figs 4B and 5A) in both maize and wheat. The labelling was also localized in the rounded vesicles near the cell wall (Figs 4C, D and 5B). These rounded vesicle‐like structures may be dictyosome‐derived vesicles (although no stacked dictyosomes were seen), which are known to transport mucilage polysaccharides across the cells up to the root periphery (Northcote and Pickett‐Heaps, 1966). The cells towards the interior of the section did not show intense labelling in their cell walls or intercellular spaces compared with the peripheral cells (Figs 4E and 5C). This indicates that mucilage may be synthesized in the interior cells and then transported to peripheral cells across the cell walls. The specificity of the fucose‐directed antibody towards mucilage and not towards wall polysaccharide is also highlighted here. Kirby and Roberts (1971) using [3H]fucose, and Vermeer and McCully (1981) using fucose‐directed fluorescein isothiocyanate (FITC) Lotus purpureas lectin, were able to localize fucose residues in the outer and tangential walls of peripheral cells. Roy and Vian (1991) used UeA I lectin to trace the exocytic pathway of mucilage from Golgi apparatus to the exterior of the cell. Their results indicated that at least one population of Golgi‐derived vesicles is directly involved in transport of mucilage to the exterior of the cell and accumulation of mucilage in pockets between the plasma membrane and the outer tangential cell wall. Their double labelling method using the cellulose‐directed antibody, CBH I, along with the fucose‐directed UeA I lectin, showed the presence of these fucose‐rich polysaccharides within the cell wall. These fucose‐rich polysaccharides, which are also localized in the present study using anti‐fucose antibodies, cannot be confused with other cell wall polysaccharides like xyloglucans, which are known to have terminal fucose residues, as the cell walls of Poaceae are known to lack xyloglucans with terminal fucose residues (Smith and Harris, 1999).
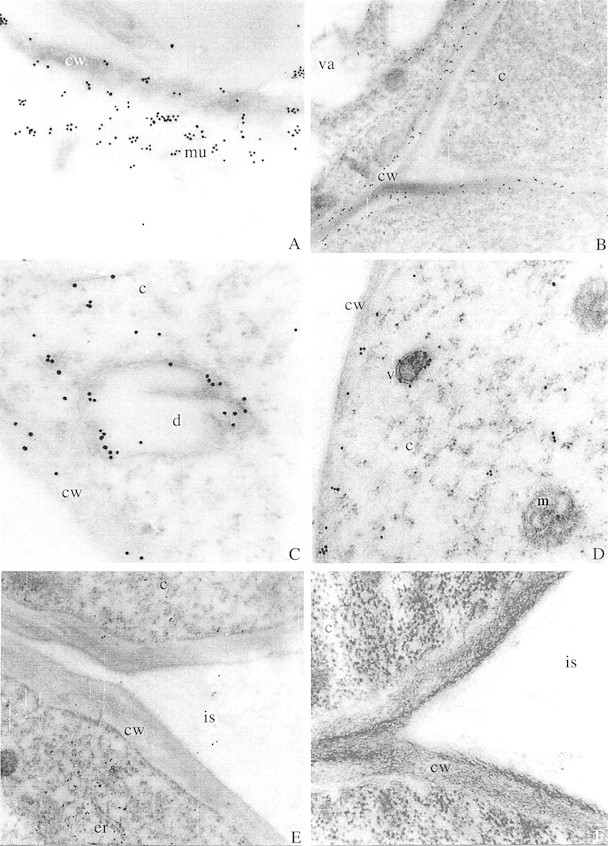
Fig. 4. Root tip sections of maize seedlings labelled with antiserum raised against BSA‐fucose and detected with GAR‐antibody labelled with 15 nm gold particles. A, Outermost layer of the root tip section of maize showing heavy labelling in the exuded mucilage. ×31 000. B, Junction of three cells in root tip section of maize showing heavy labelling in cell walls. ×16 800. C, Dictyosome‐derived vesicle like structure near the cell wall in root tip section of maize. Note heavy labelling associated with the membrane towards the sides. ×43 000. D, A vesicle near the cell wall of maize root tip section showing heavy labelling. ×31 000. E, Cells towards the interior of the transverse section of maize root tip showing little labelling. ×16 000. F, Control section of maize root in which anti‐BSA‐fucose antiserum was replaced with the pre‐immune rabbit serum. ×31 000. cw, Cell wall; c, cytoplasm; d, dictyosome‐like structure; er, endoplasmic reticulum; is, intercellular space; m, mitochondria; mu, mucilage; v, vesicle; va, vacuole.
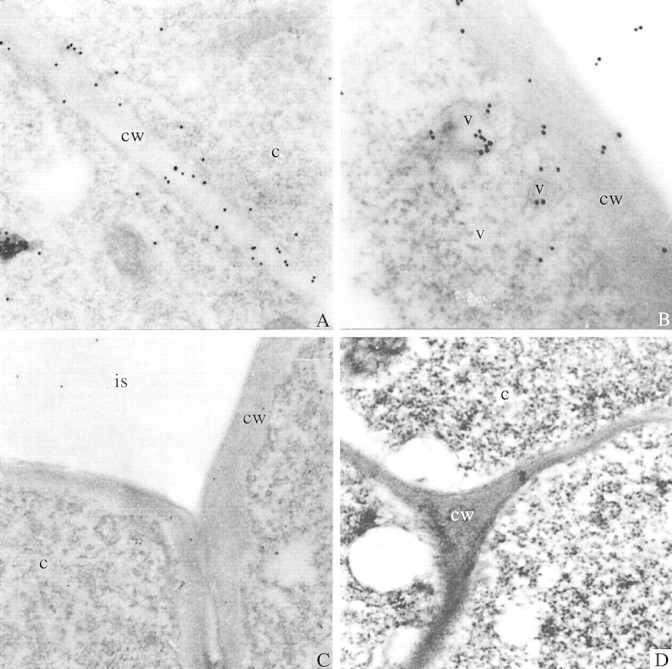
Fig. 5. Root tip sections of wheat seedlings labelled with antiserum raised against BSA‐fucose and detected with GAR‐antibody labelled with 15 nm gold particles. A, Detection of fucose in the cell wall of wheat root tip. ×31 000. B, Two vesicles near the cell wall of wheat root tip showing very heavy labelling. ×43 000. C, Cells towards the interior of the transverse section of wheat root tip showing very little labelling. ×16 800. D, Control section of wheat root in which anti‐BSA‐fucose antiserum was replaced with pre‐immune rabbit serum. ×31 000. cw, Cell wall; c, cytoplasm; is, intercellular space; d, dictyosome‐like structure; v, vesicle.
The control sections treated with pre‐immune rabbit serum and stained with the second antibody alone did not show gold labelling (Figs 4 F, 5D). No labelling was noted in the vacuoles, mitochondria or nucleus of the cells. This clearly illustrates that immunogold localization of fucose in the mucilage polysaccharide using anti‐fucose antiserum is specific and reliable.
Another interesting observation from the present study is that both maize and wheat showed a similar number of gold particles (32·6 ± 5·95 and 36·7 ± 7·33, respectively) per unit area (1 µm2) in the walls of peripheral cells, although different amounts of fucose have been reported in root mucilage of maize and wheat (Wright and Northcote, 1974; Chaboud, 1983; Bacic et al., 1986). An earlier study by Chaboud and Rougier (1984) showed that, although rice root cap mucilage contains very low amounts of fucose (5 %) compared with glucose (38 %), labelling with UeA lectin (specific for α‐l‐fucosyl residues) was stronger than with con A (specific for α‐d‐glucosyl/α‐d‐mannosyl residues). This may be because fucosyl residues are terminal and are accessible while glucosyl residues are inaccessible to lectin binding or are present in β conformations. Wright et al. (1976) showed that only 17 % of fucose residues could be removed from maize mucilage by α‐l‐fucosidase. Thus, it appears that, although maize mucilage has a higher fucose content, only a fraction is available to the fucose‐specific antibodies. Given the similar number of fucosyl residues in wheat and maize obtained in the present study, it is also evident that, although plants differ in their total fucose content the quantity of terminal fucose residues does not change drastically. Fucosyl residues have been implicated in attachment of Phytophthora zoospores to roots of Z. mays (Hinch and Clarke, 1980). Terminal fucosyl residues may have an important function in plant defence, and metabolic processes appear to ensure their availability in a functional form in similar quantities, even in distant taxa.
The technique developed in the present study has a distinct advantage over radiolabelling in terms of direct localization by microscopy. It also avoids the problem of spurious autofluorescence of plant cell walls, which occurs with fluorescein‐labelled lectin‐tagged localization. Roy and Vian (1991) used UeA I lectin to localize fucose residues and subsequently localized these epitopes with a UeA I‐directed antibody by electron microscopy using gold labelling. The present method gives a two‐step serological procedure for detection of the presence of the plant root‐derived mucilage component. Using the present technique, mucilage production and the pathway can be localized on whole root sections of plants grown under natural conditions. Moreover, sub‐cellular changes in mucilage production on plant roots subjected to biotic and abiotic stresses can also be investigated.
ACKNOWLEDGEMENTS
We thank the Ministry of Environment and Forests, Government of India, for financial assistance during this work. Ultrastructural studies were carried out at the Sophisticated Instrumentation Facility for Electron Microscopy (supported by the Department of Science and Technology, Government of India) at the All India Institute of Medical Sciences, New Delhi.
Supplementary Material
Received: 30 October 2001; Accepted: 25 November 2001.
References
- BacicA, Moody SF, Clarke AE.1986. Structural analysis of secreted root slime from maize (Zea mays L.). Plant Physiology 80: 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BowlesDJ, Northcote DH.1972. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochemical Journal 130: 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BowlesDJ, Northcote DH.1976. The size and distribution of polysaccharides during their synthesis within the membrane system of maize root cells. Planta 128: 101–106. [DOI] [PubMed] [Google Scholar]
- BuyanovskiGA, Wagner GH.1987. Carbon transfer in a winter wheat (Triticum aestivum) ecosystem. Biology and Fertility of Soils 5:76–82. [Google Scholar]
- ChaboudA.1983. Isolation, purification and chemical composition of maize root cap slime. Plant and Soil 73: 395–402. [Google Scholar]
- ChaboudA, Rougier M.1984. Identification and localization of sugar components of rice (Oryza sativa L.) root cap mucilage. Journal of Plant Physiology 116: 323–330. [DOI] [PubMed] [Google Scholar]
- DasguptaN, Kapur V, Singh KK, Das TK, Sachdeva S, Jyothisri K, Tyagi JS.2000. Characterisation of two‐component system, devR‐devS, of Mycobacterium tuberculosis Tubercle and Lung Disease 80: 141–158. [DOI] [PubMed] [Google Scholar]
- GreenJR, Northcote DH.1979. Location of fucosyl transferases in the membrane system of maize root cells. Journal of Cell Science 40: 235–244. [DOI] [PubMed] [Google Scholar]
- HainesJ, Patel PD.1997. Antibody‐ and lectin‐based assays for the rapid analysis of food‐grade gums and thickeners. Trends in Food Science and Technology 81: 395–400. [Google Scholar]
- HarrisPJ, Northcote DH.1970. Patterns of polysaccharide biosynthesis in differentiating cells of maize root tips. Biochemical Journal 120: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HinchJM, Clarke AE.1980. Adhesion of fungal zoospores to root surfaces is mediated by carbohydrate determinants of the root slime. Physiological Plant Pathology 16: 303–307. [Google Scholar]
- KerrMA, Thorpe R.1994. Immunochemistry: LabFax. Oxford: BIOS Scientific Publishers Limited. [Google Scholar]
- KirbyEG, Roberts RM.1971. The localized incorporation of 3H fucose into the cell‐wall polysaccharides of the cap and epidermis of corn roots. Autoradiographic and biosynthetic studies. Planta 99: 211–221. [DOI] [PubMed] [Google Scholar]
- McCullyM.1999. Roots in soil: Unearthing the complexities of roots and their rhizospheres. Annual Review of Plant Physiology and Plant Molecular Biology 50: 695–718. [DOI] [PubMed] [Google Scholar]
- NorthcoteDH, Pickett‐Heaps JD.1966. A function of the Golgi apparatus in polysaccharide synthesis and transport in root‐cap cells of wheat. Biochemical Journal 98: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OsbornHMI, Lochey F, Mosley L, Read D.1999. Analysis of polysaccharides and monosaccharides in the root mucilage of maize (Zea maize L.) by gas chromatography. Journal of Chromatography A 831: 267–276. [Google Scholar]
- PaullRE, Jones RL.1975. Studies on the secretion of maize root cap slime. III. Histochemical and autoradiographic localization of incorporated fucose. Planta 127: 97–110. [DOI] [PubMed] [Google Scholar]
- PazurJH.1998. Anti‐carbohydrate antibodies with specificity for monosaccharide and oligosaccharide units of antigens. Advances in Carbohydrate Chemistry and Biochemistry 51: 201–264. [DOI] [PubMed] [Google Scholar]
- PazurJH, Liu B, Witham TF.1994. Isolation and characterization of sets of anti‐l‐fucose and anti‐bovine serum albumin antibodies directed against a glycoconjugate of l‐fucose and bovine serum albumin. Journal of Protein Chemistry 13: 59–66. [DOI] [PubMed] [Google Scholar]
- RougierM.1976. Secretion de polysaccharides dans l’apex radiculaire de mais: etude radioautographique par incorporation de fucose trite. Journal of Microscopy 26: 161–166. [Google Scholar]
- RougierM, Chaboud A.1985. Mucilages secreted by roots and their biological function. Israel Journal of Botany 34: 129–146. [Google Scholar]
- RougierM, Kieda C, Monsigny M.1979. Use of lectin to detect the sugar components of maize root cap slime. The Journal of Histochemistry and Cytochemistry 27: 878–881. [DOI] [PubMed] [Google Scholar]
- RoyS, Vian B.1991. Transmural exocytosis in maize root cap: Visualization by simultaneous use of a cellulose‐probe and a fucose‐probe. Protoplasma 161: 181–191. [Google Scholar]
- SmithBG, Harris PJ.1999. The polysaccharide composition of poales cell walls: Poaceae cell walls are not unique. Biochemical Systematics and Ecology 27: 33–53. [Google Scholar]
- TraoreO, Groleau‐Renaud V, Plantureux S, Tubeileh A, Boeuf‐Tremblay V.2000. Effect of root mucilage and modelled root exudates on soil structure. European Journal of Soil Science 51: 575–581. [Google Scholar]
- VermeerJ, McCully ME.1981. Fucose in the surface deposits of axenic and field‐grown roots of Zea mays L. Protoplasma 109: 233–248. [Google Scholar]
- WalzelH, Bremer H, Neels P, Jonas L, Brock J.1991. Preparation of neoglycoprotein using a homobifunctional reagent and its applicability for protein blotting and electron microscopy. Biomedica et Biochimica Acta 50: 151–157. [PubMed] [Google Scholar]
- WillatsWGT, Knox JP.1999. Immunoprofiling of pectic polysaccharides. Analytical Biochemistry 268: 143–146. [DOI] [PubMed] [Google Scholar]
- WillatsWGT, Steele‐King CG, McCartney L, Orfila C, Marcus SE, Knox JP.2000. Making and using antibody probes to study plant cell walls. Plant Physiology and Biochemistry 38: 27–36. [Google Scholar]
- WrightK.1975. Polysaccharides of root‐cap slime from five maize varieties. Phytochemistry 14: 759–763. [Google Scholar]
- WrightK, Northcote DH.1974. The relationship of root‐cap slimes to pectins. Biochemical Journal 139: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WrightK, Northcote DH.1975. An acidic oligosaccharide from maize slime. Phytochemistry 14: 1793–1798. [Google Scholar]
- WrightK, Northcote DH, Davey RM.1976. Preparation of rat epididymal α‐l‐fucosidase free from other glycosidases: its action on root‐cap slime from Zea mays L. Carbohydrate Research 47: 141–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.