Abstract
The size (length and diameter) and number of leaf primordia of winter buds of Nothofagus antarctica (G. Forster) Oerst. shrubs were compared with the size and number of leaves of shoots derived from buds in equivalent positions. Buds developed in two successive years were compared in terms of size and number of leaf primordia. Bud size and the number of leaf primordia per bud were greater for distal than for proximally positioned buds. Shoots that developed in the five positions closest to the distal end of their parent shoots had significantly more leaves than more proximally positioned shoots of the same parent shoots. The positive relationship between the size of a shoot and that of its parent shoot was stronger for proximal than for distal positions on the parent shoots. For each bud position on the parent shoots there were differences in the number of leaf primordia per bud between consecutive years. The correlations between the number of leaf primordia per bud and bud size, bud position and parent shoot size varied between years. Only shoots produced close to the distal end of a parent shoot developed neoformed leaves; more proximal sibling shoots consisted entirely of preformed leaves. Leaf neoformation, a process usually linked with high shoot vigour in woody plants, seems to be widespread among the relatively small shoots developed in N. antarctica shrubs, which may relate to the species’ opportunistic response to disturbance.
Key words: Bud structure, preformation, shoot growth, neoformation, branching, leaf primordia, Nothofagusantarctica, ñire
INTRODUCTION
The expression of a vascular plant’s architecture results from the primary growth and branching of each of its axes (Hallé et al., 1978; Caraglio and Barthélémy, 1997). These two processes depend upon the activity of meristems located at the distal end of each axis and/or at leaf axils, and involve the differentiation of organ primordia from meristems, i.e. organogenesis, and the extension of these primordia into fully developed organs (Champagnat et al., 1986). In many temperate plants, organ primordia remain dormant in buds and develop into mature organs only after a period of time. Organs developed in this way are referred to as preformed organs. Organogenesis and organ extension may also take place sequentially, without an intervening dormancy period, and the resulting organs are termed neoformed.
Although the number of studies describing plant architecture has increased in the last decades (Hallé et al., 1978; Barthélémy, 1986; Barthélémy et al., 1989, 1999; Costes, 1993; Caraglio, 1996), little is known about the role played by preformation and neoformation in the growth of trees (but see Remphrey and Powell, 1984; Davidson and Remphrey, 1994; Puntieri et al., 2000; Sabatier and Barthélémy, 2000; Souza et al., 2000). For instance, although differences in the length and number of leaves among shoots derived simultaneously from a common parent shoot have been noted (Nicolini, 1998; Puntieri et al., 1998; Sabatier et al., 1998; Barthélémy et al., 1999; Stecconi et al., 2000), it is virtually unknown whether such differences can arise from variations among these shoots in preformation and/or neoformation capabilities. Moreover, information concerning both the co‐occurrence of preformation and neoformation within the same shoot system, and between‐year differences in bud composition for the same species, is virtually non‐existent (but see Remphrey and Davidson, 1994).
The information available indicates that a large proportion of all shoots developed by trees from temperate and temperate‐cold regions consists of organs preformed in the growing season previous to that of shoot extension (e.g. Remphrey and Davidson, 1994; Puntieri et al., 2000; Sabatier and Barthélémy, 2000; Souza et al., 2000). A minor proportion of the shoots of these species develops neoformed organs following the extension of preformed organs, which may enable plants to profit from favourable conditions late in the growing season (Davidson and Remphrey, 1994; Puntieri et al., 2000; Souza et al., 2000). Some studies on trees from different families have pointed at a connection between shoot position and the numbers of preformed and neoformed organs it includes (Macdonald and Mothersill, 1983; Macdonald et al., 1984; Remphrey and Powell, 1984; Davidson and Remphrey, 1994; Thorp et al., 1994; Puntieri et al., 2000; Sabatier and Barthélémy, 2000; Souza et al., 2000). One of the conclusions arising from these studies is that trunk and main branches of young trees are the most likely locations for shoots with preformed and neoformed organs. The absence of this type of shoot in a young tree of these species indicates low tree vigour. This information feeds plant‐simulation models currently applied in forestry, agriculture and ecology (Reffye et al., 1989, 1991, 1995; Guédon and Costes, 1997; Reffye and Houllier, 1997). However, only a few species have been studied from this perspective.
Nothofagus antarctica (G. Forster) Oerst. is a woody species whose range extends from 37°S, in the northern Patagonian Andes, to approx. 56°S, in Tierra del Fuego, Argentina (Dimitri, 1972; Cabrera, 1976; Correa, 1984). It forms dense and extensive populations in valley bottoms as well as in wetland margins in both Chile and Argentina. This species can grow as a tree up to 15 m tall, but has a strong tendency to develop as a 1–3‐m‐tall shrub, with evenly sized axes diverging from the soil surface (Ramírez et al., 1985). Despite little reproduction by seed (Premoli, 1991), N. antarctica plays an important role in the post‐fire restoration of plant communities because of its ability to regenerate vegetatively from burned stumps by coppice and root suckers (McQueen, 1976; Donoso, 1993; Veblen et al., 1996). As in other Nothofagus species (Puntieri et al., 1998; Barthélémy et al., 1999), axis extension in N. antarctica is a rhythmic process (Stecconi et al., 2000): periods of axis extension (in spring and summer) alternate with periods of constant axis length (in autumn and winter). Most shoot apices die after shoot extension and further increases in the length of an axis take place through the development, in the following growing season, of a relay shoot from one of the most distal axillary buds. One year after its extension period, each shoot develops axillary branches (including the relay shoot) whose vigour increases acropetally (Stecconi et al., 2000).
In the present study we analysed shoots of N. antarctica shrubs for: (1) the extent of preformation and neoformation; (2) the number of preformed leaves of buds developed in two consecutive years; (3) the size (length and diameter) and number of leaves of buds and shoots according to their position on the parent shoot; (4) the value of a set of variables as predictors of the number of leaf primordia of buds; and (5) the relationship between the size (length and number of leaves) of a shoot and the size of its parent shoot.
MATERIALS AND METHODS
Study site and plants
A Nothofagus antarctica population was delimited within 2000 m2 of the Centro de Salmonicultura, Universidad Nacional del Comahue, San Carlos de Bariloche, Argentina (41°10′S, 71°25′W, 880 m altitude). The soil in this area is derived from volcanic ash (Andisol) and is characterized by a high content of superficial organic matter and has a slightly acidic pH (Scoppa, 1998). Mean temperature ranges between 2 and 4 °C and between 14 and 16 °C for the coldest and warmest months, respectively. Precipitation reaches 500–600 mm between May and August, and 50–100 mm between November and February (Conti, 1998).
Woody plant cover in the study site consists mostly of N. antarctica shrubs (up to 2·5 m in height), although other native and exotic woody species grow sparsely. A fire destroyed all above‐ground plant structures in this area in 1978, after which N. antarctica shrubs re‐sprouted. This population was selected for the present study due to the homogeneity of its shrubs. The structure of these shrubs resembles that observed in other fire‐affected populations of this species.
Data collection
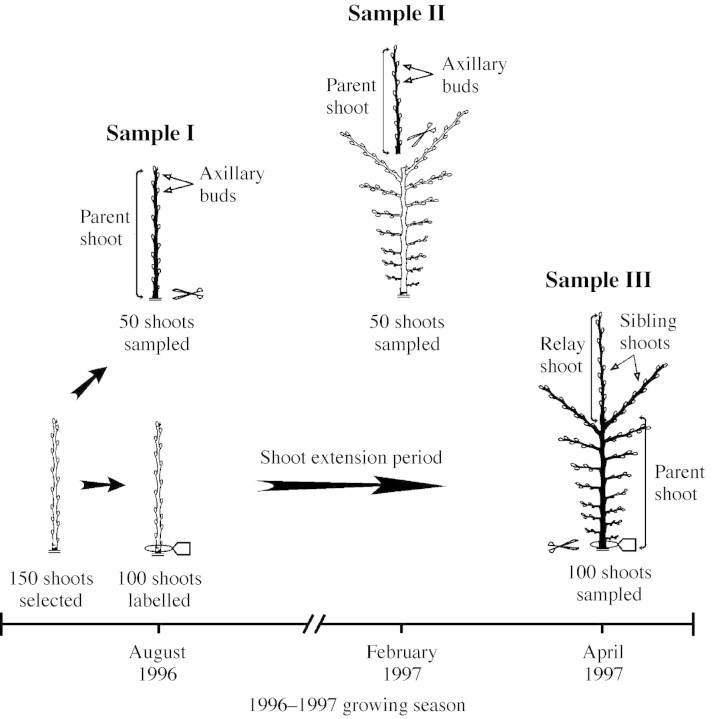
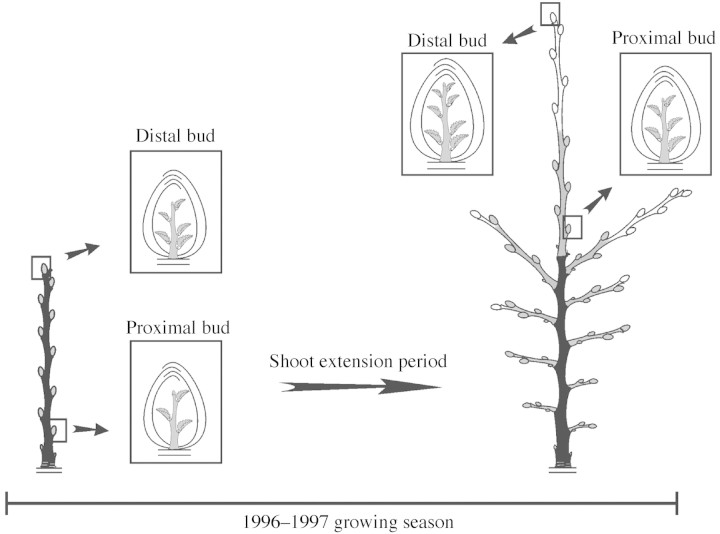
Nothofagus antarctica sprouts between 8 and 22 years old (mean age = 13·8 years, s.e. = 3·5, sample size = 96), derived from burned stumps, were selected. Three samples of shoots located at the distal end of these sprouts were taken. In August 1996 (winter), during the dormancy period of N. antarctica, 150 shoots that had extended in the 1995–1996 growing season were selected. Each of these shoots consisted of an unbranched stem with an axillary bud on most of its nodes. Fifty of these shoots were cut immediately, so as to record the organs preformed in their buds (Fig. 1). The remaining 100 shoots were labelled and the length and diameter of each of their buds measured non‐destructively to the nearest 0·1 mm with digital callipers. The latter shoots were cut after the end of the 1996–1997 growing season (April 1997), when those axillary buds measured during the winter had developed into shoots [Fig. 1; see Stecconi et al. (2000) for a study on the growth dynamics of these shoots]. These two samples were performed with the aim of assessing the proportion of preformed and neoformed organs of the shoots that developed in both samples. An independent set of 50 shoots extended in the 1996–1997 growing season was sampled in February 1997 (Fig. 1), about 1 month after the end of the extension of these shoots (Stecconi et al., 2000). By the time they were sampled, these shoots were bearing axillary buds. The objective of this sample was to compare the number of preformed organs and the size of buds developed in two consecutive years. Henceforth, samples taken in August 1996, February 1997 and April 1997 will be referred to as samples I, II and III, respectively. Bud‐bearing shoots of samples I and II, and shoot‐bearing shoots of sample III, will be termed parent shoots (Fig. 1), whereas axillary shoots derived from sample III parent shoots will be termed sibling shoots (Fig. 1). For each sample III parent shoot, the sibling shoot developed from one of its two most distal nodes following the line of growth of the parent shoot was termed the relay shoot.
Fig. 1. Diagrammatic representation of bud‐bearing and shoot‐bearing parent shoots of sample I (August 1996), sample II (February 1997) and sample III (April 1997). Shoots in black represent the sampled shoots.
For each parent shoot of each sample and for each sibling shoot of sample III, shoot length (to the nearest mm), number of leaves and apex condition (dead or persistent) were recorded. The number of leaves per shoot was determined by counting either leaves on the stem or scars left on the stem by leaves that had abscised. Previous studies on this and other species of Nothofagus show that the number of cataphylls per shoot is relatively constant (most frequently four) and that their position in a shoot is always limited to the proximal end of each shoot (Puntieri et al., 1998; Barthélémy et al., 1999; Stecconi et al., 2000). For these reasons, cataphylls were not counted in the present study. Hereafter, the word ‘leaf’ will be used in reference to a foliage (i.e. green) leaf. A shoot apex was considered dead if the terminal bud was absent or if it was dark‐brown in colour, dehydrated or damaged. Shoots severely damaged by exogenous factors, such as herbivory, were excluded from the analyses. The nodes of each parent shoot were rank‐numbered, with 1 being located at the distal end of the parent shoot.
Parent shoots of samples I and II were preserved in 96 % ethanol for at least 2 weeks. After that period, the length and diameter of each bud of each parent shoot were measured to the nearest 0·1 mm with callipers. Bud length was measured from the point of insertion of the basal cataphylls of the bud to the distal end of the bud (Fig. 2A). Bud diameter was measured at the point of maximum bud width (Fig. 2A). The number of leaf primordia of each bud was determined by manual dissection under a stereomicroscope (Olympus SZ30; Fig. 2B). As in the case of shoots, cataphylls, which constitute the external cover of buds, were not included in this count. One or two buds sometimes occur close to the proximal end of some shoots, but due to their small size (<0·5 mm long) it was not possible to dissect them. Leaf primordia were visible as pegs on either side of the preformed shoot (at 60×). The length of the preformed shoot primordium of each bud was measured from the insertion of its most proximal cataphylls up to the shoot’s distal end (Fig. 2B) by fitting a graduated ocular to the stereomicroscope.
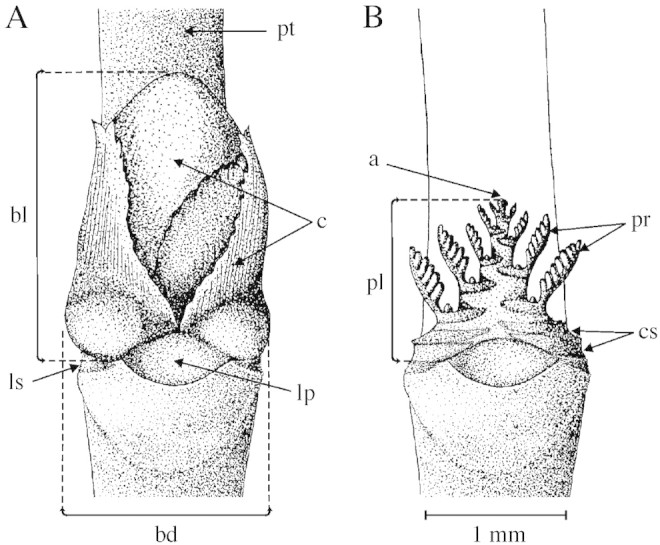
Fig. 2. Illustrations of a closed bud (A) and a dissected bud (B) of Nothofagus antarctica. a, Preformed‐shoot apex; bd, bud diameter; bl, bud length; c, cataphylls; cs, cataphyll scars; lp, subtending‐leaf petiole scar; ls, subtending‐leaf stipule scar; pl, preformed‐shoot length; pr, leaf primordium; pt, parent shoot.
Data analysis
The length and number of leaves of parent shoots were compared among samples with one‐way ANOVA followed by paired comparisons with the Tukey–Kramer method (Sokal and Rohlf, 1981).
Data concerning buds of samples I and II (i.e. bud length, bud diameter, preformed shoot length and number of leaf primordia) and sibling shoots of sample III (i.e. shoot length, basal diameter and number of leaves) were averaged, within each sample, for each node rank number on the parent shoot. These averages were obtained only for those positions represented in at least ten parent shoots of each sample (positions 1–12). Changes in the descriptive variables of buds, preformed shoots and sibling shoots, according to position on the parent shoot, were described by functions fitted by least‐squares regressions. In each case, linear, quadratic and cubic functions on raw data and a linear function on log–log transformed data were fitted. One of these functions was selected for each variable after examination of the residuals and the significance of each equation term (Sokal and Rohlf, 1981).
The number of leaf primordia of sample I buds was compared with the number of leaves of sample III sibling shoots using a one‐way ANOVA for each position (1–12) on the parent shoots (significance levels were adjusted for the number of comparisons performed using Bonferroni’s procedure).
Stepwise regression analyses (forward selection procedure; Sokal and Rohlf, 1981) were performed to find the best set of predictor variables for the number of leaf primordia (dependent variable) of sample I and sample II buds. Bud length, diameter and position on the parent shoot, and the length and number of leaves of the parent shoot were the independent variables in these analyses. The same analysis was used to find the best set of predictor variables for the length and number of leaves of the relay shoot of sample III parent shoots. In this case, the independent variables included in each analysis were: length and diameter of buds that originated the relay shoot, and length and number of leaves of the parent shoot. For each position on the parent shoots of sample III, the coefficient of determination (r2) relating the length of the parent shoot to the length of the sibling shoot in that position was computed.
RESULTS
Parent shoots
On average, parent shoots were longer in sample II than in samples I and III, and were similar in length for the latter two samples (F = 24·8, P < 0·001, Table 1). The mean number of leaves of parent shoots did not differ significantly between samples (F = 0·6, P = 0·56). All parent shoots had a dead apex after their extension.
Table 1.
Mean (± s.e.; sample size in parentheses) length and number of leaves of parent shoots of samples I (August 1996), II (February 1997) and III (April 1997)
| Sample | Length (mm) | Leaves |
| I | 67·8 ± 2·19a (50) | 12·3 ± 0·31a (50) |
| II | 88·6 ± 3·29b (50) | 12·6 ± 0·26a (50) |
| III | 68·8 ± 1·54a (95) | 12·7 ± 0·21a (95) |
Means followed by the same superscript letter are not statistically different (P > 0·05).
Length and diameter of buds and sibling shoots
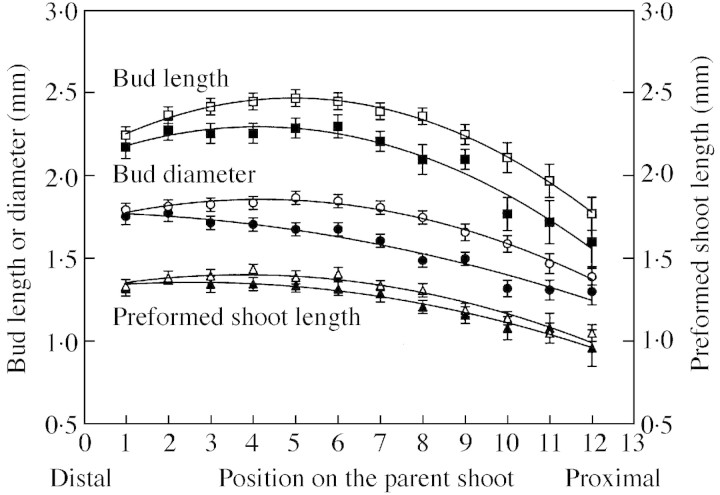
Quadratic functions were selected to describe the changes along the parent shoots of the length and diameter of buds and the length of shoots preformed in buds of samples I and II (Fig. 3; Table 2). These functions indicate slight increases or invariability in the measures describing bud and preformed shoot size from the distal end to intermediate positions of the parent shoot, and indicate decreases towards the proximal end of the parent shoot (Fig. 3; Table 2). For most positions, the size of buds and preformed shoots tended to be higher for sample II than for sample I parent shoots (Fig. 3).
Fig. 3. Length (squares) and diameter (circles) of buds and length of preformed shoots (triangles) corresponding to samples I (solid symbols) and II (open symbols), according to bud position ranked from the parent shoot’s distal end. Data are means ± s.e. The lines fitted to each data set are described in Table 2.
Table 2.
Functions describing variations with position on the parent shoot of bud length (mm), bud diameter (mm), preformed shoot length (mm) and number of leaf primordia for samples I and II; and variations in the length, diameter and number of leaves of sibling shoots of sample III
| Intercept | Linear | Quadratic | r 2 | n | |
| Buds of samples I and II | |||||
| Bud length | |||||
| Sample I | 2·092 | 0·101 | –0·012 | 0·14 | 536 |
| Sample II | 2·140 | 0·134 | –0·014 | 0·17 | 536 |
| Bud diameter | |||||
| Sample I | 1·777 | –0·001 | –0·004 | 0·20 | 536 |
| Sample II | 1·723 | 0·066 | –0·008 | 0·18 | 536 |
| Preformed shoot length | |||||
| Sample I | 1·333 | 0·024 | –0·005 | 0·10 | 511 |
| Sample II | 1·308 | 0·052 | –0·007 | 0·18 | 516 |
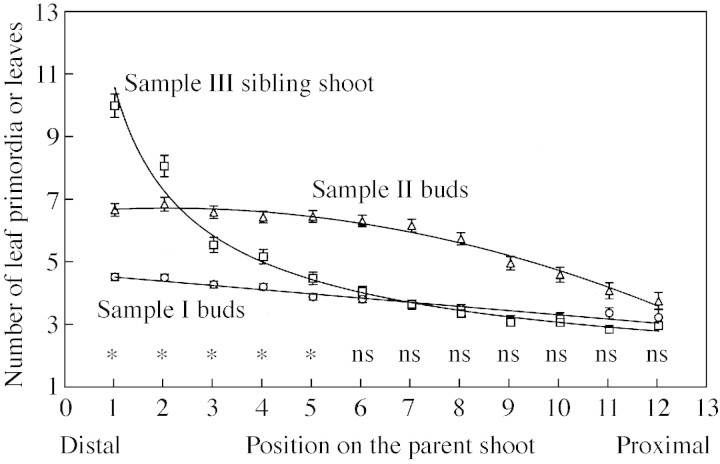
| Number of leaf primordia | |||||
| Sample I | 4·692 | –0·146 | – | 0·25 | 504 |
| Sample II | 6·546 | 0·148 | –0·034 | 0·30 | 523 |
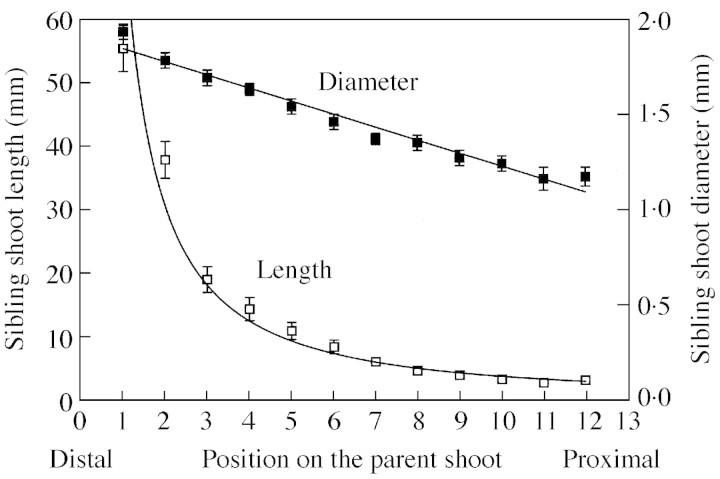
| Sibling shoots of sample III | |||||
| Length | 1·729 | –1·230 | – | 0·58 | 882 |
| Diameter | 1·950 | –0·074 | – | 0·39 | 856 |
| Leaves | 0·985 | –0·523 | – | 0·54 | 856 |
The intercept, first‐order (linear) and second‐order (quadratic) coefficients, the coefficient of determination (r2), and the number of x–y pairs (N) are indicated for each function. Italic font indicates log–log data transformation before function fitting.
A linear function fitted on log–log transformed data was chosen to describe the tendency in the length of sample III sibling shoots along the parent shoots: differences in sibling shoot length were more notable for distal than for proximal positions (Fig. 4; Table 2). On the other hand, the diameter of sibling shoots of this sample decreased linearly from the distal to the proximal end of parent shoots. All sibling shoots of sample III had a dead apex after their extension.
Fig. 4. Mean (± s.e.) length (open symbols) and diameter (solid symbols) of sample III sibling shoots, according to sibling shoot position counted from the parent shoot’s distal end. The lines fitted to each data set are described in Table 2.
Number of leaves of buds and sibling shoots
In the case of sample I, the number of leaf primordia per bud showed a moderate but significant linear decrease from distal to proximal positions on the parent shoot (Fig. 5; Table 2). For sample II, the number of leaf primordia per bud was better described by a quadratic function, indicating a higher and less variable number of primordia close to the distal end of the parent shoot and a gradually decreasing number of primordia towards the proximal end of the parent shoot. On average, two more primordia were found in sample II than in sample I buds. The number of leaves of sample III sibling shoots decreased more notably at the distal than at the proximal end of the parent shoot (a linear function was fitted to log–log transformed data; Fig. 5; Table 2).
Fig. 5. Mean (± s.e.) number of leaf primordia of buds of samples I (circles) and II (triangles) and number of leaves of sibling shoots of sample III (squares). Bud and sibling shoot positions are numbered from distal to proximal nodes on the parent shoot. The significance levels of the differences between means corresponding to samples I and III are indicated: *P < 0·05 and ns P > 0·05 (Bonferroni’s adjustment was applied). The lines fitted to each data set are described in Table 2.
The mean number of leaves of sibling shoots derived from the five positions closest to the distal end of sample III parent shoots was significantly (P < 0·05) higher than the number of leaf primordia of buds in similar positions of sample I parent shoots (Fig. 5). In more proximal positions, the mean number of leaf primordia of sample I buds did not differ significantly from the mean number of leaves of sample III sibling shoots (Fig. 5).
Parent shoot size and the structure of buds and sibling shoots
In the case of sample I buds, bud position on the parent shoot (negative effect) and parent shoot length (positive effect) were the independent variables most closely correlated with the number of leaf primordia per bud (Table 3). For sample II, bud diameter (positive effect) and, less notably, bud position on the parent shoot (negative effect; Table 4) were the independent variables most correlated with the number of leaf primordia per bud. For both samples, bud length explained a much smaller percentage of the variance of the number of leaf primordia per bud. The number of leaves of the parent shoot did not have a significant effect on the number of leaf primordia of a bud either for sample I or for sample II.
Table 3.
Summary of the predictor variables significantly related to the number of leaf primordia per bud (dependent variable) of sample I, as selected in three steps of stepwise regression
| Step | |||
| 1 | 2 | 3 | |
| Slope | 4·7 | 3·0 | 3·5 |
| Bud position | |||
| Slope | –0·15 | –0·17 | –0·17 |
| t | –12·9 | –16·8 | –17·2 |
| Parent–shoot length | |||
| Slope | 0·03 | 0·03 | |
| t | 12·7 | 13·1 | |
| Bud length | |||
| Slope | –0·21 | ||
| t | –3·1 | ||
| r 2 | 0·25 | 0·43 | 0·44 |
For each regression analysis, the regression coefficient (slope) and the student’s t‐ratio of each independent variable and the coefficient of determination of the regression (r2) are indicated.
Table 4.
Summary of the predictor variables significantly related to the number of leaf primordia per bud (dependent variable) of sample II, as selected in three steps of a stepwise regression
| Step | |||
| 1 | 2 | 3 | |
| Slope | –1·9 | –0·1 | –0·2 |
| Bud diameter | |||
| Slope | 4·4 | 3·9 | 3·3 |
| t | 30·6 | 29·5 | 13·3 |
| Bud position | |||
| Slope | –0·15 | –0·16 | |
| t | –12·3 | –12·7 | |
| Bud length | |||
| Slope | 0·57 | ||
| t | 3·2 | ||
| r 2 | 0·64 | 0·72 | 0·73 |
For each regression analysis, the regression coefficient (slope) and the student’s t‐ratio of each independent variable and the coefficient of determination of the regression (r2) are indicated.
Parent shoot length was the only variable that contributed significantly to explain the variance in the length (regression coefficient = 0·72, t = 3·3, r2 = 0·11) and number of leaves (regression coefficient = 0·07, t = 3·1, r2 = 0·10) of the relay shoot of sample III. The correlation between sibling shoot length and parent shoot length increased from distal to proximal sibling shoots (r = 0·35, 0·42, 0·52, 0·54, 0·51, 0·59, 0·66, 0·61, 0·61, 0·63, 0·66, from distal to proximal positions).
DISCUSSION
Bud structure and preformation
Given the widespread occurrence of apex death in N. antarctica shrubs (present study; Stecconi et al., 2000), the development of shoots from axillary buds is essential for both length growth and branching of each axis in this species. As in Nothofagus dombeyi and N. pumilio (both native to Patagonia), each axillary bud of N. antarctica consists of a preformed shoot with distal leaf primordia tightly enclosed by proximal cataphylls (Figs 1 and 6; Barthélémy et al., 1999; Puntieri et al., 2000; Souza et al., 2000). In all three Nothofagus species (present study; Puntieri et al., 2000; Souza et al., 2000), as well as in other trees such as Fraxinus pennsylvanica var. subintegerrima (Remphrey and Davidson, 1994), axillary buds that develop distally on a shoot tend to have more leaf primordia than more proximal axillary buds on the same shoot (Fig. 6). In contrast, in Persea spp. (Thorp et al., 1994) and Juglans regia (Sabatier and Barthélémy, 2000), the number of leaf primordia of axillary buds was shown to be constant along parent shoots.
Fig. 6. Diagrammatic representations of an N. antarctica shoot before (A) and after (B) the extension of its sibling shoots. In both cases, longitudinal sections of a distal and a proximal bud of a shoot are shown. Grey and white portions of the sibling shoots represent their preformed and neoformed portions, respectively.
The gradient in the number of leaf primordia per bud along each parent shoot found here for N. antarctica corresponded closely with gradients in the length and diameter of buds and in the length of the preformed shoot. Bud size and the number of leaf primordia per bud both varied between shoots extended in two successive growing seasons (Fig. 6). This difference could be related, at least in part, to the difference in the length of the parent shoots developed in the 1995–1996 and 1996–1997 growing seasons and to the difference in the dates of these samples. Studies on the dynamics of shoot extension in N. dombeyi and N. antarctica pointed to the relationship between daily temperatures and shoot length growth (Puntieri et al., 1998; Stecconi et al., 2000). The size and the number of leaf primordia of axillary buds could also be related to environmental conditions during bud inception, an idea supported by studies on Betula papyrifera (Macdonald et al., 1984) and Fraxinus pennsylvanica (Remphrey and Davidson, 1994). For the latter species, the number of preformed organs was found to be higher in populations subjected to conditions more favourable for tree growth (Remphrey and Davidson, 1994).
In N. antarctica shrubs, the position of a bud on its parent shoot, bud diameter and the length of the parent shoot from which a bud derives may contribute as predictors of the number of leaf primordia per bud. We found notable between‐year differences in the relevance of each of these variables as predictors of the number of leaf primordia of a bud. Such differences may be due to between‐sample differences in bud size which may, in turn, be related to parent shoot size. The present results indicate that the number of leaf primordia would be better estimated by bud position on its parent shoot and parent shoot size in the case of small buds, and by bud diameter in the case of large buds. For species of Picea, Fagus, Larix, Fraxinus and Juglans, the number of leaf primordia per bud was found to be related to bud size, parent shoot length and the position of the bud on the parent shoot or in the crown (Gill, 1971; Baxter and Cannell, 1978; Maruyama, 1983; Remphrey and Powell, 1984; Remphrey and Davidson, 1994; Sabatier and Barthélémy, 2000). The results of these studies, as well as those of the present one, thus support the notion of axis differentiation by which a tree may be viewed as a set of axis types, each one with its own morphological and physiological features (Barthélémy et al., 1997).
Sibling shoot structure and neoformation
In N. antarctica, sibling shoots develop from buds in the axil of all but the four to six most proximal leaves of their parent shoots. In the present study, the number of leaves of the five most distal sibling shoots was significantly higher than the number of leaf primordia of axillary buds in equivalent positions. These shoots extended uninterrupted (i.e. without the development of a bud during the growth period; Stecconi et al., 2000), which indicates that leaves additional to those preformed in the buds resulted from neoformation (Fig. 6).
The number of neoformed leaves per shoot decreased from sibling shoots that developed close to their parent shoot’s distal end (with about five neoformed leaves) to sibling shoots that developed more proximally on their parent shoot (Fig. 6). As mentioned above, the number of leaf primordia of axillary buds (and thus the number of preformed leaves of shoots derived from them) was related to parent shoot size. Thus, the higher probability of neoformation in sibling shoots that developed closer to the distal end of their parent shoots would explain the lower sibling shoot size/parent shoot size correlation found for distal than for proximal positions on the parent shoot.
Considering the relatively small size of the shoots studied here, it is worth remarking on their capacity to develop neoformed organs. Studies on N. dombeyi, N. pumilio, Betula papyrifera, Salix nigra, Populus trichocarpa, Fraxinus americana, Liquidambar styraciflua, Prunus armeniaca and Larix laricina found neoformation development only for trunk or main‐branch shoots of vigorous trees; relatively short shoots such as those sampled for the present study tend to be entirely preformed (Critchfield, 1960; Gill, 1971; Kozlowski, 1971; Macdonald et al., 1984; Remphrey and Powell, 1984; Brown and Sommer, 1992; Costes, 1993; Davidson and Remphrey, 1994; Puntieri et al., 2000; Souza et al., 2000).
Plants dependent upon preformation for the development of leaves may be limited in their short‐term production of leaves, so that their response to environmental conditions would be manifest in a period of time subsequent to the stimulus (Diggle, 1997). Neoformation has been regarded as a feature that provides plasticity to shoot development (Hallé et al., 1978; Geber et al., 1997). The capacity of a shoot to develop neoformed leaves may depend upon the position of the shoot along the species’ morphogenetic gradient (Barthélémy et al., 1997), but the extent of neoformation might be related to local environmental conditions during shoot extension. The weak relationship found in the present study between the size of a shoot and that of the shoot from which it is derived would support this view. Given this perspective, the widespread occurrence of neoformation in N. antarctica shrubs could be interpreted as giving this species an advantage in communities repeatedly affected by disturbance: the relatively large number of shoots with the capacity to develop neoformed leaves could profit from newly opened gaps in their proximity by differentiating and extending leaves which could fill these gaps. This could be one of the reasons for the abundance of N. antarctica in disturbed sites (Donoso, 1993; Veblen et al., 1996).
In the N. antarctica shrubs studied here, the increases in sibling shoot length and number of leaves towards the distal end of the parent shoots were more notable than those in diameter. Similar results were found for longer parent shoots of N. pumilio and N. dombeyi (Puntieri et al., 2000; Souza et al., 2000), although for these two species, parent shoots with the same number of leaves as those of N. antarctica studied here did not exhibit such sharp gradients in sibling shoot length and number of leaves.
As a result of the high frequency of apex deaths after shoot extension in the shoots of N. antarctica studied here, each axis in these shrubs has a predominantly pseudo‐monopodial construction: each year, a relay shoot is formed from one of the most distal axillary buds of a parent shoot (Caraglio and Barthélémy, 1997; Barthélémy et al., 1999; Stecconi et al., 2000). The length and number of leaves of the relay shoot was correlated with the length of its parent shoot, although only up to 11 % of the variance of the dependent variables could be explained. The number of leaves of the parent shoot and the size of the axillary bud from which the relay shoot derived were both weaker estimators of relay shoot size than parent shoot length.
ACKNOWLEDGEMENTS
We thank Javier Grosfeld, Cecilia Brion, María Sol Souza, Alfredo Passo and Camilo Mazzini for their assistance. We also acknowledge the Centro de Salmonicultura of the Universidad Nacional del Comahue where field work was carried out. This study was supported by Universidad Nacional del Comahue (B 704, Argentina), CONICET (PEI 0800/98, Argentina), CIRAD and INRA (France).
Supplementary Material
Received: 30 August 2001; Returned for revision: 30 November 2001; Accepted: 8 February 2002.
References
- BarthélémyD.1986. Establishment of modular growth in a tropical tree: Isertia coccinea Vahl. (Rubiaceae). Philosophical Transactions of the Royal Society of London Series B 313: 89–94. [Google Scholar]
- BarthélémyD, Caraglio Y, Costes E.1997. Architecture, gradients morphogénétiques et âge physiologique chez les végétaux. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architecture des plantes. Paris: INRA Editions, 89–136. [Google Scholar]
- BarthélémyD, Edelin C, Hallé F.1989. Architectural concepts for tropical trees. In: Holm‐Nielsen LB, Baslev H, eds. Tropical forest: botanical dynamics, speciation and diversity London: American Press, 89–100. [Google Scholar]
- BarthélémyD, Puntieri J, Brion C, Raffaele E, Marino J, Martinez P.1999. Características morfológicas y arquitecturales de las especies de Nothofagus Blume (Fagaceae) en el norte de la Patagonia argentina. Boletín de la Sociedad Argentina de Botánica 34: 29–38. [Google Scholar]
- BaxterSM, Cannell MGR.1978. Branch development on leaders of Picea sitchensis Canadian Journal of Forest Research 8: 121–128. [Google Scholar]
- BrownCL, Sommer HE.1992. Shoot growth and histogenesis of trees possessing diverse patterns of shoot development. American Journal of Botany 79: 335–346. [Google Scholar]
- CabreraAL.1976. Regiones fitogeográficas Argentinas. Buenos Aires: Editorial Acme S.A.C.I. [Google Scholar]
- CaraglioY.1996. Le développement architectural du Merisier. Forêt‐entreprise 107: 72–80. [Google Scholar]
- CaraglioY, Barthélémy D.1997. Revue critique des termes relatifs à la croissance et à la ramification des tiges des végétaux vasculaires. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architecture des végétaux Paris: INRA Editions, 11–87. [Google Scholar]
- ChampagnatP, Barnola P, Lavarenne S.1986. Quelques modalités de la croissance rythmique endogène des tiges chez les végétaux ligneux. Naturalia Monspeliensia (supplement): 279–302. [Google Scholar]
- ContiHA.1998. Características climáticas de la Patagonia. In: Correa MN, ed. Flora Patagónica VIII (I) Buenos Aires: INTA, 31–47. [Google Scholar]
- CorreaMN.1984. Fagaceae. In: Correa MN, ed. Flora Patagónica IVa Buenos Aires: INTA, 4–11. [Google Scholar]
- CostesE.1993. Architecture aérienne de l’Abricotier en développement libre. Acta Botanica Gallica 140: 249–261. [Google Scholar]
- CritchfieldWB.1960. Leaf dimorphism in Populus trichocarpa American Journal of Botany 47: 699–711. [Google Scholar]
- DavidsonCG, Remphrey WR.1994. Shoot neoformation in clones of Fraxinus pennsylvanica in relation to genotype, site and pruning treatments. Trees 8: 205–212. [Google Scholar]
- deReffyeP, Houllier F.1997. Modelling plant growth and architecture: some recent advances and applications to agronomy and forestry. Current Science 73: 984–992. [Google Scholar]
- deReffyeP, Dinouard P, Barthélémy D.1991. Modélisation et simulation de l’architecture de l’orme du Japon Zelkova serrata (Thunb.) Makino (Ulmaceae): la notion d’axe de référence. Naturalia Monspeliensia (Supplement): 251–266. [Google Scholar]
- deReffyeP, Edelin C, Jaeger M.1989. La modélisation de la croissance des plantes. La Recherche 207: 158–207. [Google Scholar]
- deReffyeP, Houllier F, Blaise F, Barthélémy D, Dauzat J, Auclair D.1995. A model simulating above and below ground tree architecture with agroforestry applications. Agroforestry Systems 30: 175–197. [Google Scholar]
- DigglePK.1997. Extreme preformation in alpine Polygonum viviparum: an architectural and developmental analysis. American Journal of Botany 84: 154–169. [PubMed] [Google Scholar]
- DimitriMJ.1972. La región de los bosques Andino‐Patagónicos. Buenos Aires: INTA. [Google Scholar]
- DonosoC.1993. Bosques templados de Chile y Argentina. Variación, estructura y dinámica. Santiago de Chile: Ed Universitaria, Conaf. [Google Scholar]
- GeberM, de Kroon H, Watson MA.1997. Organ preformation in mayapple as a mechanism for historical effects on demography. Journal of Ecology 85: 211–223. [Google Scholar]
- GillAM.1971. The formation, growth and fate of buds of Fraxinus americana in Central Massachussetts. Harvard Forestry Papers 20: 1–16. [Google Scholar]
- GuédonY, Costes E.1997. Modélisation de la croissance d’un axe végétatif. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architecture des végétaux Paris: INRA Editions, 173–185. [Google Scholar]
- HalléF, Oldeman RAA, Tomlinson PB.1978. Tropical trees and forest. Berlin: Springer‐Verlag. [Google Scholar]
- KozlowskiTT.1971. Growth and development of trees. I. Seed germination, ontogeny and shoot growth. London: Academic Press. [Google Scholar]
- McQueenDR.1976. The ecology of Nothofagus and associated vegetation in South America. Tuatara 22: 38–68. [Google Scholar]
- MacdonaldAD, Mothersill DH.1983. Shoot development in Betula papyrifera I. Short‐shoot organogenesis. Canadian Journal of Botany 61: 3049–3065. [Google Scholar]
- MacdonaldAD, Mothersill DH, Caesar JC.1984. Shoot development in Betula papyrifera III. Long‐shoot organogenesis. Canadian Journal of Botany 62: 437–445. [Google Scholar]
- MaruyamaK.1983. Shoot characteristics as a function of bud length in Japanese beech trees. Journal of the Japanese Forestry Society 65: 43–51. [Google Scholar]
- NicoliniE.1998. Architecture et gradients morphogénétiques chez de jeunes hêtres (Fagus sylvatica L. Fagaceae) en milieu forestier. Canadian Journal of Botany 76: 1232–1244. [Google Scholar]
- PremoliAC.1991. Morfología y capacidad germinativa en poblaciones de Nothofagus antarctica (Forster) Oerst. del noroeste andino patagónico. Bosque 12: 53–59. [Google Scholar]
- PuntieriJ, Barthélémy D, Martinez P, Raffaele E, Brion C.1998. Annual‐shoot growth and branching patterns in Nothofagus dombeyi (Fagaceae). Canadian Journal of Botany 76: 673–685. [Google Scholar]
- PuntieriJ, Souza MS, Barthélémy D, Brion C, Nuñez M, Mazzini C.2000. Preformation, neoformation, and shoot structure in Nothofagus dombeyi (Nothofagaceae). Canadian Journal of Botany 78: 1044–1054. [Google Scholar]
- RamírezC, Correa DM, Figueroa H, San Martín J.1985. Variación del hábito y hábitats de Nothofagus antarctica en el centro‐sur de Chile. Bosque 6: 55–73. [Google Scholar]
- RemphreyWR, Davidson CG.1994. Shoot preformation in clones of Fraxinus pennsylvanica in relation to site and year of bud formation. Trees 8: 126–131. [Google Scholar]
- RemphreyWR, Powell GR.1984. Crown architecture of Larix laricina saplings: shoot preformation and neoformation and their relationships to shoot vigour. Canadian Journal of Botany 62: 2181–2192. [Google Scholar]
- SabatierS, Barthélémy D.2000. Bud structure in relation to shoot morphology and position on the vegetative annual shoots of Juglans regia L. (Juglandaceae). Annals of Botany 87: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SabatierS, Barthélémy D, Ducousso Y, Germain E.1998. Modalités d’allongement et morphologie des pousses annuelles chez le noyer commun, Juglans regia L. cv. ‘Lara’ (Juglandaceae). Canadian Journal of Botany 76: 1253–1264. [Google Scholar]
- ScoppaCO.1998. Los Suelos. In: Correa MN, ed. Flora Patagónica VIII (I) Buenos Aires: INTA, 15–30. [Google Scholar]
- SokalRR, Rohlf FJ.1981. Biometry. 2nd edn. New York: Freeman and Co. [Google Scholar]
- SouzaMS, Puntieri J, Barthélémy D, Brion C.2000. Bud content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae). Annals of Botany 85: 547–555. [Google Scholar]
- StecconiM, Puntieri J, Barthélémy D.2000. Annual shoot‐growth in Nothofagus antarctica (G. Forster) Oersted (Nothofagaceae) from northern Patagonia. Trees 14: 289–296. [Google Scholar]
- ThorpTG, Aspinall D, Sedgley M.1994. Preformation of node number in vegetative and reproductive proleptic shoot modules of Persea (Lauraceae). Annals of Botany 73: 13–22. [Google Scholar]
- VeblenTT, Donoso C, Kitzberger T, Rebertus AJ.1996. Ecology of southern Chilean and Argentinean Nothofagus forests. In: Veblen TT, Hill RS, Read J, eds. The ecology and biogeography of Nothofagus forests Yale: Yale University Press, 293–353. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.