Abstract
We examined the relationship between morphological characteristics of anthers and fertility in japonica rice cultivars subjected to high temperature (37·5/26 °C day/night) at flowering. Percentage fertility was negatively correlated with the number of cell layers that separated the anther locule from the lacuna that formed between the septum and the stomium. The cell layers consisted of the remaining septum and degraded tapetum, and serve to keep the adjacent two locules closed. Anther dehiscence therefore requires the rupture of the cell layers. We conclude that the tight closure of the locules by the cell layers delayed locule opening, and decreased fertility at high temperatures.
Key words: Anther dehiscence, anther size, high temperature tolerance, lacuna between septum and stomium, Oryza sativa L., rice, septum, tapetum
INTRODUCTION
Rice is grown mainly in tropical and subtropical zones, and a high temperature at flowering can induce floret sterility and can limit grain yield (Osada et al., 1973; Satake and Yoshida, 1978; Matsushima et al., 1982). Since the 1980s, an increase in the concentration of greenhouse gases, such as carbon dioxide, in the atmosphere is thought to have been responsible for increasing the air temperature (Hansen et al., 1984). Amongst other things, global warming is expected to result in the occurrence of high temperature‐induced floret sterility in rice.
Crop scientists have attempted to assess the effects of increasing temperature and high carbon dioxide in the atmosphere on the growth and yield of rice using simulation models (Boote et al., 1994; Horie et al., 1996, 1997; Matthews et al., 1997). Horie et al. (1996) suggested that the anticipated high temperature would induce floret sterility and increase the instability of the rice yield even in temperate regions. These authors also showed that adoption of high temperature‐tolerant cultivars is one of the most effective countermeasures to maintain high productivity and stability of rice under the anticipated climate in temperate regions (Horie et al., 1996).
The main cause of floret sterility induced by high temperatures at flowering is anther indehiscence (Satake and Yoshida, 1978; Mackill et al., 1982; Matsui et al., 1997a, b, 2001). High temperatures at flowering inhibit swelling of the pollen grains (Matsui et al., 2000b), which is the driving force behind anther dehiscence in rice (Matsui et al., 1999a, b). Anthers of high temperature‐tolerant cultivars dehisce more easily than those of susceptible cultivars and contribute to pollination under high‐temperature conditions (Satake and Yoshida, 1978; Mackill et al., 1982; Matsui et al., 2000b, 2001). However, the factors determining the degree of anther dehiscence are unknown.
Here, we examine the relationship between morphological characteristics of the anther and floret tolerance to high temperature at flowering in japonica rice cultivars in relation to sterility. We also investigate the morphological characteristics of anthers in tolerant cultivars and discuss why their anthers dehisce so readily.
MATERIALS AND METHODS
Floret sterility due to high temperatures during the flowering period
Data concerning floret tolerance to high temperatures at flowering have been reported previously (Matsui et al., 2001). Nine japonica rice cultivars, including two tolerant to, and two susceptible to, high temperature were used (Table 1). Seeds were sown from March to June 1995 so that panicles would emerge late in August. Seedlings at the 5·0–5·5 leaf stage were transplanted in a circular pattern into 4‐l pots, with 20 seedlings per pot, and were grown outdoors under submerged soil conditions (Experimental Farm of Kyoto University, Osaka, Japan). Each pot was provided with 0·4 g N, 0·4 g P2O5 and 0·4 g K2O as a top dressing about 45 d before heading (about 15 d before panicle initiation). Tillers were removed during the vegetative stage as they appeared.
Table 1.
Tolerance of rice cultivars to high temperature at flowering based on the studies of Matsui et al. (2001)
| Percentage fertility under high temperature | |||||
| Control | |||||
| Cultivar | 35·0/26·0 °C* | 37·5/26·0 °C | 40·0/26·0 °C | Tolerance to high temperature | |
| 1 | Nipponbare | 94·6 | 85·3 | 35·0 | Tolerant |
| 2 | Akitakomachi | 93·4 | 80·0 | 50·3 | Tolerant |
| 3 | Aichinokaori | 96·1 | 78·2 | 19·8 | Moderate |
| 4 | Yumehikari | 91·5 | 74·3 | 22·9 | Moderate |
| 5 | Kinmaze | 95·4 | 65·2 | 22·2 | Moderate |
| 6 | Akihikari | 92·6 | 65·9 | 13·2 | Moderate |
| 7 | Aoinokaze | 91·4 | 58·4 | 19·9 | Moderate |
| 8 | Minamihikari | 92·7 | 45·7 | 19·2 | Susceptible |
| 9 | Hinohikari | 93·4 | 44·1 | 13·7 | Susceptible |
* Day/night temperature
Sun‐lit phytotrons were used for high‐temperature treatments. Plants at the middle heading stage were exposed to 37·5 °C for 6 h (1000–1600 h) for six consecutive days. The night‐time temperature (1800–0800 h) was 26·0 °C. Relative humidity was 60 % in the daytime (1000–1600 h) and 80 % at night. Air temperature and humidity were altered gradually between 0800 and 1000 h, and between 1600 and 1800 h. The treatments started at 1800 h and ended at 1800 h, 6 d later. Three pots of each cultivar were used for each treatment.
The fertility of all florets on panicles on which florets started to open on the first day of treatment and finished opening before the last day of treatment was examined at maturity. More than four panicles (over 150 florets) were examined per pot. Means of the three pots were used for regression analysis.
Morphological characteristics of anthers
The same nine cultivars were grown in 2000 under the conditions described above. On the day before florets opened, the third florets from the top of the first branches were fixed in FAA after removing the lemmas. Six florets per cultivar were fixed.
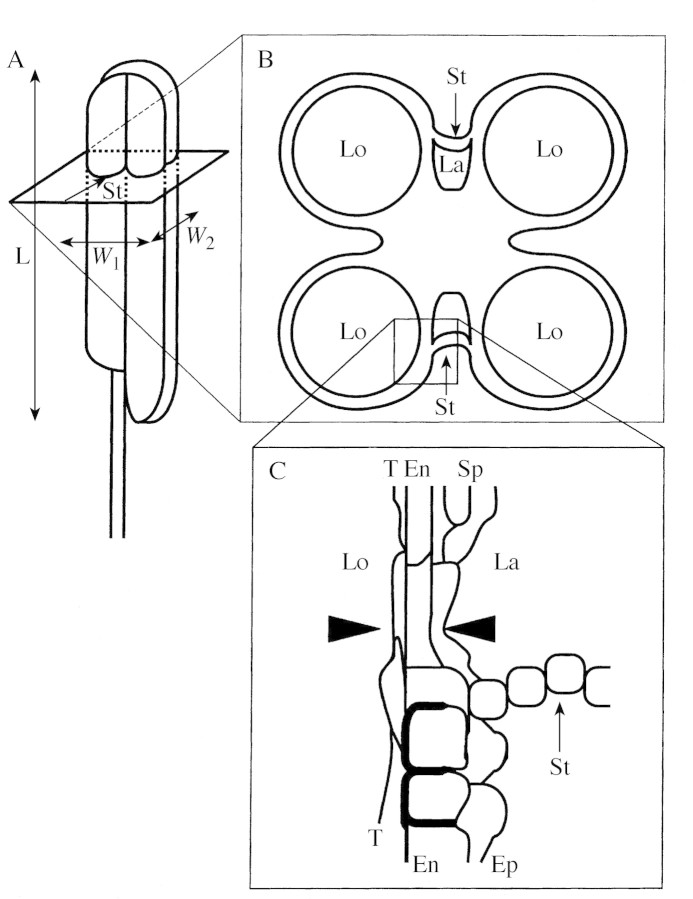
Three florets (18 anthers) per cultivar were used for measurement of anther sizes. The length and width of anthers were measured with a micrometer under a stereomicroscope. Width was taken as the mean of measurements made parallel and at right angles to the stomium (Fig. 1A).
Fig. 1. Measurement of anther characteristics. A, Exterior of rice anther. Anther length (L) was taken as the length of the long locule. Anther width was calculated as the mean width taken in two directions [(W1+W2)/2]: one parallel to the stomium (W1) and the other (W2) at right angles. B, Schematic transverse section of anther. C, Magnification of B around the cell layers separating the locule from the lacuna formed between the septum and the stomium. The number of cell layers separating the lacuna from the locule between the septum and the stomium was counted at the point where the number of cell layers was smallest (the probable breaking point when the septum opens; between arrowheads). La, Lacuna formed between the septum and the stomium; En, endothecium; Ep, epidermis; T, tapetum; Lo, locule; St, stomium.
The other three florets were dehydrated in a methanol–butanol series before being infiltrated and embedded in paraffin. Transverse sections, 8 µm thick, were cut from the florets of 18 anthers and stained with toluidine blue‐O. The number of cell layers separating the anther locule from the lacuna that formed between the septum and the stomium was counted at the point where the number of layers was smallest (probably the point that will break when the septum opens; Fig. 1C, cf. Fig. 4). The number of cell layers was recorded every 80 µm for all four locules in five sections, from 480 µm to 800 µm from the apex of the anther. Mean anther length, width, length × width and the number of cell layers were calculated for each floret. Means of the three florets of each cultivar were then used for regression analysis. Predictor valuables in the multiple regression analysis were determined by a stepwise regression procedure.
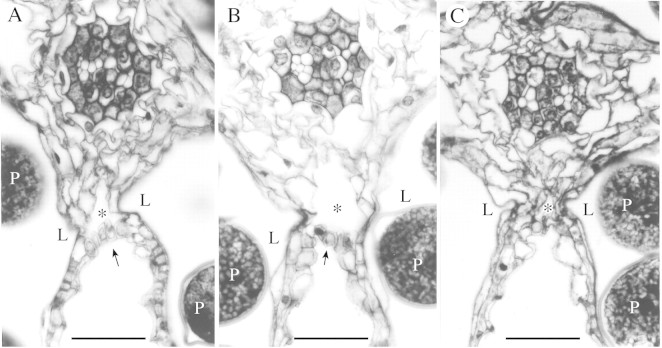
Fig. 4. Transverse section of the septum in which one or two (A, B), or three (C) cell layers separate the locule from the lacuna between the septum and the stomium. A, Degrading tapetal cells separate the lacuna from the locule (‘Nipponbare’, high‐temperature tolerant); B, degrading endothecium and degraded tapetal cells separate the lacuna from the locule (‘Akitakomachi’, high‐temperature tolerant); C, degrading tapetal cells, endocecium cells and parenchyma cells separate the lacuna from the locule (‘Minamihikari’, high‐temperature susceptible). L, Anther locule; P, pollen grain; arrowhead, stomium; *, lacuna formed between the septum and the stomium. Bars = 25 µm.
RESULTS
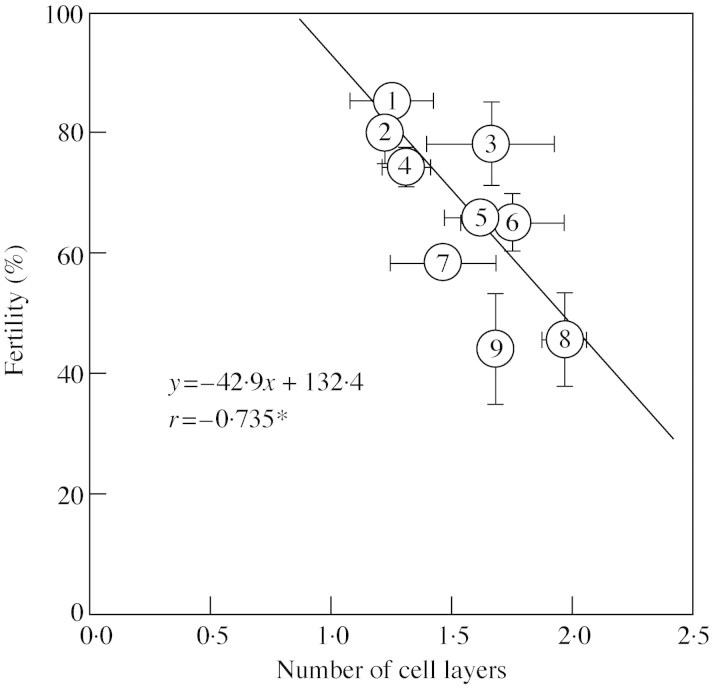
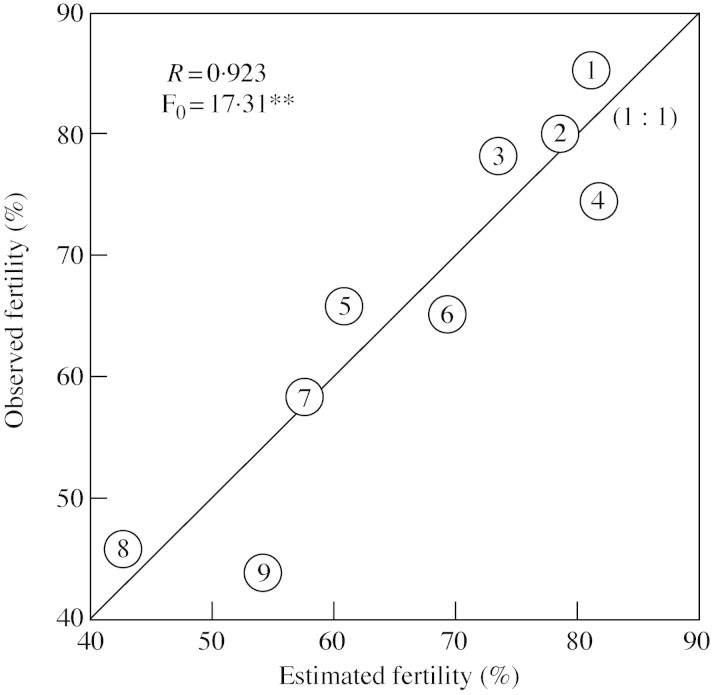
Percentage fertility under a day/night temperature regime of 37·5/26 °C was significantly correlated with the number of cell layers separating the locule from the lacuna that formed between the septum and the stomium (Fig. 2). There was no correlation between anther length, width or length × width and percentage fertility, but anther length × width was significantly correlated with the difference between measured percentage fertility and percentage fertility estimated from the number of cell layers separating the locule from the lacuna (r = 0·750, P < 0.05). Under high temperature, 85 % of the variance in the percentage fertility among the nine cultivars was explained by multiple regression with the number of cell layers and the product of anther length and width (Fig. 3).
Fig. 2. Relationship between the number of cell layers separating the locule from the lacuna formed between the septum and the stomium and percentage fertility. Numbers represent cultivars (see Table 1). Vertical bars indicate ± s.e. of three pots. Horizontal bars indicate ± s.e. of three florets.
Fig. 3. Comparison between observed and estimated fertility in rice under a daytime temperature of 37·5 °C. Numbers represent cultivars (see Table 1). The estimation was made by: y = – 57·6x1** + 160·5x2* + 12·03, where x1 and x2 represent the number of cell layers separating the locule from the lacuna between the septum and the stomium, and the product of length and width of anther, respectively. * and ** indicate significance at 5 and 1 % levels, respectively.
When the locule is separated from the lacuna between the septum and the stomium by three or more layers, then these layers consist of parenchyma cells and endothecium cells in the septum, and tapetal cells (Fig. 4). If it is separated by one or two layers only, then these consist of endothecium cells and/or degrading tapetal cells.
DISCUSSION
The difference in the sterility‐inducing daytime temperature between the most tolerant cultivar, Akitakomachi, and the most susceptible cultivar, Hinohikari, used in the present study has been reported to be about 3 °C (Matsui et al., 2001). Using a crop simulation model, Horie et al. (1996) showed that even a difference of 1·6 °C in the sterility‐inducing temperature could have a serious effect on rice yield in a temperate region under anticipated global warming. The present results suggest that the 3 °C difference in tolerance was mainly related to the number of cell layers separating the locule from the lacuna that formed between the septum and the stomium, and secondarily related to anther size.
It has been reported that anther length is positively correlated with tolerance to floret sterility induced by a low temperature at the booting stage (e.g. Hashimoto, 1961; Suzuki, 1981, 1982; Tanno et al., 1999), which is the most serious cool‐weather damage in rice. Using cultivars with a large variation in anther width, Tanno et al. (1999) showed that the product of anther length and width was correlated more closely with tolerance to low temperature at the booting stage than was length alone. The number of pollen grains in the anther is positively correlated with anther length in rice cultivars (Suzuki, 1981), and the inner wall of the anther locule in Poaceae is lined with pollen grains before the septum opens (Keijzer et al., 1996; Matsui et al., 2000a). Thus, anther width would also be positively correlated with the number of pollen grains in the anther. Since the direct cause of floret sterility induced by low temperature at the booting stage is the decreased number of germinated pollen grains caused by anther indehiscence (Shimazaki et al., 1964), it has been assumed that anther size would be correlated with tolerance to low temperature because the reduction in the number of dehisced anthers would be compensated by a large number of pollen grains per anther (Suzuki, 1981; Tanno et al., 1999). Anther size might be correlated with tolerance to high temperature‐induced sterility in a similar way, because the direct cause of spikelet sterility induced by high temperature at flowering is also a decrease in the number of germinated pollen grains caused by poor anther dehiscence (Satake and Yoshida, 1978; Mackill et al., 1982; Matsui et al., 1997a, b, 2001).
The number of cell layers separating the locule from the lacuna between the septum and the stomium was more closely related to fertility under the high‐temperature treatment than was anther size. Keijzer et al. (1996) reported that the stomium dissociates from the septum 2 d before anthesis in maize, and this dissociation ruptures the tapetum membranes. Thus, adjacent maize locules become one cavity before anthesis (Keijzer et al., 1996). In rice, the stomium dissociates from the septum before anthesis, but the locules are kept closed until anthesis by parenchyma and endothecium cells in the septum, often supported by the degrading tapetum (Matsui et al., 1999b). The lacuna that is surrounded by the septum and stomium cells is formed in this way. Since the locule remains closed until anthesis, opening of the septum (i.e. locule opening) at flowering is an indispensable process for anther dehiscence (Matsui et al., 1999b). The number of cell layers separating the locule from the lacuna between the septum and the stomium is the number of cell layers responsible for keeping the locules closed. These cell layers might be responsible for the high‐temperature susceptibility of rice through the strength of the septum and thus the degree of anther dehiscence.
The fundamental cause of poor anther dehiscence induced by high temperatures is disturbance of pollen swelling (Matsui et al., 2000b), which is the driving force for anther dehiscence (Matsui et al., 1999a, b). Although it is not known whether the disturbance is caused by water loss, water loss in the locule would impede pollen swelling because swelling occurs as a result of water migration into pollen grains. Indeed, low atmospheric humidity disturbs anther dehiscence in rice (Matsui et al., 1999b). Thus, delayed anther dehiscence at floret opening might inhibit the swelling of pollen grains through loss of water in the locule under high temperatures, and might have a feedback effect delaying dehiscence further. Therefore, the strength of the septum might be more important for high‐temperature tolerance than the size of the anther which is the dominant factor in tolerance to sterility induced by low temperature at the booting stage. However, it must be noted that the range of anther sizes used in the present experiment (1·82–2·24 mm in length and 0·405–0·455 mm in width) was not as wide as that used in studying tolerance to low temperature at the booting stage, when tolerance was attributed to anther size (e.g. anther lengths from 1·59 to 2·54 mm, and widths from 0·395 to 0·515 mm; Tanno et al., 1999). Thus, the narrow range of anther sizes may have overemphasized the importance of the number of cell layers in high‐temperature tolerance.
ACKNOWLEDGEMENT
We thank H. Kagata for technical assistance.
Supplementary Material
Received: 12 October 2001; Returned for revision: 12 December 2001; Accepted: 20 February 2002.
References
- BonnerLJ, Dickinson HG.1989. Anther dehiscence in Lycopersicon esculentum Mill. I. Structural aspects. New Phytologist 113: 97–115. [DOI] [PubMed] [Google Scholar]
- BooteKJ, Pickering NB, Baker JT, Allen LH Jr.1994. Modeling leaf and canopy photosynthesis of rice in response to carbon dioxide and temperature. International Rice Research Notes 19: 47–48. [Google Scholar]
- HansenJ, Lacis A, Rind D, Russell G, Stone P, Fung I, Ruedy R, Lerner J.1984. Climate sensitivity: analysis of feedback mechanisms. In: Hansen J, Takahashi T, eds. Climate process and climate sensitivity Washington DC: American Geophysical Union, 130–163. [Google Scholar]
- HashimotoK.1961. On size of anther in rice plant varieties. Bulletin of Hokkaido Study Meeting of Breeding and Crop Science 2: 11 (in Japanese). [Google Scholar]
- HorieT, Matsui T, Nakagawa H, Omasa K.1996. Effect of elevated CO2 and global climate change on rice yield in Japan. In: Omasa K, Kai K, Toda H, Uchijima Z, Yoshino M, eds. Climate change and plants in East Asia Tokyo: Springer‐Verlag, 39–56. [Google Scholar]
- HorieT, Centeno HGS, Nakagawa H, Matsui T.1997. Effect of elevated carbon dioxide and climate change on rice production in East and Southeast Asia. In: Oshima Y, ed. Proceedings of the International Scientific Symposium on Asian Paddy Fields Canada, Saskatchewan: College of Agriculture, University of Saskatchewan, 49–58. [Google Scholar]
- KeijzerCJ.1987. The process of anther dehiscence and pollen dispersal. I. The opening mechanism of longitudinally dehiscing anther. New Phytologist 105: 487–498. [DOI] [PubMed] [Google Scholar]
- KeijzerCJ.1999. Mechanism of angiosperm anther dehiscence, a historical review. In: Clement C, Pacini E, Audran J‐C, eds. Anther and pollen Heidelberg: Springer‐Verlag, 55–67. [Google Scholar]
- KeijzerCJ, Leferink‐ten Klooster HB, Reinders MC.1996. The mechanics of the grass flower: anther dehiscence and pollen shedding in maize. Annals of Botany 78: 15–21. [Google Scholar]
- MackillDJ, Coffman WR, Rutger JN.1982. Pollen shedding and combining ability for high temperature tolerance in rice. Crop Science 22: 730–733. [Google Scholar]
- MatsuiT, Omasa K, Horie T.1997a High temperature‐induced spikelet sterility of japonica rice at flowering in relation to air temperature, humidity and wind velocity condition. Japanese Journal of Crop Science 66: 449–455. [Google Scholar]
- MatsuiT, Namuco OS, Ziska LH, Horie T.1997b Effects of high temperature and CO2 concentration on spikelet sterility in indica rice. Field Crops Research 51: 213–219. [Google Scholar]
- MatsuiT, Omasa K, Horie T.1999a Rapid swelling of pollen grains in response to floret opening unfolds locule in rice. Plant Production Science 2: 196–199. [Google Scholar]
- MatsuiT, Omasa K, Horie T.1999b Mechanism of anther dehiscence in rice (Oryza sativa L.). Annals of Botany 84: 501–506. [Google Scholar]
- MatsuiT, Omasa K, Horie T.2000a Mechanism of septum opening in anthers of two‐rowed barley (Hordeum vulgare L.). Annals of Botany 86: 47–51. [PubMed] [Google Scholar]
- MatsuiT, Omasa K, Horie T.2000b High temperatures at flowering inhibit swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.). Plant Production Science 3: 430–434. [Google Scholar]
- MatsuiT, Omasa K, Horie T.2001. The difference in sterility due to high temperatures during the flowering period among japonica‐rice varieties. Plant Production Science 4: 90–93. [Google Scholar]
- MatsushimaS, Ikewada H, Maeda A, Honda S,Niki H.1982. Studies on rice cultivation in the tropics. 1. Yielding and ripening responses of the rice plant to the extremely hot and dry climate in Sudan. J apa n ese J ournal of Trop ical Agr iculture 26: 19–25. [Google Scholar]
- MatthewsRB, Kropff MJ, Horie T, Bachelet D.1997. Simulating the impact of climate change on rice production in Asia and evaluating options for adaptations. Agricultural Systems 54: 399–425. [Google Scholar]
- NeelamA, Sexton R.1995. Cellulase (endo β‐1,4 glucanase) and cell wall breakdown during anther development in the sweat pea (Lathyrus odoratus L.): isolation and characterization of partial cDNA clones. Journal of Plant Physiology 146: 622–628. [Google Scholar]
- OsadaA, Saciplapa V, Rahong M, Dhammanuvong S, Chakrabandho H.1973. Abnormal occurrence of empty grains of indica rice plants in the dry, hot season in Thailand. Proceedings of the Crop Science Society of Japan 42: 103–109. [Google Scholar]
- SatakeT, Yoshida S.1978. High temperature‐induced sterility in indica rice at flowering. J a p a n ese J ournal of Crop Science 47: 6–10. [Google Scholar]
- ShimazakiY, Satake T, Ito N, Doi Y, Watanabe K.1964. Sterile spikelets in rice plants induced by low temperature during the booting stage. Research Bulletin of the Hokkaido National Agricultural Experiment Station (Japan) 83: 1–9 (in Japanese with English summary). [Google Scholar]
- SuzukiS.1981. Cold tolerance in rice plants with special reference to the floral characters. I. Varietal differences in anther and stigma length and effects of planting densities on these characters. Japanese Journal of Breeding 31: 57–64 (in Japanese with English abstract). [Google Scholar]
- SuzukiS.1982. Cold tolerance in rice plants with special reference to the floral characters. II. Relations between floral characters and the degree of cold tolerance in segregating generations. Japanese Journal of Breeding 32: 9–16 (in Japanese with English abstract). [Google Scholar]
- TannoH, Xiong J, Dai L, Ye C.1999. Some characteristics of cool weather‐tolerant rice varieties in Yunnan province, China. Japanese Journal ofCrop Science 68: 508–514 (in Japanese with English abstract). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.