Abstract
A dissected‐leaved form of Ranunculus repens L. occurs in the temporary limestone lakes (turloughs) across the west of Ireland. Turloughs fill with groundwater for up to 8 months of the year. Under experimental conditions, these turlough populations demonstrated a higher rate of aerial and submerged photosynthesis than populations of the more typical broad‐leaved ruderal form. The turlough populations also had higher rates of stomatal conductance and exhibited a higher stomatal index on the upper leaf surface and a lower index on the lower leaf surface than the ruderal populations. Neither population could utilize bicarbonate to any great extent, with rates of photosynthesis under submerged conditions being only 5 % of aerial rates. Respiration under submerged conditions was significantly higher in the turlough populations than in ruderal populations, and it is hypothesized that the more dissected leaf shape of the turlough population may have a thinner boundary layer and thus enhance gas exchange in submerged conditions.
Key words: Ranunculus repens L., creeping buttercup, turloughs, amphibious plants, flooding, stomatal index, submerged photosynthesis, submerged respiration
INTRODUCTION
Flooding and submergence can have serious implications on growth and productivity. A reduction in stomatal conductance following the onset of flooding has been observed in many species (e.g. Jackson and Hall, 1987; Castonguay et al., 1993). Stomatal closure can help to reduce transpiration and thus lower the uptake of soil toxins, which are present in flooded soils (Ponnamperuma, 1984), but will also depress gas exchange (Bradford and Hsiao, 1982). This phenomenon of stomatal closure is thought to result from internal transportational problems resulting in the accumulation of assimilates or growth regulators in the leaves (Bradford and Hsiao, 1982). Complete submergence of the aerial parts of plants results in the diffusion pathway for oxygen between the atmosphere and the root being blocked (Laan and Blom, 1990). Transport of gases to the plant by diffusion is 104‐times slower in water than in air (Bowes and Salvucci, 1989; Madsen and Sand‐Jensen, 1991). The main exogenous carbon source in many freshwater systems is HCO3–, as there is virtually no free gaseous CO2 above pH 7·5 (Allen and Spence, 1981). Under saturating levels of light and CO2, photosynthetic rates of submerged plants can often be less than 5 % of those of terrestrial C3 plants (Thai et al., 1976) as many submerged plants do not have the ability to utilize HCO3– (Allen and Spence, 1981; Maberly and Spence, 1983; Sand‐Jensen, 1987). The diffusion of free CO2 and HCO3– through unstirred layers of solution at the cell surface is considered to be an important rate‐limiting process of aquatic photosynthesis (Smith and Walker, 1980). The unstirred layer in a well‐mixed solution is reported to be around 10 µm, while in more natural conditions or around bulky organs the unstirred layer can vary between 20–500 µm (Smith and Walker, 1980). The thickness of the boundary layer is also highly dependent on the morphology of the leaf, with more dissected leaves having a thinner boundary layer than broad leaves (Givinish, 1979; Smith and Walker, 1980). Many submerged macrophytes have highly dissected or linear leaves (Sculthorpe, 1967).
Ranunculus repens is generally found in relatively wet habitats (Harper, 1957). This species was described as being a specialist at the interface of anaerobic mud and free water as it can endure a maximum of 7–9 d of experimental anoxia (Braendle and Crawford, 1987). Other studies considered that the survival tactics of Ranunculus repens, following experimental flooding, were intermediate between amelioration and tolerance (He et al., 1999). Amelioration of flooded conditions was demonstrated by the induction of a depth accommodation response (Ridge, 1985; He et al., 1999), whereby petioles elongated and established contact with the aerial environment. Lysigenous aerenchyma, which facilitates O2 transport to the roots (Armstrong et al., 1991), is induced by waterlogging in this species (He et al., 1999); again, an example of amelioration. However, other studies on R. repens have demonstrated that the extent of aerenchyma production increased with root age even under drained conditions (Lynn, 1998). A significant decrease in the light compensation point in submerged conditions was observed when a submergence pre‐treatment was applied (He et al., 1999); this led the authors to hypothesize that this species may possess or develop a certain degree of metabolic flood tolerance.
Populations of Ranunculus repens occur in many of the temporary lakes (turloughs) in the west of Ireland (Lynn, 1998). These populations are morphologically distinct from the more typical ruderal form which often occurs in close proximity in surrounding pastureland. The turlough form has more dissected glabrous leaves than the ruderal form, and these differences are genetically determined (Lynn and Waldren, 2001). Turlough populations must endure prolonged submergence, often in several metres of water, where any attempt at amelioration, such as ‘depth accommodation’, would not be feasible. The alkalinity (mg CaCo3 l–1) of turlough waters is often in the upper range reported for fresh waters (Lynn, 1998), and this, combined with a high pH, results in the available exogenous carbon for photosynthetic purposes being bicarbonate, with negligible concentrations of free CO2 (Allen and Spence, 1981). However, R. repens has been deemed to be a strict CO2 user in submerged conditions (He et al., 1999).
This paper investigates whether different physiological strategies are required to survive in the turlough habitat by comparing physiological characteristics of turlough and ruderal populations, the latter of which would never be exposed to submergence under natural conditions. The outcome evaluates whether the turlough populations have evolved to survive the conditions imposed by the turlough environment.
MATERIALS AND METHODS
Plant propagation
Field‐collected ramets were planted in 13 cm pots containing equal parts of sand : peat : loam enriched with 3·5 g l–1 Osmocote Plus (Sierra Chemical Company, Marysville, OH, USA) slow release fertilizer. The ramets were left to establish in a heated glasshouse (minimum temperature 12 °C) with no supplementary light, and were subsequently transferred outdoors. Ramets produced in cultivation were pinned to the compost surface to encourage root development, and connections with the mother rosette were severed when the ramet became fully rooted. All experimental ramet‐derived material was produced in cultivation.
Field‐collected seed was dried for several weeks on a laboratory bench. Seeds were germinated in a growth room (14 h day, PAR 60–100 µmol m–2 s–1, 25/22 °C day/night) on moist filter paper in glass Petri dishes. When the cotyledons emerged seedlings were transferred to an equal part sand : peat : loam compost enriched with 3·5 g l–1 Osmocote. Plants were cultivated in 13 cm pots.
Location details for the experimental populations are presented in Table 1.
Table 1.
Location details for the experimental populations of Ranunculus repens
| Population | Grid reference | Habitat type |
| Dodder | O 150 297 | Disturbed ground |
| Bull Island | O 230 370 | Dune system |
| Lough Gealáin | R 317 938 | Turlough |
| Hawkhill | M 411 025 | Turlough |
| Cooloorta | R 337 979 | Turlough |
Effect of flooding on photosynthesis in ramet‐derived stock
Populations: Dodder (ruderal), Lough Gealáin (turlough).
Eight plants from each population were subjected to flooded or drained conditions. The plants were cloned so that each plant was represented in each treatment. In the flooded treatment, plants were placed in domestic wash basins (four per basin) and the water level was maintained up to the soil surface. The plants were arranged randomly in unshaded outdoor conditions. Treatments were imposed for 8 weeks during the growing season.
During the seventh week, rates of photosynthesis and stomatal conductance were measured at four light levels (300, 900, 1500 and 1600 µmol m–2 s–1) using an LCA 4 Infra Red Gas Analyser (ADC Bioscientific, Hoddersdon, UK). Measurements were made in growth room conditions following a 1 d acclimatization period. CO2 was drawn in from ambient external conditions. Measurements were made on three leaves per plant following a predetermined 7 min acclimation to a given light regime. Measurements were calculated on a unit area basis; leaf area was estimated as the proportion of the leaf chamber covered by the leaf.
Differences in photosynthetic rates and stomatal conductance between the populations in the different water regimes and at the different light levels were analysed using ANOVA. Data were analysed using DataDesk 6·0 (Data Description Inc., Ithaca, New York, USA). As the four light levels were applied to each replicate leaf, these replicates were nested within clones. The clones were nested within populations. Bonferroni post hoc tests were used to analyse any significant interactions as the sample sizes were uneven.
Measurements of stomatal index in seed‐derived stock
Populations: Cooloorta, Lough Gealáin and Hawkhill (turlough), Dodder and Bull Island (ruderal).
Leaf impressions were made by softening a perspex slide with a drop of acetone, then pressing the leaf firmly onto the slide and allowing the perspex to solidify around the leaf surface. The leaf was then pulled off the slide leaving a negative impression. Impressions of upper and lower surfaces of six leaves (one leaf per plant) per population were made. Stomatal and epidermal cell numbers of each slide were counted on Leica microscope (10× objective) to which a JVC TIC–128 video camera was attached. The image produced was projected onto a Mitsubishi television screen. Counts were made within a 21 × 21 cm area delimited by a sheet of acetate. Five fields of view were counted per leaf surface. Stomatal index (after Salisbury, 1927) was calculated as follows:
stomatal index = stomatal number/stomatal number + epidermal cell number
The differences in stomatal indices between the habitat types and between the upper and lower surfaces were analysed using ANOVA. Replicate fields of view were nested within leaf surface, plant replicates were nested within population, and populations were nested within habitat type. Habitat type and leaf surface were crossed. The interaction was further analysed using a Scheffe post hoc test.
Chlorophyll concentrations in seed‐derived stock
Populations: Dodder (ruderal), Lough Gealáin (turlough).
Sections of leaves were removed and their area measured using a Delta T leaf area meter (Delta T, Cambridge, UK). Two leaves per plant and five plants per population were sampled. Leaves were ground in liquid nitrogen and placed in 15 ml of acetone overnight. Samples were covered with aluminium foil to exclude light to prevent the breakdown of chlorophyll a. Absorbance was read at 645, 647, 663 and 664 nm on a Pye Unicam spectrophotometer. The following formulae were used to calculate total chlorophyll and the ratio of chlorophyll a : b (Leegood, 1992):
total chlorophyll (µg mm–2) = 7·93A664 + 19·53A647
chlorophyll a (µm) = 13·19A664 – 2·57A647
chlorophyll b (µm) = 22·10A647 – 5·36A664
where Ax is absorbance at wavelength x. Results were expressed per specific leaf area.
Rates of photosynthesis and respiration in submerged seed‐derived stock
Populations: Dodder (ruderal), Lough Gealáin (turlough).
Photosynthesis and respiration of leaf material were measured in a Hansatech oxygen disc electrode (Hansatech, Pentney, UK). A small chamber enclosed in a water jacket was attached to the disc electrode. Temperature was regulated in the water jacket using a Jubalo water bath (Jubalo, Seelbach, Germany). The electrode was calibrated by allowing it to equilibrate with 2 ml of air‐saturated artificially hardened water (Table 2, very hard), circulated in the chamber by a small magnet. The amount of oxygen present in the 2 ml was measured by removing the oxygen from the solution using a few crystals of sodium dithionite, and the difference from the equilibrium trace on a chart recorder was calculated.
Table 2.
Chemical constitution (mg l–1) of solutions with increasing alkalinity (mg CaCO3 l–1)
| Chemical | Very soft | Soft | Moderately hard | Hard | Very hard |
| NaHCO3 | 12 | 48 | 96 | 192 | 384 |
| CaSO4·2H2O | 7·5 | 30 | 60 | 120 | 240 |
| MgSO4 | 7·5 | 30 | 60 | 120 | 240 |
| KCl | 0·5 | 2 | 4 | 8 | 16 |
| pH range | 6·4–6·8 | 7·2–7·6 | 7·4–7·8 | 7·6–7·8 | 8·0–8·4 |
| Alkalinity | 10–13 | 30–35 | 80–100 | 160–180 | 280–320 |
pH and alkalinity range are adapted from American Association of Public Health (1992).
Fully expanded leaf material was taken from the experimental plants. The area of these leaf samples was measured on a Delta T leaf area meter. The leaf was illuminated with saturating light, 1100 µmol m–2 s–1 (Li cor Model LI‐189 PAR sensor), provided by a tungsten filament. Rates of oxygen evolution were measured from a chart recorder following a 3 min acclimation period. The chamber was covered with aluminium foil to record dark respiration. Photosynthesis/respiration was measured on a chart recorder as the rate of oxygen evolved/consumed per unit area.
Rates of photosynthesis (corrected for dark respiration) and respiration were measured in four replicates per population at 4, 8, 12, 16 and 20 °C. Comparisons among populations subjected to the different temperatures were analysed using a two‐way factorial ANOVA.
Rates of photosynthesis (corrected for dark respiration) and respiration were also measured in four replicates per population in solutions of increasing alkalinity (Table 2). The solutions were kept at the optimal temperature (16 °C). The midpoint of the range of the alkalinity concentration was calculated to represent each water type. Comparisons among populations subjected to the different alkalinities were analysed using a two‐way factorial ANOVA.
RESULTS
Effect of flooding on photosynthesis
The turlough population had a higher rate of photosynthesis than the ruderal population at all light levels in both treatments (Fig. 1A; Table 3). Although there was a significant population × light interaction, post hoc tests did not distinguish where the different responses to light were manifested; it is likely to be due to a relatively higher increase between the two lower light levels and a relatively higher decrease in the rate of photosynthesis between the two upper light levels in the turlough population. Flooding significantly enhanced the rate of photosynthesis at all light levels in both populations. The rate of photosynthesis increased significantly between 300 and 900 µmol m–2 s–1 (post hoc test, P < 0·001), levelled off between 900 and 1500 µmol m–2 s–1 (post hoc test, P < 0·05) and there was a significant decrease in the rate of photosynthesis in both populations between 1500 and 1600 µmol m–2 s–1 (post hoc test, P > 0·001).
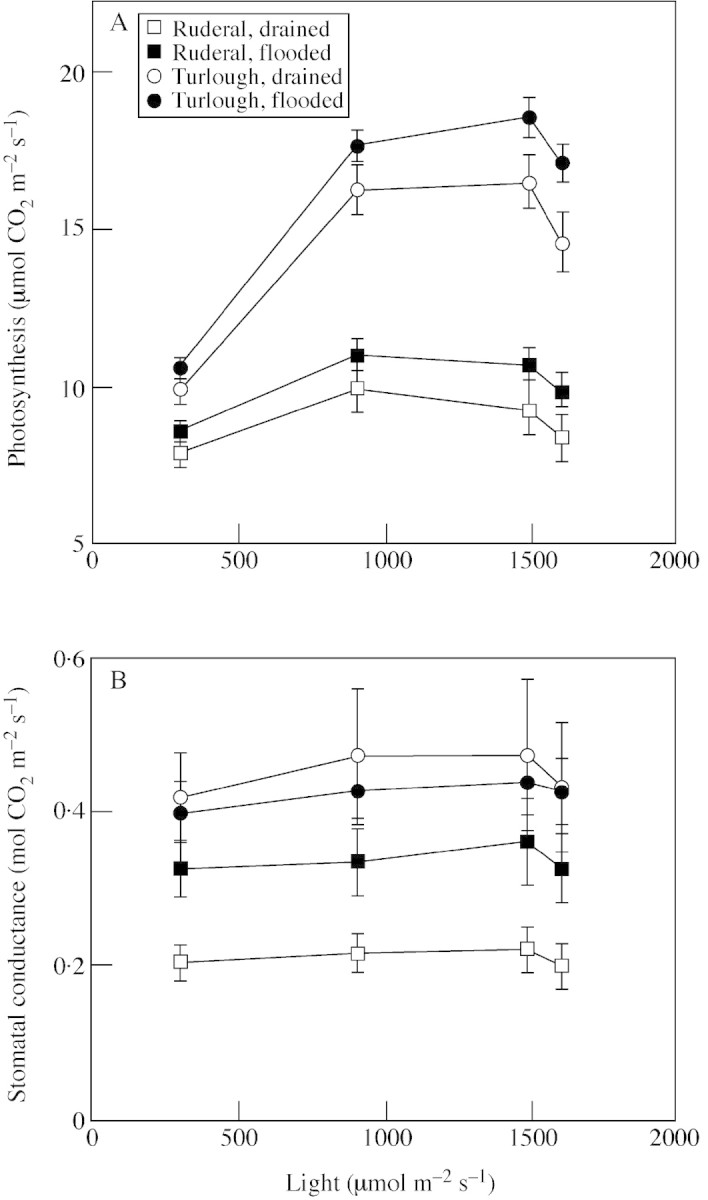
Fig. 1. The effect of flooding on photosynthesis (A) and stomatal conductance (B) in a turlough (dissected‐leaved) and ruderal (broad‐leaved) population of Ranunculus repens. Plants were flooded to the soil surface or allowed to drain freely for 7 weeks. Measurements were then made using an infrared gas analyser. Values are means of 24 replicates and bars represent s.e.m. The plants were cloned field‐collected ramets.
Table 3.
Summary of ANOVA for the effect of flooding on photosynthesis and stomatal conductance on ruderal (Dodder) and turlough (Lough Gealáin) populations of Ranunculus repens
| Dependent variables | |||
| Source of variation | d.f. | Photosynthesis | Stomatal conductance |
| Population | 1 | 52·18∗∗∗ | 8·98∗∗ |
| Clone | 14 | 1·94 | 3·99∗∗∗ |
| Treatment | 1 | 19·85∗∗∗ | 23·72∗∗∗ |
| Leaf | 32 | 3·72∗∗∗ | 2·28∗∗∗ |
| Light | 3 | 128·78∗∗∗ | 3·83∗ |
| Population × light | 3 | 43·46∗∗∗ | 0·60 |
| Clone × light | 42 | 1·42 | 2·27∗∗∗ |
| Treatment × light | 3 | 1·48 | 0·07 |
| Leaf × light | 96 | 0·26 | 0·07 |
| Population × treatment | 3 | 0·30 | 14·96∗∗∗ |
| Population × treatment × light | 1 | 0·29 | 0·04 |
Plants were either flooded or allowed to drain freely for 7 weeks.
Measurements were made at four light levels (300, 900, 1500 and 1600 µmol m–2 s–1) on three leaves per plant and eight plants per treatment per population.
*** P < 0·001; ** P < 0·01; * P < 0·05.
The turlough population had a higher stomatal conductance than the ruderal population in both treatments (Fig. 1B; Table 3). Flooding significantly increased stomatal conductance at all light levels in the ruderal population (Table 3; post hoc test, P < 0·001), but there was no significant increase in the turlough population (post hoc test, P > 0·05). Stomatal conductance increased between 300 and 900 mol m–2 s–1 in both populations, levelled off between 900 and 1500 mol m–2 s–1 and decreased at 1600 mol m–2 s–1. Although there was a significant effect of light on photosynthesis, post hoc tests were not sensitive enough to determine where the differences occurred.
Stomatal index measurements
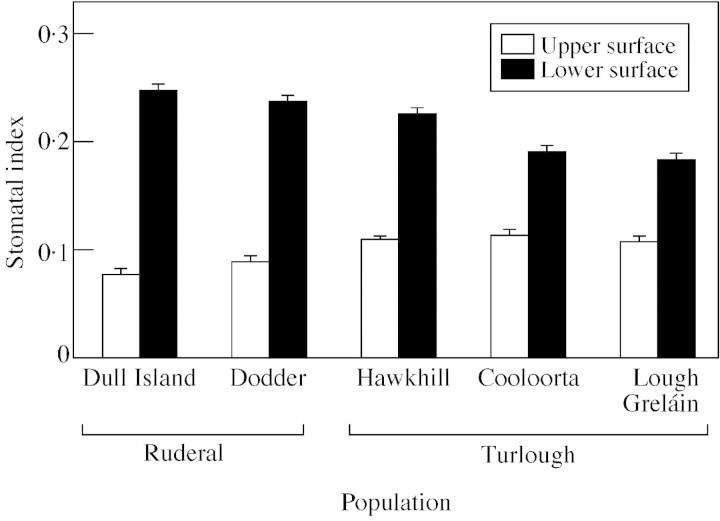
The lower leaf surface had a significantly higher stomatal index than the upper leaf surface in all populations (Fig. 2; Table 4). The turlough populations had a relatively higher index on the upper leaf surface and a relatively lower index on the lower leaf surface than the ruderal populations (Table 5). However, the differences in stomatal indices between upper and lower surfaces were significantly less marked in the turlough populations because these populations had a relatively higher stomatal index on the lower surface and a lower stomatal index on the upper surface.
Fig. 2. Stomatal index of the upper and lower leaf surfaces of ruderal (Bull Island and Dodder) and turlough (Hawkhill, Cooloorta and Lough Gealáin) populations of Ranunculus repens. Plants were grown from seed and cultivated in growth room conditions. Values are means of 30 replicates and bars represent s.e.m.
Table 4.
ANOVA of stomatal index on the upper and lower leaf surfaces of ruderal (Bull Island and Dodder) and turlough populations (Hawkhill, Cooloorta and Lough Gealáin) of Ranunculus repens
| Source of variation | d.f. | P‐values |
| Habitat type | 1 | 0·71 |
| Population | 3 | 1·69 |
| Surface | 1 | 1559·40*** |
| Surface × habitat type | 1 | 97·49*** |
| Plant | 25 | 3·28*** |
| Replicate | 8 | 0·81 |
Population was nested within habitat type, plant was nested within population, and replicate was nested within surface.
*** P < 0·001.
Table 5.
Pooled stomatal index means for habitat type on the upper and lower leaf surfaces of ruderal (Bull Island and Dodder) and turlough (Hawkhill, Cooloorta and Lough Gealáin) populations of Ranunculus repens
| Habitat type | Surface | Stomatal index | n |
| Ruderal | Lower | 0·242 ± 0·005 | 60 |
| Upper | 0·083 ± 0·004 | 60 | |
| Turlough | Lower | 0·200 ± 0·004 | 90 |
| Upper | 0·111 ± 0·002 | 90 |
Plants were grown from seed and cultivated in growth room conditions.
n = Sample size.
Errors represent s.e.m.
Chlorophyll concentrations
There were no significant differences in the chlorophyll a : b ratio or the total chlorophyll per specific leaf area (Table 6; P > 0·05) among populations.
Table 6.
Chlorophyll a : b ratio and total chlorophyll of a turlough and ruderal population of Ranunculus repens
| Population | Chlorophyll a : b | Total chlorophyll (g/SLA mm2 mg–1) |
| Ruderal | 2·89 ± 0·19 | 0·87 ± 0·07 |
| Turlough | 3·05 ± 0·12 | 0·85 ± 0·05 |
Values are the means of ten replicates, errors represent s.e.m.
Plants were grown from seed and cultivated in glasshouse conditions.
Rates of photosynthesis and respiration in submerged seed‐derived stock
The turlough population had a higher rate of respiration than the ruderal population at all temperatures (Fig. 3A; Table 7). Increasing temperature increased the rate of respiration in both populations (Fig. 3A; Table 7), although respiration in the turlough population appeared to decline at 20 °C (Fig. 3A). The turlough population also had a higher rate of photosynthesis than the ruderal population at all temperatures (Fig. 3B; Table 7). Again, the rate of photosynthesis in the turlough population appeared to decline at 20 °C (Fig. 3B).
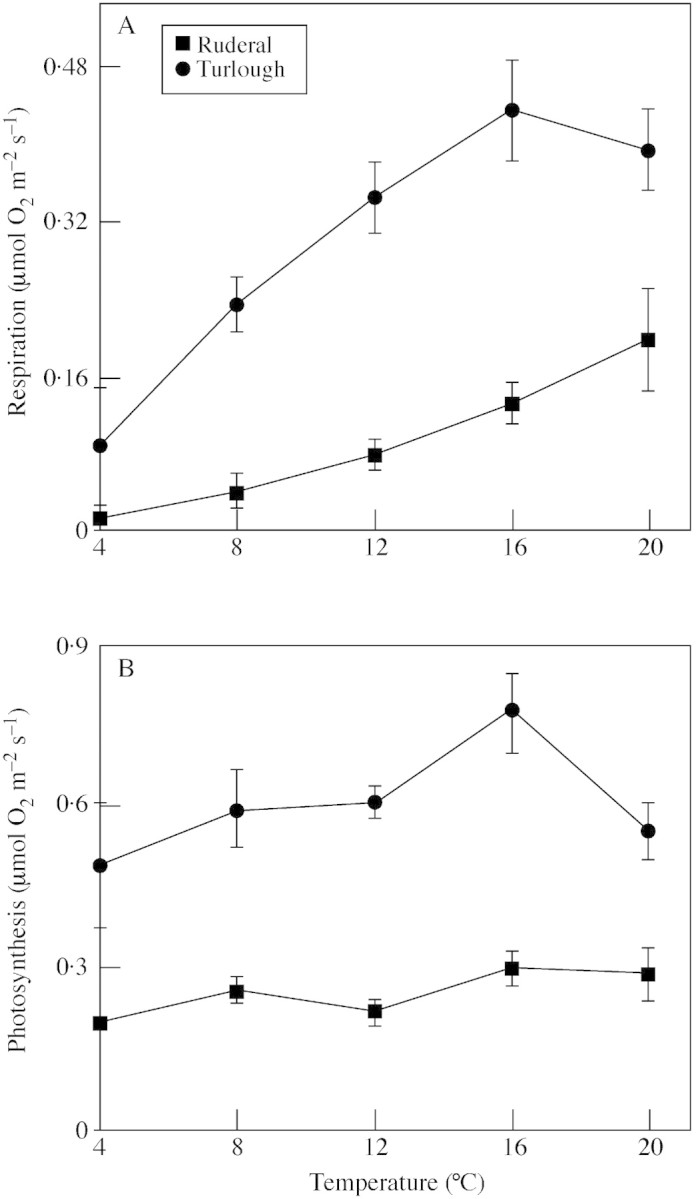
Fig. 3. The effect of temperature on the rate of respiration (A) and photosynthesis (B) in a turlough (Lough Gealáin) and ruderal (Dodder) population of Ranunculus repens. Leaf segments of known area were submerged in artificially hardened water and oxygen evolution was measured using an oxygen electrode under saturating light conditions. Plants were grown from seed. Values are means of four replicates and bars represent s.e.m.
Table 7.
ANOVA for the effect of temperature on rates of respiration and photosynthesis of submerged turlough (Lough Gealáin) and ruderal (Dodder) populations of Ranunculus repens
| Source of variation | d.f. | Respiration | Photosynthesis |
| Population | 1 | 76·12*** | 95·10*** |
| Temperature | 4 | 14·85*** | 2·86* |
| Population × temperature | 4 | 2·65 | 1·05 |
*** P < 0·001; * P < 0·05.
The turlough population had a higher rate of respiration than the ruderal population at all alkalinities (Fig. 4A; Table 8). Increasing alkalinity had no significant effect on the rate of respiration (Table 8). The turlough population had a higher rate of photosynthesis at all alkalinities than the ruderal population (Fig. 4B; Table 8). There was an effect of increasing alkalinity on the rates of photosynthesis (Table 8). The trend suggested a slight increase in the rate of photosynthesis with increasing alkalinity (Fig. 4B).
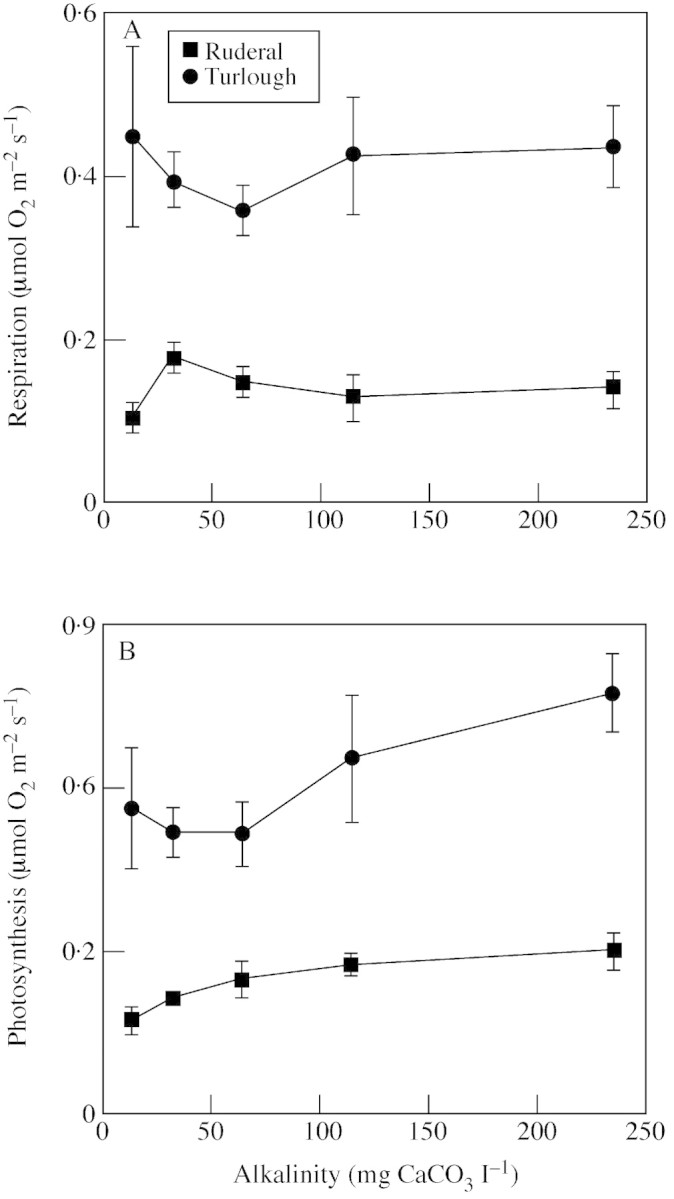
Fig. 4. The effect of alkalinity on the rate of respiration (A) and photosynthesis (B) in a turlough (Lough Gealáin) and ruderal (Dodder) population of Ranunculus repens. Leaf segments of known area were submerged in varying solutions of artificially hardened water. Oxygen evolution was measured using an oxygen electrode under saturating light conditions at 16 °C. Plants were grown from seed. Values are means of four replicates and bars represent s.e.m.
Table 8.
ANOVA for the effect of alkalinity on rates of respiration and photosynthesis of submerged turlough (Lough Gealáin) and ruderal (Dodder) population of Ranunculus repens
| Source of variation | d.f. | Respiration | Photosynthesis |
| Population | 1 | 77·34*** | 82·62*** |
| Alkalinity | 4 | 0·15 | 2·74* |
| Population × alkalinity | 4 | 0·68 | 0·84 |
*** P < 0·001; * P < 0·05.
DISCUSSION
In both populations, the rate of photosynthesis was greater in plants subjected to flooding than in those which drained freely for the same time period. This indicates that there were no internal transport problems that may have resulted in a reduction in stomatal conductance (Drew et al., 1978; Jackson and Hall, 1987; Castonguay et al., 1993) or a reduction in photosynthesis due to a negative feedback from a build‐up of photosynthates in the leaves (Barta, 1987).
Compared with the ruderal population, the turlough population had a significantly higher rate of photosynthesis at all light levels in both flooded and drained treatments. Intraspecific variation in the rate of photosynthesis has been reported in many species (Nunes et al., 1969; Patterson et al., 1977; Kumar, 1982; Hutmacher and Krieg, 1983; Wong et al., 1983; Collins et al., 1988).
In the present study, stomatal conductance was significantly higher in the turlough population. Turlough populations had a relatively higher stomatal index on the upper surface and a relatively lower index on the lower surface when compared with the ruderal populations. This may have improved the total diffusional conductance in the turlough population (Mott and Michaelson, 1991). Mott and Michaelson (1991) discussed the possibility that wider stomatal spacing in Ambrosia cordifolia, which was achieved by allocating more stomata to the upper leaf surface, may have improved total diffusional conductance. Mott and O’Leary (1984) and Kumar (1982) also reported differences in stomatal conductances between surfaces. However, Spence (1987) warned of the dangers when interpreting stomatal physiology, as stomata react to slight changes in almost every environmental parameter. There may also be errors involved in comparing leaves of different shapes because variation in stomatal characteristics may occur over the leaf surface (Smith et al., 1989). The changes in stomatal conductance across the different light levels did not mirror the rates of photosynthesis observed in either population, suggesting the involvement of other factors in the regulation of net photosynthesis. In submerged conditions stomata do not facilitate gaseous exchange (Sculthorpe, 1967), and it is therefore likely that the differences in stomatal index are related to the conditions that exist during the time when the turlough population is not submerged. Amphistomy has been associated with species where transpirational costs are not problematic (Mott et al., 1982), which is particularly the case in species that grow in damp soil. The small stature of the turlough populations and the relatively moist soils and open conditions of the turlough habitat may allow an enhanced stomatal conductance without excessive transpirational costs. The relatively higher stomatal index measured on the upper leaf of turlough populations, compared with ruderal populations, may have evolved in response to a more amphibious lifestyle.
Chlorophyll concentration did not differ between the populations. Apart from differences in internal photosynthetic mechanisms, the other possible explanation for the observed differences in photosynthetic capacity is the difference in leaf shape. Rates of diffusion across the leaf/environment interface are governed to a great extent by structural features (Lewis, 1972). The smaller, more dissected and glabrous leaf of the turlough population (Lynn and Waldren, 2001) may have a relatively thinner boundary layer than that of the ruderal population. This thinner boundary layer may reduce diffusional resistance and thus enhance photosynthesis (Raschke, 1960; Vogel, 1970; Balding and Cunningham, 1976; Gurevitch and Schuepp, 1990).
At 20 °C and 1100 µmol m–2 s–1 (saturating light), submerged photosynthesis in both populations was about 5 % of the aerial rate (measured in growth room temperatures and providing that 1 mole of CO2 released by photosynthesis measured by the IRGA is equivalent to 1 mole of O2 taken up by the process of photosynthesis measured by the oxygen electrode). These estimates, based on the chemical composition of the experimental media, indicate that neither population was able to utilize bicarbonate to any great extent, a characteristic reported for several macrophytes (Thai et al., 1976; Allen and Spence, 1981; Maberly and Spence, 1983; Laan and Blom, 1990; He et al., 1999). However, photosynthesis in the turlough population increased to an apparently greater extent in the higher alkalinities than that in the ruderal population. This suggests that the turlough population has a greater ability to utilize bicarbonate. Allen and Spence (1981) demonstrated that there was no discrete bicarbonate ‘user’ and ‘non‐user’ category, but that at a given alkalinity there was a gradation of bicarbonate use. Alternatively, Laan and Blom (1990) stated that in solutions of increasing bicarbonate concentration, the absolute amount of dissolved CO2 increases, although most of it is shifted into the bicarbonate form due to the corresponding increase in pH. The increase in photosynthesis at higher alkalinities may therefore have been due to an increase in the absolute amount of dissolved CO2, although this increase is likely to have been very small.
Respiration measurements are difficult to interpret, as the respiratory activity of plants in the light varies between 25 and 100 % of the dark respiratory activity due to an inhibition of respiration during photosynthesis (Krömer, 1995). The rate of respiration increased with increasing temperature to an apparently greater extent than did photosynthesis. It is possible that the low rate of photosynthesis observed under submerged conditions may be insufficient to cover the costs of maintenance respiration at higher temperatures, and whole plant systems may have to rely on reserves under these conditions. Compared with ruderal populations, turlough populations of Ranunculus repens had higher concentrations of total non‐structural carbohydrates in their roots (Lynn, 1998), thus enhancing their chances of survival following extensive bouts of submergence.
The consistently higher rates of respiration measured in the turlough population compared with the ruderal population reinforce the hypothesis that differences in gas exchange are a result of structural rather than biochemical attributes. These differences may be attributable to characteristics of the leaves, with the turlough population achieving higher rates of photosynthesis because they have more dissected glabrous leaves, and thus a smaller boundary layer around leaves.
These findings suggest that differences in leaf morphology between turlough and more typical populations of Ranunculus repens have resulted in enhanced gas exchange. This characteristic may lead to increased concentrations of storage carbohydrates for use in times of inundation when rates of photosynthesis are low in turlough populations.
ACKNOWLEDGEMENTS
Thanks to Drs Mike Williams and John Clifton‐Brown for technical assistance. This research was funded by the National Heritage Council of Ireland. D.E.L. was in receipt of a Forbairt research studentship.
Supplementary Material
Received: 22 October 2001; Returned for revision: 8 February 2002; Accepted: 28 February 2002.
References
- AllenED, Spence DHN.1981. The differential ability of aquatic plants to utilise the inorganic carbon supply in fresh waters. New Phytologist 87: 269–283. [Google Scholar]
- AmericanAssociationofPublicHealth.1992. In: Clesceri CS, Greenberg AE, Eaton AD, Franson MAH, eds. Standard methods for the examination of water and waste water. Washington: American Association of Public Health. [Google Scholar]
- ArmstrongW, Justin SHFW, Beckett PM, Lythe SM.1991. Root adaptation to waterlogging. Aquatic Botany 39: 57–73. [Google Scholar]
- BaldingFR, Cunningham GL.1976. A comparison of heat transfer characteristics of simple and pinnate leaf models. Botanical Gazette 137: 65–74. [Google Scholar]
- BartaAL.1987. Supply and partitioning of assimilates to roots of Medicago sativa L. and Lotus corniculatus L. under anoxia. Plant, Cell and Environment 10: 151–156. [Google Scholar]
- BowesG, Salvucci ME.1989. Plasticity in the photosynthetic carbon metabolism of submersed aquatic macrophytes. Aquatic Botany 34: 233–266. [Google Scholar]
- BradfordKJ, Hsiao TC.1982. Stomatal behaviour and water relations of waterlogged tomato plants. Plant Physiology 70: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BraendleR, Crawford RMM.1987. Rhizome anoxia tolerance and habitat specialization in wetland plants. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific Publications. [Google Scholar]
- CastonguayY, Nadeau P, Simard RR.1993. Effects of flooding on carbohydrate and ABA levels in roots and shoots of alfalfa. Plant, Cell and Environment 16: 695–702. [Google Scholar]
- CollinsRP, McNally SF, Simpson DA, Jones MB.1988. Studies on infraspecific variation in Cyperus longus L. from Europe. New Phytologist 110: 279–289. [Google Scholar]
- DrewMC, Sisworo EJ, Saker LR.1978. Alleviation of waterlogging damage to young barley plants by application of nitrate and a synthetic cytokinin, and comparison between the effects of waterlogging, nitrogen deficiency and root excision. New Phytologist 82: 312–329. [Google Scholar]
- GivinishT.1979. On the adaptive significance of leaf form. In: Solbrig OT, Jain S, Johnson GB, Raven PH, eds. Topics in plant population biology. London: Macmillan. [Google Scholar]
- GurevitchJ, Schuepp PH.1990. Boundary layer properties of highly dissected leaves: an investigation using an electrochemical fluid tunnel. Plant, Cell and Environment 13: 783–792. [Google Scholar]
- HarperJL.1957. Biological flora of the British Isles: Ranunculus acris L., R. repens L., and R. bulbosus L. Journal of Ecology 45: 289–342. [Google Scholar]
- HeJB, Bogemann GM, van de Steeg HM, Rijnders JGHM, Voesenek LACJ, Blom CWPM.1999. Survival tactics of Ranunculus species in river floodplains. Oecologia 118: 1–8. [DOI] [PubMed] [Google Scholar]
- HutmacherRB, Krieg DR.1983. Photosynthetic rate control in cotton. Stomatal and nonstomatal factors. Plant Physiology 73: 658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JacksonMB, Hall KC.1987. Early stomatal closure in waterlogged pea plants is mediated by abscisic acid in the absence of foliar water deficits. Plant, Cell and Environment 10: 121–130. [Google Scholar]
- KrömerS.1995. Respiration during photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 46: 45–70. [Google Scholar]
- KumarPA.1982. Correlation between photosynthetic rate and other physiological characteristics in seven tobacco cultivars. Photosynthetica 16: 564–567. [Google Scholar]
- LaanP, Blom CWPM.1990. Growth and survival responses of Rumex species to flooded and submerged conditions: the importance of shoot elongation, underwater photosynthesis and reserve carbohydrates. Journal of Experimental Botany 41: 775–783. [Google Scholar]
- LeegoodRC.1992. Carbon metabolism. In: Hall DO, Scurlock JMO, Bolhar‐Nordenkampf HR, Leegood RC, Long SP, eds. Photosynthesis and production in a changing environment – a field and laboratory manual. New York: Chapman and Hall. [Google Scholar]
- LewisMC.1972. The physiological significance of variation in leaf structure. Science Progress 60: 25–51. [Google Scholar]
- LynnDE.1998. Morphological and physiological variation in the turlough form of Ranunculus repens. PhD Thesis, University of Dublin. Ireland. [Google Scholar]
- LynnDE, Waldren S.2001. Morphological variation in populations of Ranunculus repens from the temporary limestone lakes (Turloughs) in the West of Ireland. Annals of Botany 87: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MaberlySC, Spence DHN.1983. Photosynthetic inorganic carbon use by freshwater plants. Journal of Ecology 71: 705–724. [Google Scholar]
- MadsenTV, Sand‐Jensen K.1991. Photosynthetic carbon assimilation in aquatic macrophytes. Aquatic Botany 41: 5–40. [Google Scholar]
- MottKA, O’Leary JW.1984. Stomatal behaviour and CO2 exchange characteristics in amphistomatous leaves. Plant Physiology 74: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MottKA, Michaelson O.1991. Amphistomy as an adaption to high light intensity in Ambrosia cordifolia (Compositae). American Journal of Botany 78: 76–79. [Google Scholar]
- MottKA, Gibson AC, O’Leary JW.1982. The adaptive significance of amphistomatic leaves. Plant, Cell and Environment 5: 455–460. [Google Scholar]
- NunesMA, Bierhuizen JF, Ploegman C.1969. Studies on productivity of coffee. III. Differences in photosynthesis between four varieties of coffee. Acta Botanica Neerlandica 18: 420–424. [Google Scholar]
- PattersonDT, Bunce JA, Alberte RS, Van Volkenburgh E.1977. Photosynthesis in relation to leaf characteristics of cotton from controlled and field environments. Plant Physiology 5: 384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PonnamperumaFN.1984. Effect of flooding on soils. In: Kozlowski TT, ed. Flooding and plant growth. London: Academic Press. [Google Scholar]
- RaschkeK.1960. Heat transfer between the plant and the environment. Annual Review of Plant Physiology 11: 111–126. [Google Scholar]
- RidgeI.1985. Ethylene and petiole development in amphibious plants. In: Roberts JA, Tucker GA, eds. Ethylene and plant development. London: Butterworths. [Google Scholar]
- SalisburyEJ.1927. On the causes and ecological significance of stomatal frequency, with special reference to woodland flora. Philosophical Transactions of the Royal Society B 431: 1–65. [Google Scholar]
- Sand‐JensenK.1987. Environmental control of bicarbonate use among freshwater and marine macrophytes. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific Publications. [Google Scholar]
- SculthorpeCD.1967. The biology of aquatic vascular plants. Ireland: Edward Arnold. [Google Scholar]
- SmithFA, Walker NA.1980. Photosynthesis by aquatic plants: effects of unstirred layers in relation to assimilation of CO2 and HCO3– and to carbon isotopic discrimination. New Phytologist 86: 245–259. [Google Scholar]
- SmithS, Weyers JDB, Berry WG.1989. Variation in stomatal characteristics over the lower surface of Commelina communis leaves. Plant, Cell and Environment 12: 653–659. [Google Scholar]
- SmitsAJM, Laan P, Thier RH, van der Velde G.1990. Root aerenchyma, oxygen leakage patterns and alcoholic fermentation ability of the roots of some nymphaeid and isoetid macrophytes in relation to the sediment type of their habitat. Aquatic Botany 386: 3–17. [Google Scholar]
- SpenceRD.1987. The problem of variability in stomatal responses, particularly aperture variance, to environmental and experimental conditions. New Phytologist 107: 303–315. [DOI] [PubMed] [Google Scholar]
- ThaiK, Haller WT, Bowes G.1976. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiology 58: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VogelS.1970. Convective cooling at low airspeeds and the shapes of broad leaves. Journal of Experimental Botany 21: 91–101. [Google Scholar]
- WongJHH, Randall DD, Nelson CJ.1983. Photosynthesis in tall fescue. IV. Carbon assimilation pattern in two genotypes of tall fescue differing in net photosynthesis rates. Plant Physiology 72: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.