Abstract
It is known that roots can respond to patches of fertility; however, root proliferation is often too slow to exploit resources fully, and organic nutrient patches may be broken down and leached, immobilized or chemically fixed before they are invaded by the root system. The ability of fungal hyphae to exploit resource patches is far greater than that of roots due to their innate physiological and morphological plasticity, which allows comprehensive exploration and rapid colonization of resource patches in soils. The fungal symbionts of ectomycorrhizal plants excrete significant quantities of enzymes such as chitinases, phosphatases and proteases. These might allow the organic residue to be tapped directly for nutrients such as N and P. Pot experiments conducted with nutrient‐stressed ectomycorrhizal and control willow plants showed that when high quality organic nutrient patches were added, they were colonized rapidly by the ectomycorrhizal mycelium. These established willows (0·5 m tall) were colonized by Hebeloma syrjense P. Karst. for 1 year prior to nutrient patch addition. Within days after patch addition, colour changes in the leaves of the mycorrhizal plants (reflecting improved nutrition) were apparent, and after 1 month the concentration of N and P in the foliage of mycorrhizal plants was significantly greater than that in non‐mycorrhizal plants subject to the same nutrient addition. It seems likely that the mycorrhizal plants were able to compete effectively with the wider soil microbiota and tap directly into the high quality organic resource patch via their extra‐radical mycelium. We hypothesize that ectomycorrhizal plants may reclaim some of the N and P invested in seed production by direct recycling from failed seeds in the soil. The rapid exploitation of similar discrete, transient, high‐quality nutrient patches may have led to underestimations when determining the nutritional benefits of ectomycorrhizal colonization.
Key words: Hebeloma syrjense P. Karst., ectomycorrhizas, nutrient patches, soil heterogeneity, mycelium, exo‐enzymes, organic nutrients, competition for nutrients, chlorophyll meter
INTRODUCTION
Soil is an innately heterogeneous environment that roots must explore in order to acquire nutrients and water (Jackson and Caldwell, 1993; Stark, 1994; Farley and Fitter, 1999). Under experimental conditions, roots have been shown to respond to patches of fertility through increased branching of higher order laterals (see Robinson, 1994). However, root proliferation is often too slow to exploit resources fully (Eissenstat and Caldwell, 1988; Jackson and Caldwell, 1989). Although many studies have involved the introduction of inorganic nutrient patches into the soil (or other growth substrate), in natural ecosystems nutrient patches are likely to be associated with decomposing organic residues. In unfertilized soils, organic patches are experimentally much more appropriate than those involving the addition of mineral salts. The composition of organic patches that might be exploited by ectomycorrhizal fungi has been suggested previously (Tibbett et al., 1998b; Tibbett, 2000), and include faeces, dead seeds and fruits, pollen or fungal, bacterial and invertebrate necromass (Hutchison and Barron, 1997; Zackrisson et al., 1999). These may be broken down and the products leached, immobilized or chemically fixed by the soil microbiota before the patches are invaded by the root system (see van Vuuren et al., 1996; Hodge et al., 1998, 1999).
Fungal mycelia are capable of rapid and comprehensive exploration and colonization of nutrient patches (Boddy, 1999). These capabilities, combined with the physiological plasticity of mycelium (Cairney and Burke, 1996), make fungi potentially more efficient than plant roots at foraging for and exploiting discrete resources. A recent study pursuing this concept for pollen with ectomycorrhizal [Paxillus invoalutus (Fr.) Fr.] Betula pendula Roth. concluded that fungus could scavenge effectively for N and P in the pollen (Perez‐Moreno and Read, 2001).
Where roots lack the morphological plasticity required to respond to transient nutrient patches, mycorrhizal fungi may be important (Tibbett, 2000). The association of plant roots with mycorrhizal fungi may provide the autotroph with a more favourably distributed and extensive system of absorbing organs (Smith and Read, 1997) and with a greater chance of encountering fertile microsites not available to the root system alone. Where improvements in plant nutrition have resulted from ectomycorrhizal symbiosis, the provision thereby of an increased surface area for nutrient absorption has been used to explain these improvements (Harley, 1969). There is now increasing evidence that extra‐radical mycelium can also play a direct role in the mobilization through exo‐enzymes, absorption and transport of key nutrients from the soil into the host plant (Bending and Read, 1995a, b; Perez‐Moreno and Read, 2000, 2001). The high physiological and morphological plasticity of the extra‐radical mycelium of the ectomycorrhizas seems to suit it for the exploitation of soil nutrient heterogeneity. This may be particularly important for exploiting the high concentrations of nutrients found in some organic resources.
If ectomycorrhizal plants have access to these high quality but transient resources, the fungus may make a major contribution to plant nutrition in otherwise infertile soils. To investigate this possibility, ectomycorrhizal and non‐ectomycorrhizal willow plants were grown under nutrient‐starved conditions. Dead seeds were added as a nutrient treatment. The N and P status of the foliage was subsequently compared for mycorrhizal and non‐mycorrhizal treatments.
MATERIALS AND METHODS
Preliminary axenic trial
A strain of Hebeloma syrjense P. Karst. Syrjäntympönen which had been shown to grow well on amino acids and peptides and to produce extracellular proteases was selected for this study (strain W; Tibbett et al., 1999). To be certain that this strain was able to utilize the nutrient reserves in dead seeds, its ability to grow on autoclaved bean seeds in vitro was tested. Six autoclaved cotyledons were placed in 9 cm diameter Petri dishes containing 25 ml water agar. The centre of each cotyledon was inoculated with 4‐mm plugs of strain W cut from the actively growing edge of mycelium maintained on modified Melin Norkrans (MMN) medium. Mycelial plugs were carefully placed on the cotyledon to ensure there was no contact between the inoculum and the agar. Petri dishes were incubated for 50 d at 22 °C. Cotyledons were prepared for the scanning electron microscope (SEM). Contents of Petri dishes were fixed with 2·5 % glutaraldehyde in 0·1 m phosphate buffer. After two washes in 0·1 m phosphate buffer, cotyledons, cut from the agar, were dehydrated for 30 min in each of 20, 40, 60, 80 and 100 % ethanol. Samples were then critical‐point dried with liquid CO2, attached to SEM stubs (using Leit‐C carbon cement) and sputter coated with gold to a thickness of 60 nm with a Polcron ES 300 freeze drier sputter coater. Prepared cotyledons were observed and imaged with a Cam Scan Series 3/30 SEM.
Plant and fungal material
Flat‐sided sterile glass jars (2 l) containing 800 ml of heat sterilized (93 °C for 1 h) pure sphagnum peat and horticultural vermiculite (50 : 50) were prepared. Cuttings from an individual Salix hybrid from Yorkshire, UK, were trimmed to three to five nodes (approx. 8 cm) and inserted into the substrate to a depth of 4 cm, and the substrate adjusted to field capacity. To prevent mycorrhizal colonization from exogenous spores, 500 ml transparent plastic cups were placed over the tops of the jars to form a tight seal. Light was excluded from the root zone by covering the bottom half of the jars in black (photographic grade) plastic bags. Plants were transferred to an air filtered growth room at 16 °C under a 16 h photoperiod (photon flux density 250 µmol m2 s–1). Roots were inoculated with an ectomycorrhizal fungus after 60 d. Fungal inoculum was prepared in flat‐bottomed flasks (1 l), each containing 250 ml of growth medium described elsewhere (Tibbett et al., 1998a) and were inoculated with 10 × 7 mm plugs of H. syrjense cut from the actively growing edge of mycelia maintained on MMN medium. After 50 d growth in the dark at 22 °C, the mycelia were transferred to a sterile salt solution containing (l–1) 200 mg (NH4)2HPO4, 125 mg KH2PO4, 100 mg MgSO4.7H2O, 50 mg CaCl.6H2O and 13 mg FeEDTA. After mixing in a food processor (5 s), 20 ml of this hyphal spawn was injected in small aliquots close to root tips in each of 12 randomly selected jars. A further 12 jars were inoculated with autoclaved spawn as non‐mycorrhizal controls.
Growth of mycorrhizal willows
The mycorrhizal status of plants was measured by counting numbers of colonized root tips visible at the side of the glass jars. After 140 d, when mycorrhizas were well established and mycelia extensive, the plastic cups were removed from the jars, and N and S added as NH4NO3 and MgSO4 (25 mg per plant). Subsequent watering was with deionized water as required every 3–4 d. After 230 d, severe symptoms of nutrient deficiency were apparent, and included leaf necrosis and reduced mycorrhizal mycelium. An addition of P‐free nutrient (25 ml per jar at 1 g l–1) (Vitafeed 101; Vitax Ltd, Leicester, UK) reversed these symptoms. The photoperiod was then reduced to 4·5 h and the growth chamber cooled from 16 to 6 °C over 40 d. After the production of a dormant apical bud the plants were exposed to 4 °C in complete darkness for 5 d. Subsequently, temperature and photoperiod were gradually increased to 20 °C and 16 h, respectively. All plants reached bud break after 25 d of increased light and temperature. After 330 d, symptoms of nutrient deficiency again became apparent, but less so in the mycorrhizal plants.
One year after mycorrhizal inoculation, plants were up to 0·5 m tall. Six autoclaved bean cotyledons (approx. 400 mg d. wt per cotyledon, 4·5 % N, 1·3 % P) were inserted between the glass and the substrate of six mycorrhizal willow plants and six non‐mycorrhizal willow plants. This gave a treatment structure of mycorrhizal plants with (+M+B) and without (+M–B) seeds, and non‐mycorrhizal plants with (–M+B) and without (–M–B) seeds, each replicated six times. After a further 35 d, chlorophyll measurements (SPAD units) were taken on 15 leaves from each plant using a Minolta SPAD‐502 chlorophyll meter, and the mean recorded. SPAD units are a nominal scale based on the optical density difference at two wavelengths: 650 mm (peak chlorophyll absorbance) and 940 mm (non‐chlorophyll absorbance) (Minolta, 1989; Shapiro, 1999). The foliage was then harvested, dried (80 °C), weighed and analysed for N and P content.
Nutrient analysis
For N, leaf samples were digested for 24 h in 10 ml conc. H2SO4 using a catalyst made up of one part Se, six parts CuSO4.H2O and 100 parts K2SO4 in a Gerhardt Kjeldatherm, and made up to 100 ml with distilled H2O. NH4+ was released by the addition of 30 % NaOH. This was distilled into 0·1 m H2SO4 using a Buchi 321 distiller. Distillate was back titrated with 0·2 m NaOH for NH4+ determination. For P, ashed (500 °C) leaf samples were dissolved in 10 ml 6 m HCl and boiled to dryness. The sediment was re‐dissolved in 10 ml 2 m HCl on a hot plate, made up to 50 ml and filtered (Whatman No. 40). Phosphate in solutions was analysed on a Cobas Myra auto‐analyzer using the ammonium molybdate method. Data were analysed by two‐way ANOVA, and means compared by least significant difference tests at the 5 % level.
RESULTS
Preliminary axenic trial
The fungus grew rapidly into, around and through the bean cotyledon. This was evident from the SEM images of the surface and interior of the cotyledons (Fig. 1). A weft of mycelium and rhizomorph‐like structures was visible on the cotyledon surface and the interior was flush with hyphae which surrounded individual cells.
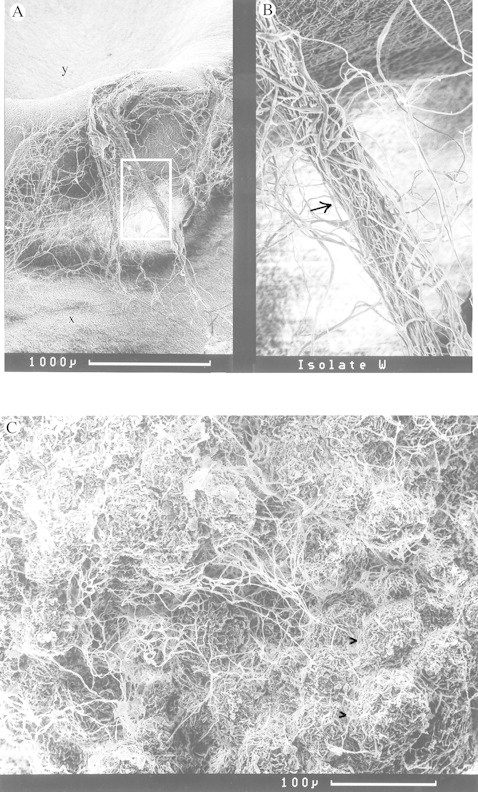
Fig. 1. A, Scanning electron micrographs of bean cotyledon surface colonized by mycelium of Hebeloma syrjense (x). Rhizomorph‐like structures (boxed, and enlarged in B and arrowed) can be seen extending from the original inoculum plug (y) to the cotyledon surface densely covered by mycelium. C, SEM of interior of bean cotyledon invaded by mycelium of H. syrjense. Individual cells (arrowed bulbous structures) are surrounded and obscured by a dense net of hyphae.
Mycorrhizal willows
Mycorrhizas were evident at the side of the jars after 40 d, and increased to a mean of 34 per jar after 1 year. Non‐mycorrhizal treatments remained free of mycorrhizal colonization throughout the experiment. There were no obvious differences in the mycorrhizal and non‐mycorrhizal treatments, an observation borne out by subsequent analysis.
Organic patch addition
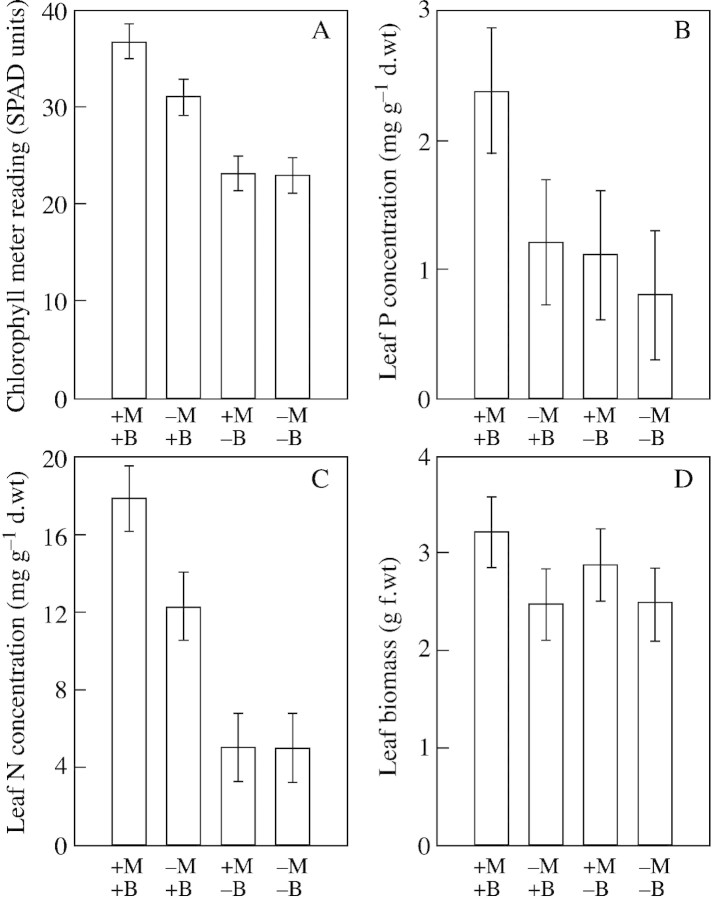
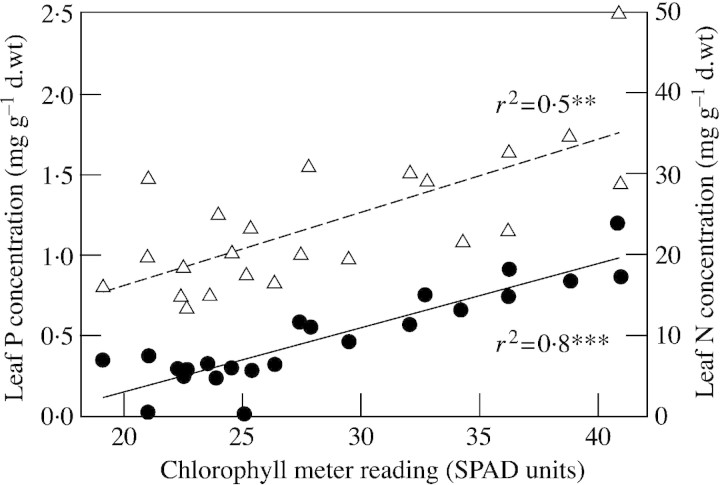
Seven days after the introduction of nutrient patches there was a visible response in the growth of the trees. Most striking was a clear change in the colour of the foliage (Figs 2 and 3). These colour differences remained discernible until harvest. Plants with mycorrhizas and nutrient patch (cotyledon) addition (+M+B) rapidly acquired denser, darker foliage, while non‐mycorrhizal plants without nutrient patch addition (–M–B) remained thin and spindly with pale, partially senescent leaves, and were clearly under nutrient stress. Treatments with either cotyledons only (–M+B), or mycorrhizas only (+M–B) showed an intermediate response. These visual observations were in agreement with chlorophyll meter readings, and N and P leaf tissue concentrations (Fig. 4A–C), as +M+B plants had significantly (P < 0·05) higher values for each measurement. Chlorophyll readings correlated well with N and P concentrations (Fig. 5). Leaf biomass was only slightly greater in the +M+B treatment compared with –M treatments (Fig. 4D).

Fig. 2. Ectomycorrhizal (with Hebeloma syrjense) (+M) and non‐ectomycorrhizal (–M) willow trees (Salix sp.) with (+B) and without (–B) organic nutrient patch addition, showing typical growth and colour differences at harvest. Note bean cotyledons can be seen heavily colonized with mycelium in +M+B treatment only (white patches in substrate in jars).

Fig. 3. Aerial view of ectomycorrhizal (with Hebeloma syrjense) (+M) and non‐ectomycorrhizal (–M) willow trees (Salix sp.) with (+B) and without (–B) organic nutrient patch addition, showing typical growth and colour differences at harvest. Hidden label (top left) reads +M+B.
Fig. 4. Chlorophyll meter readings (SPAD units, optical density difference at two wavelengths: 650 mm and 940 mm) (A), and leaf tissue P (B) and N (C) concentrations, and leaf biomass (D) in mycorrhizal (+M) and non‐mycorrhizal (–M) willows with (+B) and without (–B) organic nutrient patch addition. Bars = 95 % LSD.
Fig. 5. Correlation between chlorophyll meter readings and leaf tissue N (circles) and P (triangles) concentrations in willow trees (Salix sp.).
DISCUSSION
Evidence that ectomycorrhizal fungi are closely involved in decomposition processes in the soil is provided by their production of exo‐enzymes, almost certainly involved in liberating nutrients from organic substrates (Bending and Read, 1995b; Leake and Read, 1997; Perez‐Moreno and Read, 2001). In ericoid mycorrhizal systems, the ability of the fungal symbiont to utilize N from mycorrhizal necromass has also been demonstrated. (Kerley and Read, 1997, 1998). Nutrient patch exploitation by arbuscular mycorrhizal fungi has been debated (Tibbett, 2000; Hodge 2001), and recently demonstrated for one fungus (Hodge et al., 2001).
The ability of H. syrjense to utilize cotyledons as a sole source of nutrients was clear from the preliminary axenic trial in which cotyledons were penetrated and individual cells in the interior of the seed were surrounded by mycorrhizal hyphae. By mobilizing resources stored in the cotyledon (presumably C, N and P), the fungus grew almost as well on the water agar as would have be expected on a standard laboratory growth medium such as potato dextrose agar.
The cotyledons added as nutrient patches to the substrate of mycorrhizal plants seemed almost exclusively to be invaded by hyphae of the symbiont, and were seen to be connected directly to mycorrhizal roots by the mycelial network. The colonization of the cotyledons was so extensive that it can be seen as white flecks in the jar of the +M+B willow in Fig. 2. This occurred despite non‐sterile conditions and resulting competition from free‐living saprotrophs. Hebeloma spp. are known to secrete enzymes capable of hydrolysing the proteins and phytate found in seeds (Tibbett et al., 1998a, 1999), and the ability to exploit such an organic resource was anticipated (see Tibbett et al., 1998b). However, the rapid and apparently comprehensive utilization of the cotyledon (while still of high resource quality), at the expense of other free‐living microorganisms that colonized the cotyledons heavily in the –M jars, was unexpected. This indicates that ectomycorrhizal fungi may be very effective in utilizing high quality substrates even when in direct competition with microorganisms lacking a carbon supply from an autotrophic partner. Recent studies have concluded that an active microbial community may outcompete roots for organic N (van Vuuren et al., 1996; Hodge et al., 1998, 1999). However, in some circumstances, ectomycorrhizal fungi may be able to compete effectively with the wider microbiota for nutrients in high quality patches.
It is important to note that in the current experiment, in contrast to most others cited, the root system was well developed prior to the introduction of nutrient patches, and that a developing root system may have responded differently. An established mycorrhizal root system (in which fungus and root explore the same volume of soil) into which organic nutrient patches are placed may provide a more suitable ‘natural ecosystem’ scenario than experiments with pre‐existing patches towards which an immature developing mycorrhizal root system can extend. Developed root and mycorrhizal systems provide stable circumstances in which to determine the effect of nutrient patch addition as the sole abiotic and biotic factor. Developing root and mycorrhizal systems are more representative of disturbed systems and would favour roots active during seedling establishment and growth.
Despite 335 d of mycorrhizal colonization, the effect of mycorrhizas only (+M–B vs. –M–B) was not significantly different for any parameter measured (Fig. 4). However, the effect of mycorrhizas and organic nutrient patch addition (+M+B vs. +M–B) accounted for a significant increase in plant nutrient status within 30 d. This suggests the nutritional benefits of ectomycorrhizal symbiosis may have been underestimated when only blanket organic or mineral nutrition is considered. These data suggest that access to both temporally and spatially discrete high quality organic nutrient patches may have been overlooked and could be important in the functioning of mycorrhizal symbiosis.
A key question is what proportion of the nutrients added were transferred via the mycelium and symbiotic organs to the leaves of the host tree. As known amounts of N and P were added to the substrate in the cotyledons, the percentages of these amounts that were transferred to the leaves can be calculated from the difference between +B and –B treatments. Mycorrhization had a striking effect on transfer of N and P from nutrient patches into the foliage. Twelve per cent of the N and 8 % of the P added were transferred in the mycorrhizal plants, compared with only 7 % of the N and 1 % of the P when plants were non‐mycorrhizal. This is considerable given the time‐frame (35 d) and the likelihood that a significant fraction of N and P was retained in the lower parts of the plant and the mycelium. Only 29 % N and 25 % P were transferred to whole plants of B. pendula in symbiosis with P. involutus after 115 d growth with pollen as the source of nutrients (Perez‐Moreno and Read, 2001). The appropriate biochemical quality of seeds as a mycorrhizal substrate (susceptible to phosphatases and proteases), and their introduction into an established root and mycorrhizal system, probably account for the remarkable soil‐to‐leaf transfer. While we recognize pot‐based experiments favour observations of such transfer compared with natural ecosystems, we suggest this is an important mechanism in returning nutrients invested in seed production to the autotroph, especially in nutrient‐poor systems.
Since the substrate was not sterile, cotyledons added to non‐mycorrhizal jars were subject to opportunistic invasion and degradation by free‐living bacteria and fungi, and the non‐mycorrhizal plants were probably able to utilize the products of this degradation, including N and P. Despite this, free‐living micoorganisms were apparently not good competitors against mycorrhizal H. syrjense.
The greater difference in P transfer (compared with N transfer) from cotyledons to leaves when the plants were mycorrhizal may have been a consequence of a high demand for P since no P fertilizer was ever added. The enhanced uptake of relatively immobile PO4– ions has long been recognized as a feature of mycorrhizal colonization (see Harley, 1969).
The effect of prior mycorrhizal colonization appears to have been more important to leaf growth than the subsequent organic nutrient addition, despite the nutritional benefits of the latter. As only a short growing period was allowed after the introduction of the cotyledons, there was a small response in leaf growth in the +B treatments compared with the large changes in leaf nutrient status and colour. A longer period after the addition of cotyledons would almost certainly have led to discernible growth responses. For both P and N concentration in the leaves, the chlorophyll meter, and to some extent the human eye, proved remarkably predictive (Figs 2, 3 and 5).
We conclude that plants unable to form mycorrhizas in a soil prolific with mycorrhizal mycelium may have difficulty in acquiring nutrients from transient and discrete organic sources, as these will rapidly disappear into the micro‐ and macrobiota. Trees and shrubs colonized by mycorrhizal fungi possessing mycelia of high extracellular enzyme activity, and hence capable of rapidly exploiting organic nutrients, should benefit most in soils of low mineral nutrient status, such as those of arctic, alpine and tropical savannah environments. Compared with soils of temperate ecosystems, the native organic matter is largely recalcitrant, and annual leaf litter inputs are generally smaller in amount and poorer in quality. Inputs of high quality resources (e.g. dead seeds, insects and pollen) may make up an important part of the nutrient supply to ectomycorrhizal trees in such soils.
ACKNOWLEDGEMENTS
We would like to thank AFS Taylor for supplying the strain of Hebeloma syrjense used in this study. This work was funded by the Nuffield Foundation and the BBSRC, UK.
Supplementary Material
Received: 13 November 2001; Returned for revision: 7 January 2002; Accepted: 1 March 2002.
References
- BendingGD, Read DJ.1995a The structure and function of the vegetative mycelium of ectomycorrhizal plants V. Foraging behaviour and translocation of nutrients from exploited litter. New Phytologist 130: 401–409. [Google Scholar]
- BendingGD, Read DJ.1995b The structure and function of the vegetative mycelium of ectomycorrhizal plants. VI. Activities of nutrient mobilising enzymes in birch litter colonised by Paxillus involutus (Fr.) Fr. New Phytologist 130: 411–417. [Google Scholar]
- BoddyL.1999. Saprotrophic chord forming fungi: meeting the challenge of heterogeneous environments. Mycologia 91: 13–32. [Google Scholar]
- CairneyJWG. Burke RM.1996. Physiological heterogeneity within fungal mycelia: an important concept for a functional understanding of the ectomycorrhizal symbiosis. New Phytologist 134: 685–695. [DOI] [PubMed] [Google Scholar]
- EissenstatDM. Caldwell MM.1988. Seasonal timing of root growth in favourable microsites. Ecology 69: 870–873. [Google Scholar]
- FarleyRA. Fitter AH.1999. Temporal and spatial variation in soil resources in a deciduous woodland. Journal of Ecology 20: 67–69. [Google Scholar]
- HarleyJL.1969. The biology of mycorrhiza. Plant Science Monographs 2nd edn. London: Leonard Hill. [Google Scholar]
- HodgeA.2001. Foraging and the exploitation of soil nutrient patches: in defence of roots. Functional Ecology 15: 416. [Google Scholar]
- HodgeA, Campbell CD, Fitter A. 2001. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413: 297–299. [DOI] [PubMed] [Google Scholar]
- HodgeA, Robinson D, Griffiths BS, Fitter AH.1999. Nitrogen capture by plants grown in N‐rich organic patches of contrasting size and strength. Journal of Experimental Botany 50: 1243–1252. [Google Scholar]
- HodgeA, Stewart J, Robinson D, Griffiths BS, Fitter AH.1998. Root proliferation, soil fauna and plant nitrogen capture from nutrient rich patches in soil. New Phytologist 139: 479–494. [DOI] [PubMed] [Google Scholar]
- HutchisonLJ, Barron GL.1997. Parasitism of pollen as a nutritional source for lignicolous basidiomycota and other fungi. Mycological Research 101: 191–194. [Google Scholar]
- JacksonRB, Caldwell MM.1989. The timing and degree of root proliferation in fertile‐soil microsites for three cold‐desert perennials. Oecologia 81: 149–153. [DOI] [PubMed] [Google Scholar]
- JacksonRB, Caldwell MM.1993. The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 74: 612–614. [Google Scholar]
- KerleySJ, Read DJ.1997. The biology of mycorrhiza in the Ericacae: XIX. Fungal mycelium as a nitrogen source for the ericoid mycorrhizal fungus Hymenoscyphus ericae (Read) Korf. & Kernan and its host plants. New Phytologist 136: 691–701. [DOI] [PubMed] [Google Scholar]
- KerleySJ, Read DJ.1998. The biology of mycorrhiza in the Ericacae: XX. Plant and mycorrhizal necromass as nitrogenous substrates for the ericoid mycorrhizal fungus Hymenoscyphus ericae and its host. New Phytologist 139: 353–360. [DOI] [PubMed] [Google Scholar]
- LeakeJR, Read DJ.1997. Mycorrhizal fungi in terrestrial habitats. In: Wicklow DT, Söderström B, eds. The Mycota vol. 4: environmental and microbial relationships Berlin Heidelberg: Springer‐Verlag, 281–301. [Google Scholar]
- Minolta.1989. SPAD 502 owners manual. Ramsey New Jersey: Minolta Corporation. [Google Scholar]
- Perez‐MorenoJ, Read DJ.2000. Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytologist 145: 301–309. [Google Scholar]
- Perez‐MorenoJ, Read DJ.2001. Exploitation of pollen by mycorrhizal mycelial systems with special reference to nutrient cycling in boreal forests. Proceedings of the Royal Society of London, Series B 268: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RobinsonD.1994. The responses of plants to non‐uniform supplies of nutrients. New Phytologist 127: 635–674. [DOI] [PubMed] [Google Scholar]
- ShapiroCA.1999. Using a chlorophyll meter to manage nitrogen applications to corn with high nitrate irrigation water. Communications in Soil Science and Plant Analysis 30: 1037–1049. [Google Scholar]
- SmithSE, Read DJ.1997. Mycorrhizal symbiosis. London: Academic Press. [Google Scholar]
- StarkJM.1994. Causes of soil nutrient heterogeneity. In: Caldwell MM, Pearcy RW, eds. Exploitation of environmental heterogeneity by plants San Diego: Academic Press, 255–284. [Google Scholar]
- TibbettM.2000. Roots, foraging and the exploitation of soil nutrient patches: the role of mycorrhizal symbiosis. Functional Ecology 14: 397–399 [Google Scholar]
- TibbettM, Sanders FE, Cairney JWG.1998a The effect of temperature and inorganic phosphorus supply on growth and acid phosphatase production in arctic and temperate strains of ectomycorrhizal Hebeloma spp. in axenic culture. Mycological Research 102: 129–135. [Google Scholar]
- TibbettM, Sanders FE, Cairney JWG, Leake JR.1999. Temperature regulation of extracellular proteases in ectomycorrhizal fungi (Hebeloma spp.) grown in axenic culture. Mycological Research 103: 707–714. [Google Scholar]
- TibbettM, Sanders FE, Minto SJ, Dowell M, Cairney JWG.1998b Utilisation of organic nitrogen by ectomycorrhizal fungi (Hebeloma spp.) of arctic and temperate origin. Mycological Research 102: 1525–1532. [Google Scholar]
- vanVuurenMMI, Robinson D, Griffiths BS.1996. Nutrient inflow and root proliferation during the exploitation of a temporally and spatially discrete source of nitrogen in the soil. Plant and Soil 178: 185–192. [Google Scholar]
- ZackrissonO, Nilsson M‐C, Jäderlund A, Wardle DA.1999. Nutritional effects of seed fall during mast years in boreal forest. Oikos 84: 17–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.