Abstract
Using fluorescence microscopy, deposition of pollen on stigmas and pollen tube growth in the gynoecium of Sagittaria potamogetifolia Merr., a monoecious species with an apocarpous gynoecium, were observed. The maximum rate of pollination averaged 83·9 ± 4·7 %, and the number of pollen grains per stigma ranged from zero to 30. Pollen tubes grew through one stigma to the base of the ovary at almost the same speed, but generally only one of the pollen tubes then turned towards the ovule and finally entered the nucellus through the micropyle. The other pollen tubes grew through the ovary base and the receptacle tissue into ovules of adjacent carpels whose stigmas were not pollinated or which had been pollinated later. This phenomenon is termed pollen tube ‘reallocation’ by the authors. To verify the direct effect of the phenomenon on fruit set, artificial pollination experiments were conducted in which two or more pollen grains were placed onto only one stigma in each gynoecium; frequently more than one fruitlet was obtained from each flower treated. The reallocation of pollen tubes among pistils in the gynoecium could effect fertilization of ovules of unpollinated pistils and lead to an increase in sexual reproduction efficiency. It would, to some extent, also increase pollen tube competition among pistils of the whole gynoecium.
Key words: Sagittaria potamogetifolia Merr., pollination, pollen tube growth, fruit set, reproductive efficiency
INTRODUCTION
Deposition of pollen on stigmas, pollen germination and growth of pollen tubes to ovules are generally regarded as necessary factors that influence the success of sexual reproduction in angiosperms. The pollination rate therefore influences the amount of fruit set. An important question in plant reproductive ecology is whether female reproductive success is frequently limited by insufficient receipt of pollen (Burd, 1994). If pollination rates in gynoecia are low, as in Liriodendron chinense Sarg., an apocarpous species, fruit‐set efficiency is also likely to be low (Huang et al., 1998). In cases where pollen deposition is difficult, plants might possess strategies to produce enough competitive seeds, as in the Malpiphiaceae (Anderson, 1980) and in Callitriche (Philbrick, 1984) in which pollen tubes follow a strange growth route. The similar routes of pollen tube growth in these species should be investigated with a view to improving the efficiency of fruit set in apocarpous species.
Sagittaria potamogetifolia Merr., a helophytic herb found only in China, is a member of the monocotyledonous family Alismataceae. It is monoecious with unisexual flowers, an apocarpous gynoecium (159 ± 37 carpels per pistillate flower), entomophilous pollination, and is self‐compatible and shows extremely efficient fruit set (nearly 100 % on fine days) (Wang and Chen, 2001). The majority of pollinators are bees of the family Tiphiidae and Bombidae, and outcrossing rates estimated in seven natural populations ranged from 50·0 to 92·8 % (Wang and Chen, 1998, 2001). Despite some research into pollination mechanisms and mating systems, little is known about other aspects of its reproductive biology. The present research aims to reveal the cause of the extremely high fruit‐set efficiency in S. potamogetifolia through observation of the route of pollen tube growth from the stigma to the ovule and through artificial pollination, and to investigate pollen tube competition in this species.
MATERIALS AND METHODS
Plant material and study site
Plants were collected from a marsh in Chaling County, Hunan Province, China (26°50′N, 113°40′E) in April 1999, when the weather was fine and insect pollinators were flying normally. Ten pistillate flowers were collected at random at each of nine different times after flower opening (0·5, 1·0, 1·5, 2·0, 2·5, 3·0, 3·5, 4·0 and 4·5 h after flower opening). Specimens were fixed in formalin–acetic acid–alcohol (FAA).
Counting pollen grains on stigmas and monitoring pollen tube growth
After rinsing with water, the fixed flowers were cleared in KOH (5 %) at room temperature until most tissues had become transparent. They were then rinsed in water and stained for over 1 h with aniline blue (0·1 % in 1/30 mol l–1 K3PO4), according to Kho and Baer’s (1968) method modified by Guo et al. (1990) and Tangmitcharoen and Owens (1997). The number of pollen grains on stigmas was counted, and pollen tube growth in carpel tissue was examined under a fluorescence microscope (Olympus BX‐60). Where necessary, bright field microscopy was used to examine the number of pollen grains on stigmas. The remaining fixative solution and 5 % KOH solution were examined under a microscope to determine whether pollen grains had been removed from stigmas during the treatment. The paraffin method was used to obtain longitudinal sections of the whole gynoecium to show the transmitting tissue in each carpel, and the route of pollen tube growth among carpels.
Controlled pollinations
To verify the direct relationship between pollen tube growth and fruit set, two controlled pollination experiments were performed in the field: (1) pistillate flowers were enclosed in paper bags to prevent the flowers from receiving any pollen; (2) with the help of a stereomicroscope, more than ten pollen grains or exactly two grains from another plant were artificially deposited onto only one stigma in the gynoecium of a pistillate flower immediately after it had flowered. In each treatment, eight to 20 pistillate flowers on different plants were used. Fruit set was recorded 15 d after the treatments.
RESULTS
Deposition of pollen
Flowers of S. potamogetifolia opened between 0600–0700 h. The flowering period of a single pistillate flower is 2 d (opening daily/closing at night). The stigmas were receptive from 0700 to 1300 h during the first day of flower opening. The pollination rate and the number of pollen grains deposited on stigmas were both zero within 2 h after flower opening. According to data obtained in natural populations, on fine days when flower‐visiting insects could fly normally, the pollination rate increased sharply from 3 h after flower opening and almost reached its peak value (80–85 %) at 4 h after flower opening, when the number of pollen grains that had landed on each stigma averaged six to seven. However, this number was highly variable, ranging from zero to 30 (Table 1).
Table 1.
. Pollination rate and the number of pollen grains per stigma in gynoecia of Sagittaria potamogetifolia Merr. at different times after flower opening in natural populations
| Hours after pistillate flower | Pollination rate (%) | Pollen grains per stigma | ||||
| opening | Mean | s.d. | N* | Mean | s.d. | N † |
| 0·5 | 0 | 0 | 7 | 0 | 0 | 88 |
| 1·0 | 0 | 0 | 7 | 0 | 0 | 80 |
| 1·5 | 0 | 0 | 6 | 0 | 0 | 69 |
| 2·0 | 0 | 0 | 7 | 0 | 0 | 84 |
| 2·5 | 24·1 | 36·1 | 9 | 1·7 | 3·9 | 136 |
| 3·0 | 50·5 | 27·8 | 8 | 3·5 | 6·5 | 122 |
| 3·5 | 69·0 | 17·0 | 9 | 4·4 | 5·3 | 136 |
| 4·0 | 83·9 | 4·7 | 9 | 6·6 | 5·8 | 139 |
| 4·5 | 81·0 | 10·7 | 8 | 6·5 | 6·3 | 115 |
*Number of pistillate flowers.
†Number of single pistils.
Pollen tube growth
In all S. potamogetifolia material observed, the rate of pollen germination on the stigma was 100 %; since the pollen grains could have derived from staminate flowers of the same plant or from other plants within the natural population, no pollen selection occurs on the stigma (Wang and Chen, 2001). Although no pollen was observed to have landed on stigmas within 2 h of flower opening, some pollen tubes had reached ovaries, or even entered ovules, 2·5 h after flower opening, suggesting that pollen tubes grow very rapidly in this species, and that the establishment of domination in pollen tube competition depends mainly on the temporal order of pollen deposition.
Carpels of S. potamogetifolia were stained with 0·1 % water‐soluble aniline blue and observed under a fluorescence microscope. As pollen grains and pollen tubes emitted very strong fluorescence, the pathways of growing pollen tubes could be easily observed. Three types of pollen tube growth could be distinguished. (1) If a stigma was pollinated with only one pollen grain, the pollen tube would extend through tissue in the endocentric side of pistil, turn towards the eccentric side near the ovary base and reach the micropyle from beneath. The pollen tube tip then entered the nucellus (Fig. 1A). (2) When more than one pollen grain was deposited on a stigma, all pollen grains germinated and no pollen tubes stopped growing. All pollen tubes reached the basal part of ovary, where one or two pollen tubes extended towards the ovule. Only one pollen tube tip entered the nucellus through the micropyle (Fig. 1B). Most pollen tubes continued to grow, as evidenced by the fact that pollen tubes could be seen growing out through the ovary base soon after the pistil was removed from the receptacle (Fig. 1B). In natural conditions, pollen tubes entered receptacle tissue (Fig. 1C). These pollen tubes grew with changes in orientation until they reached the basal part of other pistils and entered their ovules (Fig. 1D). (3) If some stigmas were pollinated later than others, a pollen tube from an adjacent pistil could reach the nucellus before a pollen tube growing down the late‐pollinated stigma. The pollen tube from the adjacent pistil would ‘occupy’ this nucellus (Fig. 1E), while the pollen tube from the late‐pollinated stigma would continue to grow and enter receptacle tissue through the ovary base.
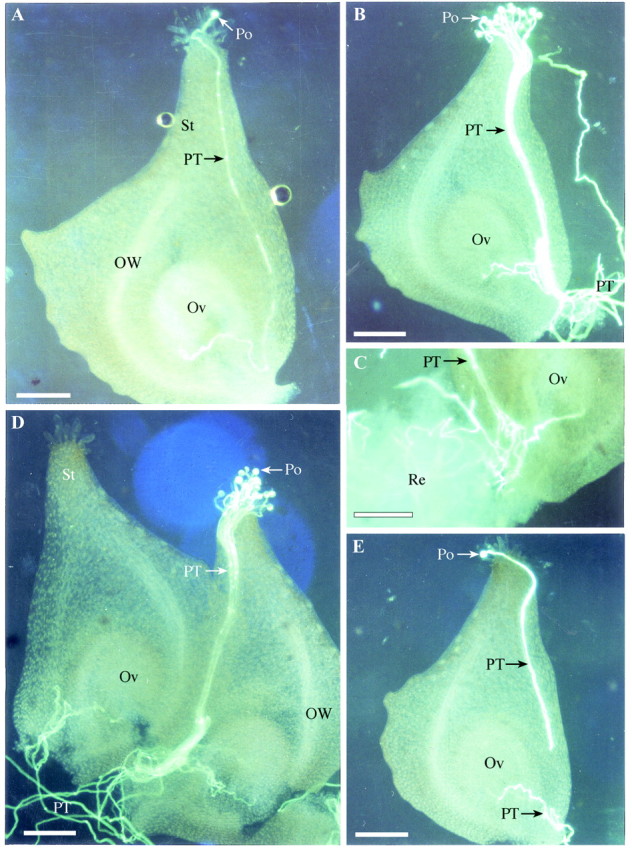
Fig. 1. Pollen tube growth in the pistils of Sagittaria potamogetifolia Merr., observed with fluorescence microscopy. A, Route of pollen tube growth in a pistil pollinated with one pollen grain. B, More than ten pollen grains were deposited on a stigma. All grains germinated, but only one or two pollen tubes extended to the ovule while the others continued to grow and passed through the base of the ovary. C, Pollen tubes entered receptacle tissue soon after they grew out through the ovary base. D, Redundant pollen tubes in one pistil entered an unpollinated pistil. E, The nucellus of a pistil was occupied by a pollen tube from another pistil before its own pollen tube reached the ovule. Po, Pollen; PT, pollen tube; St, style; Ov, ovule; OW, ovary wall; Re, receptacle tissue. Bars = 100 µm.
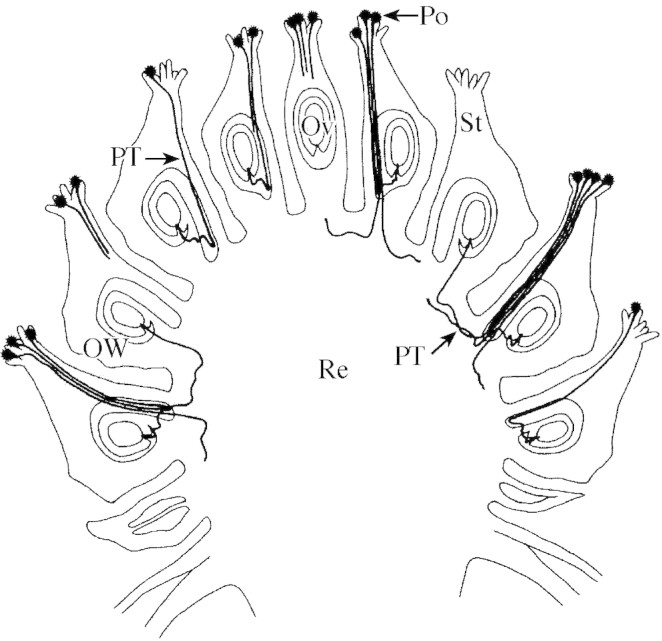
Pollen tube growth in the gynoecium of S. potamogetifolia was also observed using the paraffin method (Fig. 2). The styles were not found to be hollow. Pollen tubes grew through intercellular gaps in the parenchyma, reaching the receptacle through the endocentric side of the ovary wall. The pollen tubes then extended in a transverse direction in the shallow layer of receptacle tissue, or turned towards other ovules that had not been entered by a pollen tube.
Fig. 2. Longitudinal section through a pistillate flower of Sagittaria potamogetifolia Merr., showing the reallocation of pollen tubes in the gynoecium. Po, Pollen; PT, pollen tube; St, style; Ov, ovule; OW, ovary wall; Re, receptacle tissue.
This phenomenon of redundant pollen tubes in one pistil entering other pistils in which the ovule had not been entered by a pollen tube is termed pollen tube ‘reallocation’ by the authors. According to the authors’ preliminary observations, pollen tube reallocation also occurs in other members of the Alismataceae, such as Sagittaria pygmaea Miq., S. trifolia Linn. and Ranalisma rostratum Stapf. But the phenomenon was not observed in Liriodendron chinense Sarg., a multi‐carpel species whose fruit‐set efficiency is remarkably low (Huang et al., 1998). Further studies of the universality and the significance of the reallocation of pollen tubes in multi‐carpel plants are needed.
Fruit‐set related to pollination
Although there are 100–200 stigmas in each gynoecium of S. potamogetifolia, controlled pollination onto only one stigma could be done with the aid of a stereomicroscope. More than ten fruitlets could be obtained when only one stigma in the gynoecium of a pistillate flower was pollinated artificially with 20–30 pollen grains (Table 2; Fig. 3A), indicating that pollen tube reallocation did lead to unpollinated carpels developing into fruitlets. When the stigma was pollinated with two pollen grains, only one or two fruitlet(s) could be obtained (Table 2; Fig. 3A and B), strongly suggesting that fruitlet formation from carpels around the pollinated carpel could be explained only by pollen tube reallocation, not by a change in the level of plant growth substances in pollinated carpels. That the number of fruitlets formed is less than the number of pollen grains used in artificial pollination experiments might be due to inviability of some pollen grains. In the bagged experiment, fruitlet formation was never observed, suggesting that no agamospermy takes place, and that pollination is critical for fruit set.
Table 2.
Number of fruitlets obtained from a gynoecium of Sagittaria potamogetifolia Merr. following artificial pollination of one stigma
| Number of pollen grains pollinated | Number of pistillate flowers treated | Frequencies of fruitlets (%) | ||||
| 0 | 1 | 2 | 3–10 | >10 | ||
| 0 | 12 | 100 | 0 | 0 | 0 | 0 |
| 2 | 19 | 21·1 | 47·4 | 31·5 | 0 | 0 |
| 20–30 | 8 | 0 | 0 | 0 | 0 | 100 |
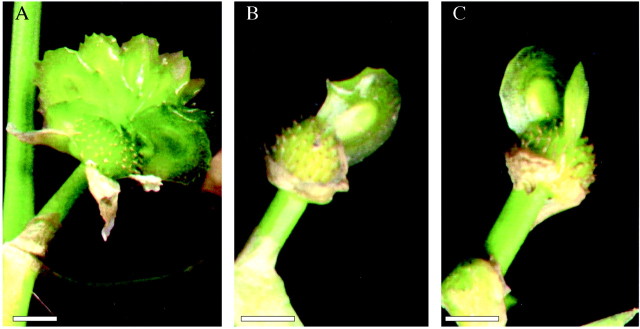
Fig. 3. Fruit set after controlled pollination of only one stigma in the gynoecium of Sagittaria potamogetifolia Merr. A, More than ten fruitlets were obtained from the gynoecium in which only one stigma was pollinated with 20–30 pollen grains. B and C, One or two fruitlet(s) were obtained from the gynoecium in which only one stigma was pollinated with two pollen grains. Bar = 1 mm.
DISCUSSION
Uneven pollination
Deposition of pollen on stigmas of S. potamogetifolia is uneven as a consequence of several features of the gynoecium and the behaviour of insect pollinators, including (1) the fact that each gynoecium contains a large number of apocarpous carpels (159 ± 37 carpels per pistillate flower) (Wang and Chen, 2001); (2) the surface of the pollen grains is rough and highly cohesive (Argue, 1974), which is an adaptation to insect pollination; (3) variation in the abundance or composition of pollinator fauna (see review by Burd, 1994); and (4) the receptive period of the stigma and the duration of insect activity are short. According to observations made in three habitats (Chaling in Hunan Province, Dongxiang in Jiangxi Province and Wuyishan in Fujian Province), there were 1·7 ± 2·2, 2·4 ± 2·3 and 1·5 ± 1·5 insect visits to pistillate flowers per hour, respectively. Within the receptive period, each pistillate flower might be visited by pollinators only three to five times on average, and the pollinator remains on pistillate flowers for a remarkably short period only (Wang and Chen, 2001). The pollination rate of S. potamogetifolia would drop further if the weather is bad and insects cannot fly.
Significance of pollen tube reallocation
To raise fruit‐set efficiency.
The pollination rate in pistillate flowers of S. potamogetifolia is only 80–85 %, but the fruit‐set frequencies can approach 100 % (Wang and Chen, 2001). No agamospermy takes place as proven by the bagged experiment, which indicated that seed formation was dependent on successful fertilization. The results of the present study show that the reallocation of pollen tubes among carpels in a gynoecium could effect fertilization of ovules in unpollinated pistils. In unpollinated pistils, pollen tubes enter over 85 % of ovules within 4·5 h of flower opening; this leads to a comparatively higher value of fruit‐set efficiency than expected given the low pollination rate.
To enhance pollen tube competition.
Mulcahy and Mulcahy (1987) proposed that angiosperms have taken advantage of the greatly increased pollen tube competition through carpel enclosure and insect pollination. Despite its potential, pollen competition is quite rare in natural populations (Mulcahy and Mulcahy, 1987). But a significant degree of pollen tube competition takes place in some species in which pollen is not a limiting factor in seed production (Snow, 1986). The reallocation of pollen tubes in S. potamogetifolia ensures that fast‐growing pollen tubes are the first to reach the nucellus of other carpels whose stigmas have been pollinated but whose pollen tubes grow slowly. The range of pollen tube competition has expanded from one carpel, or one pistil, to the whole gynoecium, thus the competition system becomes fiercer.
Mechanism of tropism in the unusual route of pollen tube growth
When more than one pollen grain lands on a stigma whose ovary contains only one ovule, usually only one pollen tube can successfully enter the ovule while the other pollen tubes stop growing. Cases of strange pollen tube growth have been reported. Anderson (1980) reported that pollen in the tiny cleistogamous flowers of the Malpiphiaceae germinated inside the indehiscent anther. The pollen tubes then grew down through the filament, into the receptacle, up into the carpels and into the nucellar beak of the ovule. Philbrick (1984) described pollen tubes of Callitriche traversing large segments of vegetative tissue within a plant to effect self‐fertilization. In Zannihcellia palustris, when one pollen tube entered the ovule, the others could continue to grow around the ovule (Guo et al., 1990). In the present study, the redundant pollen tubes of S. potamogetifolia can penetrate the ovary base and grow in the shallow layer of receptacle tissue, before turning towards ovules of other carpels. The success of these pollen tubes in turning towards ovules of other carpels is probably due to chemotropic substances from unpollinated carpels.
Pollen tubes serve to deliver sperm cells to the ovule and thus initiate the crucial process of seed development. For successful fertilization, pollen must germinate on the stigma, grow through the style, and find and penetrate the ovule micropyle. These processes require the growing pollen tube to undergo numerous changes in growth orientation. The cues in the pistil that signal pollen tube orientation are believed to be electrical, mechanical and chemical, but their nature is not well understood (Malho and Trewavas, 1996). Chemotropic substances in pistils are the subject of much research. Many experiments have suggested that the calcium gradient in the pistil is related to tropic growth of pollen tubes (Hepler, 1997; Wilhelmi and Preuss, 1997). Other biochemical factors may include lipids (Wolters‐Arts et al., 1998), gradients in glucose (Reger et al., 1992) and in water (Lush et al., 2000), and the glycosylation gradient of TTS a floral transmitting tissue‐specific glycoprotein (Wu et al., 1995). Sagittaria potamogetifolia would be an ideal experimental material for researching the mechanism of tropic growth of pollen tubes because of the strange growth route discovered in this study.
ACKNOWLEDGEMENTS
We thank Professor You‐Hao Guo and Dr Qing‐Feng Wang of Wuhan University for their suggestions. The research was supported by the National Scientific Foundation of China (NSFC 30170157, 30070078) and the Visiting Scholar Foundation of the Key Laboratory in the University of China.
Supplementary Material
Received: 11 June 2001; Returned for revision: 20 December 2001; Accepted: 6 March 2002.
References
- AndersonWR.1980. Cryptic self‐fertilization in the Malpighiaceae. Science 207: 892–893. [DOI] [PubMed] [Google Scholar]
- ArgueCL.1974. Pollen studies in the Alismataceae (Alismaceae). Botanical Gazette 135: 338–344. [Google Scholar]
- BurdM.1994. Bateman’s principle and plant reproduction: The role of pollen limitation in fruit and seed set. The Botanical Review 60: 83–139. [Google Scholar]
- GuoY‐H, Sperry R, Cook CDK, Cox PA.1990. The pollination ecology of Zannichellia palustris L. (Zannichelliaceae). Aquatic Botany 38: 341–356. [Google Scholar]
- HeplerPK.1997. Tip growth in pollen tubes: calcium leads the way. Trends in Plant Science 2: 79–80. [Google Scholar]
- HuangS‐Q, Guo Y‐H, Chen J‐K.1998. Pollination rates and pollen tube growth in a vulnerable plant, Liriodendron chinense (Hemsl.) Sarg. (Magnoliaceae). Acta Phytotaxonomica Sinica 36: 310–316 (in Chinese). [Google Scholar]
- KhoY, Baer J.1968. Observing pollen tube by means of fluorescence. Euphytica 17: 298–302. [Google Scholar]
- LushWM, Spurck T, Joosten R.2000. Pollen tube guidance by the pistil of a solanaceous plant. Annals of Botany 85 (supplement A): 39–47. [Google Scholar]
- MalhoR, Trewavas AJ.1996. Localized apical increases of cytosolic free calcium control pollen tube orientation. The Plant Cell 8: 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MulcahyDL, Mulcahy GB.1987. The effects of pollen competition. American Scientist 75: 44–50. [Google Scholar]
- PhilbrickCT.1984. Pollen tube growth within vegetative tissues of Callitriche (Callitrichaceae). American Journal of Botany 71: 882–886. [Google Scholar]
- RegerBJ, Chaubal R, Pressey R.1992. Chemotropic response of pearl millet pollen tube. Sexual Plant Reproduction 5: 47–56. [Google Scholar]
- SnowAA.1986. Pollination dynamics in Epilobium canum (Onagraceae): consequences of gametophytic selection. American Journal of Botany 73: 139–151. [DOI] [PubMed] [Google Scholar]
- TangmitcharoenS, Owens JN.1997. Floral biology, pollination, pistil receptivity, and pollen tube growth of teak (Tectona grandis Linn f.). Annals of Botany 79: 227–241. [Google Scholar]
- WangX‐F, Chen J‐K.2001. Floral expression, pollination mechanism and mating system of Sagittaria potamogetifolia Acta Phytoecologica Sinica 25: 155–160 (in Chinese). [Google Scholar]
- WangX‐F, Chen J‐K.1998. Quantitative estimates of outcrossing rates in a natural population of Sagittria potamogetifolia Journal of Wuhan University (Natural Science Edition) 44: 217–220 (in Chinese). [Google Scholar]
- WilhelmiLK, Preuss D.1997. Pollen tube guidance in flowering plants. Plant Physiology 113: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters‐ArtsM, Lush WM, Mariani C.1998. Lipids are required for directional pollen‐tube growth. Nature 392: 818–821. [DOI] [PubMed] [Google Scholar]
- WuH, Wang H, Cheung AY.1995. A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell 82: 395–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.