Abstract
Wounding of trees by debarking during the vegetative period sometimes results in the formation of callus tissue which develops over the entire wound surface or on parts of it. This light and transmission electron microscopy study of living lime trees found that the formation of such a surface callus is subdivided into three stages. During the first stage, numerous cell divisions take place in regions where differentiating xylem remains at the wound surface after debarking. This young callus tissue consists of isodiametric parenchymatous cells. Cambium cells, sometimes also remaining at the wound surface, collapse and do not contribute to callus formation. During the second stage, cells in the callus undergo differentiation by forming a wound periderm with phellem, phellogen and phelloderm. In the third stage, a cambial zone develops between the wound periderm and the xylem tissue laid down prior to wounding. This process is initiated by anticlinal and periclinal divisions of a few callus cells only. Later this process extends tangentially to form a continuous belt of wound cambium. Subsequently, this cambium produces both wound xylem and wound phloem and thus contributes to further thickening.
Key words: Debarking, wound reactions, cambium, surface callus, tissue differentiation, Tilia sp., light microscopy, electron microscopy
INTRODUCTION
Wound reactions of trees have been the subject of numerous investigations at the macroscopic and microscopic levels (e.g. Shigo, 1984; Rayner and Boddy, 1988; Liese and Dujesiefken, 1996; Pearce, 1996; Schwarze et al., 2000). Depending on the depth of wounding, reactions can be restricted to the bark or can also be initiated in the xylem. In the bark, a ligno‐suberized layer is formed primarily around the wound, thus rapidly protecting unaffected tissues against water loss and invasion by microorganisms. Subsequently, a wound periderm develops close to this layer (Mullick, 1977; Biggs, 1985; Trockenbrodt, 1994; Oven et al., 1999). The affected cambium at the edge of a wound reacts with the formation of callus tissue to close the opened surface of the stem (Liese and Dujesiefken, 1996; Oven and Torelli, 1999; Grünwald et al., 2002). Wound reactions in the xylem are characterized by the formation of a boundary layer, also called a marginal zone or reaction zone, which is visible mainly as a distinctly discoloured zone and which also protects healthy tissues (Shigo, 1984; Liese and Dujesiefken, 1996). The main mechanism involved in boundary layer formation is occlusion of vessels by phenolic compounds synthesized in the parenchyma and secreted into vessels through pits, or by the formation of tyloses (e.g. Schmitt and Liese, 1990, 1994; Pearce, 2000).
However, wounds produced during the wood formation season by totally removing the bark tissue from the trunk but leaving the cambium or the outermost differentiating xylem mechanically unaffected may lead to the formation of a callus tissue over the whole wound surface (Zhengli and Keming, 1988; McDougall and Blanchette, 1996; Kielbaso and Hart, 1997; Dujesiefken et al., 2001). This type of wounding may be caused by traffic accidents along streets or careless logging in forests. This phenomenon of large‐scale reactions at the wound surface has been known for about 200 years and has been studied at both macroscopic and tissue levels. It has been variously described as ‘reproduction of new bark and wood tissue’ (Hartig, 1844), ‘surface or superficial callus growth’ (Sharples and Gunnery, 1933), ‘direct cambium to bark development’ (Fenner, 1949), ‘Wundbekleidung’ (Fink, 1999) or just ‘surface callus’ (Dujesiefken et al., 2001). In the following description, the term surface callus is used for this type of wound response whose fine structure and developmental stages are still unknown.
The present paper describes the formation of surface callus on stem wounds of lime trees as observed by light and electron microscopy. Particular emphasis is given to examining the initial stages of surface callus formation, which include initiation of cell divisions after wounding, and subsequent development of a wound tissue comprising wound xylem, wound cambium and wound phloem.
MATERIALS AND METHODS
Investigations were carried out using three lime trees (Tilia sp.), each approx. 50 years old, growing in a forest near Hamburg, Germany. The trees were wounded during a 4‐week period in June 1999. Wounds were made in a helical pattern at stem heights between 40 and 200 cm. These wounds, measuring 10 cm2, were produced by using a saw to cut the bark through to the cambium and then removing it from the trunk by tapping with a hammer. Thereafter, the wounds were covered by 0·5 mm thick, black polyethylene plastic wraps. These wraps were pinned using steel needles to healthy bark along the edges of the wounds.
Microscopy was carried out on samples taken after response periods ranging from 1 to 4, 8 to 11 and 13 to 16 weeks. Callus tissue that remained attached to the xylem laid down prior to wounding (sample size 10 × 10 × 5 mm) was carefully removed from the wound surface with a chisel and razor blades. For light microscopy (LM), these samples were cut into 4 × 4 × 4 mm pieces, fixed for 1–3 d in a phosphate‐buffered solution of 37 % formaldehyde, dehydrated in ethanol and embedded in glycolmethacrylate (Technovit 7100). Transverse sections were cut with a rotary microtome at a thickness of about 6 µm and stained for 1·5 h with standard Giemsa solution.
The samples removed for transmission electron microscopy (TEM) had a final size of 2 × 2 × 5 mm. After fixation for 2–3 d in Karnovsky’s paraformaldehyde/glutaraldehyde solution, washing in 0·1 m cacodylate buffer (pH 7·2), postfixation in 2 % aqueous OsO4 for 12 h, washing in buffer and dehydration in a graded series of acetone, specimens were embedded in Spurr’s low viscosity epoxy resin. Ultrathin transverse sections were double‐stained with uranyl acetate and lead citrate and examined with a Philips CM 12 TEM at an accelerating voltage of 80 or 100 kV.
RESULTS
The bark was easy to remove mechanically from the wood, leaving a whitish, moist and sometimes fibrous tissue on the wound surface (Fig. 1A). The wounds, which were covered with plastic wraps immediately after wounding (Fig. 1B), rapidly showed reactions over most of their surface. The reactions were visible with the naked eye and showed discolouration as little as 1 week after wounding. Some parts were greenish to brownish, while other parts remained white (Fig. 1C). These colour differences were more intense 2 weeks after wounding (Fig. 1D). After 3 weeks, the white parts were distinctly thickened, indicating early stages of callus formation on the wound surface (Fig. 1E). In contrast, the dark areas did not thicken even 9 weeks after wounding and could easily be distinguished from areas of callus formation (Fig. 1F).

Fig. 1. Macroscopic view of a wound surface in lime. A, Fresh wound; B, wound covered with black plastic wrap; C, discolouration on some parts of the wound surface after 1 week; D, intense discolouration in the centre and on the left‐hand side of the wound after 2 weeks; E, 3 weeks after wounding a bright callus tissue develops (arrows); F, after 9 weeks nearly half the wound is covered with bright surface callus tissue (arrows); arrowhead indicates place of sampling for LM and TEM.
Microscopy revealed that in most cases wounding resulted in separation of the bark from the xylem within the zone of differentiating xylem (Fig. 2A and B). In some cases, cambium and the innermost phloem, or cambium alone, remained on the wound surface. In other wounds, incompletely differentiated xylem was exposed (Fig. 2C and D).
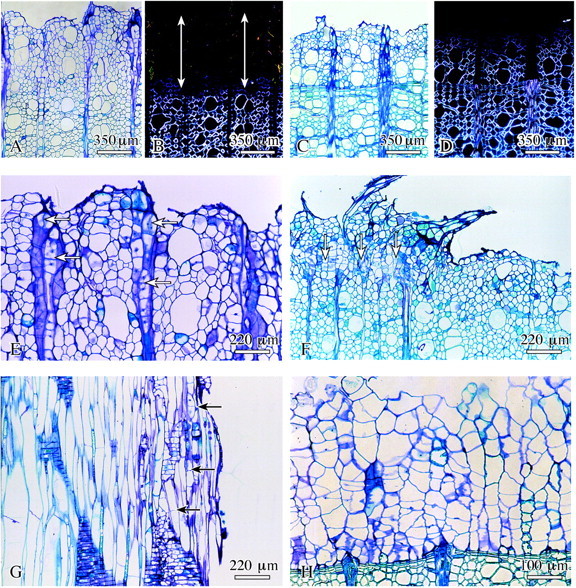
Fig. 2. Early stage of surface callus development in lime. A, C, E–H, Bright‐field microscopy; B and D, polarized light. A and B, Layer of xylem cells without birefringence as a marker for missing secondary wall (arrows); C and D, nearly all cells on the wound surface show birefringence under polarized light showing that these cells have already formed a secondary wall; E, proliferation and cell division of the ray parenchyma cells (arrows); F, young callus tissue (arrows) on parts of the wound surface; G, formation of transverse walls in fibres close to the wound surface (arrows); H, complete layer of surface callus cells directly attached to previous year’s latewood. A–F and H, Transverse sections; G, radial.
After response periods of a few days, those wounds in which the cambium and innermost phloem remained attached to the xylem showed a few rows of collapsed cells on their surface. More detailed analyses revealed that these rows consisted of collapsed innermost phloem and cambium. Inside of this zone, undifferentiated xylem cells without secondary walls proliferated through mitotic activity (Fig. 2E). Ray parenchyma cells also divided and proliferated, thus actively contributing to callus formation (Fig. 2F).
In addition, longitudinal sections showed that the axially elongated cells of the differentiation zone of the inner xylem had started to insert transverse walls (Fig. 2G). By continued cell divisions and enlargement of daughter cells, this tissue with its isodiametric cells rapidly expanded in both radial and tangential directions. It became evident that undifferentiated xylem cells at the stage of primary wall formation were involved in callus formation. As a consequence, an exclusively parenchymatic tissue without vessels, fibres and ray structures developed directly adjoining the latewood of the previous year’s growth ring (Fig. 2H), or next to current‐year earlywood laid down prior to wounding in which cells already had secondary walls. The cells of the collapsed zone did not participate actively in callus formation, but obviously provided protection to the underlying tissue. Bacteria often colonized this zone, but were not found in the living tissues.
During this developmental stage the callus tissue was found (during preparation for microscopy) to be quite soft. Electron microscopy revealed that individual cells of this early callus tissue had a large vacuole and a narrow layer of cytoplasm attached to a thin primary wall (Fig. 3A). Cell divisions occurred frequently, indicating rapid enlargement (Fig. 3B–D). The plane of the newly formed walls was without a preferred orientation, leading to the formation of an unstructured tissue. In addition, numerous intercellular spaces were characteristic of this young callus tissue (Fig. 3E). Later, these spaces became progressively occluded by fibrillar and granular substances secreted from the neighbouring callus cells through their thin primary walls. Sometimes callus cells degenerated and finally collapsed; neighbouring cells simultaneously enlarged and rapidly filled this gap (Fig. 3F and G).
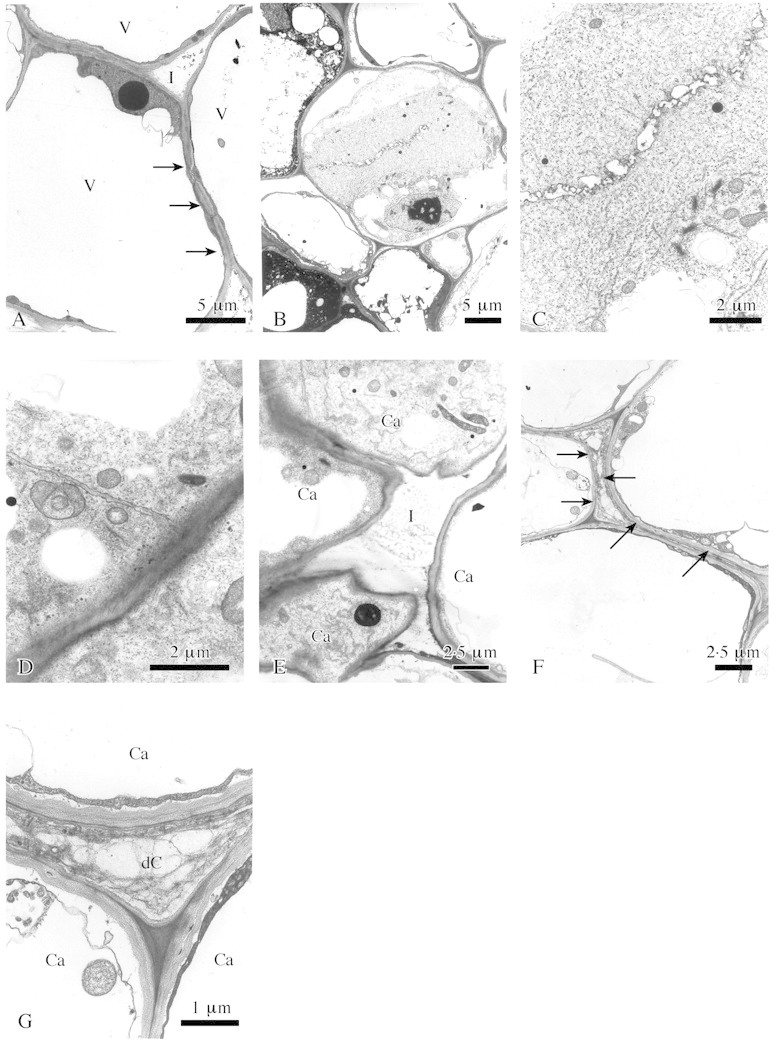
Fig. 3. Fine structure of surface callus in lime. A, Callus cells with large vacuoles (V), thin primary walls and a thin plasma layer (arrows). I, Intercellular space. B, Early stage of cell division. C, Vesicle stage of wall formation, detail of B. D, Lateral extension of the young wall completed. E, Callus cells (Ca) separated by an intercellular space (I). F, Degenerated callus cell (arrows) between surrounding turgescent cells. G, Detail of F. Ca, Callus cells; dC, degenerated cell. Transverse sections; TEM.
Another series of samples was taken from white surface regions 4–8 weeks after wounding. Parenchyma cells just inside the collapsed zone had started to deposit a thin suberin‐like layer onto their primary walls. Additionally, dark‐staining phenolic compounds were regularly observed in the vacuoles of these cells (Fig. 4A). A few rows further inwards, simultaneous cell divisions were observed in some cells, and the newly formed walls were strictly tangential. The shape of these cells changed from isodiametric to radially flattened (Fig. 4B). This process of differentiation was first observed 4–8 weeks after wounding in some localized regions; it later extended tangentially until a continuous band was formed (Fig. 4C). This band could be clearly identified as the phellogen of a developing wound periderm on the basis of its structure and position (Fig. 4D). Wound periderm formation was completed in the following weeks by the deposition of a phellem to the outside and a phelloderm to the inside of the phellogen (Fig. 4E and F). Phellem cells developed a thick suberin‐like inner wall layer with several plasmodesmata, and had phenolic constituents in their vacuoles (Fig. 4A and D).
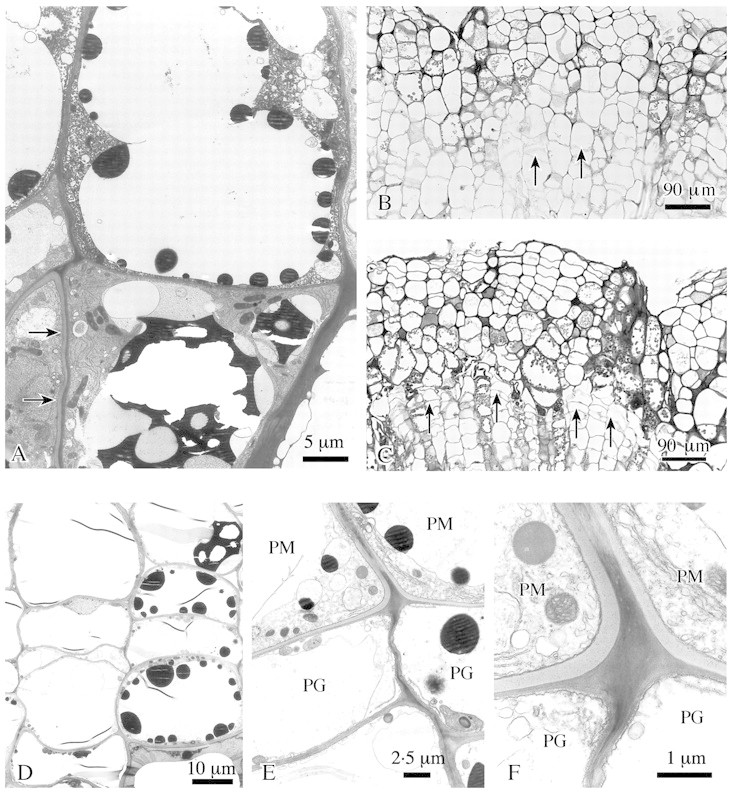
Fig. 4. Formation of wound periderm in lime. A, In outer callus cells suberin‐like layers are attached to the cell wall (arrows) and dark staining substances are deposited in vacuoles. B and C, A phellogen develops inside the outer callus tissue, some cells insert tangential walls (arrows) (B); later a complete tangential layer of flattened phellogen cells is formed (arrows) (C). D, Detail of phellogen formation: isodiametric callus cells change into flattened cells by insertion of tangential walls. E, Young phellem cells (PM) with suberized additional wall layer and phellogen cells (PG). F, Detail of E. Phellogen (PG) and phellem (PM) cells, the latter with suberin‐like layer being formed. Transverse sections; B and C, LM; A, D–F, TEM.
Just after completion of the wound periderm, a new meristematic tissue began to form in inner layers of callus (Fig. 5A). Initially, a few callus cells divided by producing radial and tangential walls, leading to a reduction in cell size and changes in shape. Thereafter, these modified cells appeared tangentially flattened (Fig. 5B and C). No intercellular spaces occurred in this tissue portion. In addition, longitudinal sections showed a distinct axial elongation of these cells (Fig. 5D). Again, this process was initiated in a few regions, later extending tangentially to build up a complete layer of wound cambium.
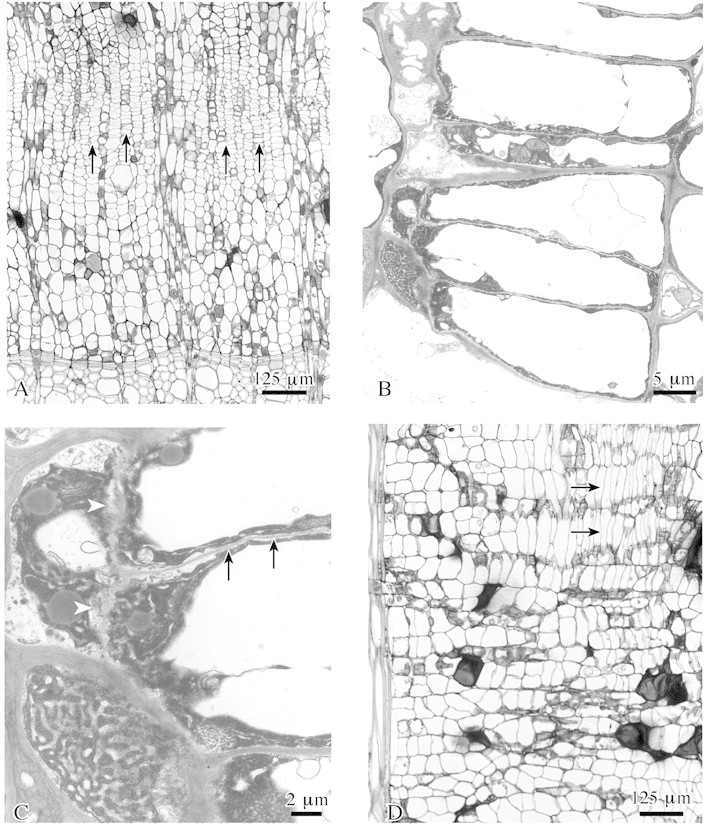
Fig. 5. Wound cambium formation within surface callus tissue in lime. A, A new wound cambium develops (arrows) close to previous year’s xylem. B, Radially flattened wound cambium cells develop from isodiametric callus cells by insertion of tangential and radial walls. C, Detail of B showing formation of tangential (arrow) and radial (arrowheads) walls. D, Cambial cells are axially elongated (arrows) as compared with callus cells. A–C, transverse sections; D, radial section. A and D, LM; B and C, TEM.
As a consequence of numerous cell divisions as well as differentiation of wound periderm and wound cambium, a pronounced surface callus with structural and functional subdivision covered the large‐scale wounds. During the vegetative period, it took 8–11 weeks until formation of wound cambium was complete.
Samples taken after response periods of 13–16 weeks typically showed wound xylem and wound phloem laid down by the wound cambium (Fig. 6A–G). The wound xylem characteristically contained fewer and smaller vessels, and also more axial parenchyma cells as compared with regular xylem. Additionally, stages in ray reconstitution were observable (Fig. 6B–E). A zone of cells with thin secondary walls and without vessels was regularly located as a kind of scar between the wound xylem and the regular xylem laid down prior to wounding, representing the callus tissue initially developed at the wound surface (Fig. 6A and B). On the outside, the surface callus then contained a fully differentiated periderm characterized by several rows of phellem cells with a thick suberized inner wall layer (Fig. 6F and G). The surface callus was well developed within a response period of up to 4 months.
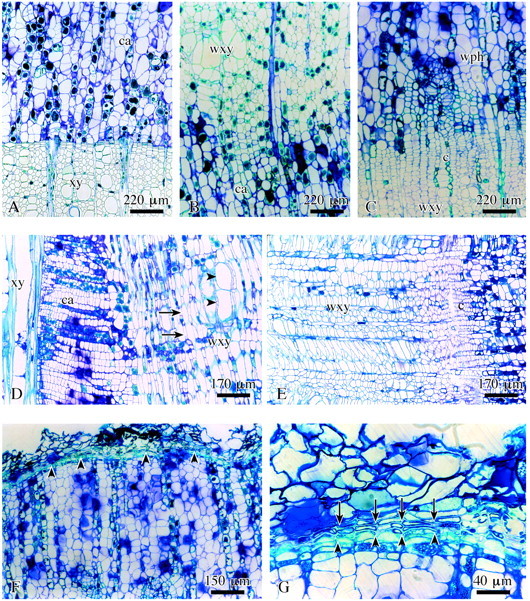
Fig. 6. Structure of completed surface callus in lime. A, Callus cells (ca) close to previous year’s xylem (xy). B, Wound xylem (wxy) adjacent to the callus tissue. C, Wound tissue: xylem, cambium (c), phloem (wph). D, Wound xylem with short fibres (arrows) and vessels (arrowheads) in radial view. E, Wound cambium (c) cells are axially shortened as compared with regular cambium cells. F, Wound periderm formation (arrowheads) completed on the outside of the surface callus. G, Detail of F showing inner phellogen layer (arrowheads) and distinctly suberized phellem cells (arrows) of the wound periderm. A–C, LM; F and G, transverse sections; D and E, radial sections.
DISCUSSION
Formation of a surface callus is a tree’s response to large‐scale superficial wounding. This phenomenon, known as a botanical peculiarity for more than 200 years (Dujesiefken et al., 2001), has been observed in various broad‐leaved tree species in climatically different areas (e.g. Trécul, 1853; Noel, 1968; Novitskaja, 1998). However, the fine structural processes and the developmental stages have not previously been described.
The present study revealed that if debarking occurs during the vegetative period, then it is mostly undifferentiated young xylem cells that remain on the wound surface and start to proliferate and divide, signalling the onset of surface callus formation over the entire area of the wound, or parts of it. In cases where only cells that have already started forming secondary walls remain on the surface, a callus tissue cannot form. There are a few examples where completely differentiated axial and ray parenchyma cells are reported to have proliferated (Sharples and Gunnery, 1933; Fisher, 1981). The cambium is often removed together with the bark, but when retained it forms a layer of collapsed cells protecting the undifferentiated xylem cells underneath. Hence cambium is not directly involved in surface callus formation if wounding occurs during the vegetative period. The wound depth and the number of cells that remain on the wound surface and that are able to divide are related to the growth of a surface callus.
Trécul (1853) described a new tissue coming out of the body of maple, walnut, horse chestnut and elm after removal of a large area of bark in the springtime. After only 1–3 d, Brown and Sax (1962) observed cell divisions in springtime wounds of poplar and pine; callus cells then grew mostly from proliferating ray cells. Similar results were reported for Hibiscus and Hevea (Sharples and Gunnery, 1933). Various tree species in Asia are also known to grow callus from undifferentiated cells on the wound surface (Zhengli and Keming, 1988).
After 2–3 weeks, the experimental trees had formed a multi‐layered tissue of isodiametric parenchyma cells with many intercellular spaces. The cells were characterized by large vacuoles, a thin cytoplasmic layer around the wall and a thin primary wall. Other tree species, e.g. Hevea, Hibiscus and Populus also produced a layer of isodiametric callus cells with intercellular spaces on the barkless wound surface (Sharples and Gunnery, 1933; Brown and Sax, 1962). Structurally, these cells looked similar to callus cells along the rim of a wound (Kucera, 1971), on cuttings (Stoll, 1874), callus from the pith (Barker, 1953), on leaves (Koizumi, 1983) or in vitro (Barnett, 1978).
During callus growth, remarkable changes take place in the tissue 3–4 weeks after wounding: the outer cells form suberized walls and phenolic substances are deposited in vacuoles. The subsequent callus tissue on the inside is altered into a tangentially oriented belt of flattened cells: the initiation of a phellogen. After 8 weeks, a fully functional wound periderm has formed, consisting of a complete phellogen forming phellem externally and phelloderm internally. The phellem is clearly suberized and contains phenolic substances in its vacuoles. Brown and Sax (1962) observed a similar reorganization in poplar after 2–3 weeks.
The restructuring of the outer surface callus cells can be compared with the formation of a ligno‐suberized layer in the bark formed after wounding (Oven et al., 1999). A necrophylactic periderm develops (Mullick, 1977; Biggs, 1985), which later merges into the original periderm at the rim of the wound. According to Oven et al. (1999), the formation of such a ligno‐suberized layer in the bark is a precondition for the growth of a wound periderm. Therefore, restructuring of the outer surface callus as well as the formation of a wound periderm are necessary for continuing surface callus development.
Following establishment of the wound periderm, a wound cambium is formed in lime trees by the inner surface callus tissue, i.e. close to the regular xylem. This was also shown to occur in poplar and pine as well as in two tropical species (Brown and Sax, 1962; Noel, 1968). However, in some subtropical tree species in Asia, the wound periderm and the wound cambium form almost simultaneously (Zhengli and Keming, 1988). The wound cambium develops by inserting radial and tangential walls into parenchymatic callus cells in the course of several cell divisions. This often occurs simultaneously at several places, merging into a continuous band of cambium. If a tree does not rapidly succeed in forming such a continuous band of cambium, bundle‐like structures of cambium cells are visible from where a tangentially extending cambium later originates until a continuous band is finally formed (Noel, 1968). The present study shows that the fusiform initials of the wound cambium are distinctly shorter than those in the regular cambium (Larson, 1994; Farrar and Evert, 1997a, b). They are, at first, only able to form xylem cells similar to wound xylem (De Vries, 1876; Sharon, 1973; Smith, 1980; Rademacher et al., 1984); only after 2 weeks are functional phloem cells produced (Brown and Sax, 1962).
Between the xylem that is already complete at the time of wounding and the xylem that originates from the wound cambium there is a zone of parenchyma where only a few cells develop a secondary wall. This tangentially oriented tissue, which remains as a kind of a scar, is located where the differentiating xylem existed at the time of wounding. It is similar to a barrier zone with regard to its position as well as to its structure (e.g. Sharon, 1973; McGinnes et al., 1977; Moore, 1978; Mulhern et al., 1979; Smith, 1980; Lowerts et al., 1986; Wilkes, 1986). Tangential stem shakes may occur later in such areas (McGinnes et al., 1971; Shigo, 1972; Phelps et al., 1975).
By removing the bark, the stress conditions in the cambial zone are altered. If pressure is imposed artificially on meristematic tissue, regular xylem formation is induced in the wound area in spite of the damage to the cell structure (Brown and Sax, 1962). This is possibly an explanation for the formation of undifferentiated callus tissue on the wound surface in the absence of stress. A cambium in the inner callus begins to emerge only after a wound periderm has developed, possibly as a result of increasing radial pressure due to the increasingly thick tissue layer and the wound periderm. Since the pressure under the growing callus is presumably less than normal, at first a structurally altered wound xylem is established, and xylem formation only slowly becomes normal (De Vries, 1876; Sharon, 1973; Smith, 1980; Rademacher et al., 1984; Liese and Dujesiefken, 1996).
To conclude, the development of a surface callus is usually clearly divided into three stages: an initial stage of parenchyma cell formation (first stage) and two stages of restructuring, namely the formation of a wound periderm in the outer callus (second stage) and the subsequent formation of a wound cambium in the inner tissue (third stage). The surface callus is only fully developed when this wound cambium has formed. Then, a fully functional tissue of bark, cambium and wood will develop on the surface from which bark and most of the cambium had previously been removed.
ACKNOWLEDGEMENTS
We thank the German Federal Environmental Foundation in Osnabrück for financial support, Dr Adya P. Singh, presently at the Chonnan National University in Kwangju, Korea, for improving the English expression, and Tanja Potsch and Christina Waitkus for technical assistance.
Supplementary Material
Received: 17 December 2001; Returned for revision: 5 February 2002; Accepted: 26 February 2002.
References
- BarkerWG.1953. Proliferative capacity of the medullary sheath region in the stem of Tilia americana American Journal of Botany 40: 773–778. [Google Scholar]
- BarnettJR.1978. Fine structure of parenchymatous and differentiated Pinus radiata callus. Annals of Botany 42: 367–372. [Google Scholar]
- BiggsAR.1985. Suberized boundary zones and the chronology of wound response in tree bark. Phytopathology 75: 1191–1195. [Google Scholar]
- BrownCL, Sax K.1962. The influence of pressure on the differentiation of secondary tissues. American Journal of Botany 49: 683–691. [Google Scholar]
- DeVriesH.1876. Über Wundholz. Flora 59: 2–8, 17,–25, 38,–45, 49,–55, 81,–88, 97,–108, 113,–121, 129–139. [Google Scholar]
- DujesiefkenD, Stobbe H, Kowol T.2001. Der Flächenkallus – eine Wundreaktion von Bäumen nach Rücke‐ und Anfahrschäden. Forstwissenschaftliches Centralblatt 120: 80–89. [Google Scholar]
- FarrarJJ, Evert RF.1997a Seasonal changes in the ultrastructure of the vascular cambium of Robinia pseudoacacia Trees 11: 191–202. [Google Scholar]
- FarrarJJ, Evert RF.1997b Ultrastructure of cell division in the fusiform cells of the vascular cambium of Robinia pseudoacacia Trees 11: 203–215. [Google Scholar]
- FennerC.1949. Shade method of direct cambium‐to‐bark development. National Shade Tree Conference, Proceedings 18: 131–138. [Google Scholar]
- FinkS.1999. Pathological and regenerative plant anatomy. Berlin, Stuttgart: Gebrüder Bornträger. [Google Scholar]
- FisherJB.1981. Wound healing by exposed secondary xylem in Adansonia (Bombacaceae). IAWA Bulletin n.s. 2: 193–200. [Google Scholar]
- GrünwaldC, Stobbe H, Schmitt U.2002. Entwicklungsstufen der seitlichen Wundüberwallung von Laubgehölzen (Developmental stages of callus formation at the wound edges of broad‐leaved trees). Forstwissenschaftliches Centralblatt (in press). [Google Scholar]
- HartigT.1844. Bericht über gelungene Versuche zur Reproduktion neuer Holz‐ und Rindenschichten aus dem Holzkörper der Bäume. Verhandlungen des Vereins zur Beförderung des Gartenbaus in den königlich preussischen Staaten 17: 329–331. [Google Scholar]
- KielbasoJ, Hart JH.1997. Wundverschluβmittel im Test. Baumzeitung 31: 170–173. [Google Scholar]
- KoizumiM.1983. Relationship between wound‐healing process of Citrus leaf tissues and successful infection through wounds by Xanthomonas campestris pv. citri (Hasse) Dye. Annals of Phytopathology Society Japan 49: 352–360. [Google Scholar]
- KuceraLJ.1971. Wundgewebe in der Eibe (Taxus baccata L.). Vierteljahrschrift der Naturforschenden Gesellschaft in Zürich 116: 445–467. [Google Scholar]
- LarsonPR.1994. The vascular cambium. Development and structure. Berlin, Heidelberg, New York: Springer‐Verlag. [Google Scholar]
- LieseW, Dujesiefken D.1996. Wound reactions of trees. In: Raychaudhuri SP, Maramorosch K, eds. Forest trees and palms: diseases and control. New Delhi, Oxford: IBH Publishing. [Google Scholar]
- LowertsG, Wheeler EA, Kellison RC.1986. Characteristics of wound‐associated wood of yellow‐poplar (Liriodendron tulipifera L.). Wood Fiber Science 18: 537–552. [Google Scholar]
- McDougallDN, Blanchette RA.1996. Polyethylene plastic wrap for tree wounds: a promotor of wound closure on fresh wounds. Journal of Arboriculture 22: 205–210. [Google Scholar]
- McGinnesEA Jr, Chang CI‐J, Wu KY‐T. 1971. Ring shakes in some hardwood species: the individual tree approach. Journal of Polymer Science, Part C 36: 153–176. [Google Scholar]
- McGinnesEA Jr, Phelps JE. Szopa PS, Shigo AL. 1977. Wood anatomy after tree injury – a pictorial study. University of Missouri Arboricultural Experiment Station, Research Bulletin 1025. [Google Scholar]
- MooreKE.1978. Barrier‐zone formation in wounded stems of sweetgum. Canadian Journal of Forest Research 8: 389–397. [Google Scholar]
- MulhernJ, Shortle WC, Shigo AL.1979. Barrier zone in red maple: an optical and scanning microscope examination. Forest Science 25: 311–316. [Google Scholar]
- MullickDB.1977. The non‐specific nature of defence in bark and wood during wounding, insect and pathogen attack. Recent Advances in Phytochemistry 11: 395–441. [Google Scholar]
- NoelARA.1968. Callus formation and differentiation at an exposed cambial surface. Annals of Botany 32: 347–359. [Google Scholar]
- NovitskajaLL.1998. Regeneration of bark and formation of abnormal birch wood. Trees 13: 74–79. [Google Scholar]
- OvenP, Torelli N.1999. Response of the cambial zone in conifers to wounding. Phyton 39: 133–137. [Google Scholar]
- OvenP, Torelli N, Shortle WC, Zupancic M.1999. The formation of ligno‐suberized layer and necrophylactic periderm in beech bark (Fagus sylvatica L.). Flora 194: 137–144. [Google Scholar]
- PearceRB.1996. Antimicrobial defences in the wood of living trees. New Phytologist 132: 203–233. [Google Scholar]
- PearceRB.2000. Decay development and its restriction in trees Journal of Arboriculture 26: 1–11. [Google Scholar]
- PhelpsJE, McGinnes EA Jr, Lieu PJ‐Y.1975. Anatomy of xylem tissue formation associated with radial seams and cracks in black oak. Wood Science 8: 397–405. [Google Scholar]
- RademacherP, Bauch J, Shigo AL.1984. Characteristics of xylem formed after wounding in Acer, Betula and Fagus IAWA Bulletin n.s. 5: 141–151. [Google Scholar]
- RaynerADM, Boddy L.1988. Fungal decomposition of wood. Its biology and ecology. Chichester, New York: J. Wiley and Sons Ltd. [Google Scholar]
- SchmittU, Liese W.1990. Wound reaction of the parenchyma in Betula IAWA Bulletin n.s. 11: 413–420. [Google Scholar]
- SchmittU, Liese W.1994. Wound tyloses in Robinia pseudoacacia L. IAWA Journal 15: 157–160. [Google Scholar]
- SchwarzeFWMR, Engels J, Mattheck C.2000. Fungal strategies of wood decay in trees. Berlin, Heidelberg, New York: Springer‐Verlag. [Google Scholar]
- SharonEM.1973. Some histological features of Acer saccharum wood formed after wounding. Canadian Journal of Forest Research 3: 83–89. [Google Scholar]
- SharplesA, Gunnery H.1933. Callus formation in Hibiscus rosa‐sinensis L. and Hevea brasiliensis Müll. Arg. Annals of Botany 47: 827–839. [Google Scholar]
- ShigoAL.1972. Ring and ray shakes associated with wounds in trees. Holzforschung 26: 60–62. [Google Scholar]
- ShigoAL.1984. Compartmentalization: a conceptual framework for understanding how trees grow and defend themselves. Annual Review of Phytopathology 22: 189–214. [Google Scholar]
- SmithDE.1980. Abnormal wound formation following fall and spring injuries in Black Walnut. Wood Science 12: 243–251. [Google Scholar]
- StollR.1874. Über die Bildung des Kallus bei Stecklingen. Botanische Zeitschrift 32: 737–742, 753,–768, 785–800. [Google Scholar]
- TréculA.1853. Acroissement des végéteaux dicotylédonés ligneux, reproduction du bois et de l’écorce par le bois décortiqué. Annal de Science Naturel, 3. Série Botanique 19: 157–192. [Google Scholar]
- TrockenbrodtM.1994. Light and electron microscopic investigations on wound reactions in the bark of Salix caprea L. and Tilia tomentosa Moench. Flora 189: 131–140. [Google Scholar]
- WilkesJ.1986. Host attributes affecting patterns of decay in a regrowth Eucalypt forest. V. Barrier zones. Holzforschung 40: 37–42. [Google Scholar]
- ZhengliL, Keming C.1988. Differentiation of secondary xylem after girdling. IAWA Bulletin n.s. 9: 375–383. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


