Abstract
The various pollen dispersal units (PDU) found in orchids are discussed together with possible evolutionary trends and the consequences for germination and fertilization. Orchids with monad and tetrad pollen form more complex dispersal units by means of pollenkitt, elastoviscin, a callosic wall, common walls or a combination of these. Evolutionary trends include (1) from pollenkitt to elastoviscin; (2) from monad to tetrads and multiples of tetrads; (3) from partially dehydrated (<30 %) to partially hydrated (>30 %) pollen; and (4) from monad pollen to PDUs with many pollen grains. The biological consequences concern both male and female reproductive systems. Some features of the male side are present in all orchids irrespective of the pollen dispersal unit, whereas other characters are found only in orchids with pollinia; the same applies for the female counterpart. Pollen grains of orchids with pollinia germinate at least 24 h after pollination because the pollen grains/tetrads must swell and make space for the growth of pollen tubes.
Key words: Review, pollen, compound pollen, pollen dispersal, pollination, Orchidaceae
INTRODUCTION
Angiosperm pollen may be dispersed in different ways, either as single units or united in various manners (clumps, tetrads or multiples of tetrads). This variability is probably a consequence of (1) pollination on a stigma; (2) a long style, i.e. the distance separating the stigma and female gametes; (3) the increased number of ovules per ovary (in some cases, as in orchids, more than 10 000 ovules per ovary); and (4) the evolution of animals as pollen vectors (Mulcahy, 1979; Friis et al., 1988; Ottaviano and Mulcahy, 1989).
The term PDU (pollen dispersal unit) was introduced by Pacini (1997) to indicate the different ways in which pollen is presented to dispersal agents and whether it travels individually or en masse as ‘compound pollen’ (Knox and McConchie, 1986). Compound pollen may be united by means of (1) viscous fluids, generally derived from tapetal activity/degeneration; (2) filaments of various origin and composition but usually continuous with the exine, and generally known as viscin threads if they consist of sporopollenin; and (3) common walls (Pacini and Franchi, 1999a; Hesse et al., 2000). Pollen may be dispersed singly or in units of two up to more than a million grains (Schill et al., 1992). Thirteen types of PDUs have been recognized (Pacini and Franchi, 1998). Some of the most common PDUs in orchids were first recognized by early plant embryologists (Reichenbach, 1852; Hofmeister, 1861).
The number of grains per pollen dispersal unit and the number of ovules per ovary have genetic and ecological consequences: the more grains/PDU, the greater the probability that seeds in a fruit will have the same male parent; the greater the amount of pollen adhering to the stigma, the greater the male competition; and the greater the number of ovules/ovary, the greater the female competition, especially if only a few pollen grains adhere to the stigma (Pacini and Franchi, 1999b).
Monocot pollen exhibits many of the PDU types, but some, especially the different kinds of pollinia, are found exclusively in the family Orchidaceae (Pacini and Franchi, 2000). The present paper is one of a series studying different aspects of PDU and considers all the types now recognized in the family Orchidaceae as well as various biological aspects of dispersal of pollen en masse.
CONCEPTS AND NOMENCLATURE
Before discussing the different PDU types, some terms pertaining to orchid anthers (e.g. Dressler, 1981; Yeung, 1987a) are defined.
Pollinarium: the complete set of pollinia from an anther, with the associated viscidium and stipe, are transported as a pollination unit.
Pollinium: a more or less compact and coherent mass of pollen, usually consisting of massulae, i.e. some tens of tetrads united together and attached to a caudicle.
Viscidium: a viscid, sticky part of the rostellum dispersed with the pollinia as a unit; it serves to attach pollinia to pollinators.
Stipe: a non‐viscid band or strap of columnar tissue connecting pollinia to the viscidium. The stipe is a cellular tissue derived from the column, and should not be confused with caudicles.
Caudicle: a slender, mealy or elastic extension of the pollinium or a mealy portion at one end of the pollinium; the structure is part of the pollen mass, and is produced within the anther.
Elastoviscin: a highly viscous fluid found only in Orchidaceae and Asclepiadaceae, i.e. in the two families that evolved pollinia independently and that also have other intermediate PDU types (for details see Wolter and Schill, 1986; Dannenbaum and Schill, 1991). Elastoviscin is derived from degeneration of tapetal cell cytoplasm and its main components are spherosomes (Wolter et al., 1988). In the anther of orchids, elastoviscin may have various locations and functions: (1) to engulf and to hold monad pollen together, as in Apostasia wallachii and Cypripedium calceolus (Schill and Wolter, 1986, Figs 1 and 2; Pacini, 2000, Fig. 2E) and attach them to the pollinator; (2) to hold massulae together (Hesse and Burns‐Balogh, 1984) and attach them to the pollinator; (3) to hold the massulae of a pollinium to the caudicle, as in Epidendrum ibaguense (Blackman and Yeung, 1983b); and (4) to form the viscidium (Yeung, 1987c), i.e. act a small cushion at one pole of the stipes which functions to attach the pollinium to the pollinator (Proctor and Harder, 1994, Fig. 1; Pacini, 2000, Fig. 2i).
Because pollenkitt and elastoviscin share some functions and originate from the tapetum, they can be considered homologous (Schill and Wolter, 1986; Halbritter et al., 1997). Elastoviscin, according to Schill and Walter (1986), is present in all subfamilies of orchids; however, Pterostylis concinna (Fitzgerald et al., 1994), Pterostylis plumosa (Pandolfi et al., 1993, unpubl. res.) and Neottia nidus‐avis (Buchner and Weber, 2000) have pollenkitt.
Pollenkitt and elastoviscin are both removed by acetolysis, but distinguishing between them is technically difficult. Pollenkitt is often removed during fixation and embedding for light microscopy, but is preserved by fixation and embedding procedures for TEM because osmium tetroxide binds to its lipid component (Weber, 1992; Hesse, 1993). Pollenkitt, in contrast to elastoviscin, does not totally engulf the pollen grains, although it sometimes covers and obscures minute ornamentations (Nepi and Pacini, 1993). Pollenkitt is seen under a light microscope as translucent domes or spheres, merging and detaching from the exine when the pollen is placed in water. Elastoviscin is not removed by water due to its chemical nature and enormous viscosity. Nepi and Franchi (2000) describe this and other methods to distinguish pollenkitt. Ontogenetically, there is a slight difference: plastids are involved in formation of pollenkitt (Weber, 1992) (the only known exception being Rosmarinus; Ubera Jiménez et al., 1996), but not elastoviscin (Halbritter et al., 1997).
PDU TYPES
Attempts have been made to list some of the PDU types of orchids and to match them with subfamilies (Dressler, 1981; Wolter and Schill, 1985, 1986; Yeung, 1987a; Zee and Siu, 1990; Freudenstein and Rasmussen, 1997; Johnson and Edwards, 2000), but no comprehensive list has ever been made. The criteria for recognition of PDU types are derived from experience and from the literature. There are several papers on the morphology of compound pollen of orchids (Schill and Pfeiffer, 1977; Wolter and Schill, 1986; Yeung, 1987a), but little data on monads and tetrads for one or more of the following reasons: pollen was often acetolysed, destroying diagnostically useful details; herbarium specimens were modified by desiccation and pressing; and authors glossed over certain details and published unclear drawings.
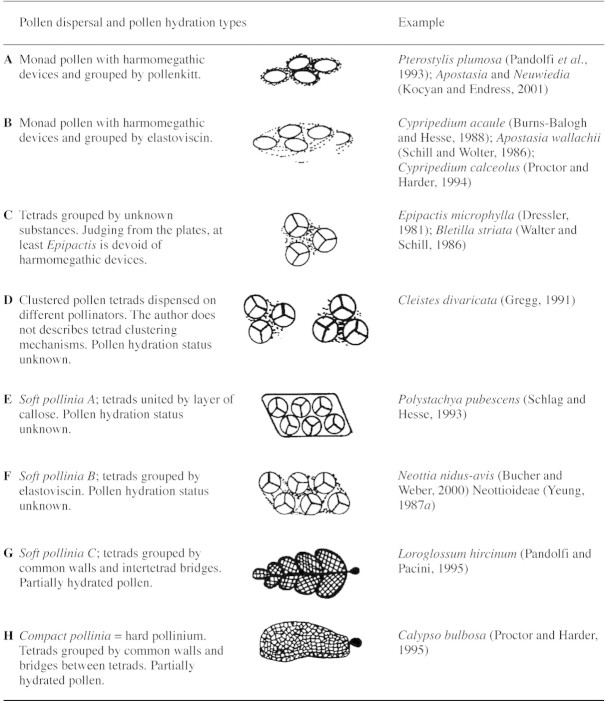
Orchid pollen is programmed to be dispersed by animals but asexual seed formation and autogamy are common (Neiland and Wilcock, 1998). Orchid PDUs have unspecialized devices (pollenkitt and elastoviscin) or specialized systems (the viscidium) to attach to pollinator bodies and PDUs therefore consist of several units. Table 1 shows the eight main PDU types in orchids and these are described below.
Table 1.
Different types of orchid pollen dispersal units together with details on structure, harmomegathy and hydration status
Monad pollen with pollenkitt
Aperture of furrow type. Found in Pterostylis plumosa (Pandolfi et al., 1993) and Pterostylis concinna (Fitzgerald et al., 1994), both Neottioids, and in Apostasia and Neuwiedia (Kocyan and Endress, 2001). The exine is reticulate and ornamentation is evident even if covered in pollenkitt (Fitzgerald et al., 1994, Fig. 13a).
Monad pollen grouped by elastoviscin
Aperture always present. It is possible to distinguish the following cases: Cypripedium acaule (Burns‐Balogh and Hesse, 1988, Fig. 6), with smooth exine and elastoviscin forming filamentous connections between grains (Fig. 2B); Apostasia wallachii (Schill and Wolter, 1986, Figs 1 and 2), with reticulate exine and elastoviscin forming thick connections between grains; and Cypripedium calceolus (Pacini, 2000, Fig. 2e), with elastoviscin totally engulfing the pollen (Fig. 2C). Nevertheless, we do not know whether these differences reflect real differences in the amount or viscosity of elastoviscin or whether they are due to methods of preparation or observation (light microscope, scanning electron microscope).
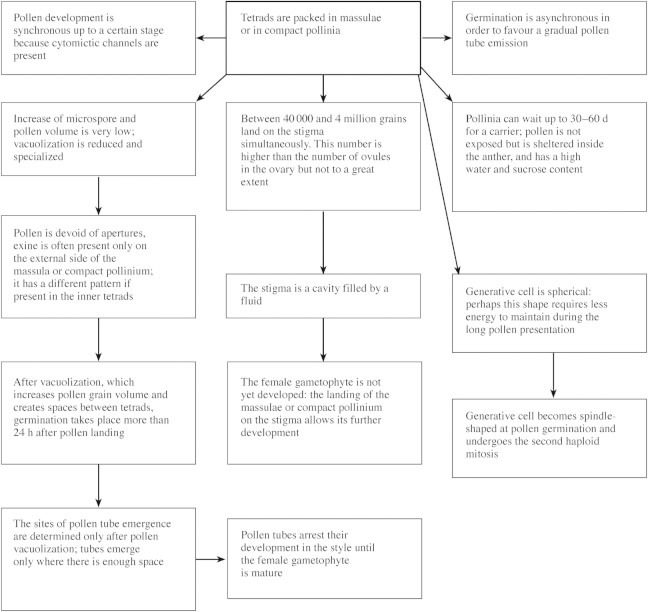
Fig. 1. Changes in male and female gametophytes of orchids as a consequence of the formation of pollinia as PDU and reasons for delay in pollen tube emergence. Features refer to flowers with a long lifespan (modified from Pacini and Franchi, 1996).
Isolated tetrads
Epipactismicrophylla (Dressler, 1981, Fig. 3·22), with one aperture per grain; the material was acetolysed and it is not possible to recognize other details. There are two examples: Epipactis microphylla (Dressler, 1981) with an aperture, and Bletilla striata (Wolter and Schill, 1986, Fig. 105); in the latter case, the material was acetolysed and it is not possible to determine other details, e.g. whether there are pores or furrows. Unfortunately, we do not know if these tetrads are covered in pollenkitt or other viscous fluids to form clumps, but Neottia pollen grains are devoid of furrows, i.e. they lack clear harmomegathic devices.
Aggregated pollen tetrads
Cleistes divaricata has aggregated pollen tetrads (Gregg, 1991). Again, it is not known how the tetrads are held together, but it is a separate PDU category because tetrads are not individual. This species has a peculiar mechanism by which the flower dispenses pollen to different visitors. There are no data on furrows or other harmomegathic devices.
Group of tetrads united externally by a thin layer of callose: soft pollinium type A
The massulae of a pollinarium can be dispersed to different flowers (Schlag and Hesse, 1993). No data on furrows and other harmomegathic devices are available.
Tetrads grouped by elastoviscin: soft pollinium type B
This type of PDU is found in Neottia nidus‐avis (Buchner and Weber, 2000) and the Neottioideae (Yeung, 1987a). The massulae of a pollinarium can be deposited on different stigmata. There are no data on furrows and other harmomegathic devices.
Tetrads grouped by common walls forming a massula: soft pollinium type C
The external shape of the pollinium may vary from spherical to pear‐shaped (Schill and Pfeiffer, 1977). The massulae of a pollinarium may be deposited on one stigma or dispersed to different stigmata, depending on pollinator movements when visiting the flower. This is the most common PDU type of the family, and according to Wolter and Schill (1986) is present in Spiranthoideae, Orchidoideae and Epidendroideae. Harmomegathic devices are absent. Possible variations concern wall structure: (1) exine is present around all the tetrads but only the external ones have a thick exine and ornamentations, i.e. Epidendrum scutella (Cocucci and Jensen, 1969; Dressler, 1981, Fig. 3·22f); (2) exine is only present on the outer surface of external tetrads of the massulae, as in Epidendrum ibaguense (Yeung, 1987b) and Loroglossum hircinum (Pandolfi and Pacini, 1995). Other possible variations are the presence of one or two viscidia: if only one is present it implies that the pollinia are kept together by the pollinator, if two are present the pollinia may be taken separately. Two viscidia are present in Loroglossum hircinum (Pandolfi and Pacini, 1995) and one in Pleurothallis eumecocaulon (Stenzel, 2000).
Tetrads grouped in a compact pollinium
This type of PDU is found in Calypso bulbosa (Proctor and Harder, 1995). Pollinia are generally flat and there are four pollinia per pollinarium. The exine only occurs on the outer surface of external tetrads, and harmomegathic devices are absent. The pollinarium adheres to the body of the pollinator by means of the viscidium; the whole pollinarium is deposited on one stigma. A similar PDU is also present in the Asclepiadaceae (Dannenbaum and Schill, 1991), but there are fewer grains per PDU and each pollinium is pear‐shaped.
HYPOTHESIS FOR THE EVOLUTION OF PDU IN ORCHIDS
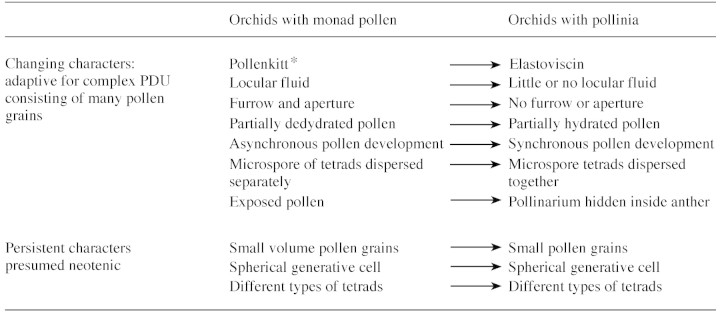
Two types of monad pollen are present in early orchids, namely that with pollenkitt and that with elastoviscin. The former is primitive because elastoviscin is clearly a modification of pollenkitt, a sticky fluid found principally in angiosperms for clumping pollen (Tables 1 and 2). The next step is from monads to tetrads. We do not know whether species with tetrads held together by pollenkitt exist, but if they do, they are only a branch in the evolution of PDUs in orchids.
Table 2.
Persistent and changing characters in pollen of primitive and more evolved orchids (characters common to early and evolved orchids are presumed to be neotenic)
* Elastoviscin engulfs monad pollen of Cypripedium acaule (Burns‐Balogh and Hesse, 1988) and Apostasia wallachii (Schill and Wolter, 1986).
The tetrad is a crucial evolutionary stage because more complex PDUs evolved from it. This is a common rule in families having more elaborate types of compound pollen such as Leguminosae s.l. and Apocynaceae (Knox and McConchie, 1986; Dannenbaum and Schill, 1991).
In extant orchids, tetrads form more elaborate PDUs by means of elastoviscin, an outer callosic wall, or a common wall in tetrads and bridges between tetrads. Elastoviscin also takes part in formation of the caudicle and viscidium (Tables 1 and 3). Pollinia form varies little (Schill and Pfeiffer, 1977) and is probably limited by the number of massulae/pollinium and the shape of the stigma cavity receiving the pollinium. The number of pollen grains/massula cannot be too great because too much time would be required to create space for pollen tube emergence. Pollen hydration and pollen tube emergence are critical phases in angiosperms, expecially when the stigma is exposed to solar radiation and air currents. In massulate orchids, unlike other angiosperms, this is not a problem because rehydration occurs inside the closed cavity of the stigma (Dannenbaum et al., 1989).
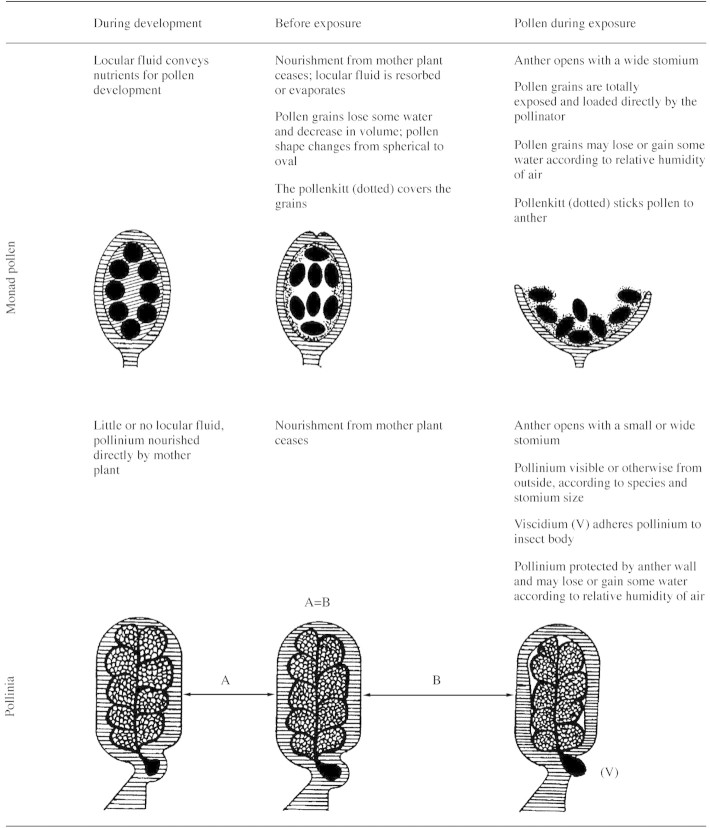
Table 3.
Semi‐diagrammatic representation of orchid anthers from development to exposure
Orchid anthers represented by horizontal lines.
Locular fluid (oblique lines) is evident only in orchids with monad pollen.
Developmental embryological studies in members of three subfamilies of Orchidaceae (Epidendroideae, Orchidoideae and Spiranthoideae) by Freudenstein and Rasmussen (1996) showed that anther devices differentiate according to the respective PDU type and the number of pollen grains.
Pandolfi et al. (1993) showed that Pterostylis plumosa, a Neottioid with monad pollen and pollenkitt, shares some features also present in massulate orchids and is therefore regarded as conservative (Table 2). These are: (1) the four grains of a tetrad stay close together until anther opening, whereas in many other species they are displaced during early microspore stage; (2) the generative cell is spherical; (3) during development, the space between microspores/pollen grains is much smaller than in other angiosperm species with monad pollen so that the volume of locular fluid is very small; (4) pollen grains are small; and (5) a small increase in volume occurs between the tetrad stage and ripe pollen stage (Fig. 1; Table 2). A list of variable and persistent characters of orchid anthers and pollen is given in Table 2.
MECHANISMS OF POLLEN COHESION
Different PDUs have different mechanisms of cohesion. Monads may be kept together by means of pollenkitt, as in Pterostylis, or by elastoviscin, as in Cypripedium and Apostasia wallachii; tetrads have common walls and mechanisms of external cohesion. Studying Epidendrum ibaguense, a species with a mealy pollinium, Yeung (1987b) recognized five mechanisms of adhesion of tetrads; these and other mechanisms are reported below.
Bilayered intine
Each tetrad forming a more complex unit has two intine layers, one for the tetrad and the other for each microspore/pollen grain (Pandolfi and Pacini, 1995).
Remains of pollen mother cell walls
This wall usually persists until middle tetrad stage (Pacini, 1994) and later disappears, being reabsorbed by the developing pollen. In orchids, however, it acts as a glue to keep tetrads together.
Fibrillar material in locular fluid during meiosis and tetrad stage
This fibrillar material generally disappears but in some orchids it persists, holding the tetrads together.
Persistence of cytomictic channels from prophase up to the end of the first haploid mitosis
These channels may be present between pollen grains of a tetrad (Yeung, 1987a, Figs 5 and 6) and/or between microspores of contiguous tetrads (Zavada, 1983, Fig. 55; Yeung, 1987c, Fig. 5; Zavada, 1990, Figs 24 and 32). They enable a flow of nutrients from the tapetum, synchronous first pollen mitosis in all microspores of the anther and synchronous pollen development (Heslop‐Harrison, 1966, 1968). Once closed, thin bridges are formed by exine and/or intine depending on the stratification mode of the tetrad walls.
Small pollen size at maturity and little increase in volume during development
Not all angiosperm pollen increases in size at the same rate. Orchid pollen has been found to increase three‐fold, while pollen of angiosperms increases by 11–20‐fold (Pandolfi et al., 1993). Indeed, if orchid pollen increased as much as that of other angiosperms it would be impossible to have a compact pollinium. This, together with the presence of different tightly packed tetrad types (see below), may explain why massulate orchids have little or no locular fluid. The smaller increase in volume of orchid pollen is due to the lack of the vacuolated stage that is so common in angiosperm pollen. A vacuolate stage is even missing in the monad pollen of the orchid Pterostylis plumosa (Pandolfi et al., 1993). Orchid pollen has only one type of vacuole which is round and small (0·1–0·5 µm) (Schlag and Hesse, 1992) and often has an electron opaque dot inside (Cocucci and Jensen, 1969, Fig. 8; Wolter and Schill, 1986, Figs 48 and 88; Brown and Lemmon, 1994, Fig. 6; E. Pacini, unpubl. res.). These vacuoles are probably derived from ribosomal turnover (E. Pacini, unpubl. res.) and are always absent in ripe angiosperm and gymnosperm pollen (Pacini, 1997; Pacini et al., 1999a). An extensive vacuolate phase only occurs in orchids, as in all angiosperms, after pollination and before pollen tube emergence (Pandolfi and Pacini, 1995).
Tetrads of different types with reduced amounts of locular fluid
Davis (1966) reported tetrad types in the various angiosperm families. Few families have more than one type. Orchids, however, have all possible tetrad types: linear, decussate, square, tetrahedral, rhomboid and T‐shaped are all reported, e.g. in Calanthe discolor which has a soft pollinium (Konta and Tsuji, 1982). In Pterostylis plumosa, which has monad pollen, Pandolfi et al. (1993) recognized only five types: T‐shaped, rhomboidal, tetrahedral, square and decussate. If the PDU is mealy or if pollinia are compact, the percentage of each tetrad type varies from the outside to the inside of the massula (Cocucci and Jensen, 1969; Pandolfi et al., 1993). Linear tetrads are sometimes only found on the outside of the pollinium, distributed radially like a palisade layer (Schill and Pfeiffer, 1977, Table 14, Fig. 1). To accommodate all tetrads, it is probably necessary to have tetrads of different types for geometrical reasons (Pandolfi et al., 1993).
When multiples of tetrads form the PDU, as in the case of Acacia polyads and Asclepiadaceae with pollinia (Kenrick and Knox, 1979; Dannenbaum and Schill, 1991), little or no space is left between the surface of the pollen and the loculus; in such cases tapetal cells retain their cell walls until degeneration (Pacini, 1997). In orchids with monad pollen, the space between the developing microspore/pollen is less than in other families with the same feature (Pandolfi et al., 1993).
Orchid anthers and pollen do not dehydrate (see below), and the pollen remains in the anther during presentation (Fig. 1; Table 3). Species with long‐lived pollinia may have devices for keeping pollen wet in the anther, as in Cucurbita pepo, in which monad pollen is exposed for 6 h only (Nepi and Pacini, 1993).
CELL WALLS
In orchids, pollen cell walls differ according to PDU type. Monads have individual exine and intine. Tetrads have common walls, intine and exine. In more complex PDUs, the intine is bilayered with the outer layer coating the tetrad and the inner layer coating individual grains (Pandolfi and Pacini, 1995). Exine structures differ according to position in the pollinium. The microspores of tetrads external to the pollinium have thicker intine and exine. In many cases, the outer tetrad exine is discontinuous with respect to contiguous tetrads, as shown when sporopollenin is stained with the fluorochrome Auramine O (Pandolfi and Pacini, 1995, Fig. 4). Exine patterns vary from outside to inside, and in some cases inner tetrads have thin, discontinuous or no exine (Cocucci and Jensen, 1969; Zee and Siu, 1990; Pandolfi and Pacini, 1995). Poor cohesion of external exine and reduced amounts of inner exine may explain why the fossil record of orchid pollen is so meagre (Wolter and Schill, 1985).
Burns‐Balogh (1983) showed the evolutionary trends of the various exine components in the different orchid subfamilies and hypothesized that there was a reduction in exine with increasing pollen grain number per PDU. The reason for this might be that tightly packed pollen impedes the distribution and penetration of nutrients in the massulae. The more pollen grains there are per pollinium, the more critical is nutrient distribution. Another mechanism favouring uniform distribution is the presence of inter‐ and intra‐tetrad cytomictic channels up to the first haploid mitosis (Heslop‐Harrison, 1966, 1968).
APERTURE AND POLLEN TUBE EMISSION
Orchids with monad pollen have a single aperture. The aperture in Pterostylis plumosa (Pandolfi et al., 1993) is very similar to that of other monocots, e.g. Liliaceae (e.g. Zavada, 1983; Halbritter and Hesse, 1993). Aperture form is more complex in appearance and structure in cypri pedioid orchids such as Paphiopedilum, Cypripedium, Phragmipedium and Selenipedium (Burns‐Balogh and Hesse, 1988). In species with tetrad pollen such as Neottia nidus‐avis (Buchner and Weber, 2000) and Epipactis microphylla (Dressler, 1981), no data exist on pollen tube emergence, but there is only one aperture per grain. Apertures seem to be absent in mealy or compact pollinia because inner tetrads have no space to form pollen tubes. Sites of pollen tube emergence are designated/determined only after tetrad displacement and creation of space for pollen tubes. When this process was followed in detail, as in Loroglossum hircinum (Pandolfi and Pacini, 1995), three stages were recognized: (1) pollen grains increase in volume through extensive vacuolization; (2) as a consequence, the relative position of microspores in tetrads changes; (3) space for pollen tube emergence is thus created. In Loroglossum hircinum, these steps take 24–48 h. Germination is asynchronous and it seems that inner tetrads germinate earlier than external ones. This asynchronous germination does not lead to further male competition because pollen tubes wait in the style until the female gametophytes are mature, at least in some Oncidiinae, and fertilization occurs 45–50 d after pollination (Clifford and Owens, 1988).
The generative cell in angiosperms is invariably spindle‐shaped (Tanaka, 1993), except in orchids. Monad pollen has a spherical generative cell (Pandolfi et al., 1993), as does pollen in pollinia (Schlag and Hesse, 1992). At least in Pterostylis plumosa, the generative cell becomes spindle‐shaped before entering the pollen tube (Pandolfi and Pacini, 1995, Figs 10 and 11). A spherical generative cell probably requires less energy to maintain than a spindle‐shaped one with its well‐organized cytoskeleton (Schlag and Hesse, 1992), especially during long exposure and in the period after pollination and pollen tube emergence (Table 1).
HARMOMEGATHY AND WATER CONTENT
Harmomegathy is a term coined by Wodehouse (1935) to indicate changes in volume and shape of pollen before the anther opens, when they decrease in volume, and after landing on the stigma, when they rehydrate. These changes are possible because (1) the walls are elastic, (2) pollen usually has furrows or other adaptations to enable size changes and (3) the pollen cytoplasm has devices to survive changes in water content (Pacini, 1990).
Anthers lose water to allow pollen dispersal; pollen grains lose water and reach equilibrium with the environment so as to survive presentation (with massulate orchids this may last up to 2 months) and dispersal (rarely more than 1 d). Pollen is termed partially dehydrated (PDP), if water content is less than 30 %, and partially hydrated (PHP) if it is over 30 % (Nepi et al., 2001). PHP appears to be typical of angiosperms and has the advantage of rapid germination (5–30 min) compared with PDP which germinates after 1–6 h (Nepi et al., 2001). At dispersal, the different types can be easily distinguished because PDP is ovoid with furrows and PHP is spherical and devoid of harmomegathic devices such as furrows. PDP and PHP co‐exist in the same family and even in the same genus (Nepi et al., 2001).
Monad pollen of orchids is always partially dehydrated because of the presence of furrows or furrow‐like structures. From the literature, it is not easy to interpret the pollen hydration status of orchids having tetrad pollen, but to judge from tetrads of other angiosperm families it seems to be partially hydrated, at least in the case of Neottia nidus‐avis (Buchner and Weber, 2000) and Epipactis microphylla (Dressler, 1981, Fig. 3·22c). All species with pollinia have PHP. It is possible to state this because: (1) water percentages have been recorded as being over 30 %; (2) pollinia are not compatible with dehydration and rehydration (see above); (3) pollinia contain small vacuoles; and (4) the pollen is devoid of furrows or other harmomegathic devices. Notwithstanding the high water content of ripe pollinia, orchids do not germinate quickly because after pollination tetrads undergo extensive vacuolation, increasing in volume and creating space for pollen tube emergence.
In species with abundant locular fluid, its resorption and/or evaporation is necessary before anther opening (Pacini, 2000). In orchids with pollinia, on the other hand, there is almost no locular fluid so this process is negligible or absent, and onset of anthesis is indicated by opening of the flower and stomium (Table 3). In at least some species of Bulbophyllum, a slight loss of water occurs in pollinia during dispersal, allowing them to fit into the stigma cavity (Borba and Semir, 1999). This also avoids self‐ and cross‐pollination because pollinaria have different external shapes.
POLLEN AND FLOWER LONGEVITY
Catling and Catling (1991) observed that ‘species in which seed production is pollinator‐limited often have flowers that are relatively long‐lived, with the result that the opportunity for pollination is maximally expected’; this problem is aggravated by few flowers per inflorescence and few plants per unit surface area.
According to Endress (1994), flower anthesis in orchids lasts from a few hours to several weeks. In the case of Calypso bulbosa, it lasts 8–11 d (Proctor and Harder, 1995). Examples of species in which flower viability is extremely long include Oncidium cheirophorum, where individual flowers remain open for about 30 d, and Lemboglossum maculatum where they stay open for up to 60 d (Clifford and Owens, 1988). In principal, the length of flower anthesis depends on pollination and removal of the pollinium (Luit and Johnson, 2001). Female receptivity may last a long time, but the percentage of fruit formation varies over the period; in the case of Orchis morio, where female receptivity lasts 20 d, fruit set is 100 % when pollination occurs 4 d after anthesis (Neiland and Wilcock, 1995).
The number of grains per PDU often matches the number of ovules per ovary; in European orchids, the pollen/ovule ratio ranges from 10 : 1 to 24 : 1 (Neiland and Wilcock, 1995). In some cases, owing to the huge number of ovules per ovary, pollinated flowers remain receptive for further pollination for at least 8 d (Neiland and Wilcock, 1995).
Pollen longevity may differ depending on whether male and female receptivity is simultaneous or not. We know of only one case of long‐lived monad pollen with elastoviscin; this is Cypripedium reginae where pollen longevity lasts 4 d (Proctor, 1998), i.e. the same order as dicot monad pollen with or without pollenkitt (Pacini et al., 1997).
Pollen longevity should be considered in relation to whether the pollen is inside or outside the anther. Some orchid species with pollinia have long‐lived pollen (Dafni and Firmage, 2000). Recently, Luit and Johnson (2001) found pollinia with a mean lifespan of 24 d in Mystacidium venosum. Pollinia inside the flower were viable after more than 10 d, while those kept outside under environmental conditions were still viable after 20 d.
Pollen longevity seems to depend on the following factors: (1) carbohydrate and water content of grains at the onset of anthesis; (2) the environment during exposure and dispersal; and (3) the site where pollen presentation occurs, i.e. whether pollen is exposed or protected (Pacini et al., 1997; Dafni and Firmage, 2000; Nepi et al., 2001). Large amounts of sucrose and cytoplasmic polysaccharides are required for long viability (Speranza et al., 1997). If pollen has a high water content, i.e. more than 30 %, and simultaneously has a high percentage of sucrose and cytoplasmic polysaccharides, it resists desiccation better than pollen with a small amount of carbohydrate (Dafni and Firmage, 2000). Environmental conditions such as temperature, light intensity and relative humidity influence pollen longevity (Pacini et al., 1997).
The pollinium of the orchid Stanhopea tigrina has 95·6 µg mg–1 of sucrose, a high concentration compared with other species with long‐lived pollen (Nepi et al., 2001; unpubl. res.). There is a contradiction between the high water content, the presence of small vacuoles (even in ripe pollen) and the long lifespan of some pollinia; this is apparently because orchid pollinia remain inside the anther during pollen presentation (Fig. 1; Table 3). The water content of Stanhopea tigrina pollinia is 56·8 % in newly open flowers, dropping to 34·3 % a week later (unpubl. res.). Retention of the pollinarium inside the anther, together with the high concentration of sucrose, probably prevents proliferation of moulds, fungi and bacteria; indeed, such attacks are not mentioned in the literature. Exposed pollen is invaded by moulds, especially when the relative humidity is high (Bassani et al., 1994).
POLLEN PRESENTATION AND RELEASE
In primitive orchids the anther is oblong, as in many angiosperms, and readily recognizable, but in more evolved groups it is modified to accommodate the pollinarium and its development (Table 3) (Freudenstein and Rasmussen, 1996; Johnson and Edwards, 2000). Pollen presentation mechanisms generally depend on features of the anther’s mechanical layer, such as cells with walls having lignin thickenings. Freudenstein (1991) provided evidence of four types of endothecial thickenings in orchids and these match the main types of PDU. Orchids are generally entomophilous and rarely ornithophilous (Johnson, 1996). As pointed out by Kocyan and Endress (2001), pollen offered as a reward is unknown in orchids except Neuwiedia veratrifolia.
Pollen is presented to insects in three different ways. (1) Pollen, in monads or tetrads, is presented in the anthers, adhering to the anther by means of pollenkitt or elastoviscin until collected (Pacini, 1997), e.g. Pterostylis plumosa (Pandolfi et al., 1993) and Cypripedium calceolus (Proctor and Harder, 1994). (2) Anthers have mechanisms to dispense loosely clumped pollen tetrads in different doses according to the number of insect visits; this is similar to the situation in the Ericaceae which have poricidal anthers (Fægri and van der Pijl, 1979), e.g. Cleistes divaricata (Gregg, 1991). (3) The anther is modified to produce pollinia (Table 2). Pollen is not directly exposed and sometimes only part of the pollinium is visible through the stomium (Pacini, 2000, Fig. 2i). One or two viscidia stick the pollinium to the visitor’s body (Dressler, 1981). If pollinia are mealy, massulae may be left on different stigmas as insects visit flowers. If pollinia are compact, a single pollinarium may be deposited on a single stigma.
CONSEQUENCES OF STIGMATIC POLLEN LOAD
Orchid PDUs can deliver different stigmatic pollen loads, especially those having a mealy pollinium that can disperse different doses of pollen according to the pollinator size and its movement when visiting the flower.
Proctor and Harder (1994) experimentally manipulated the pollen load of three species with different PDU types: Cypripedium calceolus (monads with elastoviscin), Amerorchis rotundifolia (mealy pollinia) and Calypso bulbosa (compact pollinia). Pollen load affected seed number in Calypso but not in Cypripedium and Amerorchis. Unfortunately, the number of ovules per ovary was not considered.
COUNTERPART ADAPTATIONS
Even the female part adapts to an increase in the number of grains in the PDU (Fig. 1). The stigma of orchids with monad and tetrad pollen is more or less the same as that of other angiosperm species with the same PDU. This means that it becomes a cavity when the PDU is a pollinium. When the pollinium is compact, the receptive surface of the stigma has small‐ to medium‐sized papillae (Heslop‐Harrison and Shivanna, 1977) and is a fluid‐filled depression (Clifford and Owens, 1990). Another striking adaptation is stigmas that can receive PDUs of different types, i.e. a whole pollinium or a few massulae. Dannenbaum et al. (1989) have provided evidence of three main types of stigma. Type I is that of species having monad pollen, and type III (concave) can be regarded as an adaptation to accommodate different types of pollinia.
The huge number of pollen grains in PDUs such as compact pollinia sometimes means that investment in the female part is low and female gametophytes may not be ready for fertilization at the time of pollination. Investment in female development is only made after a successful compatible pollination. This implies that pollen tubes wait in the style for the female gametophyte to develop, in some cases for as long as 3 months (Cocucci and Jensen, 1971).
THE MALE CONTRIBUTION TO THE NEOTENY OF ORCHIDS
Orchids exhibit many derived features, maintaining some primitive, neotenic characters (Table 2). The best known of these is a globular embryo dispersed at a very early stage, but there are other neotenic features of the male part. Tapetal cells retain their cell walls until degeneration and pollen is dispersed in a partially hydrated state with small vacuoles. These features also occur in spores of bryophytes and some pteridophytes (Pacini et al., 1985). Lacking an extensive vacuolization phase, meiocytes of the same tetrads stay together until the anther opens and the loculus reduces and disappears. These features are considered primitive because they are just early stages of all land plant meiocytes.
FUTURE RESEARCH
Collecting pertinent data about the orchid family with its approx. 20 000 species is time‐consuming. Existing data are not uniform, and are sometimes contradictory. Some orchid groups, such as those having monad pollen, are less well known than those with massulae. We now consider possible lines of research worthy of attention.
The duration of the reproductive cycle and the length of different phases, including pollen exposure, mean pollen life, time for pollen tube emergence and any arrest of pollen tube growth need clarification. It is also not clear whether differences in the duration of pollen viability are determined only by carbohydrate composition (i.e. are biochemical) or by the environment and pollinium exposure as well (i.e. ecological and anatomical reasons).
Another aspect that came to the fore quite recently is pollen hydration status. This aspect must be investigated in species with monad and tetrad pollen because it seems that it is from these PDU types that orchid pollen becomes partially hydrated. PDUs intermediate between tetrads and pollinia are crucial and little is yet known about them. This problem is also related to carbohydrate content (Nepi et al., 2001).
The evolutionary steps from pollenkitt to elastoviscin, as well as elastoviscin composition are completely unknown. These two substances have multiple functions in monad pollen and pollinia and it would therefore be useful to know whether their composition and physicochemical properties are always the same.
The different patterns in the inner and outer tetrad walls of two species of the same genus, Epidendrum scutella (Cocucci and Jensen, 1969), and E. ibaguense (Yeung, 1987a, b) need clarification.
Another interesting aspect is the pollen/ovule ratio. Is an increase in the number of ovules correlated with an increase in pollen grains/pollinarium? This type of information only exists for a few Mediterranean orchids with the same type of pollinium (Neiland and Wilcock, 1995). As mentioned above, little is known about the consequences of different stigma pollen loads.
The study of differences in anther and ovary organization in cleistogamous and apomictic species compared with cross‐pollinated species will be important in the understanding of allocation in two different situations.
Research is necessary to determine whether a correlation exists between primitive PDUs and phytogeography. In which regions are orchids with monad pollen found today? No data are available for anther opening mechanisms and the degree of pollinium exposure, i.e. a wide or small aperture of the stomium. Monad orchids and those with pollinia are presumably different because the former have partially dehydrated pollen and the latter partially hydrated pollen. If the stomium is wide, much of the pollinium is exposed. Does this correspond with a higher sucrose/carbohydrate concentration to avoid water loss and prolong viability?
ACKNOWLEDGEMENTS
The authors are grateful to Mrs U. Schachner for bibliographical research. The research in this article was performed under ‘Ricerca d’Ateneo’, University of Siena and MURST (ex 40 %).
Supplementary Material
Received: 7 December 2001; Returned for revision: 1 February 2002; Accepted: 6 March 2002.
References
- BassaniM, Pacini E, Franchi GG.1994. Humidity stress in pollen of anemophilous and entomophilous species. Grana 33: 146–150. [Google Scholar]
- BlackmanSJ, Yeung EC.1983a Comparative anatomy of pollinia and caudicle of an orchid (Epidendrum). Botanical Gazette 144: 331–337. [Google Scholar]
- BlackmanSJ, Yeung EC.1983b Structural development of the caudicle of an orchid (Epidendrum). American Journal of Botany 70: 149–155. [DOI] [PubMed] [Google Scholar]
- BorbaEL, Semir J.1999. Temporal variation in pollinarium size after its removal in species of Bulbophyllum: a different mechanism preventing self‐pollination in Orchidaceae. Plant Systematics and Evolution 217: 197–204. [Google Scholar]
- BrownRC, Lemmon BE.1994. Pollen mitosis in the slipper orchid Cypripedium fasciculatum Sexual Plant Reproduction 7: 87–94. [Google Scholar]
- BuchnerR, Weber M.2000. PalDat – a palynological database: descriptions, illustrations, identification, and information retrieval. http://paldat.botanik.univie.ac.at/ [Google Scholar]
- Burns‐BaloghP.1983. A theory on the evolution of the exine in Orchidaceae. American Journal of Botany 70: 1304–1312. [Google Scholar]
- Burns‐BaloghP, Hesse M.1988. Pollen morphology of the cypripedioid orchids. Plant Systematics and Evolution 158: 165–182. [Google Scholar]
- CatlingPM, Catling VR.1991. A synopsis of breeding systems and pollination in North American orchids. Lindleyana 6: 187–210. [Google Scholar]
- ClémentC, Pacini E.2001. Anther plastids in angiosperms. Botanical Review 67: 54–73. [Google Scholar]
- CliffordSC, Owens SJ.1988. Post‐pollination phenomena and embryo development in the Oncidiinae (Orchidaceae). In: Cresti M, Gori P, Pacini E, eds. Sexual reproduction in higher plants Heidelberg: Springer Verlag, 407–412. [Google Scholar]
- CliffordSC, Owens SJ.1990. The stigma, style, and transmitting tract in the Oncidiinae (Orchidaceae): morphology, developmental anatomy, and histochemistry. Botanical Gazette 151: 440–451. [Google Scholar]
- CocucciAE, Jensen WA.1969. Orchid embryology: pollen tetrads of Epidendrum scutella in the anther and on the stigma. Planta 84: 215–229. [DOI] [PubMed] [Google Scholar]
- CocucciAE, Jensen WA.1971. Orchid embryology: germinating male gametophyte in Epidendrum scutella Kurtziana 6: 25–39. [Google Scholar]
- DafniA, Firmage D.2000. Pollen viability and longevity. Plant Systematics and Evolution 222: 113–132. [Google Scholar]
- DannenbaumC, Schill R.1991. Die Entwicklung der Pollentetraden und Pollinien bei den Asclepiadaceae. Biblioteca Botanica 34: 1–138. [Google Scholar]
- DannenbaumC, Wolter M, Schill R.1989. Stigma morphology of the orchids. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 110: 441–460. [Google Scholar]
- DavisGL.1966. Systematic embryology of the Angiosperms. London: John Wiley and Sons. [Google Scholar]
- DresslerRL.1981. The orchids. Natural history and classification. Cambridge, Massachusetts: Harward University Press. [Google Scholar]
- EndressPK.1994. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- FægriK, van der Pijl L.1979. The principle of pollination ecology. 3rd edn. Oxford: Pergamon Press. [Google Scholar]
- FitzgeraldMA, Barnes SH, Blackmore S, Calder DM, Knox RB.1994. Pollen development and cohesion in a mealy and hard type of orchid pollinium. International Journal of Plant Science 155: 481–491. [Google Scholar]
- FreudensteinJV.1991. A systematic study of endothecial thickenings in the Orchidaceae. American Journal of Botany 78: 766–781. [Google Scholar]
- FreudensteinJV, Rasmussen FN.1996. Pollinium development and number in the Orchidaceae. American Journal of Botany 83: 813–824. [Google Scholar]
- FreudensteinJV, Rasmussen FN.1997. Sectile pollinia and relationships in the Orchidaceae. Plant Systematic and Evolution. 205: 125–146. [Google Scholar]
- FriisEM, Chaloner W, Crane PR.1988. The origin of angiosperms and their biological consequences. Cambridge: Cambridge University Press. [Google Scholar]
- GreggKB.1991. Reproductive strategy of Cleistes divaricata (Orchidaceae). American Journal of Botany 78: 350–360. [Google Scholar]
- HalbritterH, Hesse M.1993. Sulcus morphology in some monocot families. Grana 32: 87–99. [Google Scholar]
- HalbritterH, Hesse M, Buchner R.1997. Pollen connecting threads in Gymnocalycium (Cactaceae): their origin, function, and systematic relevance. Grana 36: 1–10. [Google Scholar]
- Heslop‐HarrisonJ.1966. Cytoplasmic connections between angiosperm meiocytes. Annals of Botany 30: 221–230. [Google Scholar]
- Heslop‐HarrisonJ.1968. Synchronous pollen mitosis and the formation of the generative cell in massulate orchids. Journal of Cell Science 3: 457–466. [Google Scholar]
- Heslop‐HarrisonY, Shivanna KR.1977. The receptive surface of the angiosperm stigma. Annals of Botany 41: 1233–1258. [Google Scholar]
- HesseM.1993. Pollenkitt development and composition in Tilia platyphyllos (Tiliaceae) analysed by conventional and energy filtering TEM. Plant Systematics and Evolution suppl. 7: 39–52. [Google Scholar]
- HesseM, Burns‐Balogh P.1984. Pollen and pollinarium morphology of Habenaria (Orchidaceae). Pollen et Spores 26: 385–400. [Google Scholar]
- HesseM, Vogel S, Halbritter F.2000. Thread‐forming structures in angiosperm anthers: their diverse role in pollination ecology. Plant Systematics and Evolution 222: 281–292. [Google Scholar]
- HofmeisterW.1861. Neue Beiträge zur Kenntnis der Embryobildung der Phanerogamen. II. Monokotyledonen. Königlich sächsische Gesellschaft der Wissenschaften, Leipzig, Abhandlungen 7: 631–760. [Google Scholar]
- JohnsonSD.1996. Bird pollination in South African species of Satyrium (Orchidaceae). Plant Systematics and Evolution 203: 91–98. [Google Scholar]
- JohnsonSD, Edwards TJ.2000. The structure and function of orchid pollinaria. Plant Systematics and Evolution 222: 243–269. [Google Scholar]
- KenrickJ, Knox RB.1979. Pollen development and cytochemistry in some Australian species of Acacia Australian Journal of Botany 27: 413–427. [Google Scholar]
- KnoxRB, McConchie CA.1986. Structure and function of compound pollen. In: Blackmore S, Ferguson IK, eds. Pollen and spores: form and function Linnean Society Symposium Series 12. London: Academic Press, 265–282. [Google Scholar]
- KocyanA, Endress PK.2001. Floral structure and development of Apostasia and Neuwiedia (Apostasioideae) and their relationships to other Orchidaceae. International Journal of Plant Science 162: 847–867. [Google Scholar]
- KontaF, Tsuji X.1982. The types of pollen tetrads and their formations observed in some Orchidaceae in Japan. Acta Phytotaxonomy and Geobotany 33: 206–217. [Google Scholar]
- LuitR, Johnson SD.2001. Hawkmoth pollination of the African epiphytic orchid Mystacidium venosum, with special reference to flower and pollen longevity. Plant Systematics and Evolution 228: 49–62. [Google Scholar]
- MulcahyDL.1979. The rise of the angiosperms: a genecological factor. Science 206: 20–23. [DOI] [PubMed] [Google Scholar]
- NeilandMRM, Wilcock CC.1995. Maximisation of reproductive success by European Orchidaceae under conditions of infrequent pollination. Protoplasma 187: 39–48. [Google Scholar]
- NeilandMRM, Wilcock CC.1998. Fruit set, nectar reward, and rarity in the Orchidaceae. American Journal of Botany 85: 1657–1671. [PubMed] [Google Scholar]
- NepiM, Franchi GG.2000. Cytochemistry of mature angiosperm pollen. Plant Systematics and Evolution 222: 45–62. [Google Scholar]
- NepiM, Pacini E.1993. Pollination, pollen viability and pistil receptivity in Cucurbita pepo Annals of Botany 72: 527–536. [Google Scholar]
- NepiM, Franchi GG, Pacini E.2001. Pollen hydration status at dispersal: cytophysiological features and strategies. Protoplasma 216: 171–180. [DOI] [PubMed] [Google Scholar]
- OttavianoE, Mulcahy DL.1989. Genetics of angiosperm pollen. Advances in Genetics 26: 1–64. [Google Scholar]
- PaciniE.1990. Harmomegathic characters of Pteridophyta spores and Spermatophyta pollen. In: Hesse M, Ehrendorfer F, eds. Morphology, development and systematic relevance of pollen and spores. Plant Systematics and Evolution suppl. 5: 53–69. [Google Scholar]
- PaciniE.1994. Cell biology of anther and pollen development. In: Williams EG, Knox RB, Clarke AE, eds. Genetic control of self incompatibility and reproductive development in plants Dordrecht: Kluwer, 289–308. [Google Scholar]
- PaciniE.1997. Tapetum character states: analytical keys for tapetum types and activity. Canadian Journal of Botany 75: 1448–1459. [Google Scholar]
- PaciniE.2000. From anther and pollen ripening to pollen presentation. Plant Systematics and Evolution 222: 19–43. [Google Scholar]
- PaciniE, Franchi GG.1996. Some cytological, ecological and evolutionary aspects of pollination. Acta Societatis Botanicorum Poloniae 65: 11–16. [Google Scholar]
- PaciniE, Franchi GG.1998. Pollen dispersal unit, gynoecium and pollination. In: Owens SJ, Rudall PI, eds. Reproductive biology Kew: Royal Botanic Gardens, 183–195. [Google Scholar]
- PaciniE, Franchi GG.1999a Pollen grain sporoderm and types of dispersal units. Acta Societatis Botanicorum Poloniae 68: 362–366. [Google Scholar]
- PaciniE, Franchi GG.1999b Types of pollen dispersal units and pollen competition. In: Clément C, Pacini E, Audran JC, eds. Anther and pollen: from biology to biotechnology Berlin: Springer‐Verlag, 1–11. [Google Scholar]
- PaciniE, Franchi GG.2000. Types of pollen dispersal units in Monocots. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution Melbourne: CSIRO, 295–300. [Google Scholar]
- PaciniE, Franchi GG, Hesse M.1985. The tapetum: its form, function, and possible phylogeny in embryophyta. Plant Systematics and Evolution 149: 155–185. [Google Scholar]
- PaciniE, Franchi GG, Ripaccioli M.1999. Ripe pollen structure and histochemistry of some Gymnosperms. Plant Systematics and Evolution 217: 81–99. [Google Scholar]
- PaciniE, Franchi GG, Lisci M, Nepi M.1997. Pollen viability related to type of pollination in six angiosperm species. Annals of Botany 80: 83–87. [Google Scholar]
- PandolfiT, Pacini E.1995. The pollinium of Loroglossum hircinum (L.) Rich. (Orchidaceae) between pollination and pollen tube emission. Plant Systematics and Evolution 196: 141–151. [Google Scholar]
- PandolfiT, Pacini E, Calder DM.1993. Ontogenesis of monad pollen Pterostylis plumosa (Orchidaceae Neottioideae). Plant Systematics and Evolution 186: 175–185. [Google Scholar]
- ProctorHC.1998. Effect of pollen age on fruit set, fruit weight, and seed set in three orchid species. Canadian Journal of Botany 76: 420–427. [Google Scholar]
- ProctorHC, Harder LD.1994. Pollen load, capsule weight, and seed production in three orchid species. Canadian Journal of Botany 72: 294–255. [Google Scholar]
- ProctorHC, Harder LD.1995. Effect of pollination success on floral longevity in the orchid Calypso bulbosa (Orchidaceae). American Journal of Botany 82: 1131–1136. [Google Scholar]
- ReichenbachHG.1852. De pollinis Orchidearum genesi ac structura. Leipzig: ???. [Google Scholar]
- SchlagM, Hesse M.1992. The formation of the generative cell in Polystachya pubescens (Orchidaceae). Sexual Plant Reproduction 5: 131–137. [Google Scholar]
- SchlagM, Hesse M.1993. Morphogenesis of the sporoderm in Polystachia pubescens (Orchidaceae). Grana 32: 22–28. [Google Scholar]
- SchillR, Pfeiffer W.1977. Untersuchungen an Orchideenpollinien unter besonderer Berücksichtigung ihrer Feinskulpturen. Pollen et Spores 19: 5–118. [Google Scholar]
- SchillR, Wolter M.1985. Ontogeny of elastoviscin in the Orchidaceae. Nordic Journal of Botany 5: 575–580. [Google Scholar]
- SchillR, Wolter M.1986. On the presence of elastoviscin in all subfamilies of the Orchidaceae and the homology to pollenkitt. Nordic Journal of Botany 6: 321–324. [Google Scholar]
- SchillR, Dannenbaum C, Neyer P.1992. Quantitative Untersuchungen an Orchideenpollinien. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 114: 153–171. [Google Scholar]
- SperanzaA, Calzoni GL, Pacini E.1997. Occurrence of mono‐ or disaccharides and polysaccharide reserves in mature pollen grains. Sexual Plant Reproduction 10: 110–115. [Google Scholar]
- StenzelH.2000. Pollen morphology of the subtribe Pleurothallidinae Lindl. (Orchidaceae). Grana 39: 108–125. [Google Scholar]
- TanakaI.1993. Development of male gametes in flowering plants. Journal of Plant Science 106: 55–63. [Google Scholar]
- UberaJiménezJL, Hidalgo Fernandez P, Schlag MG, Hesse M.1996. Pollen and tapetum development in male fertile Rosmarinus officinalis (Lamiaceae). Grana 34: 305–316. [Google Scholar]
- WeberM.1992. The formation of pollenkitt in Apium nodiflorum (Apiaceae). Annals of Botany 70: 573–577. [Google Scholar]
- WodehouseRP.1935. Pollen grains: their structure, identification and significance in science and medicine. New York: McGraw‐Hill. [Google Scholar]
- WolterM, Schill R.1985. On acetolysis structures in the Orchidaceae – why fossil record of orchids is so rare. Grana 24: 139–143. [Google Scholar]
- WolterM, Schill R.1986. Ontogenie von Pollen, Massulae und Pollinien bei den Orchideen. Tropische und subtropische Pflanzenwelt 56: 1–93. [Google Scholar]
- WolterM, Seuffert C, Schill R.1988. The ontogeny of pollinia and elastoviscin in the anther of Doritis pulcherrima (Orchidaceae). Nordic Journal of Botany 8: 77–88. [Google Scholar]
- YeungEC.1987a Development of pollen and accessory structures. In: Arditti J, ed. Orchids biology: reviews and perspectives. Vol. IV Itacha, NY: Comstock Publishing Associates, 197–225. [Google Scholar]
- YeungEC.1987b Mechanisms of pollen aggregation into pollinia in Epidendrum ibaguense (Orchidaceae). Grana 26: 47–52. [Google Scholar]
- YeungEC.1987c The development and structure of the viscidium in Epidendrum ibaguense H.B.K. (Orchidaceae). Botanical Gazette 148: 149–155. [Google Scholar]
- ZavadaMS.1983. Comparative morphology of monocot pollen and evolutionary trends of apertures and wall structures. Botanical Review 49: 331–379. [Google Scholar]
- ZavadaMS.1990. A contribution to the study of pollen wall ultrastructure of orchid pollinia. Annals of the Missouri Botanical Garden 77: 785–801. [Google Scholar]
- ZeeSY, Siu IHP.1990. Studies on the ontogeny of the pollinium of a massulate orchid (Peristylus spiranthes). Review of Palaeobotany and Palynology 64: 159–164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.