Abstract
Maturation of potato (Solanum tuberosum L.) tuber native and wound periderm and development of resistance to periderm abrasion were investigated utilizing cytological and histochemical techniques. Both native and wound periderm consist of three different tissues: phellem, phellogen and phelloderm. It was previously determined that the phellogen walls of immature native periderm are thin and prone to fracture during harvest, leading to periderm abrasion (excoriation). Phellogen walls thicken and become less susceptible to fracture upon maturation of the periderm, leading to resistance to excoriation. We now demonstrate that phellogen cells of immature wound periderm also have thin radial walls and that wound periderm abrasion is due to fracture of these walls. Maturation of the wound periderm is also associated with an increase in the thickness of the phellogen radial walls. Histological analysis with ruthenium red and hydroxylamine–FeCl2, which stain unesterified and highly methyl‐esterified pectins, respectively, indicates that the phellogen cell walls of native and wound periderm differ significantly regardless of the stage of maturity. Results obtained by staining with ruthenium red and hydroxylamine–FeCl2 imply that phellogen cell walls of immature native periderm contain methyl‐esterified pectin, but are lacking in unesterified (acidic) pectins. Maturation of native periderm is accompanied by an apparent increase in unesterified pectins in the walls of phellogen cells, which may allow for the strengthening of phellogen cell walls via calcium pectate formation. Histological staining of the phellogen walls of wound periderm, on the other hand, implies that these walls are deficient in pectins. Moreover, maturation of wound periderm is not accompanied by an increase in unesterified pectins in these walls. Since peroxidase is known to catalyse the cross‐linking of cell wall polymers, we stained native and wound periderm for the presence of peroxidase utilizing guaiacol as a substrate. Peroxidase staining was strong in the phellogen walls of both immature and mature native periderm and we could not detect any differences in staining between them. Peroxidase staining was weak in the phellogen walls of immature wound periderm and was not detectably different in mature wound periderm. Peroxidase data imply that there are distinct differences between native and wound periderm, though our data do not indicate that changes in peroxidase activity are involved in the development of resistance to periderm abrasion that occurs upon maturation of the periderm. However, we cannot rule out the involvement in this process of peroxidase isozymes that have low affinity for the substrates utilized here.
Key words: Guaiacol, histochemistry, pectin, periderm, peroxidase, phellem, phelloderm, phellogen, potato, ruthenium red, Solanum tuberosum L., wound‐healing
INTRODUCTION
Potato (Solanum tuberosum L.) native periderm forms an effective barrier around the tuber that protects it from infection and dehydration. An immature periderm can make the tuber susceptible to skinning (excoriation of the skin) during harvest, which renders the tuber vulnerable to dehydration and disease while in storage (Lulai and Orr, 1995; Lulai and Corsini, 1998). The potato periderm is made up of three tissues: phellem, phellogen and phelloderm (Reeve et al., 1969). The phellem (or cork) forms a series of layers at the outermost level of the periderm, and is derived from the phellogen layer (or cork cambium) underneath it. As phellem cells develop, they become suberized and then die, forming a protective layer. The phelloderm cells form the innermost tier of the periderm, and are similarly derived from the phellogen layer which is located directly above them. The phellogen is a single layer of meristematic cells derived from the hypodermis early during development of the tuber (Artschwager, 1924; Peterson and Barker, 1979). An immature periderm has a phellogen layer made up of cells with thin radial walls which fracture easily, allowing the phellem (skin) to scuff off (Lulai and Freeman, 2001).
A tuber with damaged skin, or a tuber cut for seed, will develop a wound periderm which shares many characteristics with native periderm. The wound response starts with the formation of a closing layer of suberin at the wound site, followed by the development of a new periderm under the closing layer (Thomson et al., 1995; Lulai, 2001). The wound periderm arises from a new phellogen layer derived from parenchyma cells under the wound site (Lulai, 2001). The wound periderm is derived from this actively dividing phellogen just as the native periderm is derived from the phellogen while it is meristematically active. Immature wound periderm and immature native periderm are both susceptible to excoriation until fully mature, and abrasion of either type of periderm renders the tuber vulnerable to dehydration and infection (Lulai, 2001). The biochemical processes responsible for the maturation of both native and wound periderm are poorly understood, and very little research on this subject has been published. In this paper we examine immature wound periderm to determine which part of the periderm is responsible for its susceptibility to excoriation. We also use established histological procedures to examine and compare the basic biochemical changes in cell wall structure (especially that of phellogen) associated with both native and wound periderm maturation. Specifically, we examined changes in unesterified pectin with ruthenium red and changes in methyl‐esterified pectin with hydroxylamine‐ferric chloride staining. Peroxidase activity, which is responsible for catalysing many cross‐linking reactions in the cell wall, was also examined.
MATERIALS AND METHODS
Native periderm material
Solanum tuberosum L. ‘Russet Burbank’ tubers used for analysis of native periderm were grown in field plots in Florida and in North Dakota using standard cultural practices. Tubers harvested early in the growing season were susceptible to skinning and were therefore considered to be immature. This was confirmed by mechanically testing skinning susceptibility as described previously (Lulai and Orr, 1993). Tubers from this harvest that were stored for at least 4 weeks under 96 ± 2 % relative humidity at 21 ± 2 °C in the dark were resistant to skinning and were therefore considered mature. Tubers harvested late in the growing season were also resistant to skinning and were therefore considered mature. Results from mature tubers were similar regardless of whether they were analysed after being stored until maturity or analysed when harvested mature.
Wound periderm material
Solanum tuberosum L. ‘Russet Burbank’ tubers used for analysis of wound periderm were grown in field plots in Minnesota and North Dakota using standard cultural practices. Wound periderm was formed by cutting mature tubers in half and shaving off a 0·75‐mm slice from the cut surface with an industrial steel blade to ensure a level wound surface. A wound periderm was allowed to develop under optimal conditions (Morris et al., 1989), at 21 ± 2 °C and 96 ± 2 % relative humidity in the dark for the desired time period (either 8 d for immature periderm, or 31–42 d for mature periderm). Wound periderm that was less than 2 weeks old was susceptible to skinning and was therefore considered to be immature. Wound periderm that was more than 3 weeks old was resistant to skinning and was therefore considered to be mature.
Microscopy and histology
Tissue blocks (1·0 × 0·5 × 0·3 cm) which included the periderm were cut from tubers and hand‐sectioned with a razor blade. In the case of wound periderm, blocks were removed from the periderm formed from perimedullary tissue while avoiding the pith and either tuber end. Blocks were fixed for at least 2 h in FAA (50 % ethanol, 5 % acetic acid, 10 % formalin) before they were sectioned with a razor blade and examined and/or stained. Fixation of periderm tissue with FAA did not alter histological staining of cell walls compared with staining of fresh tissues. Sections were stained with 0·02 % ruthenium red to detect unesterified (acidic) pectin, or with hydroxylamine‐ferric chloride to detect methyl‐esterified pectin (Harris et al., 1994). In some cases samples were de‐esterified with 0·1 m Na2CO3 (overnight at 4 °C) prior to staining. Samples were examined using standard light microscopy with a Zeiss Axioskop 50 microscope, and either photographs were taken with a Zeiss MC‐100 camera, or images were recorded with a Zeiss Axiocam digital camera. Autofluorescence was examined utilizing UV epifluorescent illumination provided by an HBO 50 W (L2) mercury short arc lamp (exciter filter G‐365, chromatic beam splitter FT‐395, barrier filter LP‐429). All of the samples were examined by UV epifluorescence to determine the location of the phellogen layer. All experiments were repeated at least three times with different samples.
Staining for peroxidase was evaluated in situ utilizing periderm sections cut from fresh blocks which were fixed for precisely 2 h in FAA to immobilize the enzyme without diminishing peroxidase activity (Krishnamurthy, 1999). Fixed sections were incubated in 0·05 % hydrogen peroxide and one of the following substrates: 0·2 % guaiacol, 2 mm4‐chloro‐1‐naphthol or 0·05 % tetramethylbenzidine (TMB). Experiments were repeated at least three times with different samples.
Phellogen radial cell wall thickness was measured in wound periderm utilizing digital microscopy. The width the of radial walls of 21–23 phellogen cells from both immature and mature periderm was measured utilizing a software‐embedded stage micrometer. Data are presented as mean ± standard error (n = number of sample measurements). Student’s t‐test was used to test for significant differences (P < 0·01) between sample measurements for immature and mature periderm.
RESULTS
Native and wound periderm anatomy
Potato tuber native periderm and immature wound periderm were very similar in basic organization (Fig. 1). In both types, the periderm was made up of phellem, phellogen and phelloderm tissues. However, there were differences in the number of cell layers in these tissues. The number of cell layers in the phellem was usually greater in native periderm (Fig. 1A) than in wound periderm (Fig. 1B). On the other hand, the number of cell layers in the phelloderm was usually greater in wound periderm compared with native periderm. Figure 1C shows that the intense UV‐autofluorescence characteristic of suberized walls does not extend beyond the radial walls of the phellem cells closest to the phellogen layer in wound periderm. Weaker autofluorescence is evident along the lower tangential wall of the phellem cells adjacent to the phellogen layer. Fracturing during periderm abrasion occurs just under the innermost phellem layer, which indicates that breakage occurs in the phellogen layer (Fig. 1D). Figure 2 shows phellogen cells of wound periderm under greater magnification. Phellogen radial walls of immature wound periderm were much thinner than phellogen walls of mature wound periderm (Fig. 2). On average, phellogen radial walls of mature periderm were 145 % thicker than those of immature periderm (Fig. 2).
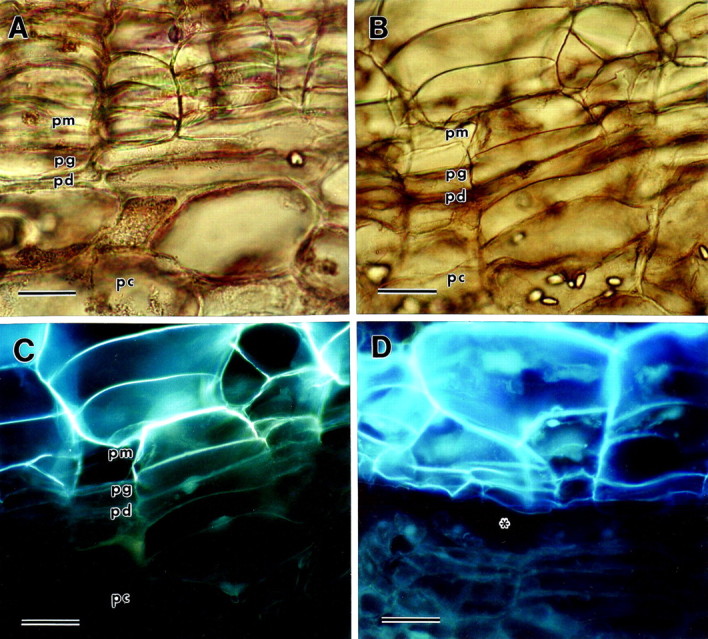
Fig. 1. Light micrographs of potato periderm sections viewed under brightfield or UV epifluorescence (pm, phellem; pg, phellogen; pd, phelloderm; pc, parenchyma underlying the periderm). A, Mature native periderm viewed under brightfield. Bar = 30 µm. B, Immature wound periderm viewed under brightfield. Bar = 60 µm. C, The same immature wound periderm viewed under UV epifluorescence. Note that the autofluorescence of the phellem tissue ends where the phellogen cell layer begins. Bar = 60 µm. D, Excoriated immature wound periderm viewed under UV epifluorescence, demonstrating that wall fracture occurs in the phellogen layer, just under the phellem. The area of the fracture is indicated by an asterisk. Bar = 60 µm.
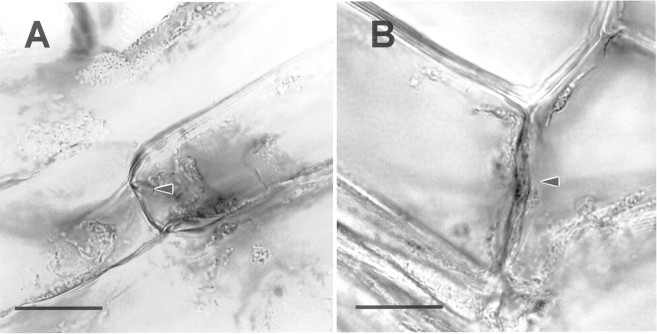
Fig. 2. Light micrographs showing phellogen cells of wound periderm. Arrowheads indicate radial walls. A, Immature wound periderm. B, Mature wound periderm. Bar = 2 µm. Note that the phellogen radial wall of the mature periderm is thicker than that of the immature periderm. Mean phellogen radial wall width for immature periderm = 146 + 27 nm (n = 21) and for mature periderm = 357 + 31 nm (n = 23). Means are significantly different at P = 0·01.
Staining for pectins in native periderm
Ruthenium red (which stains unesterified pectin) stained the walls of phelloderm in immature native periderm, but did not stain the walls of phellogen or phellem cells (Fig. 3A). In comparison, the walls of the parenchyma cells underneath the periderm stained intensely with ruthenium red. Chemical de‐esterification with 0·1 m Na2CO3 resulted in an increase in overall staining with ruthenium red (Fig. 3B). Both tangential and radial phellogen cell walls stained with ruthenium red after the periderm tissue was chemically de‐esterified. The phellem cell walls also stained after chemical de‐esterification, though not as intensely. Ruthenium red stained both phelloderm and phellogen cell walls in mature native periderm (Fig. 3C). Both tangential and radial phellogen cell walls stained intensely, but staining of phellem cell walls was very weak. The walls of parenchyma cells underneath the periderm stained intensely. De‐esterification with 0·1 m Na2CO3 resulted in an increase in the staining of phellem cell walls, many of which stained intensely (Fig. 3D). Ruthenium red stained some of the walls of phellem cells but left other phellem walls unstained. Similar results were obtained by de‐esterification with 4 % NaOH for 20–30 min (data not shown). Hydroxylamine–FeCl2 (which stains methyl‐esterified pectin) weakly stained the walls of phellem, phellogen and phelloderm cells in both immature and mature native phellogen (data not shown). The walls of the parenchyma cells underneath the periderm stained more intensely with hydroxylamine–FeCl2 than the walls of the periderm in both immature and mature native periderm (data not shown).
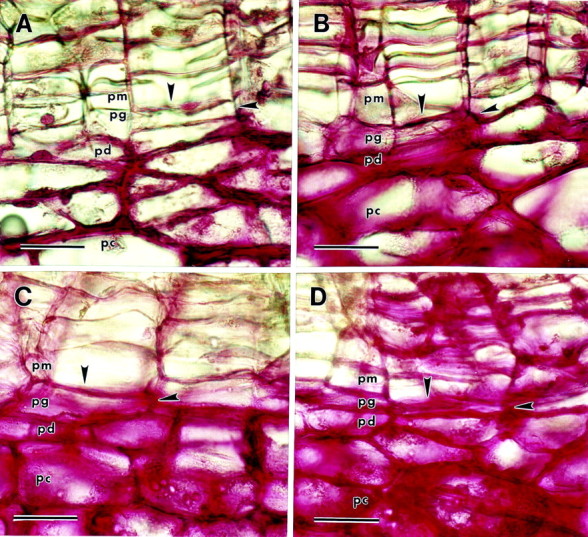
Fig. 3. Ruthenium red staining of native periderm sections (pm, phellem; pg, phellogen; pd, phelloderm; pc, parenchyma underlying the periderm). Arrowheads indicate phellogen upper tangential and radial cell walls. A, Immature periderm. The only cell walls of the periderm that are stained are the lower tangential and radial walls of the phelloderm layer. B, Immature periderm de‐esterified with 0·1 m Na2CO3 before staining. All three tissues of the periderm are stained. C, Mature periderm. Phellogen and phelloderm cell walls are stained; phellem walls are unstained. D, Mature periderm de‐esterified with 0·1 m Na2CO3 before staining. All three tissues of the periderm are stained. Bar = 40 µm.
Staining for pectins in wound periderm
Ruthenium red did not stain the walls of phellem, phelloderm or phellogen cells in immature wound periderm (Fig. 4A). In contrast, the parenchyma cells underneath the periderm did stain. De‐esterification with 0·1 m Na2CO3 increased the intensity of staining in the parenchyma cells underneath the periderm but did not alter the staining of phelloderm or phellogen walls (Fig. 4B). However, chemical de‐esterification allowed for some weak, uneven staining of some walls in the phellem. In mature wound periderm, ruthenium red did not stain phellogen cell walls, although phelloderm cell walls did stain (Fig. 4C). Staining of phellem cells was weak and uneven, with most of these walls being unstained. Chemical de‐esterification allowed for increased staining of phellem walls, though staining was still not uniform or consistent. Ruthenium red stained many, though not all, of the radial and tangential phellogen cell walls after chemical de‐esterification (Fig. 4D). Hydroxyl amine–FeCl2 staining was very weak in both immature and mature wound periderm sections, and there was no significant staining in any of the cell types in wound periderm (data not shown).
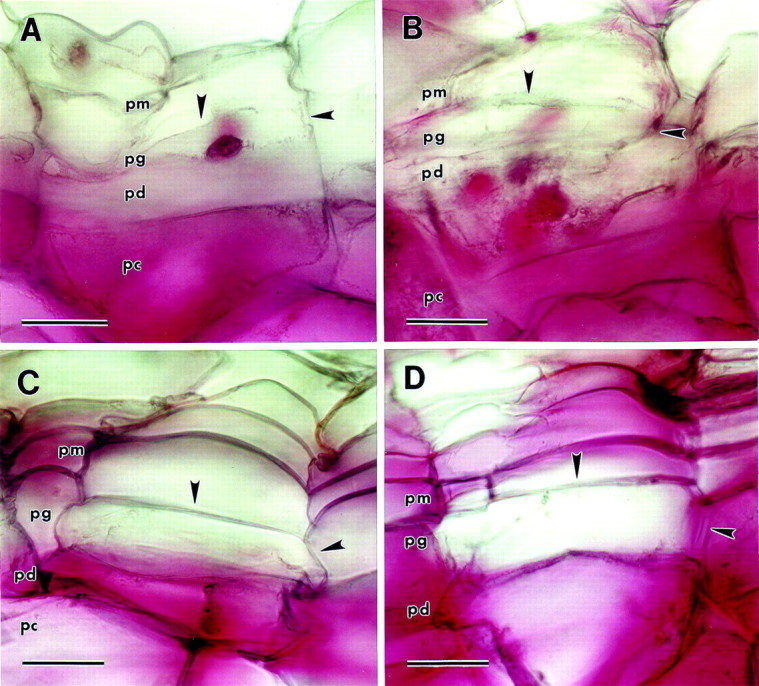
Fig. 4. Ruthenium red staining of wound periderm sections (pm, phellem; pg, phellogen; pd, phelloderm; pc, parenchyma underlying the periderm). Arrowheads indicate phellogen upper tangential and radial cell walls. A, Immature wound periderm. No periderm cells walls are stained, though the walls of the parenchyma cells underneath the periderm are stained intensely. B, Immature wound periderm de‐esterified with 0·1 m Na2CO3 before staining. No periderm cells are stained, although the walls of the parenchyma cells underneath the periderm are stained intensely. C, Mature wound periderm. Phelloderm cell walls are stained, but phellogen cell walls are not stained. Phellem cell wall staining is weak and sporadic. D, Mature wound periderm de‐esterified with 0·1 m Na2CO3 before staining. All three periderm cell types are stained, though staining of phellem and phellogen cell walls is uneven and weak. Bar = 40 µm.
Staining for peroxidase activity in native and wound periderm
Peroxidase staining with 0·2 % guaiacol as the substrate resulted in reddish‐brown reaction product in periderm cell walls (Fig. 5). In both immature and mature native periderm, staining was intense throughout the periderm, including the phellogen walls (Fig. 5A and B). Staining was also intense in the underlying parenchyma cells (Fig. 5). There was no staining of the walls of phellem or phellogen cells in immature wound periderm, though the phelloderm cell walls stained weakly (Fig. 5C). In contrast, cell walls of the parenchyma tissue underneath the periderm stained intensely (Fig. 5C). The staining pattern in mature wound periderm was more intense in the phellem layer, but there was no significant staining of the cell walls of the phellogen, with the exception of the upper tangential walls shared with the adjacent phellem layer (Fig. 5D). Staining was weak and scattered in the phelloderm layer, and was most intense in cell walls of the parenchyma underlying the periderm (Fig. 5D). There was no staining of native periderm in the absence of added hydrogen peroxide, indicating that the reactions that resulted in coloured product were probably catalysed by peroxidase (Fig. 5E). The dark brown/black colouration in the upper phellem and in the netting above the phellem is natural pigment and does not represent peroxidase staining. Results of staining of wound periderm without added hydrogen peroxide were similar to the results shown for native periderm (data not shown). Staining patterns with guaiacol were very similar to staining patterns with the substrates 4‐chloro‐1‐naphthol and TMB (data not shown).
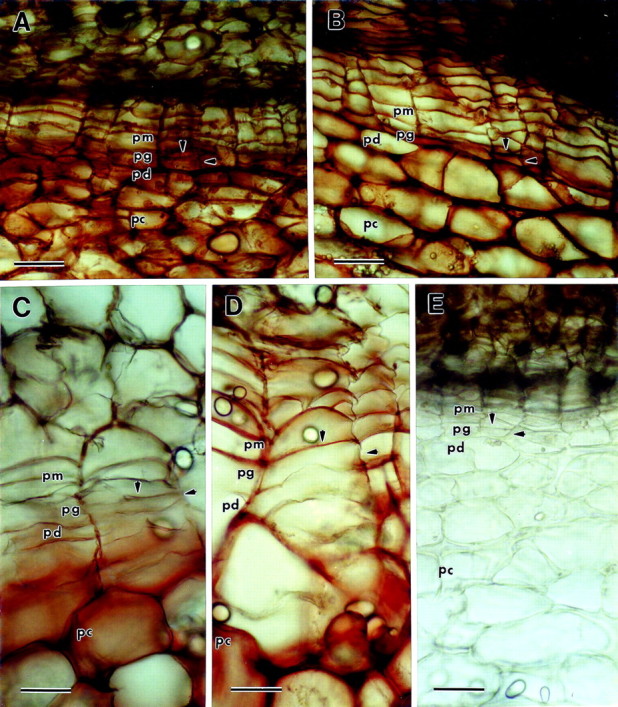
Fig. 5. Peroxidase staining utilizing guaiacol as the substrate. Hydrogen peroxide was added just prior to the guaiacol unless otherwise indicated (pm, phellem; pg, phellogen; pd, phelloderm; pc, parenchyma underlying the periderm). Arrowheads indicate phellogen upper tangential and radial cell walls. A, Immature native periderm. The entire periderm is stained red‐brown, including phellogen cell walls. B, Mature native periderm. The entire periderm is stained red‐brown including phellogen cell walls. C, Immature wound periderm. There is no staining in the phellem or phellogen layers, though the walls of the phelloderm are stained weakly. In contrast, red‐brown staining is intense in the cell walls of the parenchyma underneath the periderm. D, Mature wound periderm. Phellem cell walls are stained red‐brown, but the radial and lower tangential phellogen cell walls are unstained. There is some weak scattered staining in the phelloderm. E, Mature native periderm without hydrogen peroxide added prior to staining. There is no staining, demonstrating the specificity of the reaction for peroxidase (the brown/black colouration in the upper phellem and netting above the phellem is natural colouration and does not represent peroxidase staining). Bar = 60 µm.
DISCUSSION
Native and wound periderm of ‘Russet Burbank’ tubers were very similar in organization, both consisting of phellem, phellogen and phelloderm layers (Fig. 1). Phellem cells were identified by their rectangular shape and arrangement in columnar rows, and by the fact that their walls autofluoresce under UV‐illumination (Lulai and Morgan, 1992). This columnar pattern is a result of their origin from periclinal divisions in the phellogen cell layer (Artschwager, 1924) and the autofluorescence of phellem cell walls is due to the presence of aromatic suberin polymers (Bernards and Lewis, 1998). Phellogen cells were identified as the first row of cells under the autofluorescent phellem layer, and were generally narrower than the phellem cells above them (Fig. 1).
Immature periderm is characterized by a meristematically active phellogen layer, while mature periderm is characterized by a meristematically inactive phellogen layer (Lulai and Freeman, 2001). It has sometimes been asserted that maturity, and therefore susceptibility to skinning, of wound periderm is dependent on the thickness of the phellem layer (Morris et al., 1989). However, our research clearly shows that the scuffing off or excoriation of immature wound periderm is due to separation of the phellem from the underlying phellogen layer, just as is the case with immature native periderm (Lulai and Freeman, 2001). Fracture of the wound periderm upon excoriation always occurs in the phellogen layer, just underneath the phellem tissue (Fig. 1D). The radial walls of phellogen cells of immature wound periderm are considerably thinner, and by inference more fragile, than the corresponding walls in mature wound periderm (Fig. 2). These observations suggest that fracturing of the radial walls of the phellogen cells is responsible for periderm scuffing or excoriation in immature wound periderm. These observations are also similar to those reported for native periderm (Lulai and Freeman, 2001).
Ruthenium red primarily stains unesterified galacturonan blocks of pectins, as the binding site requires two negative charges 0·42 nm apart (Sterling, 1970). Methyl‐esterification of the carboxyl groups of adjacent galacturonic acid residues eliminates the negative charges required for binding (Sterling, 1970). Ruthenium red can therefore be effectively used to differentiate between unesterified and esterified pectins.
In immature native periderm sections, ruthenium red stained walls of phelloderm cells, but did not stain walls of either phellem or phellogen cells (Fig. 3A). Ruthenium red stained walls of the parenchyma cells underneath the periderm and this staining was especially intense at cell junctions. This is consistent with the abundance of unesterified pectins reported to be found at cell junctions between parenchyma cells of both flax (Jauneau et al., 1997) and potato (Marty et al., 1995; Bush and McCann, 1999; Bush et al., 2001). Both radial and tangential walls of phellogen cells stained after chemical de‐esterification, implying that pectins are present in these walls but that they are highly methyl‐esterified. It is interesting to note that immunolabelling for unesterified pectin was reported to be poor in the walls of meristematic cells of stolens that were developing into tubers, whereas labelling for highly esterified pectins was much stronger in these cells (Bush et al., 2001). Phellem cell walls also stained after chemical de‐esterification, though staining was erratic, with some walls staining and others not (Fig. 3B). The increased staining of phellem cells after chemical de‐esterification could either be due to the presence of highly methylated pectin, or due to de‐esterification of suberin polymers, which would be expected to break the ester linkages which bind the polyaliphatic and polyaromatic domains of suberin together (Kolattukudy and Espelie, 1985). The depolymerization of this water‐resistant barrier could have allowed ruthenium red increased access to any pectins present in the phellem cell walls, and thereby increased the staining intensity. It was recently reported that potato phellem walls labelled poorly with antibodies specific for both esterified and unesterifed pectin (Bush et al., 2001). However, this research utilized small nascent tubers that had recently formed from stolon and may not have contained fully suberized phellem cells. For this reason, a direct comparison with our research may not be appropriate.
In contrast to immature native periderm, ruthenium red stained the walls of phellogen cells in addition to the walls of phelloderm cells in mature native periderm (Fig. 3C). This staining differential implies that the amount of unesterified pectins increased in phellogen cell walls during maturation of the periderm and loss of phellogen mitotic activity. Ruthenium red staining of phellem cells was also poor in mature periderm, but once again this may be due to pectins being masked by the barrier created by suberin polymers present in these walls. Chemical de‐esterification of mature native periderm allowed for an intensification of ruthenium red staining of the phellem walls, either due to the presence of highly methyl‐esterified pectins, or possibly due to the stripping of masking suberin aliphatic polymers from these walls (Fig. 3D).
The hydroxylamine–FeCl2 staining method is based on the ability of alkaline hydroxylamine HCl to react with the methyl ester groups of pectins, the product of which is then reacted with ferric ions to produce a reddish‐brown precipitate (Reeve, 1959). The staining intensity is therefore dependent on the degree of methyl esterification of any pectins present. Chemical de‐esterification of both immature and mature periderm sections before hydroxylamine–FeCl2 treatment prevented the staining reaction, supporting the premise that methylated groups were stained by this method (data not shown). Treatment of both immature and mature native periderm sections with hydroxylamine–FeCl2 resulted in staining of phellem, phellogen and phelloderm cell walls (data not shown). This staining pattern implies that the walls of all three cell types in the native periderm contain methylated pectins, and therefore supports the similar conclusions based on ruthenium red staining of chemically de‐esterified immature periderm.
The most likely explanation for the differences in the staining patterns of immature and mature native periderm is that maturation of native periderm is accompanied by an increase in unesterified pectin in the walls of phellogen cells. This increase in unesterified pectin may be responsible for the development of resistance to radial wall fracture in mature periderm. Unesterified pectin has the ability to impart rigidity to the cell wall by cross‐linking via calcium bridges to form calcium pectate (Jarvis, 1984; Thakur et al., 1997). It is generally accepted that in most cells pectin is synthesized in the Golgi apparatus and transported into the wall in a highly methyl‐esterified form. Pectin methylesterases (PMEs) present in the wall can catalyse the de‐esterification of these polymers, allowing cross‐links to form which strengthen the wall in association with the loss of meristematic activity of differentiated cells (Goldberg et al., 1996). In support of this theory, a recent paper reported that a specific basic PME isoform found in the cambium of a hybrid aspen is associated with the cessation of meristematic activity and the establishment of dormancy in this species (Micheli et al., 2000). Because pectins are methyl‐esterified before they are transported into the cell wall, the walls of young, immature cells tend to be characterized by highly esterified pectins, which prevents cross‐linking via calcium ions. Older, fully differentiated cells have more rigid walls that are characterized by a low degree of esterification which allows for calcium cross‐linking of pectin polymers (Goldberg et al., 1989). These characteristics are in agreement with our data, which suggest that the phellogen walls of immature native periderm contain methyl‐esterified pectins, but are lacking in unesterified pectin. Phellogen walls of mature native periderm, however, apparently contain both types of pectin, thereby allowing calcium pectate to strengthen these walls and make them resistant to fracture.
During differentiation of vascular cambium, actively dividing cambial cells undergo changes in cell wall structure that are similar to phenomena that we found in the phellogen cell walls of potato during periderm development. The walls of actively dividing vascular cambium are characterized by a lack of both unesterified pectins and a lack of calcium ions needed to form calcium pectate (Lachaud et al., 1999). In cell derivatives of vascular cambium that are destined to become phloem, cell walls are thickened and become enriched in unesterified pectins and calcium ions (Guglielmino et al., 1997; Lachaud et al., 1999). These reports are consistent with our data implying that thickening of cell walls in inactive phellogen is associated with an increase in unesterified pectin.
The lack of staining of immature wound periderm cell walls with ruthenium red implies a lack of unesterified pectins in the walls of all three types of periderm cells (Fig. 4A). In contrast, the walls of the underlying parenchyma cells stained strongly with ruthenium red. Chemical de‐esterification before staining intensified the staining of the parenchyma cell walls under the periderm, but had little effect on staining of the periderm cell walls (Fig. 4B). These results imply that the walls of immature wound periderm cells are lacking in both esterified and unesterified pectins. It is also possible that pectin was present in these walls, but was too scarce to be detected with ruthenium red.
Walls of phelloderm and phellem cells did stain with ruthenium red in mature wound periderm, though staining was very weak and uneven in phellem walls (Fig. 4C). Phellogen cell walls, however, did not react with ruthenium red. Chemical de‐esterification of mature wound periderm resulted in an intensification of ruthenium red staining of phellem and phellogen cell walls, though staining was still weak and irregular and some walls were not stained at all (Fig. 4D). These results imply that maturation of wound periderm is not accompanied by an increase in unesterified pectins in the radial walls of phellogen cells, despite the thickening of these walls. Maturation does seem to be accompanied by an increase in esterified pectin, though the sporadic and interrupted nature of the staining makes this conclusion uncertain.
Hydroxylamine–FeCl2 staining of immature and mature wound periderm was very poor for all three cell types (data not shown). These results imply that there was very little methyl‐esterified pectin present in walls of phelloderm, phellogen and phellem cells in immature and mature wound periderm. This conclusion is supported by the lack of ruthenium red staining in chemically de‐esterified immature wound periderm cells (Fig. 4B), but not by the ruthenium red staining of de‐esterified mature wound periderm (Fig. 4D). However, with the exception of phelloderm cell walls, ruthenium red staining in de‐esterified mature wound periderm was sporadic and uneven, and therefore not definitive. It is also unclear whether a specific type of esterification pattern is required for hydroxylamine–FeCl2 staining and the minimum amount of pectin that is required for detectable staining.
Peroxidases are known to be involved in the cross‐linking of a number of cell wall polymers including suberin (Espelie et al., 1986), extensin (Cooper and Varner, 1983) and feruloylated hemicelluloses (Tan et al., 1991). Despite the involvement of peroxidases in the cross‐linking of numerous cell wall polymers, we were not able to discern any changes in peroxidase staining of phellogen cell walls occurring during either native or wound periderm maturation. Peroxidase staining was strong in the cell walls of both immature and mature native periderm, including the phellogen layer (Fig. 5A and B). We could not detect any significant differences in peroxidase staining between immature and mature native periderm, although this does not exclude the possibility that there are subtle differences in peroxidase activity involving specific isozymes.
There was very little peroxidase staining in the cell walls of phellogen and phellem layers of immature wound periderm; this was in stark contrast to the strong staining present in the underlying cortical parenchyma cell walls and the moderate staining present in cell walls of the phelloderm layer (Fig. 5C). In comparison with immature wound periderm, there was an increase in peroxidase staining in mature wound periderm, but this was primarily restricted to the phellem layer (Fig. 5D). There was no significant change in peroxidase staining in walls of the phellogen layer, however. The increased peroxidase staining found in the phellem cell walls of mature wound periderm may be due to suberization of these walls, which is reported to be dependent on an anionic peroxidase (Espelie et al., 1986). It is possible that peroxidase isozymes that are not specific for any of the three substrates we tested are involved in cell wall changes in the phellogen layer. Therefore, we cannot presume that no peroxidases are involved in the maturation of phellogen cell walls. It is also worth noting that peroxidase staining was strong with all three substrates in wound‐healing tissue during the development of the closing layer before wound periderm formation (data not shown). This is not unexpected because peroxidase is believed to be involved in the suberization process necessary for closing layer formation (Espelie et al., 1986).
In conclusion, excoriation of immature potato wound periderm is due to fracture at the phellogen cell layer and this susceptibility to excoriation is probably due to the thinness of the radial walls of meristimatically active phellogen cells in the immature wound periderm. The radial walls of inactive phellogen cells from mature wound periderm are thicker and therefore less likely to fracture compared with those of active phellogen cells. In addition, we have discovered significant differences between native and wound periderm in their cell wall biochemistry, despite similarities in basic organization. The most striking differences are in the walls of their phellogen cells, both inactive and active. The walls of active phellogen cells from immature native periderm apparently contain methyl‐esterified pectins, but are lacking in unesterified pectins. Upon maturation of the native periderm, walls of the inactive phellogen cells contain unesterified pectins in addition to methyl‐esterified pectins. In contrast, the walls of active phellogen cells from immature wound periderm are apparently deficient in both types of pectin, although we cannot be certain that low levels of pectin were not present. Furthermore, maturation of wound periderm is apparently not associated with an increase in unesterified pectin in phellogen walls. It is unclear whether maturation of wound periderm is accompanied by changes in methyl‐esterified pectin in phellogen cell walls. Peroxidase activity is greater in native periderm at both stages of development compared with wound periderm, but in neither type of periderm could we discern any histological changes in peroxidase activity associated specifically with phellogen maturation in either native or wound periderm. Peroxidases that do not utilize any of the three substrates we tested may still be involved in phellogen maturation.
A summary of the reactions of all three periderm cell types in native and wound periderm to the stains used in this study is provided in Table 1. Further research is being conducted to determine the specific changes in cell wall structure of phellogen cells associated with both native and wound periderm maturation.
Table 1.
Summary of the reactions of each cell type in native and wound periderm to ruthenium red, hydroxylamine–FeCl2 and peroxidase staining
| Ruthenium red† | |||||
| Cell type | Periderm* | Untreated | De‐esterified | Hydroxylamine | Peroxidase |
| Phellem | Imm Native | – | + | + | ++ |
| Mat Native | – | + | + | ++ | |
| Imm Wound | – | – | – | – | |
| Mat Wound | – | + | – | + | |
| Phellogen | Imm Native | – | + | + | ++ |
| Mat Native | ++ | ++ | + | ++ | |
| Imm Wound | – | – | – | – | |
| Mat Wound | – | + | – | – | |
| Phelloderm | Imm Native | + | ++ | + | ++ |
| Mat Native | ++ | ++ | + | ++ | |
| Imm Wound | – | – | – | + | |
| Mat Wound | ++ | ++ | – | + | |
*Imm, Immature; Mat, mature; –, weak or no staining; +, moderate staining; ++, strong staining.
†For ruthenium red, the first reaction is for untreated periderm and the second reaction is for periderm de‐esterified with 0·1 m Na2CO3 prior to staining.
ACKNOWLEDGEMENTS
We thank Jeff Miller and Marty Glynn for excellent technical assistance, and Tom Freeman and Alan White for helpful comments on revising this manuscript. Mention of a trademark, proprietary product or vendor does not constitute endorsement by the United States Department of Agriculture.
Supplementary Material
Received: 12 September 2001; Returned for revision: 21 January 2002; Accepted: 19 March 2002
References
- ArtschwagerE.1924. Studies on the potato tuber. Journal of Agricultural Research 27: 809–835. [Google Scholar]
- BernardsMA, Lewis NG.1998. The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry 47: 915–933. [DOI] [PubMed] [Google Scholar]
- BushMS, McCann MC.1999. Pectic epitopes are differentially distributed in the cell walls of potato (Solanum tuberosum) tubers. Physiologica Plantarum 107: 201–213. [Google Scholar]
- BushMS, Marry M, Huxham IM, Jarvis MC, McCann MC.2001. Developmental regulation of pectic epitopes during potato tuberisation. Planta 213: 869–880. [DOI] [PubMed] [Google Scholar]
- CooperJB, Varner JE.1983. Insolubilization of hydroxylproline‐rich cell wall glycoprotein in aerated carrot root slices Biochemistry and Biophysics Research Communications 112: 161–167. [DOI] [PubMed] [Google Scholar]
- EspelieKE, Franceschi VR, Kolattukudy PE.1986. Immuno cytohemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound‐healing potato tuber tissue. Plant Physiology 81: 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GoldbergR, Morvan C, Jauneau A, Jarvis MC.1996. Methyl‐esterification; de‐esterification and gelation of pectins in the primary wall. In: Visser J, Voragen AGJ, eds. Pectins and pectinases Amsterdam: Elsevier Sciences B.V., 151–173. [Google Scholar]
- GoldbergR, Devillers P, Prat R, Morvan C, Michon V, Hervé du Penhoat C.1989. Control of cell wall plasticity. Relationship to pectin properties. In: Lewis NG, Paice MG, eds. Plant cell wall polymers‐ biogenesis and biodegradation Washington D.C.: American Chemical Society, 312–323. [Google Scholar]
- GuglielminoN, Liberman M, Jauneau A, Vian B, Catesson AM, Goldberg R.1997. Pectin immunolocalization and calcium visualization in differentiating derivatives from polar cambium. Protoplasma 199: 151–160. [Google Scholar]
- HarrisN, Spence J, Oparka.1994. General and enzyme histochemistry. In: Harris N, Oparka KJ, eds. Plant cell biology: a practical approach New York: IRL Press, 51–68. [Google Scholar]
- JarvisM.1984. Structure and properties of pectin gels in plant cell walls. Plant Cell and Environment 7: 153–164. [Google Scholar]
- JauneauA, Quentin M, Driouich A.1997. Micro‐heterogeneity of pectins and calcium in the epidermal and cortical parenchyma cell walls of flax hypocotyl. Protoplasma 198: 9–19. [Google Scholar]
- KolattukudyPE, Espelie KE.1985. Biosynthesis of cutin, suberin, and associated waxes. In: Takayoshi H, ed. Biosynthesis and biodegradation of wood components Orlando: Academic Press, 161–207. [Google Scholar]
- KrishnamurthyKV.1999. Light microscopic cytochemistry. In: Methods in cell wall cytochemistry Boca Raton: CRC Press, 29–149. [Google Scholar]
- LachaudS, Catesson AM, Bonnemain JL.1999. Structure and functions of the vascular cambium. Comptes Rendus de l’Academie des Sciences/ Sciences de la Vie 322: 633–724. [DOI] [PubMed] [Google Scholar]
- LulaiEC.2001. Tuber periderm and disease resistance. In: Stevenson WR, Loria R, Franc GD, Weingartner DP, eds. Compendium of potato diseases St Paul: American Phytopathological Society, 3–6. [Google Scholar]
- LulaiEC, Corsini DL.1998. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound‐healing. Physiological and Molecular Plant Pathology 53: 209–222. [Google Scholar]
- LulaiEC, Freeman TP.2001. The importance of phellogen cells and their structural characteristics in susceptibility and resistance to excoriation of potato tuber (Solanum tuberosum L.) during periderm maturation. Annals of Botany 88: 555–561. [Google Scholar]
- LulaiEC, Morgan WC.1992. Histochemical probing of potato periderm with neutral red: a sensitive cytofluorochrome for the hydrophobic domain of suberin. Biotechnic and Histochemistry 67: 185–195. [DOI] [PubMed] [Google Scholar]
- LulaiEC, Orr PH.1993. Determining the feasibility of measuring genotypic differences in skin‐set. American Potato Journal 70: 599–609. [Google Scholar]
- LulaiEC, Orr PH.1995. Porometric measurements indicate wound severity and tuber maturity affect the early stages of wound‐healing. American Potato Journa l72: 225–241. [Google Scholar]
- MartyP, Goldberg R, Liberman M, Vian B, Bertheau Y, Jouan B.1995. Composition and localization of pectic polymers in the stems of two Solanum tuberosum genotypes. Plant Physiology and Biochemistry 33: 409–417. [Google Scholar]
- MicheliF, Sundberg B, Goldberg R, Richard L.2000. Radial distribution pattern of pectin methylesterases across the cambial region of hybrid aspen at activity and dormancy. Plant Physiology 124: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MorrisSC, Forbes‐Smith MR, Scriven FM.1989. Determination of optimal conditions for suberization, wound periderm formation, cellular desiccation and pathogen resistance in wounded Solanum tuberosum tubers. Physiological and Molecular Plant Pathology 35: 177–190. [Google Scholar]
- PetersonRL, Barker WG.1979. Early tuber development from explanted stolon nodes of Solanum tuberosum var. Kennebec. Botanical Gazette 140: 398–406. [Google Scholar]
- ReeveRM.1959. A specific hydroxylamine‐ferric chloride reaction for histochemical localization of pectin. Stain Technology 34: 209–211. [DOI] [PubMed] [Google Scholar]
- ReeveRM, Hautala E, Weaver ML.1969. Anatomy and compositional variation within potatoes. I. Developmental histology of the tuber. American Potato Journal 46: 361–373. [Google Scholar]
- SterlingC.1970. Crystal‐structure of ruthenium red and stereochemistry of its pectin stain. American Journal of Botany 57: 172–175. [Google Scholar]
- TanK‐S, Hoson T,Masuda Y, Kamisaka S.1991. Correlation between cell wall extensibility and the content of difurulic and ferulic acids in cell walls of Oryza sativa coleoptiles grown under water and in air. Physiologia Plantarum 38: 397–403. [Google Scholar]
- ThakurBR, Singh RK, Handa AK.1997. Chemistry and uses of pectin – a review. Critical Reviews of Food Science and Nutrition 37: 47–73. [DOI] [PubMed] [Google Scholar]
- ThomsonN, Evert RF, Kelman A.1995. Wound healing in whole potato tubers: a cytochemical, fluorescence, and ultrastructural analysis of cut and bruise wounds. Canadian Journal of Botany 73: 1436–1450. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


