Abstract
Silica accumulation in long‐lived leaves of Sasa veitchii was investigated using the molybdenum blue method. In general, silica accumulation was rapid during spring and summer, and slow during winter. The leaves continuously accumulate silica throughout their life. The significance of these observations is discussed in relation to two hypotheses for silica accumulation in plants: (1) that silica accumulation is a result of water consumption by the plant; and (2) that silica is actively accumulated to protect the plant. The results of the present study support the former hypothesis.
Key words: Sasa veitchii (Carrière) Rehder, bamboo, Poaceae, leaf, silica accumulation, ageing, sigmoid curve, molybdenum blue method
INTRODUCTION
Silica accumulation is one of the most prominent characteristics of the Poaceae. Grasses readily absorb silica in the form of silicic acid from the soil, and most silica is accumulated in the aerial parts as amorphous hydrated silica (SiO2.nH2O). Various mechanisms and functions have been proposed to explain silica accumulation in the Poaceae (Jones and Handreck, 1967; Kaufman et al., 1981; Hodson and Sangster, 1990; Epstein, 1999). Several researchers consider silica accumulation to be a result of transpiration from the surface of aerial parts because heavy deposits of silica are often observed where water movement terminates, such as leaf blades and inflorescence bracts (e.g. Frey‐Wyssling, 1930; Yoshida et al., 1962b; Jones and Handreck, 1967). Frey‐Wyssling and Mühlethaler (1965) regarded silica as waste which was unavoidably absorbed from soil during water absorption. These opinions can be generalized, explaining silica accumulation as being the result of water consumption by plants. On the other hand, others believe that silica accumulation is controlled by plants to increase the mechanical stability of their tissues (Balasta et al., 1989) and to provide protection against microorganisms (Belanger et al., 1995; Marschner, 1995) and herbivores (Jones and Handreck, 1967; McNaughton et al., 1985; Cid et al., 1989).
There are thus two different hypotheses to explain silica accumulation in plants. If silica accumulation is a result of water consumption, plants could be expected to accumulate silica not only during growth but also after maturation. If, on the other hand, silica plays an active role in the protection of plants, it could be expected that accumulation occurs during plant tissue differentiation and ceases when plants are mature.
There are several studies on silica accumulation with respect to ageing of grasses (e.g. Ishizuka and Tanaka, 1952; Handreck and Jones, 1968). These studies revealed that silica accumulation followed a sigmoid curve. Most previous studies have focused on the whole plant or organ of annual grasses, and the accumulation pattern seemed to support the protection hypothesis. However, these studies do not show clearly that plants cease to accumulate silica when they mature. The reasons are as follows: (1) annual grasses include organs at various growth stages; and (2) annual plants are short‐lived after maturation.
There are some studies on the accumulation of silica in bamboo leaves (Ueda and Ueda, 1961; Kaneko, 1995), which have long lifespans (more than 12 months) after expansion. Results of studies using bamboo are different to those of studies using annual grasses. Ueda and Ueda (1961) showed that new leaves of Phyllostachys bambusoides started to accumulate silica after expansion in June, and accumulated silica rapidly from July to September. Accumulation gradually decreased in the autumn and almost no accumulation was observed during the winter or spring of the second year; defoliation occurred in June. The silica accumulation process followed a sigmoid curve, similar to that of annual plants. In contrast, Kaneko (1995) noticed that leaves of Phyllostachys pubescens accumulated silica again from February to April in the second year and were shed in June. The different results obtained for two species of bamboo (Phyllostachys bambusoides and P. pubescens) may be due to an insufficient number of observations of second‐year leaves, as these leaves have lifespans of 12 months and were shed in the season that they accumulated silica rapidly. Leaves of Sasa veitchii have longer lifespans (about 24 months). Hence, the present study aimed to investigate silica accumulation in leaves of S. veitchii, determining the silica content throughout their lifespan using the molybdenum blue method.
MATERIALS AND METHODS
Leaves were collected from Sasa veitchii (Carrière) Rehder growing in the Botanical Garden of Tohoku University, Sendai, Japan.
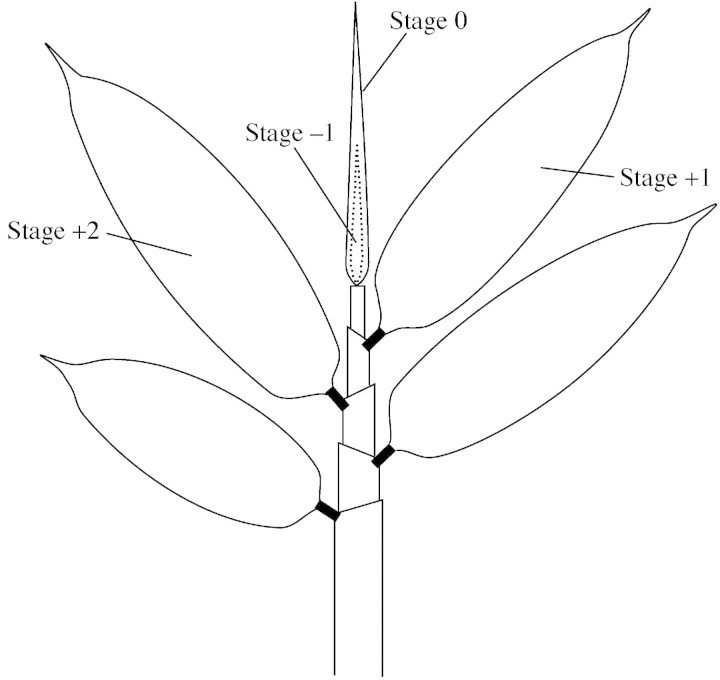
For analysis of silica accumulation in developing leaves, 20 leaf blades were collected from five newly grown culms in June 1999. Each culm has several leaves at different stages of development (Fig. 1). Four stages were distinguished on the basis of the leaf position related to the sequence of emergence of the leaves (Fig. 1): stage –1, 0, +1 and +2. To gain an indication of leaf tissue maturation, leaf blade length was measured (Fig. 2A). Leaf blades at stages –1 and 0 were growing rapidly, and those at stage +1 were over 20 cm in length (Fig. 2A). As leaf blades at stage +2 were no longer than those at stage +1, we regarded leaves at stage +1 as mature. Leaves were washed with double‐deionized water to remove adhering silica that originated from aerial dust, dried for 48 h at 80 °C and then used for determination of dry weight and silica content. Leaves were homogenized with a mill to obtain a randomized silica content in a leaf.
Fig. 1. Developing shoot with leaves at the four different stages. The exposed, rolled leaf is designated stage 0. The leaf blade of stage –1 is rolled and enclosed by the leaf blade of stage 0. Leaves at stage +1 and +2 are on the first and second nodes, respectively, below stage 0.
Fig. 2. Length of leaf blades (A) and silica content (B) of leaves at the four stages of development. Leaves of five culms were measured. Bars indicate standard deviation.
To study silica accumulation according to leaf age, 177 fully expanded leaves were collected from July 1999 to December 2000. In Sasa veitchii, new culm branches appear sympodially from the nodes every year, and each branch has leaves similar to those at the top of the older branch. In July 1999, leaves that emerged in different years (1998 and 1999) were distinguished on the basis of branching pattern related to the sequence of emergence of culm branches. Current‐year branches that emerged in 1999 had several leaves at different developmental stages, as indicated in Fig. 1, and mature leaves appeared on several nodes at the top of the branches after unfolding finished in August. Each branch that emerged in 1998 had leaves on the second, third or fourth nodes from the top of the branches, and these remaining leaves were shed during summer and autumn 2000. In June 2000, new branches appeared from the basal part of older branches that emerged in 1999. From each branch that emerged in three different years, three to five mature leaves were collected monthly. The leaf surface was washed in double‐deionized water, and one disc of about 3 cm2 was punched out from the median part between the leaf margin and midrib. Leaf discs were dried for 48 h at 80 °C for determination of dry weight and silica content.
The silica content of leaves was determined using the molybdenum blue method (Kato and Mita, 1983; Mochizuki and Terashima, 1983), which can be used to analyse small amounts of plant material. Leaves were ashed on platinum dishes. The ash was mixed with Na2CO3 and H3BO3 (5 : 1) to melt; the product was dissolved in HCl solution and transferred to a volumetric flask. Ammonium molybdate, tartaric acid and ascorbic acid were added to this solution, and the absorbance of the solution was measured at 420 and 655 nm using a photometer (UV‐1600; SHIMAZDU Co., Kyoto, Japan). The silica content was calculated per unit area and on a dry weight basis by comparing the absorbance with standard silicon solutions. A comparison of the values obtained from reference plant material (NJV 94‐4, Swedish University of Agricultural Science) using our method shows that there is no significant difference between the values.
RESULTS AND DISCUSSION
Figure 2B shows the result of silica accumulation by the samples shown in Fig. 2A. Silica accumulation starts when leaf tissue begins to differentiate. At the very young stage (stage –1), before leaf expansion, rolled leaves which are sheathed by older ones contain very little silica (less than 0·05 % SiO2 on a dry weight basis). During the course of development of leaves, the silica content increased rapidly (Fig. 2B). At stage 0, the silica content was 1·5 %. Although leaf blades at stage +1 had already reached full size, the silica content was rather low (3·1 %) compared with that of stage +2 leaves (6·3 %). This indicates that silica accumulation continues after completion of cell expansion in leaf blades.
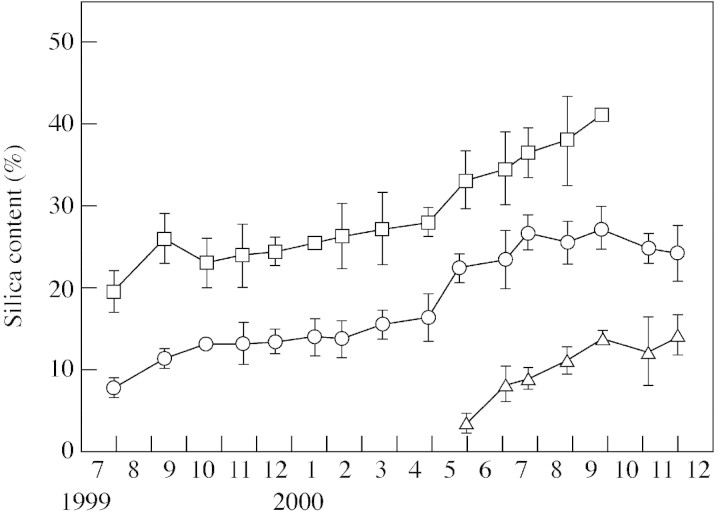
Figure 3 shows the pattern of silica accumulation in leaf blades that unfolded in three different years. In May 2000, young leaves that had emerged in the current year contained 3·4 % silica (dry weight basis). The silica content increased rapidly during spring and summer, reaching 14 % by September 2000, then stabilized during winter. Leaves that emerged in spring 1999 contained 7·9 % silica in July 1999. The silica content increased during spring and summer, reaching 15 % by winter 1999. The silica content increased rapidly again during spring and summer 2000, and was 27 % in July. The silica content ranged between 24 and 26 % in winter 2000. Leaves that emerged in spring 1998 contained 20 % silica in July 1999. The silica content increased, reaching 26 % in August, and then stabilized during winter with a little variation (23–27 %). Silica content increased again during the following spring and summer, and was 41 % in September 2000. In 1999–2000, silica accumulation was slow during winter and rapid during spring and summer. This is a good indication of the sigmoid pattern of silica accumulation in the current, second and third year after leaf expansion. The pattern of silica accumulation on a unit area basis did not differ to that on a dry weight basis, as indicated in Fig. 3. Thus, leaves constantly accumulated silica until they were shed in the third year.
Fig. 3. Time‐dependent changes in silica content (on a dry weight basis) in leaves that emerged in three different years: 1998 (squares), 1999 (circles) and 2000 (triangles). Bars indicate standard deviation.
The most important finding of this study is that Sasa veitchii accumulates the most silica reported to date (41 % SiO2 on a dry weight basis) in its leaves during their 3‐year lifespan. Previous studies have reported silica contents of up to 27 % in the Poaceae (Yoshida et al., 1962a; Epstein, 1999). The highest Si content previously recorded was in leaves of Oryza sativa cultivated in artificial medium containing a high silica concentration (100 ppm; Yoshida et al., 1962a). In contrast, leaves of O. sativa cultivated in the field have lower silica contents (8–14 %; Takahashi and Miyake, 1976a, b). Thus, the 41 % silica accumulation found in this study is the highest recorded amount of silica accumulated by grasses or bamboos, whether cultivated or wild.
The second important finding of the present study is that leaves of Sasa veitchii accumulate silica continuously throughout their life (about 24 months), not only during the developing stage, but also after maturation. Ueda and Ueda (1961) showed that leaves of Phyllostachys bambusoides accumulate silica for about 6 months after expansion. Kaneko (1995) showed that leaves of P. pubescens continue to accumulate silica throughout their life (12 months) after expansion. The long progressive accumulation of silica by S. veitchii confirms that leaves can accumulate silica continuously until they are shed.
The third important result of this study is that the sigmoid pattern of silica accumulation is repeated three times during the lifespan of Sasa veitchii leaves. Such a sigmoid silica accumulation pattern is well known in many grasses (e.g. Ishizuka and Tanaka, 1952; Handreck and Jones, 1968) and also in bamboos such as Phyllostachys bambusoides (Ueda and Ueda, 1961) and P. pubescens (Kaneko, 1995). The major difference between our results and those of previous studies is that the sigmoid pattern is repeated in every year during the lifespan of leaves. This indicates that silica accumulation is not the result of plant growth process but instead is the result of physiological plant activity.
This study has confirmed that long‐lived leaves of bamboo accumulate silica continuously, even after they reach maturity, thus supporting the first of the two hypotheses, namely that silica accumulation is the result of water consumption.
Supplementary Material
Received: 10 January 2002; Returned for revision: 25 January 2002; Accepted: 25 March 2002
References
- BalastaMLFC, Perez CM, Juliano BO, Villareal CP, Lott JNA, Roxas DB.1989. Effects of silica level on some properties of Oryza sativa straw and hull. Canadian Journal of Botany 67: 2356–2363. [Google Scholar]
- BelangerRR, Bowen PA, Ehret DL, Menzies JG.1995. Soluble silicon. Its role in crop and disease management of greenhouse crops. Plant Disease 79: 329–336. [Google Scholar]
- CidMS, Detling JK, Brizuela MA, Whicker AD.1989. Patterns in grass silicification: response to grazing history and defoliation. Oecologia 80: 268–271. [DOI] [PubMed] [Google Scholar]
- EpsteinE.1999. Silicon. Annual Review of Plant Physiology and Plant Molecular Biology 50: 641–664. [DOI] [PubMed] [Google Scholar]
- Frey‐WysslingA.1930. Vergleich Zwischen der Ausscheidung von Kieselsäure und Kalziumsalzen in der Pflanze. Berichte der Deutschen Botanischen Gesellschaft 48: 184–191. [Google Scholar]
- Frey‐WysslingA, Mühlethaler K.1965. Ultrastructural plant cytology. Amsterdam: Elsevier Publishing Company. [Google Scholar]
- HandreckKA, Jones LHP.1968. Studies of silica in the oat plant IV. Silica content of plant parts in relation to stage of growth, supply of silica, and transpiration. Plant and Soil 24: 449–459. [Google Scholar]
- HodsonMJ, Sangster AG.1990. Techniques for the microanalysis of higher plants with particular reference to silicon in cryofixed wheat tissues. Scanning Microscopy 4: 407–418. [Google Scholar]
- IshizukaY, Tanaka A.1952. Biochemical studies on the life history of rice plants. Journal of the Science of Soil and Manure, Japan 23: 23–28 (in Japanese). [Google Scholar]
- JonesLHP, Handreck KA.1967. Silica in soils, plants, and animals. Advances in Agronomy 19: 107–149. [Google Scholar]
- KanekoS.1995. Seasonal change of nutrient concentrations in Phyllostachys bambusoides and Phyllostachys pubescens Bamboo Journal 13: 27–33 (in Japanese). [Google Scholar]
- KatoK, Mita N.1983. Chemical analysis in deep‐sea sediments. Tsukuba: Geological Survey of Japan (in Japanese). [Google Scholar]
- KaufmanPB, Dayanandan P, Takeoka Y, Bigelow WC, Jones JD, Iler R.1981. Silica in shoots of higher plants. In: Simpson TL, Volcani BE, eds. Silicon and siliceous structures in biological systems New York: Springer, 409–449. [Google Scholar]
- McNaughtonSJ, Tarrants JT, McNaughton MM, Davis RH.1985. Silica as a defense against herbivory and a growth promoter in African grasses. Ecology 66: 528–535. [Google Scholar]
- MarschnerH.1995. Mineral nutrition of higher plants. San Diego: Academic Press. [Google Scholar]
- MochizukiT, Terashima S.1983. Chemical analysis of manganese nodules. Tsukuba: Geological Survey of Japan (in Japanese). [Google Scholar]
- TakahashiE, Miyake Y.1976a Distribution of silica accumulator in plant kingdom (1). Journal of the Science of Soil and Manure, Japan 47: 296–300. [Google Scholar]
- TakahashiE, Miyake Y.1976b Silica content in plants among organs and growth stage. Journal of the Science of Soil and Manure, Japan 47: 338–341. [Google Scholar]
- UedaK, Ueda S.1961. Effect of silicic acid on bamboo‐growth. Bulletin of the Kyoto University Forests 33: 79–99 (in Japanese). [Google Scholar]
- YoshidaS, Ohnishi Y, Kitagishi K.1962a Chemical forms, mobility and deposition of silicon in rice plant. Soil Science and Plant Nutrition 8: 15–21. [Google Scholar]
- YoshidaS, Ohnishi Y, Kitagishi K.1962b Histochemistry of silicon in plant. II. Localization of silicon within rice tissues. Soil Science and Plant Nutrition 8: 36–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.