Abstract
Coffea arabica L. plantlets obtained ex vitro after sowing somatic embryos produced in a bioreactor in horticultural substrate were compared with those obtained in vitro from the same embryo population under conventional culturing conditions on semi‐solid media. The intensity and quality of aerial and root system development were compared. Shoot emergence was more efficient in vitro but rooting frequencies were low. In contrast, all ex vitro‐regenerated embryos rooted. The cotyledon area of mature embryos produced in a bioreactor positively affected plantlet development when regeneration was carried out ex vitro. Embryos with an intermediate cotyledon area (0·86 cm2) had the highest rates of plant conversion ex vitro (63 %), and also resulted in vigorous plantlets. Mortality was higher in nursery conditions, but better plant development was obtained. The quality of plantlets produced under ex vitro conditions was reflected in better growth of the aerial and root systems, and also by similar morphological, mineral and water status characteristics to seedlings. Unlike roots formed on semi‐solid media, those produced in soil were branched, fine (30–50 % had a diameter of less than 0·5 mm) and they bore root hairs. Leaves of plantlets regenerated ex vitro had a histological structure similar to that of seedling leaves, and a lower stomatal density (100 vs. 233 mm–2). Moreover, they were more turgid, as indicated by higher pressure potential (ψP) (0·91 vs. 0·30 MPa) and relative water content values (97 vs. 93 %). Furthermore, under in vitro conditions, leaves had larger stomata which were abnormally round and raised. Direct sowing of germinated somatic embryos resulted in the rapid production of vigorous plantlets under ex vitro conditions, whilst removing the need for problematical and costly conventional acclimatization procedures.
Key words: Acclimatization, histology, in vitro, plant regeneration, rooting, somatic embryogenesis, water characteristics
INTRODUCTION
In genetic improvement schemes, multiplication of elite materials by somatic embryogenesis prevents genetic recombination and the need for long, expensive conventional selection cycles. It is commonly accepted that somatic embryogenesis could be used commercially once semi‐automatic propagation processes in a liquid medium have been established, along with efficient plantlet regeneration procedures that are reproducible, require little manpower and that are consequently inexpensive.
In somatic embryogenesis, plantlet regeneration usually takes place on semi‐solid media, and plantlets are acclimatized once they have a few pairs of leaves and a root system. However, ex vitro weaning causes substantial stress and is a very problematical stage that often entails considerable losses. In fact, micropropagated plants are grown under conditions of high relative humidity, low light intensity and limited gas exchange with the outside environment (Kozai, 1991). Studies carried out on plants propagated by organogenesis have shown that micropropagation induces profound anatomical, morphological and physiological modifications in plantlets that could have a negative effect on the acclimatization stage and the initial phases of plantlet development (Donnelly et al., 1985; Mohammed and Vidaver, 1991; Díaz‐Pérez et al., 1995). For instance, compared with those of seedlings or acclimated plantlets, leaves of in vitro‐regenerated plantlets have little epicuticular wax (Grout and Aston, 1977; Sutter and Langhans, 1982) and stoma functioning is often altered (Wetzstein and Sommer, 1982; Marìn et al., 1988). It has been shown that there are deficient vascular connections between the root system and the stem (Grout and Aston, 1977), that roots formed in vitro are often non‐functional and that it is often advantageous to eliminate them at the time of acclimatization to induce new ex vitro rooting (Debergh and Maene, 1981).
Existing data on plantlets produced by somatic embryo genesis mostly relate to germination frequency, conversion into plantlets and survival rates during acclimatization, or brief descriptions of their appearance (Gmitter and Moore, 1986; Fujii et al., 1989; Roberts et al., 1990; Webster et al., 1990; Aberlenc‐Bertossi et al., 1999). Apart from work on the photosynthetic capacity of such plantlets (Rival et al., 1998), few data are available on the anatomy and physiology of plantlets derived from somatic embryos. Moreover, a link between these parameters and successful transfer ex vitro remains to be established.
Direct sowing on horticultural substrate of Coffee arabica somatic embryos mass‐produced in a bioreactor was recently proposed (Etienne‐Barry et al., 1999). This technique is attractive not only because it results in quite high rates of conversion into plantlets under ex vitro conditions (around 50–70 %), but because it also drastically reduces handling requirements, laboratory area and in vitro culturing times. The aim of this work was to assess the quality of plantlets obtained by direct sowing ex vitro of coffee somatic embryos produced in a bioreactor, and to compare it with that of plantlets obtained by conventional in vitro regeneration processes on semi‐solid media. When assessing the vigour of somatic embryos and plantlets, Senaratna (1992) recommended that measurements are not restricted to rates of germination and conversion, but that a quality index is established based on numerous morphological criteria. To this end, we assessed the vigour and quality of plantlets regenerated under in vitro and ex vitro conditions, analysing various morphological, histological and biochemical parameters of the aerial and root systems.
MATERIALS AND METHODS
Plant material and somatic embryo regeneration in a temporary immersion bioreactor
A selected Coffea arabica F1 hybrid obtained from a cross between the Caturra variety and a wild accession originating in Ethiopia (E531) (Bertrand et al., 1999) was used for all experiments involving somatic embryos and seedlings. The wild accession E531 was selected from the field collection of the CATIE Institute in Costa Rica.
The mass‐production of mature somatic embryos from pieces of young leaves using a temporary immersion bioreactor for the developmental steps has been described recently (Barry‐Etienne et al., 2002). By the end of the in vitro culture stage, each bioreactor contained around 1000 mature somatic embryos with an elongated embryonic axis and a pair of open, chlorophyllous cotyledons.
Culture conditions for plantlet regeneration in vitro on semi‐solid media and ex vitro on horticultural substrate
For in vitro regeneration of plantlets with four to five pairs of leaves, somatic embryos produced in a bioreactor were placed on semi‐solid medium with two 2‐month subcultures on EG (embryo germination) germination medium (Van Boxtel and Berthouly, 1996) supplemented with 2·5 g l–1 phytagel. Eight somatic embryos were grown in each 150 ml glass pot sealed with a plastic stopper and placed in the light (12/12 h, 50 µmol m–2 s–1).
Sowing conditions for direct transfer of somatic embryos to soil were as follows: light and moisture conditions were identical to those previously defined for fully developed in vitro plantlets (Etienne et al., 1997). Mature embryos were sown vertically on top of the substrate (two parts soil, one part sand, one part coffee pulp) which had been sterilized by chemical treatment [Dazomet (DMTT), Union carbide]. The density of somatic embryo cultures in the plastic boxes (30 × 21 × 10 cm) was approx. 3600 m–2. Cultures were placed under a transparent roof that provided 50 % shade and they were watered for 2 min twice a day.
Image analysis
Images of the root, leaf and cotyledon were acquired by a scanner (HP ScanJet, 6100C/T), digitalized by computer and analysed using the software procedures of WinRHIZO V3·9 (Instrument Regent, Quebec, Montreal, Canada). Coffee tree leaves or somatic embryo cotyledons were placed in the analysis tray of the scanner and the precise area (in cm2) was determinated by analysis after image acquisition. Root systems had to be washed and were then placed in the analysis tray with 1 cm of water. For each plant, the parameters acquired by the software were: total root length, number of root tips, average root diameter, and root length in each of the root diameter intervals (0–0·5 mm; 0·5–1 mm; over 1 mm).
Characteristics of acclimatizable mature somatic embryos
Somatic embryos suitable for direct sowing in horticultural substrate were divided into three morphological types according to cotyledon area. The average cotyledon area was measured on 50 individuals for each embryo type taken at random from six bioreactors using the image analysis procedure described previously. Fresh weight and embryonic axis length were measured on the same batch of 50 embryos. Carbohydrate content was quantified on three samples (comprising 500 mg d. wt) of embryos for each morphological type after freeze‐drying. Glucose, fructose, sucrose and mannitol were quantified by ion chromatography (Peschet and Giacalone, 1991) after sample extraction in 40 % ethanol. Starch was quantified by ion chromatography after enzymatic hydrolysis of the alcohol residue solubilized in HCl (Rickard, 1987).
Regeneration efficacy and plantlet morphology
Embryo‐to‐plantlet conversion, shoot emergence and rooting frequencies were determined 4 months after planting somatic embryos bearing small, medium and large cotyledons under both in vitro and ex vitro culture conditions. For each morphological type, the calculation involved six blocks of 80 somatic embryos regenerated on horticultural substrate and 15 pots containing eight somatic embryos regenerated on a semi‐solid medium. Morphological analyses of 30 regenerated plantlets chosen at random were carried out for each embryo type used and for each regeneration system. Analyses included fresh weight, plant size (cm), and root and aerial system dry weight (mg). The root system and leaf area were then analysed using the image analysis procedure described previously.
Leaf mineral content
Mineral analyses were carried out on three batches of ten plantlets (cotyledons and leaves) for each of the populations obtained from the in vitro and ex vitro regeneration conditions. Chemical analyses of potassium, magnesium and calcium were carried out by atomic absorption spectrophotometry (AAnalysis 100, Perkin Elmer) (Mills and Benton Jones, 1996) after wet digestion with a mixture of nitric and perchloric acids (5 : 1). Phosphorus content was determined by a colourimetric method developing molybdene blue by UV/V spectrophotometry at 660 nm (Lambda1, Perkin Elmer). Total nitrogen was determined by the Kjeldahl method (Jones and Case, 1990).
Measurement of leaf water parameters
Fresh weight, dry weight, water content (WC) and relative water content (RWC) were measured on leaves from ten representative plants under each culture condition. Each value is thus a mean with a confidence interval. RWC represents the ratio of (fresh weight–dry weight) : (satured weight–dry weight), where dry weights were determined after drying the leaf samples at 80 °C for 24 h, and satured weights were obtained after leaving the samples at 5 °C for 24 h between two water‐soaked pieces of filter paper. Water potential (ψH) was measured using a Wescor C.51 thermocouple hygrometer sample chamber (Wescor, Logan, UT, USA) and a dew point microvoltmeter (HR 33). Leaf samples were left in the measuring chamber for 3·5 h, i.e. long enough to attain vapour equilibrium. A cooling period of 20 s was used to measure the dew point and the microvoltmeter was calibrated against NaCl standards in bars at 20 °C (Lang, 1967). After measurement of ψH, leaf tissues were frozen and left again for 1·5 h in the measuring chamber before recording osmotic potential (ψS). Pressure potential (ψP) was calculated from the equation: ψH = ψS + ψP. Potentials are means of six measurements on leaves from different plants.
Histology
Cotyledons (ten for each embryo morphological type), roots and leaves (five for both in vitro and ex vitro conditions) were fixed for 24 h in a solution containing 1 % glutaraldehyde, 2 % paraformaldehyde and 1 % caffeine in a 0·2 mm phosphate buffer at pH 7·2. For histological studies, samples were dehydrated in a graded series of ethanol, embedded in a 7100 resin (LKB) and cut into 3‐µm longitudinal sections. Sections were double‐stained with PAS (periodic acid‐Schiff)–NBB (naphthol blue black). PAS specifically stains polysaccharides (walls and starch) red and NBB stains soluble and insoluble proteins blue (Fisher, 1968). For scanning electron microscopy (SEM), fixed samples were dehydrated in a graded series of ethanol (up to 100 % ethanol) and then critical point dried (Homès, 1975). Samples were coated with platinum‐gold, mounted on a slide using adhesive tape and examined in an SEM (2360 Hitachi). Stomatal density (number of stomata per mm2/leaf area) and stoma size [length (µm) × width (µm)] were determined on the abaxial leaf surface.
RESULTS
Quality of mature somatic embryos and plantlet regeneration efficiency
For somatic embryos matured in a bioreactor, three morphological types were described on the basis of the area of their cotyledon, their fresh weight and the length of their embryonic axis (Table 1). The characteristic that proved to be the best discriminator was cotyledon area, which enabled a distinction to be made between three morphological types of embryo, i.e. those with ‘small’, ‘medium’ and ‘large’ cotyledons. Embryos with ‘small’ cotyledons had a cotyledon area four times smaller than that of embryos with ‘medium’ cotyledons, and around ten times smaller than that of embryos with ‘large’ cotyledons.
Table 1.
. Morphological characterization of three somatic embryo populations obtained in a 1‐l bioreactor after 2 months’ germination
| Morphological | Morphological characteristics of mature embryos | Shoot emergence* (%) | Rooting (%) | Plantlet conversion (%) | |||||
| type of the sown mature embryos | Cotyledon area (cm2) | Length of embryo axis (mm) | Fresh weight (mg) | In vitro | Ex vitro | In vitro | Ex vitro | In vitro | Ex vitro |
| Small cotyledons | 0·22 ± 0·08c | 6·65 ± 1·8b | 15·7 ± 7c | 82 ± 13a | 47 ± 7·4b | 58 ± 25a | 100 ± 0a | 58 ± 25a | 47 ± 7·4b |
| Medium cotyledons | 0·86 ± 0·3b | 6·93 ± 2·7b | 48·9 ± 31b | 76 ± 17a | 63 ± 6·3a | 13 ± 9c | 100 ± 0a | 13 ± 9c | 63 ± 6·3a |
| Large cotyledons | 2·5 ± 1·0a | 9·73 ± 3·0a | 95·5 ± 28a | 41 ± 22b | 30 ± 6·7c | 43 ± 21b | 100 ± 0a | 43 ± 21b | 30 ± 6·7c |
Data shown are from 50 mature embryos taken from six bioreactors for each population. The rates of shoot emergence and rooting, and the rates of conversion into plantlets for each morphological type are means calculated after 4 months’ germination from eight blocks of 80 embryos transferred to horticultural substrate or from 15 bottles containing eight embryos each.
Values in the same column followed by the same superscript are not significantly different at the 5 % level according to analysis of variance and the Duncan test or Tukey HSD test for the conversion values.
* Corresponds to survival rate as all the embryos that produced a shoot survived.
After 4 months’ regeneration either in vitro or ex vitro on horticultural substrate, rates of shoot emergence were higher on semi‐solid medium in all cases (Table 1). Embryos with large cotyledons had lower rates of shoot emergence than the other two embryo types. Rooting frequency was maximum for plantlets regenerated on horticultural substrate, whilst it was two to ten times lower for plantlets regenerated on semi‐solid medium (Table 1). The best plantlet conversion efficacies were obtained ex vitro with the ‘medium cotyledon’ type and in vitro with the ‘small cotyledon’ type.
When expressed as a percentage of dry matter, glucose and fructose contents increased with somatic embryo cotyledon size, but starch content was not significantly different among the three embryo types (Table 2). When expressed on a per embryo basis, all the carbohydrate contents increased in line with the size of the cotyledons.
Table 2.
. Carbohydrate contents of the three somatic embryo morphological types at the end of the maturation phase in the bioreactor
| Carbohydrate content | ||||||||
| Morphological | Glucose | Fructose | Sucrose | Starch | ||||
| type of the sown embryos | % d. wt | mg per embryo | % d. wt | mg per embryo | % d. wt | mg per embryo | % d. wt | mg perembryo |
| Small cotyledons | 2·7 ± 0·5c | 0·06 ± 2·10–3 c | 3·4 ± 0·5b | 0·07 ± 9·10–3 c | 11·4 ± 0·05a | 0·26 ± 8·10–3 c | 24·0 ± 0·7a | 1·0 ± 2·10–2 c |
| Medium cotyledons | 3·9 ± 0·1b | 0·18 ± 3·10–3 b | 5·6 ± 0·1a | 0·26 ± 5·10–3 b | 8·8 ± 0·2c | 0·37 ± 4·10–2 b | 26·1 ± 0·3a | 2·4 ± 3·10–2 b |
| Large cotyledons | 4·9 ± 0·4a | 0·44 ± 3·10–3 a | 6·9 ± 1·1a | 0·62 ± 8·10–2 a | 9·7 ± 0·5b | 0·87 ± 5·10–2 a | 23·0 ± 2·54a | 4·1 ± 0·4a |
Results are expressed either as a percentage of dry matter or on a per embryo basis and are the means of three replicates ± s.e.
Values in the same column followed by the same superscript are not significantly different at the 5 % level according to analysis of variance and the Duncan test.
A histological study of cotyledons from the different morphological types of somatic embryos showed that small cotyledons had a compact structure with no mesophyll differentiation, and no stomata were seen on the abaxial surface. The cells were rich in starch and precipitates (Fig. 1A). Embryos with medium or large cotyledons (Fig. 1B) had characteristics similar to those of a leaf with stomata; sub‐stomatal chambers formed a lacunar parenchyma, although this was thinner than that of a mature seedling leaf (Fig. 1C). The palisade parenchyma was formed by cells that were irregular in shape and size (Fig. 1B).
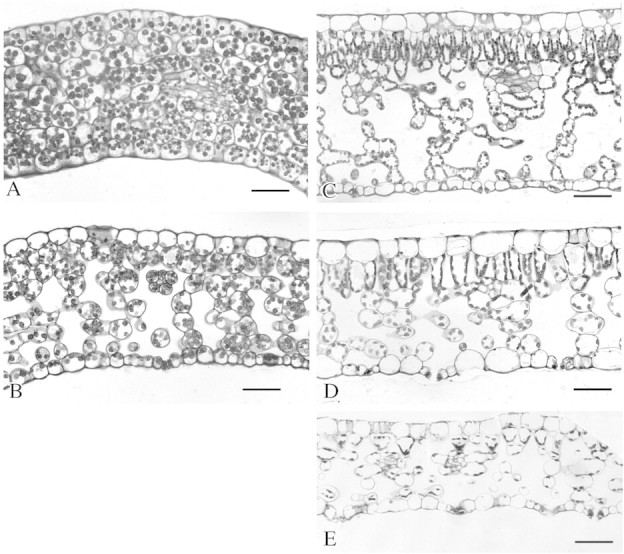
Fig. 1. Light micrographs of cross‐sections of somatic embryo cotyledons and leaves of Coffea arabica plantlets. A and B, Cross‐sections of cotyledons from mature somatic embryos with a small area (A) and with a medium or large area (B). C, Cross‐section of a leaf from a 4‐month‐old seedling. D, Cross‐section of a leaf from a plantlet regenerated ex vitro. E, Cross‐section of a leaf from a plantlet regenerated in vitro. Bar = 100 µm.
Plantlet development under in vitro or ex vitro conditions
For medium and large embryos, the best plantlet growth, evaluated by total fresh weight and size, was obtained on horticultural substrate (Table 3). These plantlets also had a larger leaf area and were more vigorous in terms of both the aerial system and the root system.
Table 3.
. Effect of regeneration conditions (in vitro or ex vitro) on development of the aerial and root systems of plantlets obtained after 4 months’ culture
| Aerial system of plantlets 4 months after sowing | Root system of plantlets 4 months after sowing | |||||||||
| Regeneration conditions | Morphological type of the sown embryos | Plantlet fresh weight (mg) | Plantlet size (cm) | Aerial system dry weight (mg) | Number of leaves | Foliar area (cm2) | Root system dry weight (mg) | Total root length (cm) | Number of root tips | Mean root diameter (mm) |
| In vitro (semi‐solid media) | Small cotyledons | 219 ± 165bc | 1·5 ± 0·3c | 42 ± 22b | 10·8 ± 3·0ab | 8·1 ± 4·9c | 7·6 ± 10b | 8·4 ± 6c | 3·6 ± 1·5c | 0·09 ± 0·02a |
| Medium cotyledons | 201 ± 166bc | 1·4 ± 0·5c | 32 ± 25cd | 11·4 ± 2·6 | 8·6 ± 7·8c | 8·6 ± 9·5b | 9·8 ± 5·8c | 5·6 ± 3·9c | 0·08 ± 0·02a | |
| Large cotyledons | 266 ± 193bc | 1·6 ± 0·6bc | 49 ± 34bc | 10·8 ± 4·6abc | 10·2 ± 9·5bc | 6·5 ± 6·5b | 10·4 ± 6·6bc | 7·7 ± 4·3bc | 0·09 ± 0·01a | |
| Ex vitro (soil) | Small cotyledons | 140 ± 49c | 1·3 ± 0·2c | 20 ± 8d | 7·4 ± 1·4c | 4·4 ± 1·6d | 5·5 ± 2·9b | 20·2 ± 8b | 13 ± 7b | 0·05 ± 0·01b |
| Medium cotyledons | 440 ± 200ab | 2·0 ± 0·7b | 63 ± 35ab | 8·6 ± 1·6bc | 12·7 ± 6·2ab | 20·5 ± 10a | 48·3 ± 21a | 32 ± 10a | 0·06 ± 0·01b | |
| Large cotyledons | 620 ± 295a | 2·9 ± 0·9a | 90 ± 48a | 8·2 ± 1·6bc | 18 ± 8·7a | 30 ± 15a | 70 ± 31a | 45 ± 18a | 0·06 ± 0·01b | |
Values are means of 30 plantlets ± s.e.
Values in the same column followed by the same superscript are not significantly different at the 5 % level according to analysis of variance and the Tukey HSD test.
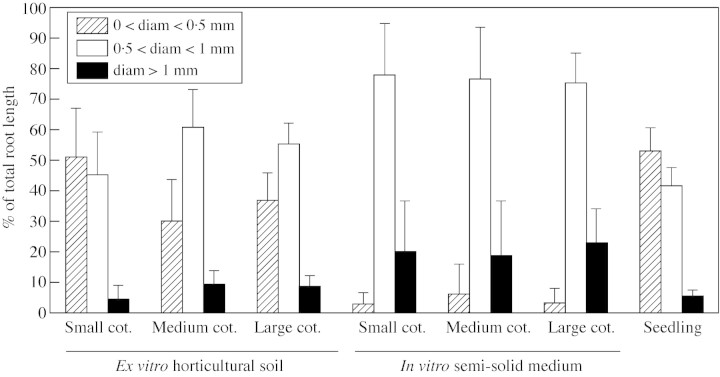
Major differences were found in the root system of 4‐month‐old plantlets depending on the regeneration conditions used (Table 3). Total root length and number of root tips showed that plantlets regenerated on horticultural substrate had a larger, more developed and more branched root system than those on a semi‐solid medium. Furthermore, as indicated by the average root diameter, roots that developed in soil were finer. For plantlets regenerated on horticultural substrate, root length distributions according to their diameter were mainly in the 0–0·5 mm (30–50 %) and 0·5 mm–1 mm intervals (44–60 %), and only 10 % of roots had a diameter over 1 mm (Fig. 3). For in vitro‐regenerated plantlets, over 70 % of roots had a diameter ranging from 0·5 to 1 mm, and 20 % of roots had a diameter >1 mm. There were very few (under 6 %) fine roots (0–0·5 mm). The study of root distribution according to diameter revealed that the root systems of plantlets obtained ex vitro were most similar to those of 4‐month‐old seedlings. SEM observations showed that the secondary roots of plantlets on horticultural substrate were fine and that they had zones rich in root hairs (Fig. 2B), whilst roots obtained under in vitro conditions were not branched, were thicker and were devoid of root hairs (Fig. 2A).
Fig. 3. Comparison of root systems by root length distribution depending on their diameter (expressed as a % of total root length) for plantlets obtained from somatic embryos with cotyledons of different areas and in in vitro and ex vitro regeneration conditions. A comparison is made with root systems of seedlings of the same age. Data relative to root lengths and diameters were obtained by scanned image analysis using WinRHIZO V3·9 software. For each treatment the values shown are means obtained from 30 plants.
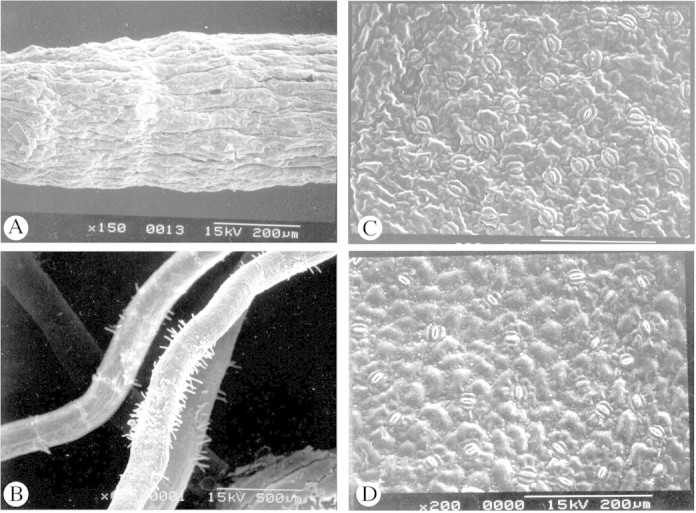
Fig. 2. SEM micrographs of roots and leaves of somatic embryo derived‐plantlets of Coffea arabica. A, Surface of a root formed in in vitro semi‐solid medium. B, Secondary roots formed on horticultural substrate. C, Abaxial surface of a leaf formed in vitro. D, Abaxial surface of a leaf formed in nursery conditions. Bars = 1 mm (A), 500 µm (B) and 200 µm (C and D).
Quality of leaves obtained under in vitro or ex vitro conditions
Histological observations showed that the leaves of plantlets obtained from somatic embryos produced in vitro or ex vitro were not as thick as seedling leaves as their spongy parenchyma was less well‐developed (Fig. 1C–E). The palisade parenchyma was weakly represented in leaves of in vitro‐regenerated plantlets and comprised cells of different shapes and sizes (Fig. 1E). In ex vitro‐regenerated plantlets, the palisade parenchyma was more like that of a seedling because of the more elongated cells, but it differed through the existence of large intercellular spaces. SEM observations showed that stomata in the leaves of plantlets regenerated on horticultural substrate had the same elliptical shape and were the same size as those of seedling leaves (Fig. 2D; Table 4). However, stomata of leaves obtained in vitro were larger, round and raised (Fig. 2C). Moreover, stomatal densities for in vitro‐regenerated plantlets were much higher than those for seedlings, whereas leaves of plantlets regenerated ex vitro had lower densities of stomata (Table 4).
Table 4.
Characteristics of the stomata and leaf water parameters in 4‐month‐old plants produced in vitro on semi‐solid media or ex vitro in horticultural substrate after direct sowing of mature somatic embryos
| Stomatal characteristics | Leaf water characteristics | |||||||
| Origin of the plantlets and regeneration conditions | Stomatal density (number mm–2) | Stomatal length (µm) | Stomatal width (µm) | Water content (%) | Relative water content (%) | ΨH (MPa) | ΨS (MPa) | ΨP (MPa) |
| Somatic embryo: in vitro | 233 ± 38 | 31 ± 2·9 | 25 ± 1·9 | 81·37 ± 0·89 | 93·07 ± 2·28 | –0·85 ± 0·12 | –1·15 ± 0·12 | 0·30 ± 0·09 |
| Somatic embryo: ex vitro | 100 ± 14 | 30·5 ± 1 | 18·5 ± 2·4 | 79·30 ± 1·45 | 97·14 ± 1·38 | –0·68 ± 0·06 | –1·60 ± 0·12 | 0·91 ± 0·15 |
| Seed: ex vitro | 167 ± 43 | 29 ± 3·0 | 17 ± 2·7 | 71·02 ± 2·29 | 96·22 ± 0·78 | –0·66 ± 0·13 | –1·89 ± 0·24 | 1·22 ± 0·36 |
Comparison with leaf characteristics of seedlings of the same age.
Stomatal density, stomatal length and width, WC and RWC are means ± s.e. of ten measurements on different plantlets; ΨH, ΨS, ΨP are means ± s.e. of six measurements on different plantlets.
Leaves of plantlets grown ex vitro were slightly less hydrated than those grown in vitro, but they had much higher RWC and ψP values than in vitro‐grown leaves, showing better turgescence under nursery conditions (Table 4). These values were similar to those measured in seedlings. In vitro‐grown leaves had a much lower osmotic potential (ψs) than that of plantlets regenerated ex vitro or that of seedlings.
Ca and Mg concentrations were lower in in vitro‐ than in ex vitro‐regenerated plantlets (Table 5). Conversely, K, P and N contents were significantly higher in plantlets obtained on semi‐solid medium. Contents of all mineral nutrients in plantlets developed on horticultural substrate were similar to those recorded in 4‐month‐old seedlings.
Table 5.
. Leaf mineral contents for plantlets obtained after 4 months of regeneration under in vitro (semi‐solid media) or ex vitro conditions (horticultural substrate)
| Origin of plantlets and | Leaf mineral content (% d. wt) | ||||
| regeneration conditions | Ca | Mg | K | P | N |
| Somatic embryo: in vitro | 0·33 ± 0·03d | 0·13 ± 0·01c | 3·16 ± 0·23a | 0·31 ± 0·01a | 4·32 ± 0·16a |
| Somatic embryo: ex vitro | 0·91 ± 0·01b | 0·38 ± 0·01a | 2·65 ± 0·05b | 0·16 ± 0·01c | 2·8 ± 0·02c |
| Seed: ex vitro | 1·02 ± 0·02a | 0·4 ± 0·01a | 2·52 ± 0·06c | 0·2 ± 0·01b | 3·02 ± 0·1b |
Results are expressed as a percentage of dry matter and are means of three replicates ± s.e.
Values in the same column followed by the same superscript are not significantly different at the 5 % level according to analysis of variance and the Duncan test.
DISCUSSION
Embryo conversion into plantlets under in vitro and ex vitro conditions
When characterizing in vitro culture conditions, mention is usually made of the heterotrophic status of the plants, the high relative humidity and the low light intensity, compared with nursery conditions. We have recently shown that it is possible to regenerate coffee plants in the nursery after direct sowing of mature somatic embryos, i.e. embryos without roots or leaves (Etienne‐Barry et al., 1999). Using this system, it is therefore possible to compare the plant conversion efficiency under ex vitro conditions with that achieved under conventional in vitro regeneration conditions on semi‐solid media.
The rate of shoot emergence was directly affected by cotyledon size under both in vitro and ex vitro conditions, and the best results were obtained with medium‐sized cotyledons (those measuring 0·86 cm2 on average). In vitro conditions led to more efficient shoot emergence, but rooting rates were low. Under nursery conditions, which are more stressful than in vitro conditions, only the most vigorous embryos could survive to the acclimatization procedure. Nevertheless, all the embryos that survived produced complete plantlets.
The histological characteristics of medium and large cotyledons from somatic embryos matured in a bioreactor are similar to those of a seedling coffee leaf. However, the palisade parenchyma and the lacunar parenchyma show differences in terms of tissue thickness and cell shape.
In contrast to in vitro culture medium, the ex vitro substrate was totally devoid of a sugar source. It was therefore possible to evaluate the relationship between the efficiency of plant regeneration and the carbohydrate reserves of mature somatic embryos. A previous study had shown that preculturing coffee somatic embryos for 2 weeks on a sucrose‐enriched medium enhanced plant regeneration after direct sowing (Etienne‐Barry et al., 1999). Given the literature on storage reserves in both zygotic and somatic embryos, it was expected that carbohydrate reserves, i.e. starch and sucrose, would have a positive effect on germination and plant conversion. However, we did not find such a relationship, indicating that carbohydrate accumulation is not a key factor in successful plant regeneration ex vitro.
Vigour and quality of plantlets regenerated under in vitro and ex vitro conditions
Plantlet development was affected by cotyledon area only when regeneration was carried out in the nursery. Under ex vitro conditions, development of the aerial and root systems was positively correlated with cotyledon area. Under in vitro conditions, where the photosynthetic activity of the cotyledons was not stimulated, the effect of cotyledon size was masked.
With the exception of embryos with small cotyledons, development of the aerial and root systems of plantlets grown in soil was clearly better than that of plantlets grown in vitro for all the parameters studied. Nevertheless, as observed for oil palm somatic embryo‐derived plantlets (Rival et al., 1998), growth was around three times less than for seedlings of the same age (data not shown). Using interior spruce [Picea glauca (Moench) Vos × Picea engelmannii Parry], Webster et al. (1990) have shown that growth rates, final height, shoot and root morphology, and frost hardiness are similar for plantlets derived from somatic embryos and from seeds. In the present work, root formation was rare in vitro, whereas it always occurred ex vitro. In addition, root systems of plantlets regenerated in soil were more extensive, more branched and had numerous fine roots, suggesting strong uptake and growth potential. A similar situation was found with in vitro‐regenerated apple (Malus domestica) plantlets (Díaz‐Pérez et al., 1995). Root systems developed ex vitro had qualitative characteristics similar to those of a seedling root system. Unlike roots formed in soil, those obtained on semi‐solid medium were thick, unbranched and devoid of root hairs. It is likely that these roots were not functional (Debergh and Maene, 1981), and/or that they were physiologically different from those formed in soil (Donnelly et al., 1985). During plantlet acclimatization on horticultural substrate, it was found that the existing root system either degenerated and was replaced by new roots (Debergh and Maene, 1981), or that it was still in place after several weeks without any structural modifications (Poole and Conover, 1983). In both cases, plantlet growth ceased. Hence, in conventional propagation procedures on semi‐solid media, it is recommended that the root system of numerous species, including coffee, be removed and a new one induced when plantlets are transferred ex vitro (Debergh and Maene, 1981; Etienne et al., 1997).
A visual inspection revealed that the leaf area produced in vitro varied little during growth, whilst under ex vitro conditions a gradual increase was seen in the area of new leaves and in internode length (data not shown). This explains the higher leaf area measurements for ex vitro‐regenerated plantlets, whereas in vitro‐regenerated plantlets had more leaves. It is likely that plantlet growth was limited by the volume of the culture vessel under in vitro conditions. This ‘crowding’ phenomenon has been noted previously on grape (Vitis vinifera) and serviceberry (Amelanchier × grandiflora ‘Princess Diana’) stems, which increased in size in line with the volume of the container (Monette, 1983; Krueger et al., 1991).
The leaves of somatic embryo‐derived plantlets developed on horticultural substrate had anatomical characteristics midway between those of leaves of plantlets regenerated in vitro and those of seedling leaves. This was also seen in micropropagated plants of Leucana leucocephale (Dhawan and Bhojwani, 1987) and Liquidambar styraciflua (Wetzstein and Sommer, 1982). Leaves of coffee plants regenerated in vitro, like those of Prunus cerasus L. (Marìn et al., 1988), had a higher stomatal density and abnormally large, round and raised stomata, which probably did not enable optimum regulation of water loss.
The leaf mineral composition of plantlets obtained from somatic embryos regenerated in horticultural substrate was similar to that recorded in seedlings of the same age, but was very different to that in in vitro‐regenerated plantlets for all mineral elements. The low Ca and Mg contents of in vitro‐regenerated plants might be related to the fragility of membranes and the absence of photosynthetic activity, respectively. The accumulation of nitrogen and phosphorus might be the result of low plant growth compared with ex vitro‐regenerated plants.
If RWC and pressure potential (ψP) values are considered, the water status of in vitro‐regenerated plants was poorer than that of plants regenerated ex vitro and that of seedlings. Likewise, a higher plant water status, as indicated by a high RWC, in transplanted in vitro apple plantlets compared with in vitro plants has been associated with higher growth rates and high net assimilation rates (Díaz‐Pérez et al., 1995). Morpho‐physiological studies on leaves of micropropagated Leucaena leucocephala plants also showed that they had little control over water losses (Dhawan and Bhojwani, 1987). These leaves had very little epicuticular wax, a substantially reduced mesophyll and little starch in the cells. The higher RWC and turgescence of plants regenerated ex vitro probably resulted from an increased ability to maintain their water balance through morphological characteristics, such as a more highly developed and more functional root system, a more normal anatomy and a lower stomatal density.
Compared with plantlets obtained ex vitro, growth of the aerial system was much lower in 4‐month‐old plantlets regenerated in vitro, and they did not have a functional root system. Moreover, their physiological condition, which was very different from that of ex vitro‐regenerated plantlets and seedlings, necessitates a tricky and labour‐intensive acclimatization phase in the glasshouse before planting out (Debergh and Maene, 1981; Etienne et al., 1997). With direct sowing of somatic embryos in horticultural substrate, even though the plantlets obtained were still small compared with seedlings (ratio of 1 : 3), they were readily acclimatized to nursery conditions and had vigorous aerial and root systems and active growth. The economic viability of this method has already been proven (Etienne‐Barry et al., 1999), and the quality of regenerated plantlets has been demonstrated in this work. Research is currently underway to improve the rate of conversion into plants after direct sowing of somatic embryos produced in a bioreactor, and also to stimulate initial plantlet growth by developing hardening conditions prior to transfer ex vitro and by optimizing horticultural practices.
ACKNOWLEDGEMENTS
We thank Carole Salazar and N. Ferrière for the histological cross‐sections. This work received financial backing in Central America from the Regional Delegation of the French Ministry of Foreign Affairs and from the scientific network PROMECAFE.
Supplementary Material
Received: 13 December 2001; Returned for revision: 13 February 2002; Accepted: 20 March 2002
References
- Aberlenc‐BertossiF, Noirot M, Duval Y.1999. BA enhances the germination of oil palm somatic embryos derived from embryogenic suspension cultures. Plant Cell Tissue and Organ Culture 56: 53–57. [Google Scholar]
- Barry‐EtienneD, Bertrand B, Schlöngvoigt A, Etienne H.2002. The morphological variability within a population of coffee somatic embryos produced in a bioreactor affects the regeneration and the development of plants in the nursery. Plant Cell Tissue and Organ Culture 68: 153–162. [Google Scholar]
- BertrandB, Aguilar G, Santacreo R, Anzueto F.1999. El mejoramiento genético en America Central. In: Bertrand B, Rapidel B, eds. Desafios de la caficultura centroamericana San José (Costa Rica): IICA Publishers, 407–456. [Google Scholar]
- DeberghPC, Maene LJ.1981. A scheme for commercial propagation of ornamental plants by tissue culture. Sciencia Horticulturae 14: 335–345. [Google Scholar]
- DhawanV, Bhojwani SS.1987. Hardening in vitro and morpho‐physiological changes in the leaves during acclimatization of micropropagated plants of Leucaena leucocephala (Lam.) de Wit. Plant Science 53: 65–72. [Google Scholar]
- Díaz‐PérezJC, Sutter EG, Shackel KA.1995. Acclimatization and subsequent gas exchange, water relations, survival and growth of microcultured apple plantlets after transplanting them in soil. Physiologia Plantarum 95: 225–232. [Google Scholar]
- DonnellyDJ, Vidaver WE, Lee KY.1985. The anatomy of tissue cultured red raspberry prior to and after transfer to soil. Plant Cell Tissue and Organ Culture 4: 43–50. [Google Scholar]
- EtienneH, Solano W, Pereira A, Bertrand B, Berthouly M.1997. Coffee in vitro plantlet acclimatization protocol. Plantations Recherche Développement 4: 304–311. [Google Scholar]
- Etienne‐BarryD, Bertrand B, Vasquez N, Etienne H.1999. Direct sowing of Coffea arabica somatic embryos mass‐produced in a bioreactor and regeneration of plants. Plant Cell Reports 19: 111–117. [DOI] [PubMed] [Google Scholar]
- FisherDB.1968. Protein staining of ribboned epon sections for light microscopy. Histochemie 16: 92–96. [DOI] [PubMed] [Google Scholar]
- FujiiJA, Slade D, Redenbaugh K.1989. Maturation and greenhouse planting of alfalfa artificial seeds. In Vitro Cellular and Develop mental Biology‐Plant 25: 1179–1182. [Google Scholar]
- GmitterF Jr, Moore GA. 1986. Plant regeneration from undeveloped ovules and embryogenic calli of Citrus: embryo production, germination, and plant survival. Plant Cell Tissue and Organ Culture 6: 139–147. [Google Scholar]
- GroutB, Aston MJ.1977. Transplanting of cauliflower plants regenerated from meristem culture. I. Water loss and water transfer related to changes in leaf wax and to xylem regeneration. Horticultural Research 17: 1–7. [Google Scholar]
- HomèsJ.1975. La préparation des tissus végétaux pour l’observation au microscope électronique à balayage. Bulletin de la Société Royale de Botanique Belge 108: 219–231. [Google Scholar]
- JonesJB, Case V.1990. Sampling, handling and analyzing plant tissue samples. In Westerman RL, ed. Soil testing and plant analysis Soil Science Society of America Book Series, Number 3. Madison: Soil Science Society of America, Inc. [Google Scholar]
- KozaiT.1991. Acclimatization of micropropagated plants. In: Bajaj YPS, ed. Biotechnology in agriculture and forestry, Vol. 17, Hightech and micropropagation Berlin: Springer‐Verlag, 127–141. [Google Scholar]
- KruegerS, Robacker C, Simonson W.1991. Culture of Amelanchier x grandiflora in a programmable micropropagation apparatus. Plant Cell Tissue and Organ Culture 27: 219–226. [Google Scholar]
- LangARG.1967. Osmotic coefficients and water potentials of sodium chloride solutions from 0 to 40 °C. Australian Journal of Chemistry 20: 2017–2023. [Google Scholar]
- MarìnJA, Gella R, Herrero M.1988. Stomatal structure and functioning as a response to environmental changes in acclimatized micro propagated Prunus cerasus L. Annals of Botany 62: 663–670. [Google Scholar]
- MillsHA, Jones JB.1996. Methods of elemental analysis. In: Jones JB, Wolf B, eds. Plant analysis handbook II. Athens: Micro‐macro Publishing. [Google Scholar]
- MohammedGH, Vidaver WE.1991. Early development of douglas‐fir plantlets following transfer to the greenhouse. Plant Science 76: 259–265. [Google Scholar]
- MonettePL.1983. Influence of size of culture vessel on in vitro proliferation of grape in a liquid medium. Plant Cell Tissue and Organ Culture 2: 327–332. [Google Scholar]
- PeschetJL, Giacalone A.1991. Un nouveau concept en analyse des sucres: La chromatographie ionique couplée à l’ampérométrie pulsée. Industrie Agricole Alimentaire 108: 1–4. [Google Scholar]
- PooleRT, Conover CA.1983. Establishment and growth of in vitro‐cultured Dieffenbachia HortScience 18: 185–187. [Google Scholar]
- RickardJ.1987. Evaluation of acid and enzyme hydrolytic method for the determination of Cassava starch. Journal of the Science of Food Agriculture 41: 373–379. [Google Scholar]
- RivalA, Beulé T, Lavergne D, Nato A, Noirot M.1998. Growth and carboxylase activities in in vitro micropropagated oil palm plantlets during acclimatisation: comparison with conventionally germinated seedlings. Advances in Horticultural Science 12: 111–117. [Google Scholar]
- RobertsDR, Sutton BCS, Flinn BS.1990. Synchronous and high frequency germination of interior spruce somatic embryos following partial drying at relative humidity. Canadian Journal of Botany 68: 1086–1090. [Google Scholar]
- SenaratnaT.1992. Artificial seeds. Biotechnical Advance s10: 379–392. [DOI] [PubMed] [Google Scholar]
- SutterEG, Langhans RW.1982. Formation of epicuticular wax and its effect on water loss in cabbage plants regenerated from shoot‐tip culture. Canadian Journal of Botany 60: 2896–2902. [Google Scholar]
- VanBoxtelJ, Berthouly M.1996. High frequency somatic embryogenesis from coffee leaves: Factors influencing embryogenesis, and subsequent proliferation and regeneration in liquid medium. Plant Cell Tissue and Organ Culture 44: 7–17. [Google Scholar]
- WebsterFB, Roberts DR, Mc Innis SM, Sutton BCS.1990. Propagation of interior spruce by somatic embryogenesis. Canadian Journal of Forest Research 20: 1759–1765. [Google Scholar]
- WetzsteinHY, Sommer HE.1982. Leaf anatomy of tissue‐cultured Liquidambar styraciflua (Hamamelidaceae) during acclimatization. American Journal of Botany 69: 1579–1586. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.