Abstract
Most studies of tiller development have not related the physiological and morphological features of each culm to its subsequent fertility. This introduces problems when trying to account for the effects of tillering on yield in crop models. The objective of this study was to detect the most likely early determinants of tiller fertility in sorghum by identifying hierarchies for emergence, fertility and grain number of tillers over a wide range of assimilate availabilities. Emergence, phenology, leaf area development and dry weight partitioning were quantified weekly for individual tillers and main culms of tillering and uniculm plants grown at one of four densities, from two to 16 plants m–2. For a given plant in any given density, the same tiller hierarchy applied for emergence of tillers, fertility of the emerged tillers and their subsequent grain number. These results were observed over a range of tiller fertility rates (from 7 to 91 %), fertile tiller number per plant at maturity (from 0·2 to 4·7), and tiller contribution to grain yield (from 5 to 78 %). Tiller emergence was most probably related to assimilate supply and light quality. Development, fertility and contribution to yield of a specific tiller were highly dependent on growing conditions at the time of tiller emergence, particularly via early leaf area development of the tiller, which affected its subsequent leaf area accumulation. Assimilate availability in the main culm at the time of tiller emergence was the most likely early determinant of subsequent tiller fertility in this study.
Key words: Sorghum bicolor L. Moench, plant density, thermal time, tiller emergence, tiller leaf area development, tiller fertility, tiller grain number, tiller hierarchy
INTRODUCTION
Plants that have the ability to tiller, such as sorghum, are often grown in regions where water supply from rainfall is limiting and variable (Neild, 1984). In such conditions, large variations in plant density are often imposed by farmers. However, in most cereals, grain yield is very stable over a wide range of plant densities as the tillering dynamics of the plant respond to the level of resources available (Seetharama et al., 1984). Indeed, Darwinkel (1978) observed only a three‐fold variation in grain yield in wheat associated with a 160‐fold variation in plant density. In sward conditions, surviving culm density was independent of a 30‐fold variation in initial sowing density (Kays and Harper, 1974; Kataoka et al., 1991). This highlights the capacity of the plant to adapt its development to the amount of resources available, leading to a large range of tillering responses. Moreover, heterogeneity among culms of the same plant has been observed for leaf appearance rate, time of maturity, final leaf number, size of individual leaf blades and stem length (Fischer and Wilson, 1975; Kirby et al., 1985; Gerik and Neely, 1987; Carberry et al., 1993; Madakadze et al., 1998; Moulia et al., 1999). Accordingly, a community of tillering plants cannot be considered a population of a unique culm type, and the ability of tillers to become fertile and to contribute to yield depends on their leaf axil of origin (Cannell, 1969a; Ishag and Taha, 1974; Masle‐Meynard and Sébillotte, 1981a; Ong, 1984; Wu et al., 1998).
Robust prediction of canopy leaf area development and grain yield in sorghum requires prediction of tiller fertility in relation to the environment (Hammer and Muchow, 1994). In conditions where tillering is affected neither by water stress nor by nitrogen stress, several authors have suggested that plant carbon balance, and in particular the availability of assimilates, drives tiller production (Mitchell, 1953; Ong and Marshall, 1979). The availability of assimilates for tillering decreases with either increasing demand by the mother culm or a reduced supply. Assimilate demand increases at high temperatures (Cannell, 1969b; Major et al., 1982; Bos and Neuteboom, 1998) in response to a high leaf growth rate (Lafarge et al., 1998). Assimilate supply is reduced by conditions of low light interception, resulting from either low incident radiation, a short photoperiod, high planting density or defoliation (Cannell, 1969b; Gerik and Neely, 1987; Bos and Neuteboom, 1998; Gautier et al., 1999). A change in light quality associated with increases in plant density has also been shown to affect tiller production. In crops grown with non‐limiting water and nutrients, Deregibus et al. (1985) and Ballaré et al. (1987) observed a decrease in the ratio of red : far‐red light and in tiller production as density increased; this occurred prior to any appreciable shading or depletion of assimilate resources. Nonetheless, under conditions that specifically favour tillering, a high tiller number per plant has been observed, e.g. 14 in modern maize (Moulia et al., 1999). These findings suggest that development should be analysed at a plant level to predict tiller emergence, but at the individual culm level to derive predictions of fertility of the emerged tillers. This latter approach would, however, require a highly detailed and elaborate system.
An alternative approach is to predict, at the plant level and in non‐limiting growing conditions, the tiller hierarchy for fertility from detailed quantitative information on tiller development. Most previous studies, however, have been qualitative and do not relate trends in tiller development to subsequent fertility of individual axes. While some authors have classified tillers as fertile, vegetative or dead, they have not studied the dynamics of each axis from emergence (Gerik and Neely, 1987; Mitchell et al., 1998). Ong (1984) related tiller survival to the number of leaves that had appeared by the start of stem elongation and to closure of the canopy, but failed to connect this with earlier developmental stages.
The objective of our study was to detect the most likely early determinants of tiller fertility in sorghum over a wide range of assimilate availabilities by identifying hierarchies for emergence and fertility of tillers and for physiological and morphological trends among plant axes. A range of assimilate availabilities was generated by varying plant density and also by removing tillers from specific plants to achieve artificially high carbon supply conditions. To avoid any other effects on tillering, such as water stress (Winkel et al., 1997), nitrogen stress (Masle‐Meynard, 1981) or phenological interactions, a single sowing date was used, and supplies of water and minerals were non‐limiting. This ensured the same conditions of photoperiod, temperature, humidity and general growing conditions for all plots.
MATERIALS AND METHODS
Plant material and growing conditions
Grain sorghum [Sorghum bicolor (L.) Moench] was grown under non‐limiting water and nitrogen supply in a field experiment at Lawes (27·34°S, 152·20°E, 90 m a.s.l.), in southeastern Queensland, Australia. A split‐plot design was established with two tiller treatments and four planting densities in three replicates, with planting density as the first split and tiller treatment the second. The four planting densities, D1–D4, were two, four, eight and 16 plants m–2, respectively. Tiller treatments included a tiller removal treatment in which tillers were systematically removed as they emerged, and the natural situation. Plots were 4 m wide and comprised eight rows spaced 0·5 m apart. Plot lengths were 10 m for D4, 20 m for D2 and D3, and 30 m for D1. Buster, a well‐adapted Australian hybrid, was chosen for its high tillering ability. The crop was sown on 23 Oct. 1998 at a depth of 20 mm using a coneseeder planter, at a target rate three to five times the required density. Plots were thinned to the required density on 8 November. Seed was dressed with Concep [Ciba Geigy, Basle, Switzerland; 1,3‐dioxolan‐2‐ylmethoxy imano (phenyl) acetonitrile] at 1·25 mg g–1, for protection against pre‐emergence herbicides. Immediately after sowing, Dual (metolachlor) at 2·4 l ha–1 and Gesaprim (atrazine) at 2·4 l ha–1 were applied to provide good weed control. When necessary, Heliothis armigera and Contarinia sorghicola were controlled by applications of Deltamethrin at 1·25 mg m–2 or Endosulfan at 73·5 mg m–2. Leaf rust was controlled by applications of Mancozeb at 160 mg m–2. There was negligible damage to the photosynthetic leaf surface throughout growth.
The soil at the experimental site was a Lawes brown black clay loam, which is a moderately fertile deep alluvial, weakly cracking vertisol (Typic Chromustert) that was well drained. Upper layers (0–0·2 m) were characterized by a pH of 7·2, a cation exchange capacity of 31·1 meq 100 g–1 and electric conductivity of 0·4 dS m–1, and deeper layers (0·4–0·6 m) by a pH of 7·6, a cation exchange capacity of 40·4 meq 100 g–1 and electric conductivity of 0·3 dS m–1. Bulk density was 1·34 at a depth of 0–0·2 m, 1·30 at 0·4–0·6 m and 1·45 at 0·9–1·2 m. Fertilizer containing potassium at 9 g m–2 as muriate of potash, phosphorous at 2·5 g m–2, and copper sulfate and zinc sulfate at 0·3 g m–2 as single superphosphate with copper and zinc was applied before sowing. A broadcast application of 24 g m–2 nitrogen as urea was made at sowing with additional applications of 6 g m–2 at initiation and anthesis. The cover crop of oats (2·5 g m–2 applied N) was removed in September 1998. Before sowing, soil nitrogen rating was 6·4 mg kg–1 soil at a depth of 0–0·2 m, 1·4 at 0·4–0·6 m and 0·1 at 0·9–1·2 m. At maturity, it was 2·1 mg kg–1 soil at 0–0·2 m, 1·5 at 0·4–0·6 m and 2·7 at 0·9–1·2 m. Overhead irrigation was applied to maintain the soil water profile at full water potential.
Climatic measurements and calculations
Air temperature (108‐L6; Campbell Scientific, Shepshed, UK) was measured in a ventilated cylinder 1·5 m above the soil. Data were stored hourly in a datalogger (CR10; Campbell Scientific) and, with the addition of minimum and maximum temperature, averaged daily. Daily temperature during the plant cycle (data not shown) varied by 12 °C (from 18 to 30 °C). Two thermal periods were distinguished, one up to 400 °Cd after emergence with a daily temperature around 21 °C, and the other subsequently, with a daily temperature around 25 °C.
Daily thermal time (δTT) was calculated from a broken linear function of the mean air temperature, depending on three parameters: base, optimum and maximal temperatures, 11, 30 and 42 °C, respectively, as reported by Hammer et al. (1993). Thermal time was then calculated by accumulating δTT from seedling emergence. The base temperature used was derived from studies on leaf appearance rate of 12 sorghum hybrids (Hammer et al., 1993) and was similar to that determined by Lafarge et al. (1998) of 10·8 °C, using leaf appearance and leaf elongation rates analysed for different leaf positions and different growing periods at two different locations. Lafarge et al. (1998) highlighted the role of meristem temperature on plant growth and development. Meristem temperature was not essential in the present study, however, as plant measurements were performed for a single growing period at weekly intervals at the plant level, and with the general goal of comparing density treatments rather than analysing temperature effects. Lafarge et al. (1998) also proposed calculating thermal time using a base temperature only, providing a correction of plant variables with vapour pressure deficit of the air. In this study, thermal time was simply calculated from three temperature parameters that took into account the detrimental effect of vapour pressure deficit by its correlation with high temperature. Such conditions rarely occurred in this study.
Phenological measurements and calculations
Seedling emergence was scored daily on 2 m per row (4 m for the lowest density) in each plot until emergence ceased. A seedling was considered to have emerged when it could be seen above the soil surface. The date of 50 % emergence, which occurred 5 d after sowing, was defined as the first day on which at least 50 % plants counted on the final day had emerged. Floral initiation of the main culm was measured under a binocular microscope (SZ 6045 TR; Olympus Optical, Tokyo, Japan) by dissecting three plants collected from the guard rows of each plot every day. Initiation was deemed to have occurred when the first row of floral primordia was visible on the shoot apex. Anthesis was noted by scoring five adjacent tagged plants in the inner rows of each plot. Each head was rated every second day by assessing how far down the head anthers were observed, in increments of 5 %. The date of anthesis was determined in each plot when an average of 50 % was reached for exerted anthers. Physiological maturity was noted on ten plants, the five tagged plants used for assessment of anthesis plus five in an adjacent row. Each head was rated every second day by assessing the presence of a black layer on individual grains. The attachment point at the seed base, through which plants translocate assimilates to the grain, turns black when the grain is mature (Eastin et al., 1973). Grains on individual heads were assessed in quartiles from the tip down. If five or more grains from the ten plants sampled had reached the black layer stage in the first quartile, the head was physiologically mature to that quartile, and was rated as 25 % mature. The date of maturity was determined in each plot when the ten main culm heads were on average 90 % mature. In each case, the mean over the three replicates provided the value for each treatment.
The production of fully expanded leaves on the main culm was recorded weekly by noting leaf ligule appearance on the five tagged plants in each plot until ligule appearance of the final leaf. A ligule was counted once it was visible above the enclosing sheath of the previous leaf. The mean of the five values gave the total number of fully expanded main culm leaves for each plot and measurement date. Leaves were numbered with a permanent marking pen, the seedling leaf being leaf number 1. When ligule appearance of the final leaf was approaching, observations were made every day. The number of dead leaves was recorded in each plot and for each individual culm of the tagged plants until the day of final harvest. A leaf was considered dead if 50 % or less of its surface was green. The number of green leaves was then determined for each culm as the difference between the total number of fully expanded leaves and the number of dead leaves.
Tiller emergence was observed weekly on the five tagged plants in each plot. The origin of each emerged tiller, defined by the main culm node from which it developed and the sheath from which it emerged, was written on one of the tiller’s leaves. For example, T1 was a tiller that developed from node 1 of the main culm and so emerged from the sheath of leaf 1. Secondary tillers (i.e. a tiller that developed from a node of another tiller) were not considered since they were rare and developed no more than two leaves. The production of fully expanded leaves was recorded for all emerged tillers on the tagged plants in the same manner as for main culms. The number of dead leaves was recorded and the number of green leaves determined in each plot and for each individual tiller of the tagged plants until the day of final harvest. A tiller was considered dead when all of its leaves were dead.
Non‐destructive morphological measurements and calculations
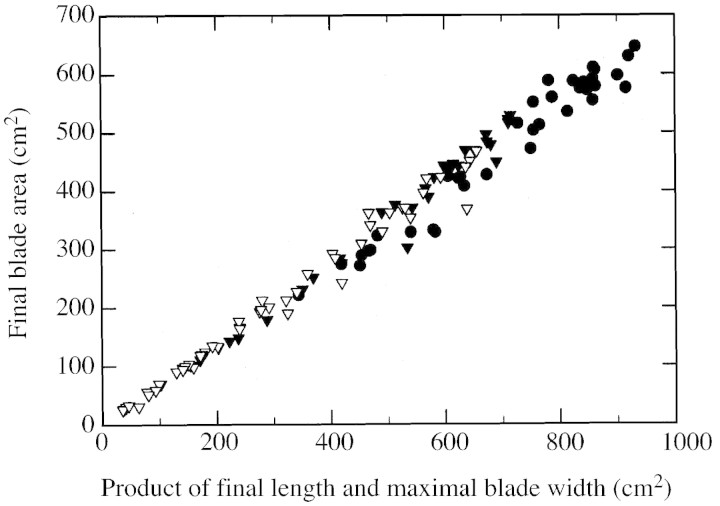
Individual mature leaf area for each culm was determined non‐destructively by measuring length and maximal width of each blade of the tagged plants. Length was measured from the ligule to the tip. Maximal width was measured between both edges of the blade where its length from the ligule corresponded roughly to one‐third of its total length. Blade area was then calculated by multiplying length, maximal width and a shape coefficient. This coefficient was determined by linear regression of the product of leaf length and maximal width on area of the same blade measured with a leaf area meter (Mk2; Delta‐T Devices Ltd, Cambridge, UK). The coefficient determined, 0·685, did not vary significantly with leaf position, culm origin or plant density (Fig. 1). It was also similar to values found by McCree et al. (1984) for sorghum (0·68), Payne et al. (1991) for pearl millet (0·68) and Keating and Wafula (1992) for maize (0·72). Height from the ground to the last visible ligule was also measured weekly on each culm of the tagged plants.
Fig. 1. Relationship between final blade area and product of final blade length and maximal blade width (measured at one‐third of the final blade length from the ligule) for main culms of sorghum grown at two plants m–2 (circles), and main culms (closed triangles) and tillers (open triangles) of sorghum grown at 16 plants m–2. Each symbol corresponds to a single leaf. Slope of the common linear regression is 0·685.
Destructive morphological measurements and calculations
Biomass accumulation was determined by sampling plants in each plot every 7 d from time of thinning to anthesis, and at mid‐grain filling and maturity. At each harvest, plants were cut at ground level from the four inner rows of each plot in a 4 m2 quadrat in D1, a 2 m2 quadrat in D2 and D3, and a 1 m2 quadrat in D4. A minimum of 0·5 m was left between each harvest area. For each sample, main culms and tillers were separated and numbered systematically. Tillers had been regularly identified and labelled in the field, around the time of emergence, to avoid any confusion at harvest. However, some difficulties in identifying tillers towards the end of the plant cycle may have introduced minor errors in tiller number associated with origin of each tiller. Moreover, measurements may have included senescent tillers during destructive harvests as it was not practical to separate senescent tillers from others with the same node of origin. For each culm at each harvest, the biomass was separated into four components: green and dead leaves, stems (which comprised sheaths and internodes) and heads (when present). Net above‐ground biomass per unit area was obtained for each component after drying samples at 80 °C until there was no further weight loss (6–7 d). Shoot dry weight of any culm was calculated as the sum of the dry weights of leaves, stems and heads. Dry heads were threshed, the grain re‐dried and weighed, and grain yield was determined. Grain number per head was calculated as grain dry weight divided by grain size, after grain size was determined by weighing 200 grains. The harvest index was calculated as grain dry weight divided by shoot dry weight. A sub‐sample of green leaves from each type of culm (roughly one‐third of the collected area, but the total area of the dry weight of samples was less than 10 g) was isolated systematically before drying. The area of these leaves was measured with a leaf area meter and their dry weight determined. For each type of culm, specific leaf area (SLA, cm2 g–1) was calculated by dividing leaf area by leaf dry weight. Total green leaf area (cm2) for the harvest area was determined from the product of total green leaf dry weight and SLA. Leaf area index (LAI) was calculated as the total green leaf area divided by the harvest area of each plot. All measurements were determined on a per plant basis by dividing each variable by the plant number per plot. Green leaves of each type of culm were analysed for N concentration at anthesis and maturity using method 7A1 for total nitrogen analysis (Bremner, 1965). Tissue nitrogen concentrations (2·96 % in main culm green leaves at anthesis and 1·93 % at maturity) indicated that non‐limiting conditions were achieved for nitrogen.
Tiller number calculations
Maximal and fertile tiller numbers per plant were calculated from non‐destructive and destructive measurements in each plot. For non‐destructive measurements, tiller number per plant was calculated weekly as the sum of all tillers counted on the five tagged plants, divided by the number of plants. Maximal tiller number corresponded to the highest calculated value. Fertile tiller number per plant was calculated at maturity as the sum of all tillers producing heads on the tagged plants, divided by the number of plants. With destructive samples, the tiller number per plant was taken to be the sum of tillers counted on the plants harvested at two sampling dates divided by the number of plants. For maximal tiller number, these dates were 23 and 30 November for D3 and D4 (around 16 plants per rep at each harvest), and 30 November and 7 December for D1 and D2 (around eight plants per rep at each harvest). For fertile tiller number, the post‐anthesis sampling dates of 14 January and 11 February were used for all densities.
RESULTS
The range of experimental conditions generated much variation in shoot dry weight per plant without affecting timing of phenological stages
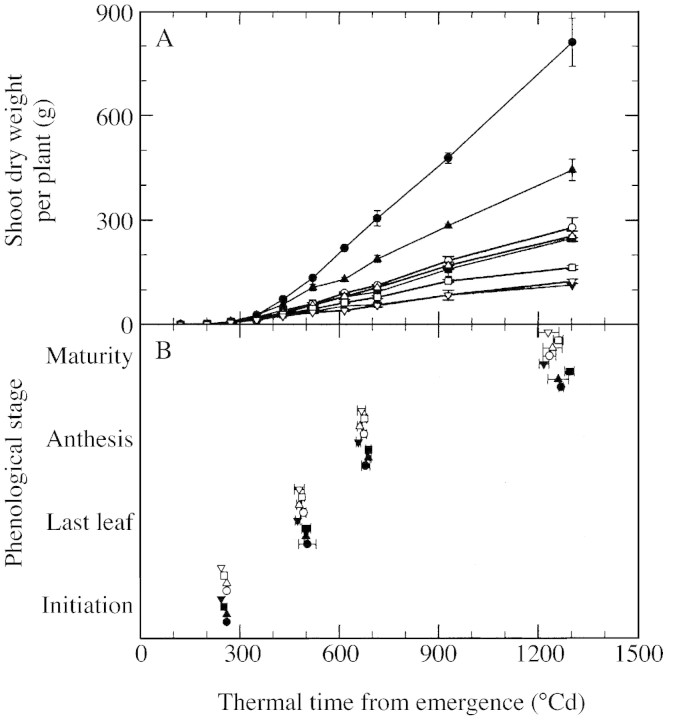
Shoot dry weight per plant increased at maturity with decreasing density, from 110 to 820 g for tillering plants, and from 110 to 285 g for uniculm plants (Fig. 2A). The timing of phenological stages did not differ appreciably across plant densities and across tillering and uniculm plants (Fig. 2B). In all treatments, panicle initiation, full expansion of the final leaf, anthesis and maturity occurred, respectively, around 280, 500, 700 and 1250 °Cd after emergence. The slight earliness of stages observed in plants at the highest density was not statistically significant, and seemed to be attributable to the final leaf number of the main culm (15·5), which was lower than that of other treatments (16·2–16·4).
Fig. 2. Change in shoot dry weight (A) and phenological stages of main culms (B) with thermal time from emergence for tillering and uniculm plants. Data are presented for tillering (closed symbols) and uniculm (open symbols) plants, grown at densities of two (circles), four (upward pointing triangles), eight (squares) and 16 (downward pointing triangles) plants m–2. In B, phenological stages were floral initiation, ligule appearance of the final leaf, anthesis and grain maturity. Vertical lines in A and horizontal lines in B represent s.e.m. of three replications.
The range of experimental conditions generated much variation in tiller number per plant and in tiller contribution to grain yield
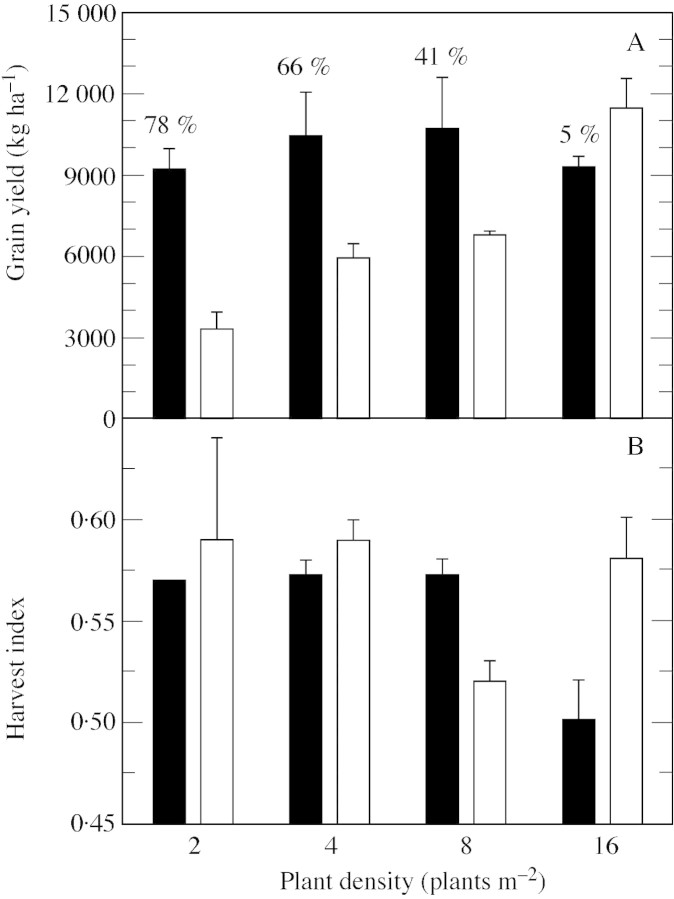
Maximal tiller number per plant increased with decreasing plant density, from 3·2 in D4 to 5·2 in D1 (Fig. 3A). Fertile tiller number per plant also increased with decreasing density, from 0·2 in D4 to 4·7 in D1. Hence, the proportion of emerged tillers that were not ultimately fertile increased with density from 9 to 93 %. It was interesting to note that in all plots, all non‐fertile tillers died progressively between full expansion of the final leaf on the main culm and plant maturity (data not shown). The density of fertile tillers, which was calculated as the difference between the density of fertile culms and the density of main culms (represented by the straight line in Fig. 3B), increased from 9·5 to 13·8 tillers m–2 from D1 to D2 and then decreased to 3·8 tillers m–2 in D4 (Fig. 3B). At the same time, mean grain dry weight per fertile culm also decreased with increasing plant density, from 80·2 to 46·8 g, but with most of the decrease (i.e. from 80·2 to 58·8 g) occurring between D1 and D2 (Fig. 3C). This generated a variation in tiller contribution to grain yield ranging from 5 to 78 % (Fig. 4A). Fertile culm (main culms + tillers) density at maturity was 11·5 culms m–2 in D1, and increased to close to 20 culms m–2 in D2, D3 and D4 (Fig. 3B). Although it did not differ significantly among treatments, grain yield of tillering sorghum tended to increase from D1 to D2 (9200 to 10 600 kg ha–1), remain constant from D2 to D3, and then decrease to 9200 kg ha–1 in D4 (Fig. 4A). Grain yield on uniculm sorghum increased significantly from 3300 to 11 500 kg ha–1 from D1 to D4. Grain yield measured in D4 was significantly higher for uniculm than for tillering sorghum, and was associated with a significant difference in harvest index of 0·08 (Fig. 4B). The harvest index was stable at around 0·58 for uniculm plants regardless of density, although the value in D3 was surprisingly low. The harvest index decreased in tillering plants with increasing density (although non‐significantly from D1 to D3), with the value recorded in D1 (0·57) approximating that of uniculm plants (Fig. 4B).
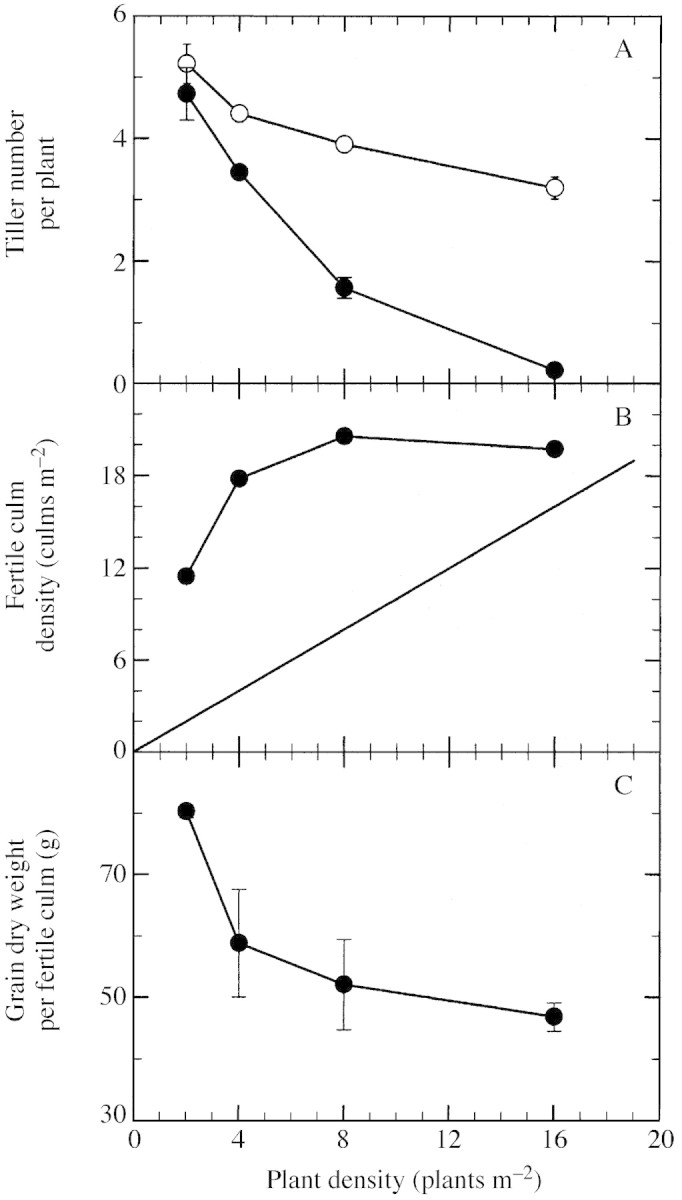
Fig. 3. Relationship with plant density of maximal (open circles) and fertile (closed circles) tiller number per plant (A), fertile culm density (circles) (B) and grain dry weight per fertile culm (C). The solid line in B corresponds to the 1 : 1 relationship between plant density and fertile culm density for a canopy formed of fertile uniculm plants only. Vertical lines represent s.e.m. of three replications.
Fig. 4. Grain yield (A) and harvest index (B) vs. plant density for tillering (black bars) and uniculm (open bars) plants. Harvest index was calculated as grain dry weight divided by shoot dry weight. Bars represent s.e.m. of three replications. Percentages shown in A represent contribution of tillers to grain yield.
The tiller types that emerged at the greatest frequency had the highest fertility rates and produced the most grain
All plants in D1 developed tiller types T3, T4 and T5 (Fig. 5A). The number of tillers that emerged per plant increased significantly from 0·32 for T1 to 1·0 for T3 and then decreased from 1·0 for T5 to 0·71 for T6. A similar pattern was observed in the other density treatments. However, emergence of T4, T5 and T6 was significantly affected by density, declining as density increased (Fig. 5A). This response largely explained variation in maximal tiller number among densities. Fertility of emerged tillers in D1 was complete from T1 to T4 (Fig. 5B). For later tillers, however, the fertile culm number per emerged culm decreased to 0·98 and 0·78 for T5 and T6, respectively. At the other densities, this fertile tiller frequency decreased significantly for tillers at leaf axils above and below 3. This decrease was greater in D2 than in D1, and greater in D3 than in D2. In D4, there were few fertile culms at any position. All main culms from either uniculm or tillering plants were fertile. Grain number per fertile culm decreased significantly as density increased and was significantly greater for main culms than for tillers at all densities (Fig. 5C). Grain number per main culm of uniculm plants was always higher than that of tillering plants: the difference was not significant at a density of 16 plants m–2 and was greatest at four plants m–2. It is interesting to note that grain number was similar among uniculm plants at densities of two and four plants m–2, indicating that 4700 grains per head could be a threshold for this variety. The same pattern was found for all densities in the variation of grain number among tillers (Fig. 5C): most grains were produced on T3 with a significant decrease on tillers from later and earlier leaf axils. This response reflected similar patterns to those observed for frequencies of emerged and fertile tillers.
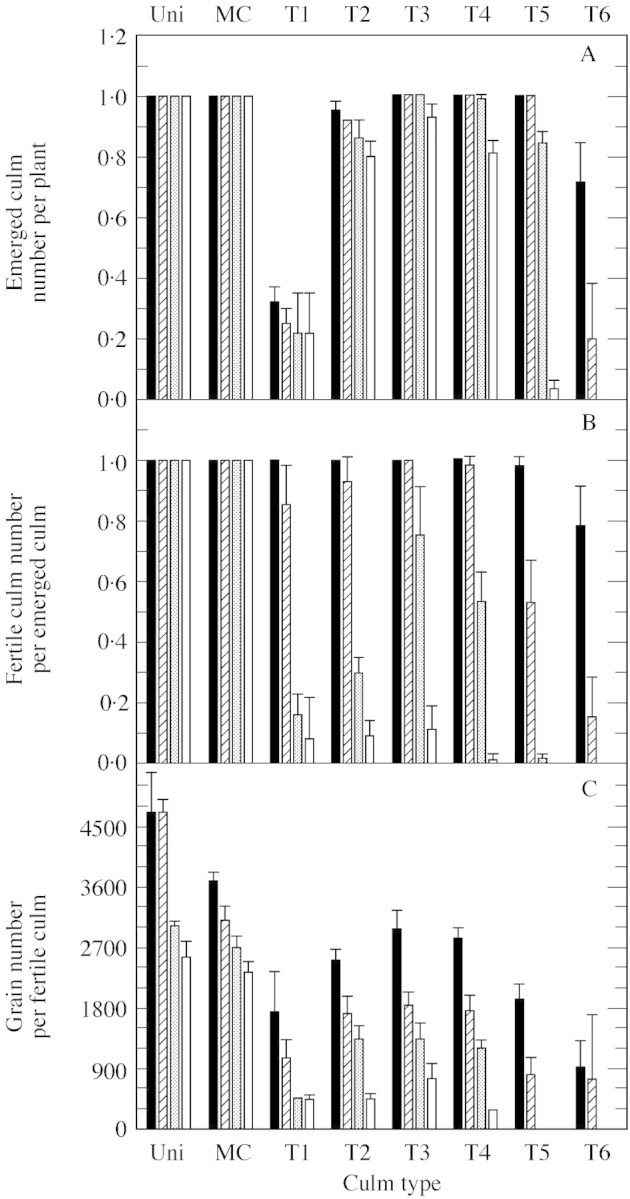
Fig. 5. Emerged culm number per plant (A), fertile culm number per emerged culm (B) and grain number per fertile culm (C) for each culm type, except secondary tillers, for plants grown at a density of two (black bars), four (hatched bars), eight (stippled bars) and 16 (open) plants m–2. Uni, Uniculm plants; MC, main culm of tillering plants; T1, tiller that emerged from the oldest subtending leaf of the main culm (leaf 1). Vertical lines represent s.e.m. of three replications.
Ligule height of the last fully emerged leaf and number of fully expanded leaves per fertile tiller varied in the same order as tiller age, whereas green leaf area and shoot dry weight per tiller varied in the same order as tiller fertility rate
Ligule height of the last fully emerged leaf and number of fully expanded leaves per fertile culm (Fig. 6A and B) were plotted against thermal time from emergence for main culm and fertile tillers from all nodes of origin (only T6 was not fully fertile, see Fig. 5B) for plants at D1. Ligule height was always greater on the main culm than for any tiller until 650 °Cd, when T1 to T4 became taller (but non‐significantly) than the main culm (Fig. 6A). Later tillers were systematically shorter. The number of fully expanded leaves on the main culm and on fertile tillers was linearly related to thermal time up to the appearance of the ligule of the final leaf (Fig. 6B). Slopes did not vary significantly across culm types. Tiller emergence occurred between 120 and 300 °Cd for T1 to T6, and emergence of T1 and T2 was simultaneous. The ligule of the final leaf of all tillers appeared simultaneously at about 650 °Cd after emergence, which was 100 °Cd later than the appearance of the final leaf on the main culm. So, the duration of leaf production (from seedling emergence to ligule appearance of the final leaf) and final leaf number of tillers decreased with increasing node of origin. In particular, leaf production duration decreased from 500 to 350 °Cd and final leaf number from 15 to ten for T1 to T6.
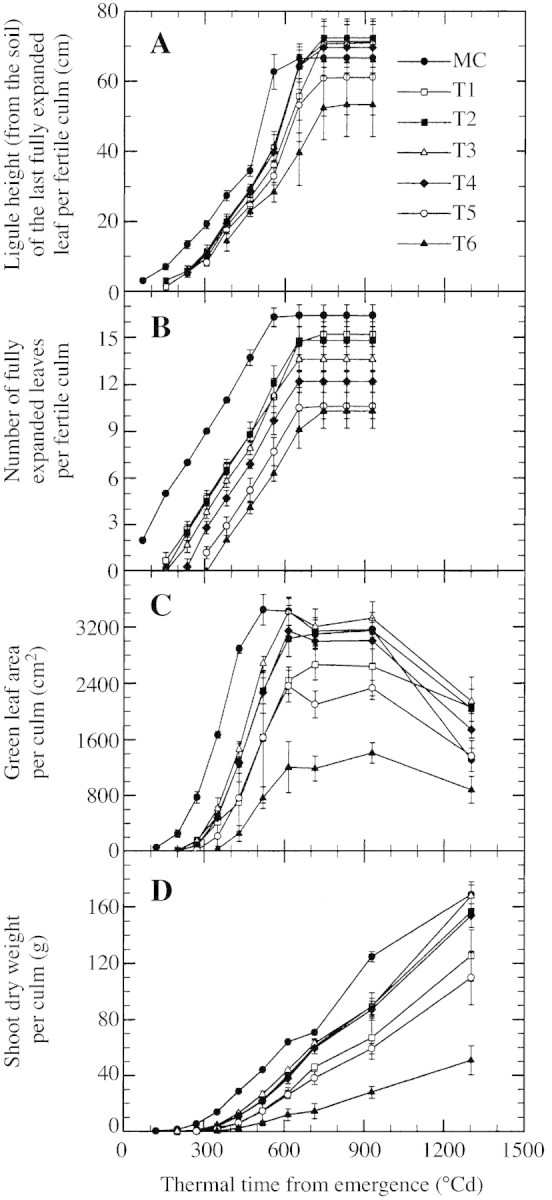
Fig. 6. Changes with thermal time from emergence in height of the ligule of the last fully expanded leaf (A), in number of fully expanded leaves (B), in green leaf area (C) and in shoot dry weight (D) for the main culm (MC, closed circles) and primary tillers T1 (open squares), T2 (closed squares), T3 (open triangles), T4 (diamonds), T5 (open circles) and T6 (closed triangles) of sorghum grown at two plants m–2. Only fertile tillers were included for A and B. In C and D, data for T6 included senescent tillers as it was not practical to separate senescent tillers from others with the same node of origin during destructive harvests. Vertical lines represent s.e.m. of three replications.
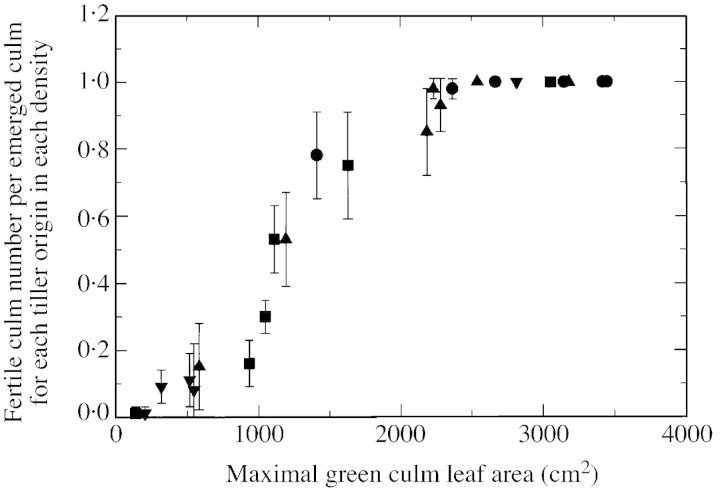
Green leaf area and shoot dry weight per culm (Fig. 6C and D) were examined for all culms of plants grown at D1. Values for T6 included fertile and senescent tillers (see Materials and Methods). Changes in visible green leaf area over time showed the same pattern across culm types, but with the maximal area varying from 2400 to 3400 cm2 when only fully fertile tiller types were considered (i.e. T1–T5; Fig. 6C). Maximal green leaf area and the time at which it was reached did not vary in the same order as tiller age, especially for T1. A critical value of maximal culm leaf area of around 2400 cm2 could be related to 100 % fertility: the maximal area was 2660 and 2360 cm2 for T1 and T5, with 100 and 98 % fertility, respectively (Figs 5B and 6C), whereas it was above 3150 cm2 for T2, T3 and T4. This relationship was confirmed when all tiller origins from plants at all density treatments were considered, although leaf areas represented both fertile and senescent tillers in cases where tillers were not fully fertile (Fig. 7): in particular in D2, maximal leaf area values of T3, T4 and T2 were 2540, 2230 and 2280 cm2, respectively, for 100, 98 and 93 % fertility. Results for dry weight at maturity were similar to those for green leaf area in that values for T3 were greater than those for T1, T2 and tillers from upper leaf axils (Fig. 6D).
Fig. 7. Fertile culm number per emerged culm vs. maximal green leaf area of that culm, for the main culm and each tiller type of sorghum grown at two (circles), four (upward pointing triangles), eight (squares) and 16 (downward pointing triangles) plants m–2. In cases where tillers were not fully fertile, green leaf area included that of senescent tillers as it was not practical to separate senescent tillers from others with the same node of origin during destructive harvests. Vertical lines represent s.e.m. of three replications.
Areas of the first leaves of tillers T1 to T4 varied among tillers in the same order as tiller fertility rate
The association between individual leaf area and leaf position produced a bell‐shaped curve for all tiller origins observed in D1, but the position of the largest leaf and its size differed (Fig. 8A). For any culm, the lower the final leaf number, the lower the position of the largest leaf. First leaves of T1 and T2 had a smaller area than those of T3 and T4, until leaf 9. Areas of the first leaves of T5 and T6 were similar to those of T4, but only until leaf 3 for T6 and leaf 6 for T5. Individual leaf area of the main culm increased with leaf position for positions 1 to 6 (Fig. 8A inset). It did not, however, change with plant density. Differences in blade leaf area of tiller leaves were associated with similar differences in both blade length and maximum width (Fig. 8B and C).
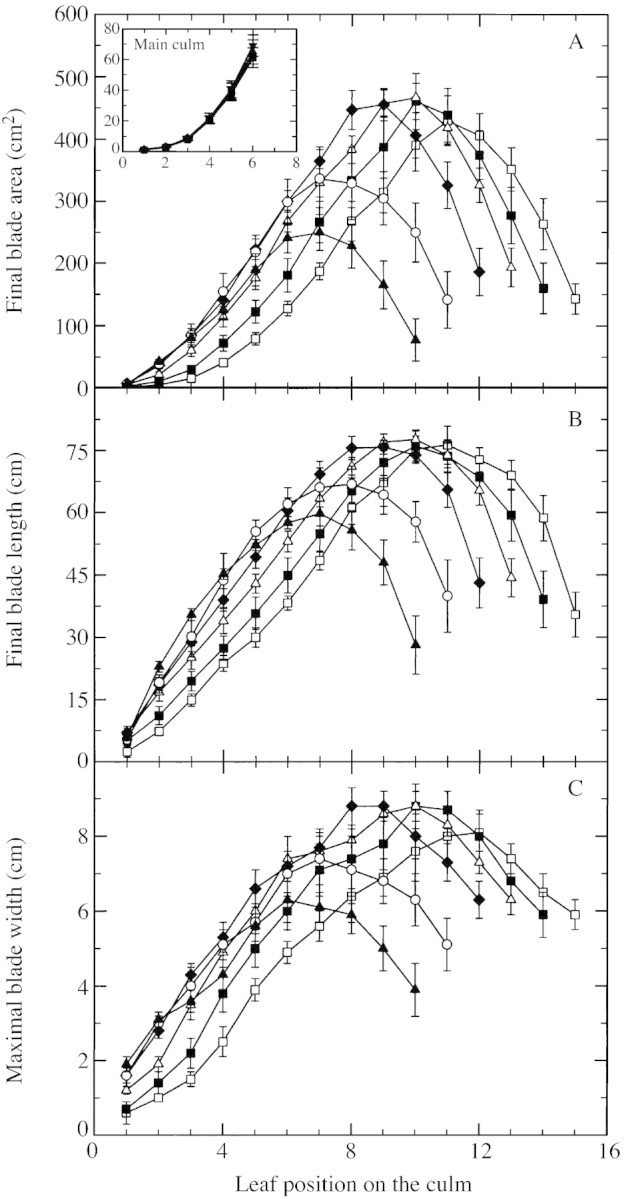
Fig. 8. Final area (A), final length (B) and maximal width (C) of individual tiller blades vs. leaf position on the culm for T1, T2, T3, T4, T5 and T6 for sorghum grown at two plants m–2. Symbols as in Fig. 6. The inset shows final blade area of main culm leaves vs. leaf position for plants grown at two (circles), four (upward pointing triangles), eight (squares) and 16 (downward pointing triangles) plants m–2.
Green leaf area and shoot dry weight per fertile culm were affected by plant density whereas number of fully expanded leaves was not
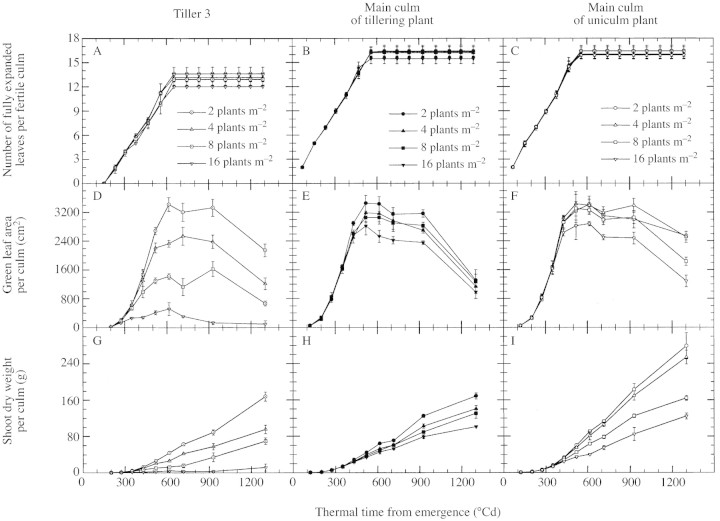
The changes with thermal time in number of fully expanded leaves, green leaf area and shoot dry weight were analysed across plant densities for three different culm types (Fig. 9). The slope of the rate of increase in number of fully expanded leaves did not change significantly with plant density for T3 or main culms (MC) of tillering and uniculm plants (Fig. 9A–C). Plant density only slightly affected final leaf number of T3. Differences among densities in green leaf area of MC in tillering plants resulted in a decrease in maximal green leaf area per culm from 3448 to 2816 cm2 as density increased (Fig. 9E). The same patterns were observed for uniculm plants, but with higher maximal values in D2 and D3 (Fig. 9F). Maximal green leaf area per culm of T3 decreased strongly with increasing density, from 3418 to 517 cm2, although some senescent tillers were included in the data for D3 and D4 (Fig. 9D). It was greater than 2400 cm2 in D2, where T3 was fully fertile (see Fig. 7). Shoot dry weight of MC at maturity varied more across density treatments for uniculm plants than for tillering plants (Fig. 9H and I). The difference between the two types of culm at a given density increased with decreasing density from 20 to 110 g, with the uniculm plants always having the greater dry weight. Shoot dry weight at maturity for T3 was similar to that for MC of tillering plants in D1, but appreciably lower in D2 (Fig. 9G and H).
Fig. 9. Change with thermal time from emergence in number of fully expanded leaves (A–C), in green leaf area (D–E) and in shoot dry weight (G–I) for tiller 3 (A, D, G; dotted symbols), main culm of tillering plants (B, E, H; closed symbols) and main culm of uniculm plants (C, F, I; open symbols) for sorghum grown at a density of two (circles), four (upward pointing triangles), eight (squares) and 16 (downward pointing triangles) plants m–2. In D and G, data corresponding to eight and 16 plants m–2 included data for senescent tillers as it was not practical to separate senescent tillers from others with the same node of origin during destructive harvests. In all other cases, data corresponded to fertile culms only. Vertical lines represent s.e.m. of three replications.
DISCUSSION
In this study on sorghum, assimilate supply in the plant seems likely to have been a key driver of tiller emergence and fertility, and light quality a key driver of tiller emergence. These findings were observed over a large range of plant densities (2–16 plants m–2) with non‐limiting water and nitrogen supply. In the tillering treatment at 16 plants m–2, where fewest tillers emerged and almost none were fertile, the dry matter difference between uniculm and tillering main culms was close to zero. Conversely, in D1, where tiller emergence and subsequent fertility were highest, shoot dry matter of uniculm plants increased substantially when compared with the main culms of the tillering treatment, whereas the maximum green leaf area was similar for both types of culm (see Fig. 6). So, surplus assimilate in the main culm was probably predisposed to tiller production rather than to increase leaf area. This induction of tillering by assimilate supply was also proposed by Mitchell (1953) and Ong and Marshall (1979), and tested by Schnier et al. (1990). In this way, Porter (1985) observed in wheat that a minimal increase in dry weight per culm was necessary to promote tiller emergence. While surplus assimilate in the main culm is a likely precursor of tiller emergence, reduced emergence of late tillers in this study was probably not due directly to depletion of available assimilates in the plant, since tiller emergence stopped in all density treatments before the LAI reached 1 (Lafarge and Hammer, 2002). Ballaré et al. (1987) reported that a decrease in tiller production was observed before the LAI was sufficiently great to effect a significant degree of mutual shading. They associated reduction in tiller emergence from upper axils with decreasing red : far‐red ratio of light in the canopy in response to increasing plant density. This was probably the case here.
However, assimilate supply did not affect rates of development. Within the range of growing conditions studied, the timing of phenological stages of the main culm was not modified by variation in plant density resulting either from variation among main density treatments imposed at thinning, or from variation of culm density imposed during the plant cycle. Similarly, Fischer and Wilson (1975) observed the same date of panicle initiation for sorghum despite varying the plant density from 1·5 to 65 plants m–2. In addition, the rate of appearance of fully expanded leaves did not vary with tiller origin or plant density in these conditions. This is consistent with the findings of Kirby and Faris (1972) on barley, Klepper et al. (1982) on wheat, Ong (1984) on millet, Skinner and Nelson (1994) on tall fescue, Wu et al. (1998) on rice and Moulia et al. (1999) on maize. It was, however, observed here only with primary tillers, as noted also by Carberry et al. (1993), as secondary tillers did not develop in this study. Nemoto et al. (1995) and Kirby et al. (1985) using rice, and wheat and barley, respectively, observed changes in leaf appearance rate with tiller origin, but generally for late‐formed axes appearing as secondary tillers.
Tillering and yield responses to density could be related to general concepts associated with interplant competition and resource capture. The high number of senescent tillers recorded at D4 was associated with significantly lower grain yield and harvest index than for uniculm plants at the same density. This indicates that some of the resources captured by tillers at D4 were probably wasted, even if senescent tillers may contribute appreciably to grain yield via assimilate translocation, as observed in barley during early stem elongation (Lauer and Simmons, 1988). Thus, grain yield was highest when senescence of tillers was avoided or reduced, as for uniculm sorghum grown at 16 plants m–2, and tillering sorghum grown at four and eight plants m–2. In D1, although tiller fertility was near complete, grain yield was significantly lower than that at D2 and D3 because resource capture was incomplete due to a non‐optimal density of fertile culms. This highlighted two distinct aspects of plant response, consistent with the tillering behaviour of grasses described by Herniaux et al. (1994). First, between two and four plants m–2, grain yield was probably affected by non‐optimal resource capture: as plant density increased, the density of fertile culms increased more than grain yield per fertile culm decreased. Secondly, between eight and 16 plants m–2, grain yield was probably affected by wastage of resource capture: as plant density increased, the density of fertile culms was nearly constant, but grain yield per fertile culm decreased slightly.
Fertility of individual tillers was possibly driven by conditions at tiller emergence, via effects on tiller growth and development, as reported by Masle‐Meynard and Sébillotte (1981b). For each plant density, a common tiller hierarchy according to tiller origin on the plant applied to frequency of tiller emergence, tiller fertility rate and grain production per tiller. For example, in D2, the hierarchy was T3 > T4 > T2 > T1 > T5 > T6. In particular, regardless of plant density, the hierarchies T3 > T2 > T1 and T3 > T4 > T5 > T6 (when present) were reported for emergence frequency as well as for fertility frequency and grain number. Such a stable tiller hierarchy has also been observed in barley by Cannell (1969a). This stability was observed here in growing conditions in which fertile tiller number per plant at maturity varied from 0·2 to 4·9 and the tiller contribution to grain yield varied from 5 to 78 %. The hierarchy was also valid over a range of senescent tillers per plant from 9 to 93 %.
Green leaf area per tiller decreased with tiller origin in a similar hierarchy to that observed for tiller emergence and fertility rate (see Fig. 6C). In particular, the tiller hierarchy for maximal green leaf area in D2 was also T3 > T4 > T2 > T1 > T5 > T6. In contrast, the hierarchies for tiller height and tiller leaf number did not match those for tiller emergence or fertility rate. So, as mutual shading increased during crop growth, the tillers most likely to survive and contribute most to grain yield were ranked according to the highest emergence frequency and greater leaf area. But these tillers were not systematically the oldest ones, in contrast to results of Ishag and Taha (1974) and Darwinkel (1978), nor the tallest ones, in contrast to observations of Cannell (1969a) and Ong (1984). Nor were they characterized by a higher rate of appearance of fully expanded leaves, as this did not vary with tiller origin or plant density.
Unfavourable conditions at tiller emergence that affected subsequent tiller leaf area development, and thus tiller fertility, could be classified into two phases.
The first phase, which ended before emergence of T3, was independent of plant density. Similar observations of low tiller emergence from the lower leaf axils compared with upper leaf axils have been reported by Downes (1968), Masle‐Meynard and Sébillotte (1981a) and Ong (1984) for sorghum, wheat and millet, respectively. The first tillers to emerge (here T1 and T2) were characterized by a small leaf area compared with T3 because of small individual leaf areas. Lauer and Simmons (1985) observed that in barley, young tillers imported their assimilates preferentially from the subtending leaf on the main culm and from the leaf immediately above it. Similarly, Peterson et al. (1982) noticed that tiller emergence in wheat was highly reduced if its subtending leaf or the one above it was excised. Hence, the rate of emergence and subsequent fertility and yield of tillers from lower axils were probably affected by the small area of the subtending leaves that led to low tiller leaf area development, as already suggested by Cannell (1969b). Masle‐Meynard and Sébillotte (1981b) and Peterson et al. (1982) observed that seed size in wheat explained the variability in vigour of different plants growing in the same conditions. This may explain why, in this experiment, of the plants grown at the same density, some had a T1 and a T2, whereas others did not.
The second phase, which started from emergence of T4 or T5 according to the treatment, was dependent on plant density. The late‐emerging tillers (T5 and T6) were characterized by a low leaf area compared with T3 due to a smaller number of leaves. This was a direct effect of the delay in their emergence, since the rate of appearance of fully expanded leaves was not affected by culm origin. This delay led to a decreasing assimilate supply available for these tillers due to their position in the canopy. Also, the larger subtending leaf of these tillers was probably unable to compensate for the increasing assimilate competition in the plant associated with elongation of internodes of the main culm. Lauer and Simmons (1985) highlighted the fact that assimilates from the main culm leaf were exported towards internodes, to the detriment of tillers, in response to the beginning of main culm elongation.
The hierarchy of tiller fertility T3 > T4 > T2 > T1 > T5 > T6 (when present) was applicable to the wide range of plant densities tested here (two to 16 plants m–2), although it may be particular to this cultivar. While this hierarchy was not observed exactly in D4 due to the effects of plant competition on development of T4, differences were too minor and fertility rates too low, indicating that the same hierarchy could be applied to the four treatments. This stability was also reported in barley by Cannell (1969a) under contrasting levels of nitrogen fertilization and plant spacing.
The results of this study provide a basis for modelling tiller dynamics in sorghum. Currently, tillering modules of sorghum crop models are poor or non‐existent. However, predictions of tillering are required to ensure such models give reliable estimates of canopy development and grain yield. A means of predicting fertile tiller number at maturity is considered in a subsequent paper (Lafarge and Hammer, 2002).
CONCLUSIONS
Assimilate supply in the main culm at the time of tiller emergence appears the most likely determinant of subsequent tiller fertility in this study. Over a wide range of plant densities, results indicated that: (1) tiller emergence was probably dependent on assimilate availability in the mother culm for lower axils, and on early perception of inter‐plant competition mediated by change in light quality for upper axils; (2) subsequent development of each tiller was affected by assimilate availability at the time of tiller emergence, which resulted from a combination of aspects of the internal environment, probably defined by the area of the subtending leaf and the mother culm developmental stage, and the external environment; and (3) tiller emergence, tiller fertility and subsequent tiller grain number could be classified in a common tiller hierarchy according to tiller origin, regardless of plant density. This hierarchy, in order of decreasing fertility, was identified here as T3 > T4 > T2 > T1 > T5 > T6, and was mostly driven by dynamics of individual tiller leaf area. The results of this study provide a basis for modelling tiller dynamics in sorghum.
ACKNOWLEDGEMENTS
We thank Peter Harland for technical assistance and Ashley Genrich for assistance in collecting data. We are grateful to the French Ministry of Foreign Affairs and the Centre International de Recherche en Agronomie pour le Développement for salary support and funding.
Supplementary Material
Received 17 September 2001; Returned for revision: 22 November 2001; Accepted: 28 March 2002
References
- BallaréCL, Sanchez RA, Scopel AL, Casal JJ, Ghersa CM.1987. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant, Cell and Environment 10: 551–557. [Google Scholar]
- BosHJ, Neuteboom JH.1998. Morphological analysis of leaf and tiller number dynamics of wheat (Triticum aestivum L.): response to temperature and light intensity. Annals of Botany 81: 131–139. [Google Scholar]
- BremnerJM.1965. Total nitrogen. In: Black CA, ed. Methods of soil analysis, Part 2. Madison, WI: American Society of Agronomy, 1149–1172. [Google Scholar]
- CannellRQ.1969a The tillering pattern in barley varieties. I. Production, survival and contribution to yield by component tillers. Journal of Agricultural Science 72: 405–422. [Google Scholar]
- CannellRQ.1969b The tillering pattern in barley varieties. II. The effect of temperature, light intensity and daylength on the frequency of occurrence of the coleoptile node and second tillers in barley. Journal of Agricultural Science 72: 423–435. [Google Scholar]
- CarberryPS, Muchow RC, Hammer GL.1993. Modelling genotypic and environmental control of leaf area dynamics in grain sorghum. II. Individual leaf level. Field Crops Research 33: 311–328. [Google Scholar]
- DarwinkelA.1978. Patterns of tillering and grain production of winter wheat at a wide range of plant densities. Netherlands Journal of Agricultural Science 26: 383–398. [Google Scholar]
- DeregibusVA, Sanchez RA, Casal JJ, Trlica MJ.1985. Tillering responses to enrichment of red light beneath the canopy in a humid natural grassland. Journal of Applied Ecology 22: 199–206. [Google Scholar]
- DownesRW.1968. The effect of temperature on tillering of grain sorghum seedlings. Australian Journal of Agricultural Research 19: 59–64. [Google Scholar]
- EastinJD, Hultquist JH, Sullivan CY.1973. Physiologic maturity in grain sorghum. Crop Science 13: 175–178. [Google Scholar]
- FischerKS, Wilson GL.1975. Studies of grain production in Sorghum bicolor (L. Moench). V. Effect of planting density on growth and yield. Australian Journal of Agricultural Research 26: 31–41. [Google Scholar]
- GautierH, Varlet‐Grancher C, Hazard L.1999. Tillering responses to the light environment and to defoliation in populations of perennial ryegrass (Lolium perenne L.) selected for contrasting leaf length. Annals of Botany 83: 423–429. [Google Scholar]
- GerikTJ, Neely CL.1987. Plant density effects on main culm and tiller development of grain sorghum. Crop Science 27: 1225–1230. [Google Scholar]
- HammerGL, Muchow RC.1994. Assessing climatic risk to sorghum production in water‐limited subtropical environments. I. Develop ment and testing of a simulation model. Field Crops Research 36: 221–234. [Google Scholar]
- HammerGL, Carberry PS, Muchow RC.1993. Modelling genotypic and environmental control of leaf area dynamics in grain sorghum. I. Whole plant level. Field Crops Research 33: 293–310. [Google Scholar]
- HerniauxP, de Leeuw PN, Diarra L.1994. Modelling tillering of annual grasses as a function of plant density: application to Sahelian rangelands productivity and dynamics. Agricultural Systems 46: 121–139. [Google Scholar]
- IshagHM, Taha MB.1974. Production and survival of tillers of wheat and their contribution to yield. Journal of Agricultural Science 83: 117–124. [Google Scholar]
- KataokaM, Ibaraki K, Tokunaga H.1991. Population regulation of rhodesgrass cultivars in a sward conditions. II. Tiller density. Journal of Applied Ecology 28: 842–854. [Google Scholar]
- KaysS, Harper JL.1974. The regulation of plant and tiller density in a grass sward. Journal of Ecology 62: 97–105. [Google Scholar]
- KeatingBA, Wafula BM.1992. Modelling the fully expanded area of maize leaves. Field Crops Research 29: 163–176. [Google Scholar]
- KirbyEJM, Faris DG.1972. The effect of plant density on tiller growth and morphology in barley. Journal of Agricultural Science 78: 281–288. [Google Scholar]
- KirbyEJM, Appleyard M, Fellowes G.1985. Leaf emergence and tillering in barley and wheat. Agronomie 5: 193–200. [Google Scholar]
- KlepperB, Rickman RW, Peterson CM.1982. Quantitative characterisation of vegetative development in small cereal grains. Agronomy Journal 74: 789–792. [Google Scholar]
- LafargeT, Hammer GL.2002. Tillering in grain sorghum over a wide range of population densities: modelling dynamics of tiller fertility. Annals of Botany 90: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LafargeT, de Raïssac M, Tardieu F.1998. Elongation rate of sorghum leaves has a common response to meristem temperature in diverse African and European environmental conditions. Field Crops Research 58: 69–79. [Google Scholar]
- LauerJG, Simmons SR.1985. Photoassimilate partitioning of main shoot leaves in field‐grown spring barley. Crop Science 25: 851–855. [Google Scholar]
- LauerJG, Simmons SR.1988. Photoassimilate partitioning by tillers and individual tiller leaves in field‐grown spring barley. Crop Science 28: 279–282. [Google Scholar]
- McCreeKJ, Kallsen CE, Richardson SG.1984. Carbon balance of sorghum plants during osmotic adjustment to water stress. Plant Physiology 76: 898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MadakadzeI, Coulman BE, Stewart K, Peterson P, Samson R, Smith DL.1998. Phenology and tiller characteristics of big bluestem and switchgrass cultivars in a short growing season area. Agronomy Journal 90: 489–495. [Google Scholar]
- MajorDJ, Hamman WM, Rood SB.1982. Effects of short duration chilling temperature exposure on growth and development of sorghum. Field Crops Research 5: 129–136. [Google Scholar]
- Masle‐MeynardJ.1981. Elaboration du nombre d’épis d’un peuplement de blé d’hiver en situation de compétition pour l’azote. I. Mise en évidence d’un stade critique pour la montée d’une talle. Agronomie 1: 623–632. [Google Scholar]
- Masle‐MeynardJ, Sébillotte M.1981a Etude de l’hétérogénéité d’un peuplement de blé d’hiver. I. Notion de structure du peuplement. Agronomie 1: 207–216. [Google Scholar]
- Masle‐MeynardJ, Sébillotte M.1981b Etude de l’hétérogénéité d’un peuplement de blé d’hiver. II. Origine des différentes catégories d’individus du peuplement; éléments de description de sa structure. Agronomie 1: 217–224. [Google Scholar]
- MitchellKJ.1953. Influence of light and temperature on the growth of ryegrass (Lolium spp.). I. Pattern of vegetative development. Physiologia Plantarum 6: 21–46. [Google Scholar]
- MitchellRB, Moser LE, Moore KJ, Redfearn DD.1998. Tiller demographics and leaf area index of four perennial pasture grasses. Agronomy Journal 90: 47–53. [Google Scholar]
- MouliaB, Loup C, Chartier M, Allirand JM, Edelin C.1999. Dynamics of architectural development of isolated plants of maize (Zea mays L.), in a non‐limiting environment: the branching potential of modern maize. Annals of Botany 84: 645–656. [Google Scholar]
- NeildRE.1984. Agroclimatology of sorghum. The Americas. In: Virmani SM, Sivakumar MVK, eds. Agrometeorology of sorghum and millet in the semi‐arid tropics. Proceedings of the International Symposium, 15–20 November 1982, ICRISAT, Patancheru, India, 115–126.
- NemotoK, Morita S, Baba T.1995. Shoot and root development in rice related to the phyllochron. Crop Science 35: 24–29. [Google Scholar]
- OngCK, Marshall C.1979. The growth and survival of severely‐shaded tillers in Lolium Perenne L. Annals of Botany 43: 147–155. [Google Scholar]
- OngCK.1984. Response to temperature in a stand of pearl millet (Pennisetum typhoïdes S. & H.). V. Development and fate of tillers. Journal of Experimental Botany 35: 150, 83–90. [Google Scholar]
- PayneWA, Wendt CW, Hossner LR, Gates CE.1991. Estimating pearl millet leaf area and specific leaf area. Agronomy Journal 83: 937–941. [Google Scholar]
- PetersonCM, Klepper B, Rickman RW.1982. Tiller development at the coleoptilar node in winter wheat. Agronomy Journal 74: 781–784. [Google Scholar]
- PorterJR.1985. Approaches to modelling canopy development in wheat. In: Day W, Atkin RK, eds. Wheat growth modelling New York: Plenum Press, 69–81. [Google Scholar]
- SchnierM, Dingkuhn M, de Datta SK, Mengel K, Wijanco E, Javellana C.1990. Nitrogen economy and canopy carbon dioxide assimilation of tropical lowland rice. Agronomy Journal 82: 451–459. [Google Scholar]
- SeetharamaN, Mahalakshmi V, Bidinger FR, Singh S.1984. Response of sorghum and pearl millet to drought stress in semi‐arid India. In: Virmani SM, Sivakumar MVK, eds. Agrometeorology of sorghum and millet in the semi‐arid tropics. Proceedings of the International Symposium, 15–20 November 1982, ICRISAT, Patancheru, India, 159–173. [Google Scholar]
- SkinnerRH, Nelson CJ.1994. Role of leaf appearance rate and the coleoptile tiller in regulating tiller production. Crop Science 34: 71–75. [Google Scholar]
- WinkelT, Renno JF, Payne WA.1997. Effect of the timing of water deficit on growth, phenology and yield of pearl millet (Pennisetum glaucum (L.) R. Br.) grown in Sahelian conditions. Journal of Experimental Botany 48: 1001–1009. [Google Scholar]
- WuG, Wilson LT, McClung AM.1998. Contribution of rice tillers to dry matter accumulation and yield. Agronomy Journal 90: 317–323. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.