Abstract
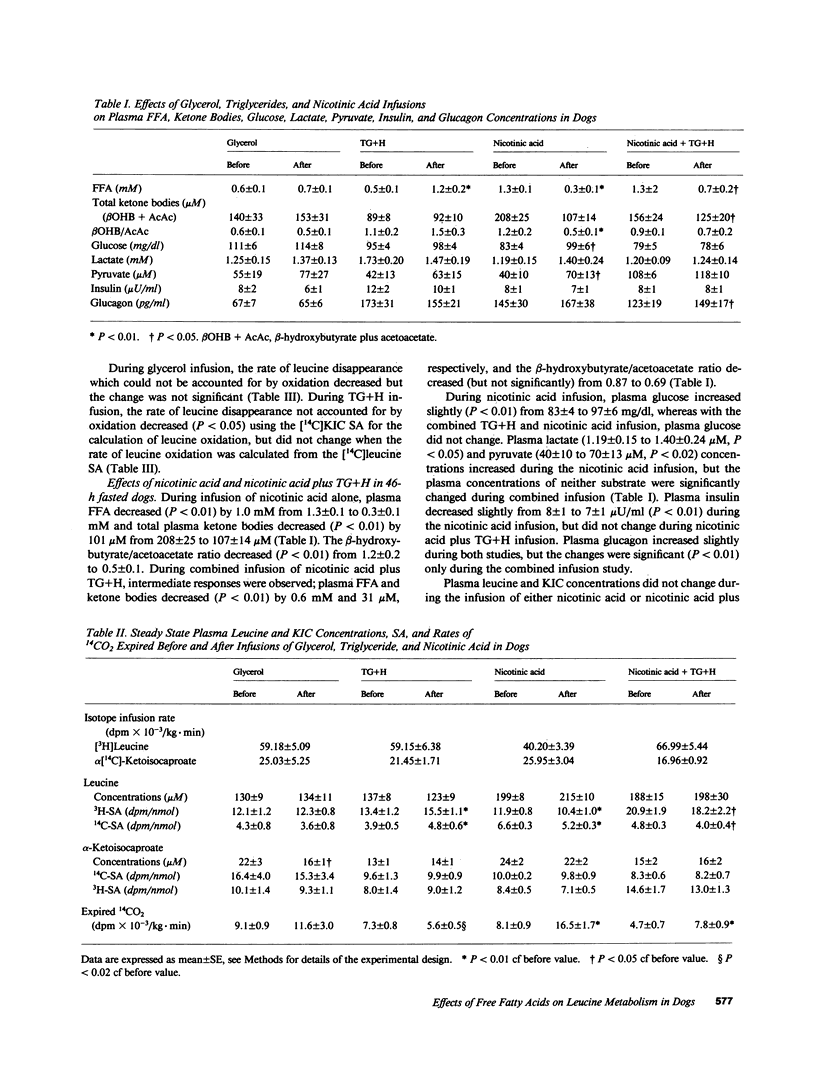
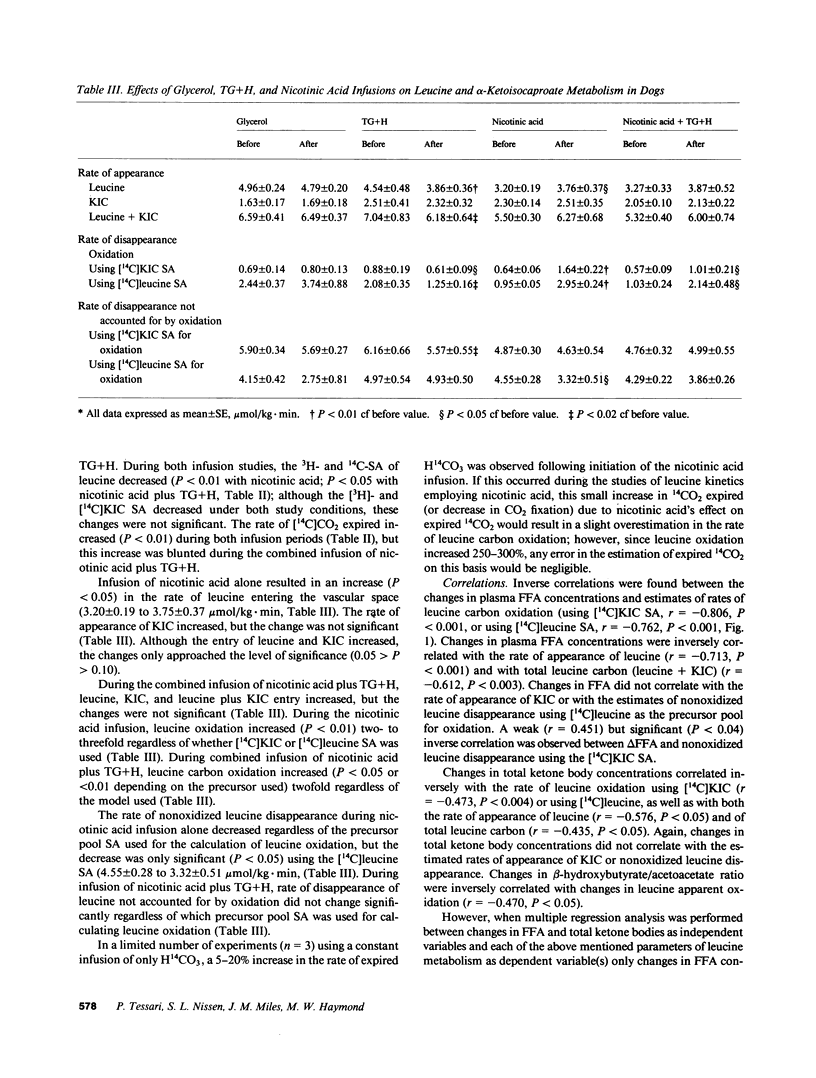
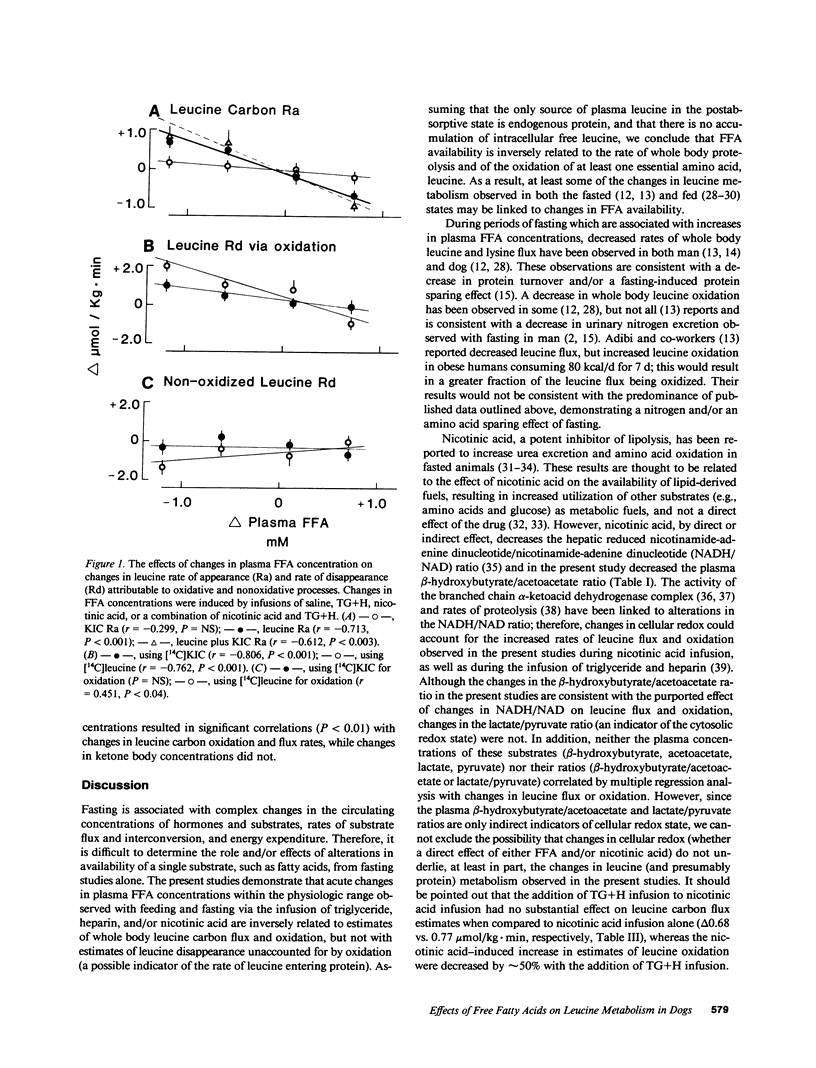
To determine the effect of fatty acid availability on leucine metabolism, 14-h fasted dogs were infused with either glycerol or triglyceride plus heparin, and 46-h fasted dogs were infused with either nicotinic acid or nicotinic acid plus triglyceride and heparin. Leucine metabolism was assessed using a simultaneous infusion of L-[4,5-3H]leucine and alpha-[1-14C]ketoisocaproate. Leucine, alpha-ketoisocaproate (KIC), and totalleucine carbon (leucine plus KIC) flux and oxidation rates were calculated at steady state. In 14-h fasted animals, infusion of triglyceride and heparin increased plasma free fatty acids (FFA) by 0.7 mM (P less than 0.01) and decreased leucine (P less than 0.01), total leucine carbon flux (P less than 0.02), and oxidation (P less than 0.05). The estimated rate of leucine utilization not accounted for by oxidation and KIC flux decreased, but the changes were not significant. During glycerol infusion, leucine and KIC flux and oxidation did not change. In 46-h fasted dogs, nicotinic acid decreased FFA by 1.0 mM (P less than 0.01) and increased (P less than 0.05) the rate of leucine and total leucine carbon flux, but did not affect KIC flux. Leucine oxidation increased (P less than 0.01) by nearly threefold, whereas nonoxidized leucine utilization decreased. Infusion of triglyceride plus heparin together with nicotinic acid blunted some of the responses observed with nicotinic acid alone. In that changes in oxidation under steady state condition reflect changes in net leucine balance, these data suggest that FFA availability may positively affect the sparing of at least one essential amino acid and may influence whole body protein metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Jefferson L. S., Rannels S. R., Williams P. E., Cherrington A. D., Lacy W. W. Role of insulin in the regulation of leucine kinetics in the conscious dog. J Clin Invest. 1982 Nov;70(5):1031–1041. doi: 10.1172/JCI110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi S. A., Stanko R. T., Morse E. L. Modulation of leucine oxidation and turnover by graded amounts of carbohydrate intake in obese subjects. Metabolism. 1982 Jun;31(6):578–588. doi: 10.1016/0026-0495(82)90096-8. [DOI] [PubMed] [Google Scholar]
- Allsop J. R., Wolfe R. R., Burke J. F. Tracer priming the bicarbonate pool. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):137–139. doi: 10.1152/jappl.1978.45.1.137. [DOI] [PubMed] [Google Scholar]
- Bistrian B. R., Sherman M., Young V. The mechanisms of nitrogen sparing in fasting supplemented by protein and carbohydrate. J Clin Endocrinol Metab. 1981 Oct;53(4):874–878. doi: 10.1210/jcem-53-4-874. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Friderici K. H., Buse J. F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972 Dec 25;247(24):8085–8096. [PubMed] [Google Scholar]
- Buse M. G., Herlong H. F., Weigand D. A. The effect of diabetes, insulin, and the redox potential on leucine metabolism by isolated rat hemidiaphragm. Endocrinology. 1976 May;98(5):1166–1175. doi: 10.1210/endo-98-5-1166. [DOI] [PubMed] [Google Scholar]
- CARLSON L. A., HAVEL R. J., EKELUND L. G., HOLMGREN A. EFFECT OF NICOTINIC ACID ON THE TURNOVER RATE AND OXIDATION OF THE FREE FATTY ACIDS OF PLASMA IN MAN DURING EXERCISE. Metabolism. 1963 Sep;12:837–845. [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Odessey R. Oxidation of amino acids by diaphragms from fed and fasted rats. Am J Physiol. 1972 Dec;223(6):1384–1391. doi: 10.1152/ajplegacy.1972.223.6.1384. [DOI] [PubMed] [Google Scholar]
- Golden M. H., Waterlow J. C. Total protein synthesis in elderly people: a comparison of results with [15N]glycine and [14C]leucine. Clin Sci Mol Med. 1977 Sep;53(3):277–288. doi: 10.1042/cs0530277. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Paxton R. Regulation of branched chain alpha-ketoacid dehydrogenase complex by phosphorylation-dephosphorylation. Minisymposium report. Fed Proc. 1985 Feb;44(2):305–315. [PubMed] [Google Scholar]
- Henson L. C., Heber D. Whole body protein breakdown rates and hormonal adaptation in fasted obese subjects. J Clin Endocrinol Metab. 1983 Aug;57(2):316–319. doi: 10.1210/jcem-57-2-316. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproate metabolism in skeletal muscle. J Biol Chem. 1978 Nov 25;253(22):8126–8133. [PubMed] [Google Scholar]
- Lundberg D. A., Nelson R. A., Wahner H. W., Jones J. D. Protein metabolism in the black bear before and during hibernation. Mayo Clin Proc. 1976 Nov;51(11):716–722. [PubMed] [Google Scholar]
- Matthews D. E., Motil K. J., Rohrbaugh D. K., Burke J. F., Young V. R., Bier D. M. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980 May;238(5):E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Schwarz H. P., Yang R. D., Motil K. J., Young V. R., Bier D. M. Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982 Nov;31(11):1105–1112. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Nissen S. L., Rizza R. A., Gerich J. E., Haymond M. W. Failure of infused beta-hydroxybutyrate to decrease proteolysis in man. Diabetes. 1983 Mar;32(3):197–205. doi: 10.2337/diab.32.3.197. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Motil K. J., Bier D. M., Matthews D. E., Burke J. F., Young V. R. Whole body leucine and lysine metabolism studied with [1-13C]leucine and [alpha-15N]lysine: response in healthy young men given excess energy intake. Metabolism. 1981 Aug;30(8):783–791. doi: 10.1016/0026-0495(81)90024-x. [DOI] [PubMed] [Google Scholar]
- Motil K. J., Matthews D. E., Bier D. M., Burke J. F., Munro H. N., Young V. R. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol. 1981 Jun;240(6):E712–E721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- Nissen S. L., Van Huysen C., Haymond M. W. Measurement of branched chain amino acids and branched chain alpha-ketoacids in plasma by high-performance liquid chromatography. J Chromatogr. 1982 Oct 8;232(1):170–175. doi: 10.1016/s0378-4347(00)86021-1. [DOI] [PubMed] [Google Scholar]
- Nissen S., Haymond M. W. Effects of fasting on flux and interconversion of leucine and alpha-ketoisocaproate in vivo. Am J Physiol. 1981 Jul;241(1):E72–E75. doi: 10.1152/ajpendo.1981.241.1.E72. [DOI] [PubMed] [Google Scholar]
- Rüdiger H. W., Langenbeck U., Goedde H. W. A simplified method for the preparation of 14 C-labelled branched-chain -oxo acids. Biochem J. 1972 Jan;126(2):445–446. doi: 10.1042/bj1260445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk W. F., Beaufrere B., Haymond M. W. Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol. 1985 Dec;249(6 Pt 1):E646–E650. doi: 10.1152/ajpendo.1985.249.6.E646. [DOI] [PubMed] [Google Scholar]
- Stacey-Schmidt C., Berg P., Haymond M. W. Use of D-glucosaminic acid as an internal standard in single-column accelerated amino acid analysis of physiological fluids. Anal Biochem. 1982 Jun;123(1):74–77. doi: 10.1016/0003-2697(82)90624-8. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Fagan J. M. Relationship of the reduction-oxidation state to protein degradation in skeletal and atrial muscle. Arch Biochem Biophys. 1982 Aug;217(1):191–201. doi: 10.1016/0003-9861(82)90493-3. [DOI] [PubMed] [Google Scholar]
- Trout D. L., Bitter H. L., Lackey W. W. Effects of nicotinic acid on the choice of substrates utilized during fasting. Biochem Pharmacol. 1967 Jun;16(6):971–978. doi: 10.1016/0006-2952(67)90269-9. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Wałajtys-Rode E., Coll K. E. Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. I. Regulation of branched chain alpha-ketoacid metabolism. J Biol Chem. 1979 Nov 25;254(22):11511–11520. [PubMed] [Google Scholar]
- Wolfe R. R., Goodenough R. D., Wolfe M. H., Royle G. T., Nadel E. R. Isotopic analysis of leucine and urea metabolism in exercising humans. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):458–466. doi: 10.1152/jappl.1982.52.2.458. [DOI] [PubMed] [Google Scholar]
- Young V. R., Havenberg L. N., Bilmazes C., Munro H. N. Potential use of 3-methylhistidine excretion as an index of progressive reduction in muscle protein catabolism during starvation. Metabolism. 1973 Nov;23(2):1429–1436. doi: 10.1016/0026-0495(73)90257-6. [DOI] [PubMed] [Google Scholar]


