Abstract
Females of Thalictrum pubescens produce stamens that contain sterile pollen, whereas males are both functionally and morphologically unisexual. This study examines the investment in stamen production by females of T. pubescens by comparing the female structures with those of their fully functional male counterparts. Stamens from females had the same biomass and contained the same amount of nitrogen and phosphorus as stamens from males. Anther size was the same in males and females, but filaments were longer in stamens from males. Females produced more pollen per anther than males, and pollen size was the same in both sexes. Within flowers, there was a positive correlation between the amount of pollen per anther and the length of anthers in males, but not in females. This would be expected if males growing in better environmental conditions or with greater vigour invested more resources in pollen production, thereby increasing fitness. Females, who receive no fitness benefits from increased pollen production, did not show this pattern. There was also evidence of a trade‐off within female flowers between the number of stamens and the number of pistils. This trade‐off was noted in conditions when variance among plants was reduced, namely in the field during a year when flower size was particularly small and in a previous glasshouse study. Therefore, it appears that when environmental variance is low, stamens are produced at the expense of producing more pistils, and hence seeds. In conclusion, stamen production does not appear to be inconsequential to females of Thalictrum pubescens.
Key words: Thalictrum pubescens, tall meadow rue, sex allocation, trade‐offs, cryptic dioecy, floral traits, sexual dimorphism
INTRODUCTION
One advantage of gender dimorphism over hermaphroditism that has long been proposed is the advantage that unisexual plants may gain over hermaphrodites by specializing in one sexual role (Darwin, 1877). Unisexuals are able to allocate the resources previously spent on the opposite sexual role more efficiently and, consequently, increase fitness. The complete separation of sexes, or dioecy, is expected to evolve when fitness gain curves accelerate as resource investment in one sexual function increases (Charnov et al., 1976). Similar resource use between the two sexual functions and intersexual competition for space and nutrients are among the factors that may contribute to this condition being met (Charnov et al., 1976; Maynard Smith, 1978; Bawa, 1980; Givnish, 1980). Even if phenotypic dimorphism is low when dioecy first arises, once dioecy has been established, further changes in resource allocation patterns are expected to occur because of sex‐specific selection (either sexual or natural selection), increasing differences between the sexes (Lloyd, 1977; Willson, 1979; Cox, 1981; Bell, 1985; Meagher, 1994; Ashman, 2000). These differences need not be adaptive in nature and may arise as a consequence of physical reproductive differences between the sexes or correlated responses to selection on other traits within one sex (Geber, 1995; Delph et al., 1996; Elle, 1998; Ashman, 1999). However, dioecy has evolved several times with at least one of the sexes maintaining full‐size sexual organs of the opposite sex, making them appear hermaphroditic whilst still being functionally unisexual. This breeding system is known as cryptic dioecy (Mayer and Charlesworth, 1991). The existence of cryptic dioecy appears to contradict the hypothesis of a critical role for the reallocation of resources in the evolution of separate sexes.
A major assumption, explicitly underlying theoretical models of sex allocation, is the existence of a fixed amount of resources available for reproduction that can be allocated in various ways among the different sexual functions (Charlesworth and Morgan, 1991). The type of resources devoted to reproduction may vary. For example, plants may invest resources that are physiological in nature, such as nutrients or photosynthate, into the production of sexual organs (Ashman and Baker, 1992). In addition, the production of reproductive structures of one sex may also impose developmental constraints on the production of reproductive structures of the other sex. For example, the number of components within each flower may be set, so that tranforming one part to a male structure precludes the production of a female structure (Geber, 1990; Bonser and Aarssen, 1996). A consequence of a fixed amount of resources would be that as resource investment in one sexual function increases, investment in the other sexual function would necessarily decrease, generating trade‐offs among sexual traits. For example, when Mazer et al. (1999) selected for increased ovule number in Spergularia marina, there was a correlated decrease in the number of anthers per flower. Such trade‐offs should further speed the evolution of sexual dimorphism.
One hypothesis, termed the Ancestry Hypothesis, which accounts for the maintenance of sterile organs in cryptically dioecious species states that the organs remain as vestiges of the ancestral state (Mayer and Charlesworth, 1991; Davis, 1997). In this case, selection is too weak to reduce the structures because the expense to the plant of producing the structures is very small or their resource requirements are non‐overlapping with other functions (Mayer and Charlesworth, 1991; Davis, 1997). Therefore, their development does not pull resources away from other uses within the flower. Although the sterile organs in some cryptically dioecious plants appear to be identical to the functional organs of the opposite sex, there may be subtle differences between them. For example, anthers of females may contain fewer pollen grains, or the amount of limiting resources, such as nitrogen or phosphorus, allocated to each anther may be lower, reducing conflicts with other functions (Mayer and Charlesworth, 1991). Alternatively, a lack of such differences between the sexes would suggest that natural selection should act to reallocate the resources more productively by reducing or removing sterile organs.
The primary goal of this investigation is to examine resource allocation to floral structures in the cryptically dioecious plant, Thalictrum pubescens, and hence to examine the role of the Ancestry Hypothesis in the maintenance of cryptic dioecy in this species. Females of this species produce stamens, thereby appearing to be hermaphroditic. Female floral structures are compared with those of their male counterparts, and resource allocation within female flowers is examined, including (1) investment of resources such as biomass, nitrogen, and phosphorus; and (2) developmental constraints such as possible meristem limitation. Thalictrum pubescens is at least partially wind pollinated so may not need to maintain resource allocation to pollen as a nutritive attractant to pollinators (Melampy and Hayworth, 1980). Davis (1997) has shown that stamens are unnecessary for successful pollination of T. pubescens females. In this study, female flowers that had sterile stamens removed did not set significantly different levels of seed compared with unmanipulated female flowers. Bawa (1980) and Givnish (1980) point out that pollination by wind or generalist insects is relatively inefficient, requiring a large input of resources for pollen production, and hence paternal costs are high. This may give females a particularly large benefit from reallocating paternal expenses. In addition, females produce several uniovulate pistils per flower, each of which develops into an achene fruit with one seed. Any reduction in the number of pistils will therefore lead to a direct reduction in the possible number of seeds each flower can produce.
MATERIALS AND METHODS
Study organism
Thalictrum pubescens (also known as T. polygamum; Park, 1992) is a summer‐flowering perennial that grows in rich woods, low thickets, swamps, wet meadows and stream banks (Keener, 1976). In this study, a population of T. pubescens located in a small, unused privately owned field adjacent to Fort Hill State Historical Park in Highland county near Hillsboro, OH, USA, was used. Females of T. pubescens produce pistillate flowers with numerous pistils and a very variable number of stamens. Each pistil has an uniovulate ovary. Each stamen from female flowers contains pollen that is inaperturate (Kaplan and Mulcahy, 1971), and hence cannot germinate when placed on Kwack’s media, whereas pollen from males germinates readily within 2–3 h (S. L. Davis pers. obs.). Stamens of females fall off before the fruit matures. Males produce only staminate flowers with stamens and no vestigial pistils. Flowers of both sexes lack petals and nectaries. Sepals are white, relatively small compared with the stamens and pistils, and fall off soon after the flower matures. The species is partially wind pollinated (Boivin, 1944; Kaplan and Mulcahy, 1971), with the primary insect visitors being Syrphid flies. Sex determination in dioecious Thalictrum spp. appears to be under nuclear control with males serving as the heterogametic sex (reviewed in Meagher, 1988).
Collection of flower material
Data were gathered both from naturally occurring field‐collected flowers and from plants grown from seed in a glasshouse.
Field‐collected flowers.
One to three flowers were collected from each of 50 females and 50 males randomly selected every year between 1993–1995 at the Hillsboro field site (except 1995, when flowers from 95 males were collected; see below). An additional collection of 25 female flowers was made in 1996. All flowers were preserved in 70 % alcohol and taken to Indiana University, USA, for morphological measurements.
In 1995, one flower from each of 95 males and 50 females was collected. One anther from each flower was removed, stored separately in a dry glass vial, and allowed to dehisce for pollen measurements.
Glasshouse‐grown plants.
Seeds were collected from 50 maternal plants growing in the Hillsboro field site in 1994. These seeds were taken to Indiana University where they were planted in shallow plastic trays filled with moist Metromix™ and stored at 4 °C for 6 weeks. They were then removed from the cold and placed in a mist room of the Indiana University glasshouse where they germinated. Germination took 10–21 d. After a further 6 weeks, ten to 15 seedlings from each maternal line that germinated were planted in individual 10 cm pots and placed in a glasshouse at the Botanical Experimental Field Station on the Indiana University campus. After 2 months, plants were transplanted to 20 cm pots and allowed to flower. The first and second flowers from each plant that bloomed were collected. One anther from each flower was placed separately in a clean, dry glass vial and allowed to dehisce for pollen measurements. The remainder of the flower was preserved in 70 % alcohol for morphological measurements. A total of 22 maternal lines had at least one male and one female flower.
Two additional fresh flowers were collected from each plant and taken to the laboratory where they were each dissected into stamens, pistils and sepals. These organs were used for biomass and nutrient concentration measurements.
Data collection
The size of the various floral organs from both field‐collected and glasshouse‐grown plants was estimated by measuring the length of each part using a micrometer under 8× magnification. One micrometer unit is equivalent to 1·25 mm, and measurements were taken to the nearest one‐tenth of a micrometer unit. Measurements taken included: number of stamens, number of pistils, anther length, filament length, ovary length, stigma length and sepal length.
The amount of pollen per anther and the size of the pollen grains were measured using an Elzone 280PC Particle Counter (Micromeritics Instruments Corporation, Norcross, GA, USA). Each anther collected in a clean, dry vial was allowed to dehisce. After the anther had dehisced, 15 ml of 1·5 % filtered saline was added to the vial. The Elzone counts the number of particles in 0·5 ml samples and gives a histogram of the size of the particles in each sample. The total amount of pollen in one anther was calculated as the mean number of pollen grains in three 0·5 ml samples multiplied by 30.
Biomass of organs and nutrient concentration within each organ type were measured using the two flowers that were collected from each plant growing in the glasshouse. Each flower was dissected into its various organs and floral parts were then oven‐dried for at least 48 h at 35 °C and weighed to 1 µg using a Sartorius microbalance. The dried floral parts were analysed for both their nitrogen and phosphorus content (one flower was used for each), following the procedures of Ashman and Baker (1992). In brief, the separated and dried parts were digested over a heating block in concentrated H2SO4. To determine nitrogen content, each sample was combined with 250 µl 10 % NaOH, 750 µl H2O and 50 µl undiluted Nesslers Ammonia colour reagent. The absorbance of each solution was read at 425 nm in a spectrophotometer. Nitrogen content was then determined by comparing the absorbance of the solution with a standard curve generated by preparing a series of solutions of known concentration. To determine phosphorus content, each sample was combined with 500 µl distilled H2O and 500 µl Reagent C. The absorbance of each solution was read at 820 nm and nitrogen content was calculated by comparing this absorbance with a standard curve generated by preparing a series of solutions of known concentration.
Statistical analysis
All statistical analyses were performed using the statistical program SPSS version 6·1 for the Macintosh.
A mixed‐model ANOVA was used to test for differences in anther and sepal size between males and females of field‐collected flowers, with anther length and sepal length as dependent variables, sex as a fixed effect and year as a random effect. Transformation did not help filament length data meet the requirements of ANOVA, so a Mann–Whitney U‐Wilcoxon Rank Sum test was used to test for differences between the sexes in this variable. The resulting probabilities were then multiplied by 3 to correct for multiple tests.
Differences in anther and filament size between males and females of glasshouse‐grown plants were tested using independent samples t‐tests. Dry mass per organ, nitrogen concentration and content, and phosphorus concentration and content for male stamens, female stamens and pistils were analysed using a one‐way ANOVA and paired orthogonal contrasts. The contrasts first tested for differences between male and female stamens and then compared stamens with pistils. Dry mass per organ was log‐transformed to meet the assumption of equality of variances.
The relationship between stamen number and pistil number within female flowers was examined using Pearson‐product moment correlation coefficients. For the field‐collected flowers, a separate correlation was calculated each year. For the glasshouse‐grown plants, different numbers of female plants flowered per family, so a Pearson‐product moment correlation coefficient was calculated using the means of maternal lines to control for maternal effects.
The amount of pollen in male vs. female anthers and the size of male vs. female pollen grains were analysed using independent samples t‐tests. The number of pollen grains per anther was log‐transformed to meet the assumption of equality of variances. Correlations between pollen content and anther size and stamen number were examined with Pearson‐product moment correlation coefficients.
RESULTS
Stamens from males vs. stamens from females
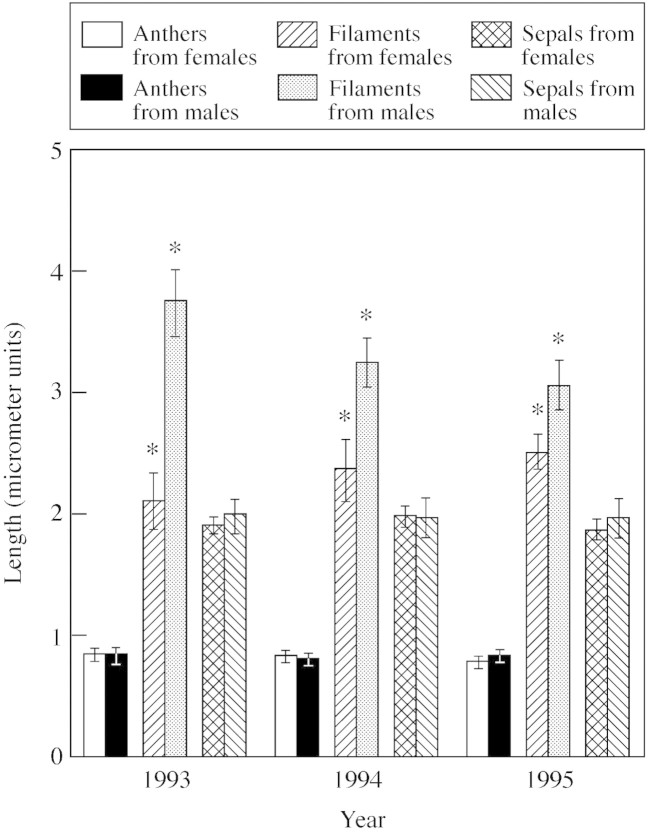
Females produced significantly fewer stamens than males in both field‐collected and glasshouse‐grown plants (Table 1). However, few differences were found in the size of floral traits common to both males and females. This was true for both field‐collected and glasshouse‐grown plants (Table 1). Stamens from males were slightly longer than those from females. This difference resulted solely from a difference in filament length. For flowers collected from the field, filaments were significantly longer in males in all years (Table 2). Anther length was the same for males and females each year (Fig. 1; Table 2). Results were the same for plants grown in the glasshouse (Table 1). With respect to overall stamen size, the anther probably represents the majority of investment in the stamen, as the filament is a thin, white strand supporting the anther. Sepal size was also the same in males and females (Fig. 1).
Table 1.
Means (s.e.) of floral traits of field‐collected and glasshouse‐grown plants of Thalictrum pubescens
| Field‐collected flowers | Glasshouse‐grown flowers | |||||
| Male stamen | Female stamen | Pistil | Male stamen | Female stamen | Pistil | |
| Number per flower | 38·19a (0·91) | 7·90b (0·21) | 12·44 (0·24) | 41·04a (2·26) | 9·32b (0·95) | 18·00 (1·49) |
| n = 123 | n = 168 | n = 168 | n = 25 | n = 22 | n = 22 | |
| Size (length) | Anther: 0·81 (0·01)a | 0·80 (0·01)a | Stigma: 1·19 (0·02) | 0·91 (0·03)a | 0·88 (0·02)a | 1·42 (0·05) |
| Filament: 3·19 (0·07)a | 2·32 (0·06)b | Ovary: 1·59 (0·02) | 4·31 (0·16)a | 3·78 (0·19)b | 1·90 (0·10) | |
| 123 | 168 | 168 | 25 | 22 | 22 | |
| Pollen grains per anther | 4128·38a | 5368·83b | NA | 3350·67a | 4162·26b | NA |
| 93 | 48 | 15 | 17 | |||
| Pollen size (µm) | 30·27 (0·35)a | 30·1196a | NA | 33·34 (0·94)a | 33·37 (0·31)a | NA |
| 37 | 24 | 15 | 17 | |||
| Mass per organ (µg) | 0·043 (0·002)a | 0·045 (0·002)a | 0·104 (0·008)b | |||
| Total per flower (µg) | 1·76 (0·11) | 0·48 (0·05) | 1·76 (0·23) | |||
| n = 28 | 30 | 31 | ||||
| Nitrogen concentration (µg mg–1) | 38·19 (1·57)a | 53·12 (12·48)a | 50·53 (2·73)a | |||
| 1·68 (0·10)a | 2·39 (0·44)a | 4·59 (0·44)b | ||||
| Total per organ (µg) | n = 20 | 21 | 21 | |||
| Phosphorus concentration (µg mg–1) | 6·90 (0·41)a | 7·99 (1·53)a | 8·87 (0·52)a | |||
| 0·30 (0·02)a | 0·38 (0·07)a | 0·78 (0·06)b | ||||
| Total per organ (µg) | 20 | 21 | 21 | |||
Size measurements for field‐collected flowers were available from several years, so these data were analysed using a mixed model ANOVA, the results of which are shown in Table 2.
Data for size and number of pollen grains per anther were analysed with independent samples t‐tests.
Traits within a half‐row that are significantly different at the P = 0·05 level are indicated by different superscripts.
Table 2.
Statistical analysis for stamen and sepal characters in males vs. females
| Variable | Factor | d.f. | MS | P |
| Anther length | Sex | 1 | <0·01 | 0·775 |
| Year | 2 | 0·03 | 0·217 | |
| Sex × year | 2 | 0·04 | 0·092 | |
| Error | 264 | 0·02 | ||
| Filament length | Sex: year 1 | n = 70 | <0·0003 | |
| Sex: year 2 | n = 104 | <0·0003 | ||
| Sex: year 3 | n = 95 | 0·0003 | ||
| Sepal length | Sex | 1 | 0·15 | 0·219 |
| Year | 2 | 0·06 | 0·504 | |
| Sex × year | 2 | 0·05 | 0·546 | |
| Error | 191 | 0·08 |
Anther length and sepal length were both tested using a mixed‐model ANOVA with sex as a fixed effect and year as a random effect.
Transformation did not help filament length meet the requirements of ANOVA, so differences between the sexes was tested for each year using a Mann–Whitney U‐Wilcoxon Rank Sum test. The resulting probabilities were then multiplied by 3 to correct for multiple tests.
Fig. 1. Mean (± 2 s.e.) size of floral parts from females and males in Thalictrum pubescens. Asterisks indicate significant differences at the 0·05 level between pairs of characters. One micrometer unit is equivalent to 1·25 mm.
No significant difference was detected between males and females of glasshouse‐grown plants in the mass of individual stamens (Table 1). In addition, there was no difference in the concentration of nitrogen or phosphorus between the two stamen types. Consequently, the total amount of nitrogen and phosphorus invested per stamen by males and females was equivalent (Table 1). Females produced more pollen per anther than males in both the field and the glasshouse (Table 1), but because males produced more stamens than females, total pollen production per flower was higher in males. Pollen grains did not differ significantly in size (Table 1).
Within female flowers
Data from glasshouse‐grown plants indicate that approx. 27 % of the biomass of a female flower that is devoted to sexual function is used to produce stamens. Each individual stamen is less than half the mass of one pistil (0·045 mg vs. 0·104 mg, respectively), with each flower having, on average, 9·32 stamens and 18·00 pistils (Table 1).
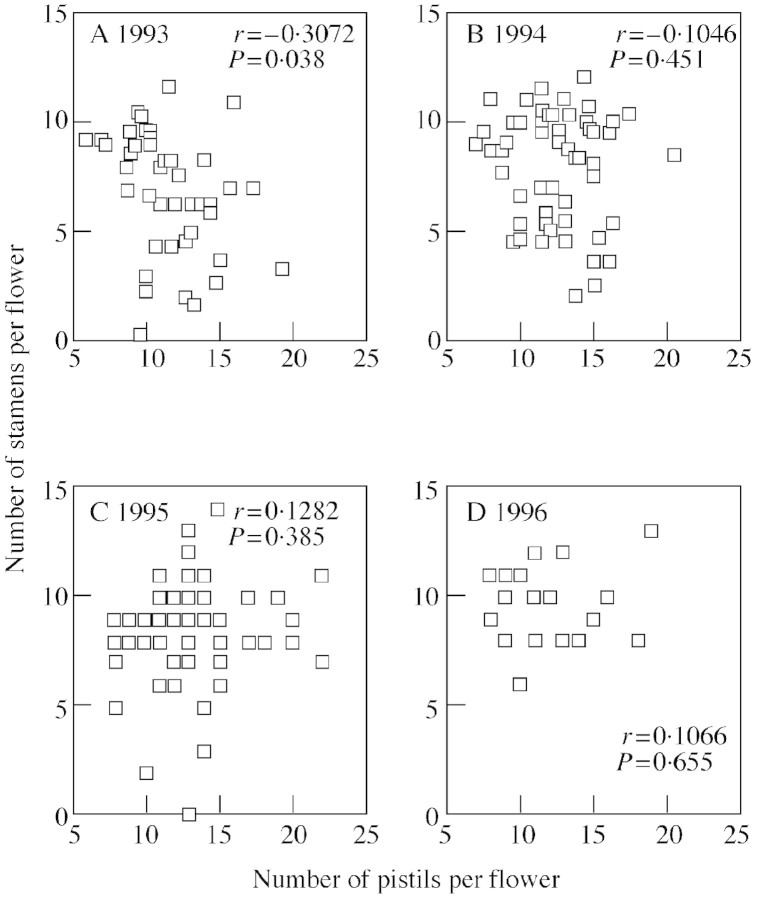
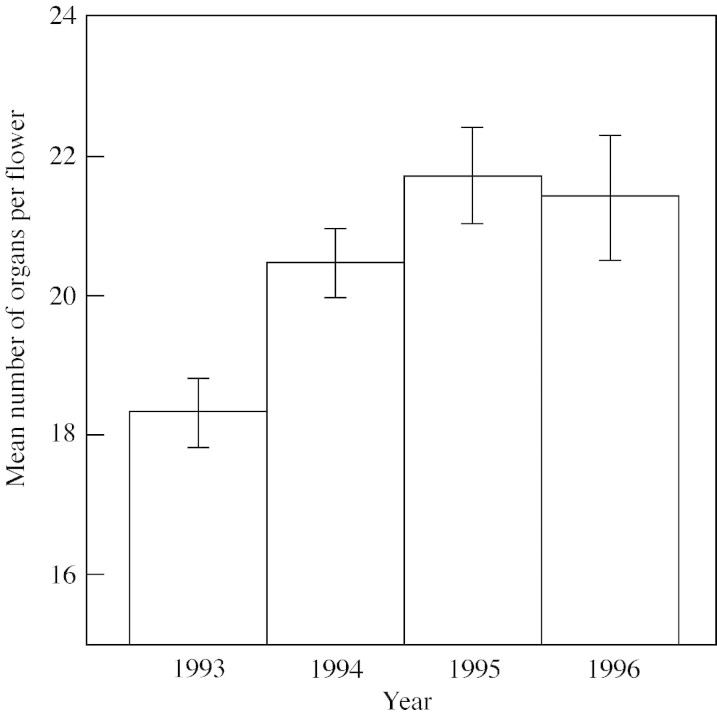
Data collected from 46 females during the first field season (1993) indicated a significant negative phenotypic correlation between stamen number and pistil number (Fig. 2A), suggesting a trade‐off between stamen production and potential seed production. However, this correlation was not significantly different from zero in any other year (Fig. 2B–D). Furthermore, if the significance level is adjusted for multiple tests by dividing the probability level of each year by four (the number of years tested), the probability level for 1993 also drops below significance. Nevertheless, there is reason to note the evidence of a trade‐off in 1993. Flowers in 1993 had significantly fewer reproductive organs than in other years (Fig. 3), indicating that resource limitation may have decreased the variance between plants, emphasizing the trade‐off found in this year.
Fig. 2. Relationship between the number of stamens and the number of pistils in female flowers of Thalictrum pubescens in 4 different years. Only in 1993 is there a significant correlation.
Fig. 3. Mean number of stamens and pistils (±1 s.e.) produced by females. Data were analysed using one‐way ANOVA, followed by a Tukey’s HSD posthoc comparison. 1993 was significantly different from all other years.
Pollen production in males vs. females
Pollen production per anther was positively correlated with anther length in males, but not in females (Fig. 4). Conversely, there was a statistically non‐significant trend for pollen production per anther to be negatively correlated with stamen number per flower in females, yet no relationship between these variables was found in males (Fig. 5).
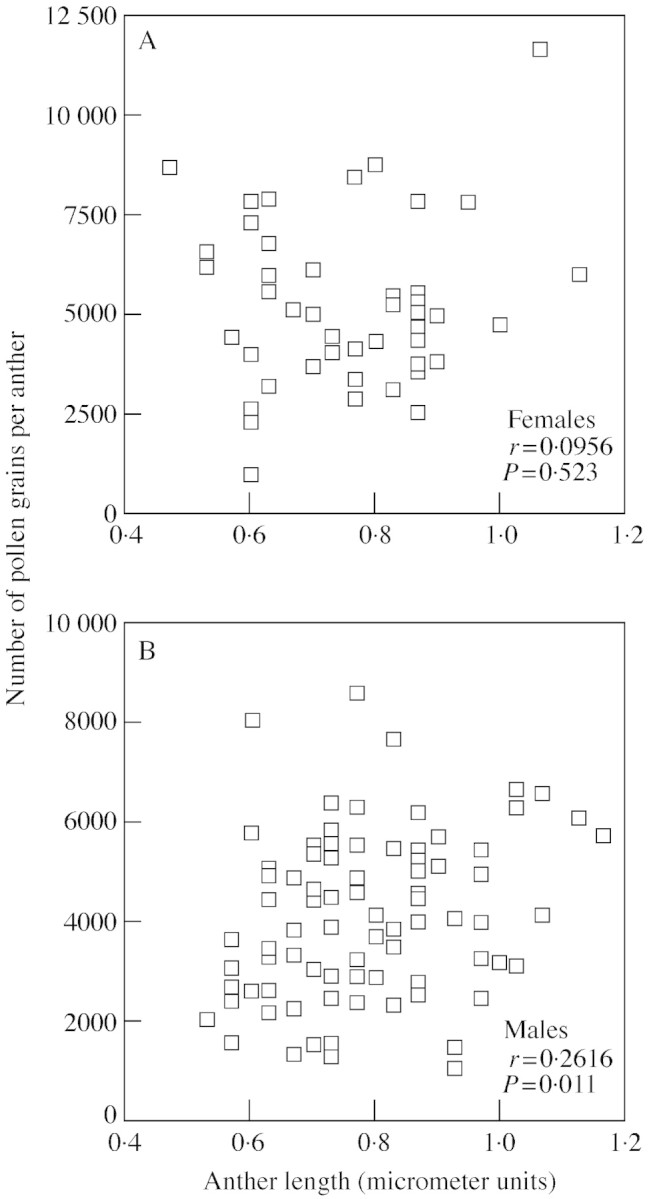
Fig. 4. Relationship between pollen production and mean anther size in females (A) and males (B). One micrometer unit is equivalent to 1·25 mm.
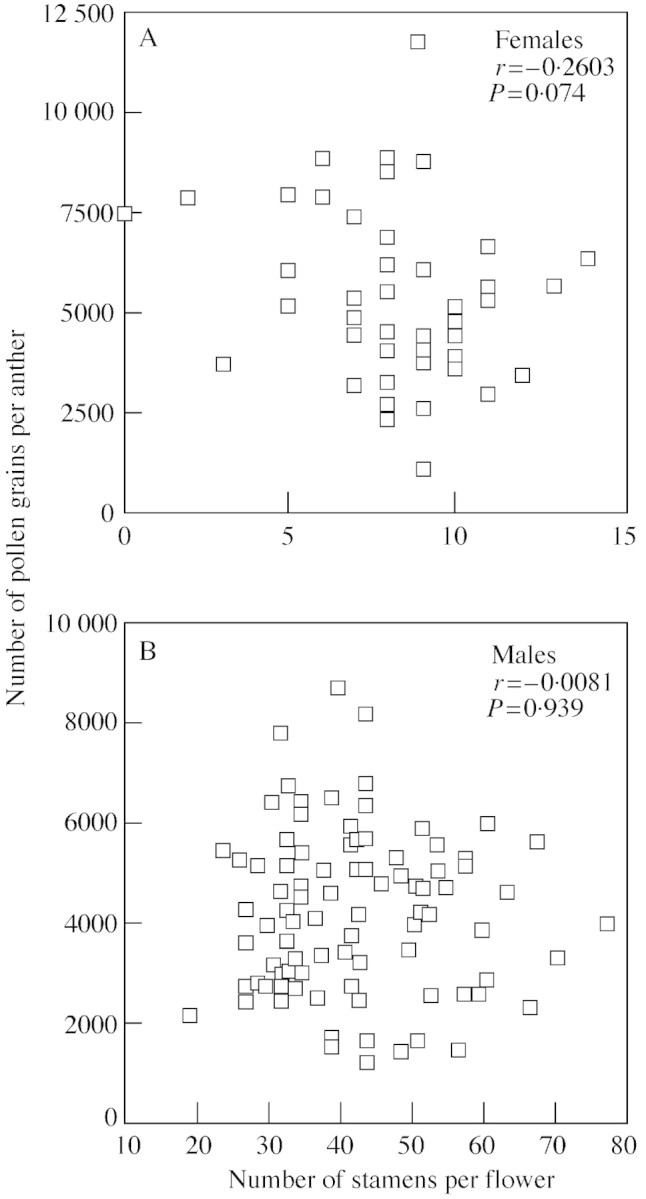
Fig. 5. Relationship between mean stamen number per flower and the number of pollen grains produced per anther in females (A) and males (B). Note that males produce many more stamens than females, and the x‐axes therefore have different scales. One micrometer unit is equivalent to 1·25 mm.
DISCUSSION
The results indicate that very little dimorphism between male and female stamens has evolved since the separation of the sexes. Individual stamens have the same mass in both sexes and contain the same amounts of nitrogen and phosphorus. Given these results and the delicate structure of the filament, the difference in filament length probably does not reflect a substantial difference in resource investment. Female anthers are the same length and contain even more pollen than those produced by males. It is, however, impossible to say whether females have increased their pollen production per anther or whether that of males has decreased since the separation of the sexes.
Some floral characters are photosynthetic and thus contribute carbon to their own production (Bazzaz et al., 1979; Galen et al., 1993; Antlfinger and Wendel, 1997). In this case it is unlikely that stamens are able to contribute to their own production. The filaments are white and consequently non‐photosynthetic. Pistils may contribute to the expense of seed production as they turn green as they mature into fruits. However, stamens have typically fallen off the flower at this point, so any extra photosynthate produced by pistils could not be diverted to stamen production.
Data collected from females in 1993 indicate the existence of a trade‐off between stamen number and pistil number. This negative correlation was significant before the correction for tests over multiple years. This suggests that females maintain stamen production at the expense of possible increased pistil production. Since each pistil may develop into one seed, decreasing pistil production directly reduces the number of seeds that can be produced. However, this correlation was not detectable in any of the following years. Negative correlations such as this are notoriously difficult to detect. Differences in overall plant quality (due to microsite differences or genetic differences at resource allocation loci) tend to skew such correlations between traits in a positive direction, as high quality plants are bigger overall and plants of lesser quality are smaller overall, hence masking possible negative correlations (Charlesworth and Morgan, 1991; Houle, 1991; O’Neil and Schmitt, 1993; Mazer and Dawson, 2001). There is evidence that this may be occurring in T. pubescens. Flowers in 1993 were significantly smaller, as measured by the number of organs per flower, than in other years, possibly reducing the effects of plant quality. Resources may have been limiting in that year, or possibly in the previous year as the plants are perennial. Therefore, although a trade‐off was found only in 1 year, it may still be biologically important when resources are low. Further evidence that environmental factors can mask this trade‐off is provided by a glasshouse study in which a significant negative phenotypic correlation between stamen number and pistil number was detected (Davis, 2001). Differences in microsite quality should also be reduced in a glasshouse. However, in this case, plant growth should not have been limited by water or nutrients and, as would be expected in better environmental conditions, the glasshouse‐grown plants had bigger flowers than those of field‐collected plants. With the reduction in environmental variance, the trade‐off between stamens and pistils could be seen.
Taken together, the field and previous glasshouse correlations indicate that females produce flowers of a certain size, with a certain number of organs within each flower, depending on the amount of resources available. The use of an organ primordium within the flower to produce a stamen reduces the number available for pistil production, and therefore reduces seed production. This is analogous to results found in Cucurbita foetidissima. Kohn (1989) concluded that there was a trade‐off between male and female function in terms of floral meristems. By not making male flowers, female plants were able to free more meristems for pistillate flowers. Because T. pubescens produces many female and male sex organs per flower, this same type of trade‐off is found on a within‐flower basis. This trade‐off occurs on the level of part number despite the large difference in mass of stamens and pistils. Female reproduction may be more resource‐limited than stamen production. Glasshouse‐grown females produced 45 % more pistils than field‐grown females (12·44–18·00 pistils per flower), but only 18 % more stamens (7·90–9·32 stamens per flower). The fact that the trade‐off could only be detected in 1 of 4 years in the field is particularly interesting. This may mean that the production of stamens in females only reduces pistil production when resources are low. During these times, however, it would be predicted that natural selection should act against stamen production in favour of pistil production and possible seed production.
The production of significantly more pollen (which was the same size in both sexes) per anther in females compared with males was an unexpected result. However, this can be explained if the hermaphroditic ancestors of T. pubescens (before the separation of sexes) were indeed constrained in the number of reproductive parts they could produce, as seen in females today. Selection may have acted to maximize the number of functional pollen grains produced by an anther of a given size. As males ceased to produce pistils, they could produce more pollen by using the resources freed from pistil production to make (1) larger anthers with more pollen or (2) more anthers per flower. In support of this hypothesis, there is no relationship between anther size and pollen production per anther in females, yet a positive correlation does exist between these variables in males. This would be expected if males of greater vigour invested more in pollen production, thereby increasing fitness. Females, who would receive no fitness benefits from increased pollen production since their pollen is sterile, do not show this pattern. Conversely, there is also a trend for a negative correlation between stamen number and pollen grains per anther in females that is not present in males. If there are resource constraints within female flowers, females that produce more anthers may have a lower maximum number of pollen grains they can produce in each anther, thereby creating the observed correlation.
The results indicate that the Ancestry Hypothesis cannot account for the maintenance of stamens in females of T. pubescens. Individual stamens appear to receive the same level of investment in both sexes. In addition, females produce stamens by sacrificing ovule production and hence possible seed production, particularly when resources are limiting. It cannot therefore be said that stamen production is inconsequential to the female plant. It has also been shown that the production of stamens does not enhance female fitness through increased pollination (Davis, 1997). When such reproductive organs lose their primary function, it has typically been thought that unless they assume a new function (such as in pollination), these organs quickly tend to be lost (Walker‐Larsen and Harder, 2001). Another possible reason for the maintenance of stamens in females in this case is that females are genetically constrained to produce stamens because of positive, between‐sex correlations among floral traits (Davis, 2001).
ACKNOWLEDGEMENTS
I thank Lynda Delph, Edmund Brodie III, Harold Lindman and Loren Rieseberg for guidance and comments on this work. Special thanks to D. J. McClellan, E. Levri, M. Levri, D. Dudle and J. Walgenbach for help planting and transplanting Thalictrum. The glasshouse staff at Indiana University helped maintain the plants. This work was supported through funds from The Indiana Academy of Sciences and The Department of Biology at Indiana University.
Supplementary Material
Received: 14 January 2002; Returned for revision: 4 March 2002; Accepted: 2 April 2002
References
- AntlfingerAE, Wendel LF.1997. Reproductive effort and floral photosynthesis in Spiranthes cernua (Orchidaceae). American Journal of Botany 84: 769–780. [PubMed] [Google Scholar]
- AshmanTL.1999. Quantitative genetics of floral traits in a gynodioecious wild strawberry, Fragaria virginiana: implications for the independent evolution of female and hermaphrodite floral phenotypes. Heredity 83: 733–741. [DOI] [PubMed] [Google Scholar]
- AshmanTL.2000. Pollinator selectivity and its implication for the evolution of dioecy and sexual dimorphism. Ecology 81: 2577–2591. [Google Scholar]
- AshmanTL, Baker I.1992. Variation in floral sex allocation with time of season and currency. Ecology 73: 1237–1243. [Google Scholar]
- BawaKS.1980. Evolution of dioecy in flowering plants. Annual Reviews in Ecology and Systematics 11: 15–39. [Google Scholar]
- BazzazFA, Carlson RW, Harper JL.1979. Contribution to reproductive effort by photosynthesis of flowers and fruits. Nature 279: 554–555. [Google Scholar]
- BellG.1985. On the function of flowers. Proceedings of the Royal Society of London B 224: 223–265. [Google Scholar]
- BoivinB.1944. American Thalictra and their Old World allies. Rhodora 46: 337–377, 391,–445, 453–487. [Google Scholar]
- BonserSP, Aarssen LW.1996. Meristem allocation: a new classification theory for adaptive strategies in herbaceous plants. Oikos 77: 347–352. [Google Scholar]
- CharlesworthD, Morgan MT.1991. Allocation of resources to sex functions in flowering plants. Philosophical Transactions of the Royal Society of London Series B 332: 91–102. [Google Scholar]
- CharnovEL, Maynard Smith J, Bull JJ.1976. Why be a herma phrodite? Nature 263: 125–126. [Google Scholar]
- CoxPA.1981. Niche partitioning between sexes of dioecious plants. American Naturalist 117: 295–307. [Google Scholar]
- DarwinCR.1877. The different forms of flowers on plants of the same species. London: Murray. [Google Scholar]
- DavisSL.1997. Stamens are not maintained as attractants to pollinators in females of cryptically dioecious Thalictrum pubescens Pursch (Ranunculaceae). Sexual Plant Reproduction 10: 293–299, 366. [Google Scholar]
- DavisSL.2001. Phenotypic and genetic correlations among floral traits in two species of Thalictrum Journal of Heredity 92: 361–366. [DOI] [PubMed] [Google Scholar]
- DelphLF, Galloway LF, Stanton ML.1996. Sexual dimorphism in flower size. American Naturalist 148: 299–320. [Google Scholar]
- ElleE.1998. Quantitative genetics of sex allocation in andromonoecious perennial, Solanum carolinense (L.). Heredity 80: 481–488. [Google Scholar]
- GalenC, Dawson TE, Stanton ML.1993. Carpels as leaves: meeting the carbon cost of reproduction in an alpine buttercup. Oecologia 95: 187–193. [DOI] [PubMed] [Google Scholar]
- GeberMA.1990. The cost of meristem limitation in Polygonum arenastrum: negative genetic correlations between fecundity and growth. Evolution 44: 799–819. [DOI] [PubMed] [Google Scholar]
- GeberMA.1995. Fitness effects of sexual dimorphism in plants. Trends in Ecology and Evolution 10: 222–223. [DOI] [PubMed] [Google Scholar]
- GivnishTJ.1980. Ecological constraints on the evolution of breeding systems in seed plants: dioecy and dispersal in gymnosperms. Evolution 34: 959–972. [DOI] [PubMed] [Google Scholar]
- HouleD.1991. Genetic covariance of fitness correlates: what genetic correlations are made of and why it matters. Evolution 45: 630–648. [DOI] [PubMed] [Google Scholar]
- KaplanS, Mulcahy D.1971. Mode of pollination and floral sexuality in Thalictrum Evolution 25: 659–668. [DOI] [PubMed] [Google Scholar]
- Keener, C.1976. Studies in the Ranunculaceae of the southeastern United States. II. Thalictrum L. Rhodora 78: 457–471. [Google Scholar]
- KohnJR.1989. Sex ratio, seed production, biomass allocation and the cost of male function in Cucurbita foetidissima HBK (Cucubitaceae). Evolution 43: 1424–1434. [DOI] [PubMed] [Google Scholar]
- LloydDG, Webb CJ.1977. Secondary sex characters in plants. Botanical Reviews 43: 177–216. [Google Scholar]
- MayerS, Charlesworth D.1991. Cryptic dioecy in flowering plants. Trends in Ecology and Evolution 6: 320–325. [DOI] [PubMed] [Google Scholar]
- MaynardSmithJ.1978. The evolution of sex. Cambridge: Cambridge University Press. [Google Scholar]
- MazerSJ, Dawson KA.2001. Size‐dependent sex allocation within flowers of the annual herb Clarkia unguiculata (Onagraceae): ontogenetic and among‐plant variation. American Journal of Botany 88: 819–831. [PubMed] [Google Scholar]
- MazerSJ, Delesalle VA, Neal PR.1999. Responses of floral traits to selection on primary sexual investment in Spergularia marina: the battle between the sexes. Evolution 53: 717–731. [DOI] [PubMed] [Google Scholar]
- MeagherTR.1994. The quantitative genetics of sexual dimorphism in Silene latifolia (Carophyllaceae). II. Response to sex‐specific selection. Evolution 48: 939–951. [DOI] [PubMed] [Google Scholar]
- MeagherTR.1988. Sex determination in plants. In: Lovett‐Doust J, Lovett‐Doust L, eds. Plant reproductive ecology: patterns and strategies. New York: Oxford University Press, 125–138. [Google Scholar]
- MelampyM, Hayworth AM.1980. Seed production and pollen vectors in several nectarless plants. Evolution 34: 1144–1154. [DOI] [PubMed] [Google Scholar]
- O’NeilP, Schmitt J.1993. Genetic constraints on the independent evolution of male and female reproductive characters in the tristylous plant Lythrum salicaria Evolution 47: 1457–1471. [DOI] [PubMed] [Google Scholar]
- ParkMM.1992. A biosystematic study of Thalictrum section Leucocoma (Ranunculaceae). PhD Thesis, Pennsylvania State University, USA. [Google Scholar]
- Walker‐LarsenJ, Harder LD.2001. Vestigial organs as opportunities for functional innovation: the example of the Penstemon staminode. Evolution 55: 477–487. [DOI] [PubMed] [Google Scholar]
- WillsonMF.1979. Sexual selection in plants. American Naturalist 113: 777–790. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.