Abstract
Eastern white pine (Pinus strobus L.) shoots from mature trees were collected from two sites of contrasting soil pH: the Glendon campus of York University in Toronto, Ontario (pH 6·7 at 40 cm); and Muskoka near Huntsville, Ontario (pH 4·2 at 40 cm). Needles of ages 1–3 years were removed from the shoots, and the percentage of ash and silica was determined for all ages. Other needles were frozen in liquid nitrogen and kept in a cryo‐biological storage system before x‐ray microanalysis. Percentages of ash and silica were higher in the needles from Muskoka. Ash and silica increased with needle age for trees from the Muskoka site, but less so at the Toronto site. Of the 13 elements (Na, Mg, Al, Si, P, S, Cl, K, Ca, Mn, Fe, Cu and Zn) detected by microanalysis, Mn, Fe, Cu and Zn were detected in small amounts in the epidermis, endodermis and transfusion tissue (the layer of tracheids and parenchyma immediately surrounding the vascular bundles), and K, P, S and Cl were almost ubiquitous in distribution. Sodium was occasionally detected in the transfusion tissue, and magnesium was concentrated in the endodermal cells. The epidermal walls, transfusion tissue and endodermis were major sites of calcium localization. Silicon was concentrated in the extreme tips of the needles in all tissues, but particularly in the transfusion tissue, and more so in the Muskoka samples. Microanalysis revealed a higher Al content in the Muskoka needles, that Al was concentrated in the needle tips and that the transfusion tissues were major sites of accumulation.
Key words: Eastern white pine, Pinus strobus, needles, mineral deposition, aluminium, silicon, calcium
INTRODUCTION
In recent years it has become increasingly evident that mineral distribution in leaves is much more heterogeneous than was previously thought. In a review of these phenomena, Karley et al. (2000) suggested that knowledge of the systems controlling ion accumulation could show how plants respond to either a lack of nutrients or to ion toxicity. Although these authors were mainly referring to differential ion accumulation in cereal leaves, there has also been some progress in other leaf types. The conifers are a particularly interesting group to study as unlike cereals their leaves or needles are retained for several years.
The majority of studies of mineral distribution in the needles of conifers have been conducted on the economically important genera Picea (spruce) and Larix (larch). Microanalytical studies of spruce needles have been performed by Godde et al. (1988, 1991) and Stelzer et al. (1990). In an investigation of needles from the North American species white spruce (Picea glauca), growing in two soils of contrasting acidity, a total of 11 elements was detected (Sangster et al., 1997; Hodson and Sangster, 1998). Three papers have examined mineral localization in the needles of larch species (Stelzer et al., 1993; Gierth et al., 1998; Sangster et al., 2001). There has been little microanalytical work on the needles of any pine species. Bacic et al. (1999) found that the surfaces of P. halepensis needles contaminated with calcareous cement factory dust had heavy deposits of amorphous wax. Pyatt (1999) detected a total of 21 elements, including heavy metals, in the needles and stems of Corsican pine from uncontaminated and contaminated sites in the Mount Olympus area of Cyprus.
This report is the first detailed investigation of mineral distribution within the pine needle. Eastern white pine (Pinus strobus L.) was selected because of its ecological and economic significance in North America. It is a dominant component of the transition forest of eastern N. America, where originally it formed virtually pure stands in the Great Lakes—St Lawrence and Appalachian Regions (Hibbs, 1982). The aim of the present work was to determine the elemental localization in different age classes of needles of healthy, undamaged P. strobus trees growing at two sites of contrasting soil pH.
MATERIALS AND METHODS
Site descriptions
White pine samples were collected from two sites (Hodson and Sangster, 1998). The Toronto (neutral pH) site is located in the Forestry Station on the Glendon Campus of York University in Toronto, Ontario, Canada (43°43′N; 79°3′W). The soil is an alfisol (brown soil) above Palaeozoic strata. The Muskoka (acid pH) site is located 225 km to the north in Arrowhead Provincial Park, approx. 8 km from Huntsville, Ontario, in the Muskoka‐Haliburton Highlands (45°23′N; 79°12′W). The soil is a spodosol (podzol) formed on fine sand.
Field collection procedures and sample storage
Eastern white pine (Pinus strobus L.) trees were sampled in October, at the end of active uptake and growth. At both sites, healthy trees approx. 10 m tall and showing no visible signs of leaf damage were selected for sampling. Branches at a height of approx. 4 m were removed and the branch bases submerged in water during transport to the laboratory. Needle lengths were measured for each site. Needles were separated into three age classes: year 1, year 2 and year 3 (Toronto trees did not retain their needles after year 2). Samples of year 1 and year 2 needles were excised from the branches and were maintained in a cryo‐biological storage unit (Locator Aid‐Barnstead Thermolyne Ltd, Dubuque, Iowa, USA) at the temperature of liquid nitrogen until required. Soil samples were collected beneath the trees at both sites and analysed as in Hodson and Sangster (1998).
Gravimetric analyses
Fresh samples of needles of all age classes were excised from the branches and oven‐dried at 80 °C for 24 h. Percentages of ash and silica were determined in triplicate using the method described by Hodson and Sangster (1998), and data were subjected to ANOVA.
Scanning electron microscope (SEM) and x‐ray microanalysis of ashed material
Samples of ash were mounted using double‐sided tape on carbon planchets which in turn were mounted on carbon blocks. The samples were coated with carbon (Edwards model E12E) and examined in a JEOL 6400 SEM. Further details of analysis, calibration and conditions are given by Hodson and Sangster (1993).
Cryo‐SEM and x‐ray microanalysis
Needles were divided into basal, middle and tip sections, and frozen segments were prepared for examination in the cryo‐SEM following the procedures used by Hodson and Sangster (1998). Quantitative data were obtained for Al and Si in the following five tissues in needles in each age class from both sites: the epidermis outer tangential wall (OTW); the hypodermis OTW; the mesophyll chlorenchyma radial walls; the endodermis OTW; and the transfusion tissue walls and cytoplast (the transfusion is the layer immediately surrounding the vascular bundles). Windows of 0·16 keV were centred around the various peaks. Beryllium and ultrathin windows were used to collect counts for 100 s with the beam centred on the cell walls at a magnification of 2000×. A raster size equivalent to a scan area at specimen level of 25 mm2 was employed at an accelerating voltage of 15 kV. Counts were periodically equilibrated with a cobalt standard spectrum. At least five replicate analyses of each tissue area were performed. Data were transformed using a ZAF‐PB program, and expressed in mmol kg–1.
RESULTS
Soil and needle analysis
Full details of the soils are presented in Hodson and Sangster (1998). Soil pH readings at the Toronto site were higher than those at the Muskoka site (6·7 and 4·2, respectively, at 40 cm soil depth), and in both cases soil pH was lowest near the surface. Mean needle length was 85 mm (n = 10) at Muskoka and 91 mm at Toronto.
Percentage ash and percentage silica
Table 1 presents the content of ash and silica (SiO2) in needles from trees at the Toronto and Muskoka sites. For trees at the Muskoka site, increases in ash (P < 0·001) and silica (P < 0·05) with needle age were observed, whereas at the Toronto site, ash content increased only slightly (but significantly, P < 0·05) from year 1 to year 2 needles and the silica content remained constant. Percentages of ash and silica were generally higher (both P < 0·05) in needles from the Muskoka site.
Table 1.
Ash and silica contents of needles of Pinus strobus from two sites in Ontario, Canada
| Site | Needle age class (year) | Soil pH (40 cm) | % Ash ± s.d. | % Silica ± s.d. |
| Toronto | 1 | 6·7 | 2·22 ± 0·11 | 0·17 ± 0·05 |
| 2 | 2·44 ± 0·08 | 0·17 ± 0·06 | ||
| Muskoka | 1 | 4·2 | 2·28 ± 0·05 | 0·30± 0·06 |
| 2 | 3·06 ± 0·13 | 0·42 ± 0·19 | ||
| 3 | 3·55 ± 0·08 | 0·49 ± 0·12 |
Data are means of three determinations.
Cryo‐SEM and x‐ray microanalysis
Figure 1A shows a representative secondary electron image of the tip of a year 2 needle from the Toronto site in which Pinus leaf anatomy is evident in transverse section. The two outermost tissues, the epidermis and hypodermis, possess extremely thickened walls. The inner tissues, which have thinner walls, include the large chlorenchyma cells of the mesophyll and the uniseriate endodermis that surround the conducting tissues of the needle. The transfusion tissue (a layer of tracheids and parenchyma), in turn, immediately surrounds the internal vascular bundle. The corresponding silicon x‐ray distribution image (Fig. 1B) demonstrates that Si is heavily localized in the following tissues: the thickened walls of the outer layers, especially the epidermis; the walls of the mesophyll chlorenchyma; and the transfusion tissue of the conducting region.
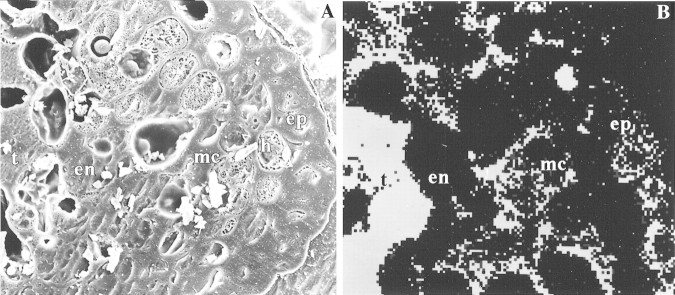
Fig. 1. Silicon localization in the outer region of a year 2 needle of eastern white pine, Toronto site, as determined by x‐ray microanalysis. Micrographs illustrate the frozen, planed face of a transverse section from 1 mm behind the tip; carbon coated. A, Secondary electron image showing the thickened epidermal and hypodermal layers surrounding the mesophyll chlorenchyma. Inwards, the uniseriate endodermis borders the large transfusion tissue cells. 750×. B, A silicon x‐ray distribution image of a similar region to that shown in A on the same section. 750×. ep, Epidermis; h, hypodermis; mc, mesophyll chlorenchyma; en, endodermis; t, transfusion tissues; v, vascular cylinder.
Figure 2 includes secondary electron and matching x‐ray distribution images for the outer and central regions of transverse sections of year 2 needles from the Muskoka site. Microanalysis of the outer region (Fig. 2A) indicates that: (1) Si is localized primarily in the thickened epidermal and hypodermal walls, in the mesophyll walls and throughout the transfusion tissue where extremely high levels occur (Fig. 2B); (2) Al also reaches its highest levels in the transfusion tissue (Fig. 2C); and (3) Ca concentration is highest in the transfusion tissues and in hypodermal and epidermal cell walls (Fig. 2D). In the centre of the needle (Fig. 2E), the x‐ray distribution map (Fig. 2F) indicates that Si is localized most intensely in the transfusion tissue and in the outer encircling row of endodermal cells.
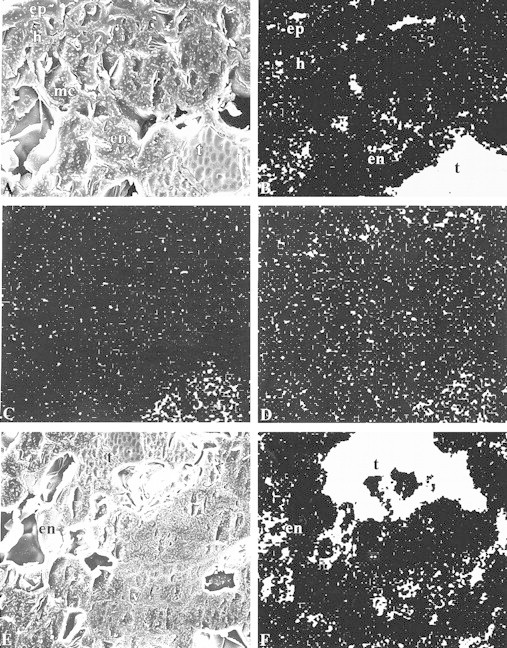
Fig. 2. Mineral localization in the outer region of year 2 needles of Eastern white pine, Muskoka site, as determined by x‐ray microanalysis. Micrographs illustrate the frozen, planed face of transverse sections from 2 mm behind the tip; carbon coated. A, Secondary electron image showing the thick‐walled epidermis and hypodermis lying outside of the mesophyll chlorenchyma tissue. The endodermis surrounds the stele in contact with the large outer transfusion tissues. 675×. B, Matching silicon x‐ray distribution image for A. 675×. C, Matching aluminium x‐ray distribution image. 675×. D, Matching calcium x‐ray distribution image 675×. E, Inner tissues of a year 2 needle. Secondary electron image, 450×. F, Matching silicon x‐ray distribution image for E. 450×. For abbreviations, see Fig. 1.
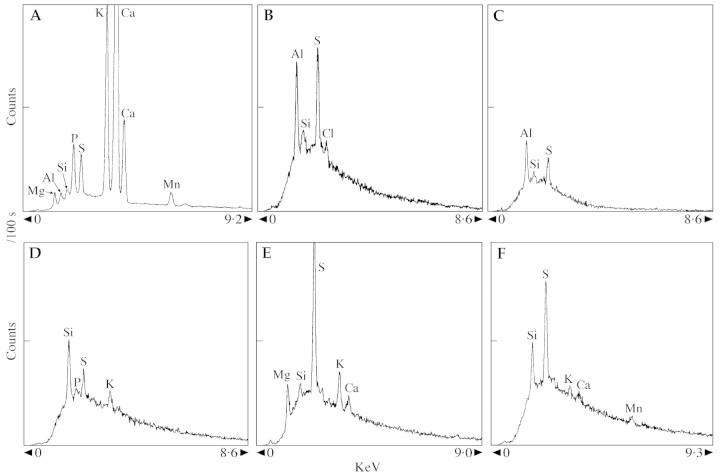
Representative spectra from analyses of various tissues and needle sections are presented in Fig. 3 for ashed (Fig. 3A) and frozen hydrated needles (Fig. 3B–F). The dominant peaks in Fig. 3A, an analysis of an ashed year 2 needle, are Ca and K, and other notable features are peaks for Al, Si and the distinct Mn peak. Microanalyses of frozen hydrated sections from the base of a year 1 needle from the Muskoka site indicate higher Al, Si and S levels in the outer tangential wall of the epidermis (Fig. 3B) than in the hypodermal OTW (Fig. 3C) [both spectra have the same vertical scale (VS): 1000 cps]. In Fig. 3D, the radial wall of the mesophyll chlorenchyma cell exhibits a higher Si peak than does the OTW of the endodermis (Fig. 3E) but the Mg and Ca peaks for the latter are notable as is the high S peak [the two spectra are comparable in terms of VS (2000 cps), needle age (1 year), section position (tip) and site (Toronto)]. The radial wall of a xylem vessel of a year 2 needle (tip, 2000 cps) from the Muskoka site (Fig. 3F) exhibits moderately sized Si and S peaks and a small Mn peak.
Fig. 3. Representative energy dispersive x‐ray analysis spectra from tissues of Eastern white pine needles, collected at the Muskoka site (A–C and F) and the Toronto site (D and E). Full vertical scales (VS) in counts per second are variable according to the area analysed. Horizontal spectral ranges are in keV. Transverse cryo‐sections were taken at 2 mm both from the base (B and C) and the tip (D–F) of frozen hydrated needles. Ashed needles were also analysed (A). Accelerating voltages were 20 kV (A and F) and 15kV (B–E). A, Epidermis of an ashed, year 2 needle, VS = 16000. B, Epidermal OTW of a year 1 needle (cryo‐section), VS = 1000. C, OTW of the hypodermis of a year 1 needle, VS = 1000. D, Radial wall, mesophyll, year 1 needle, VS = 2000. E, ITW, endodermis, year 1 needle, VS = 2000. F, Radial wall, xylem vessel, year 2 needle, VS = 2000.
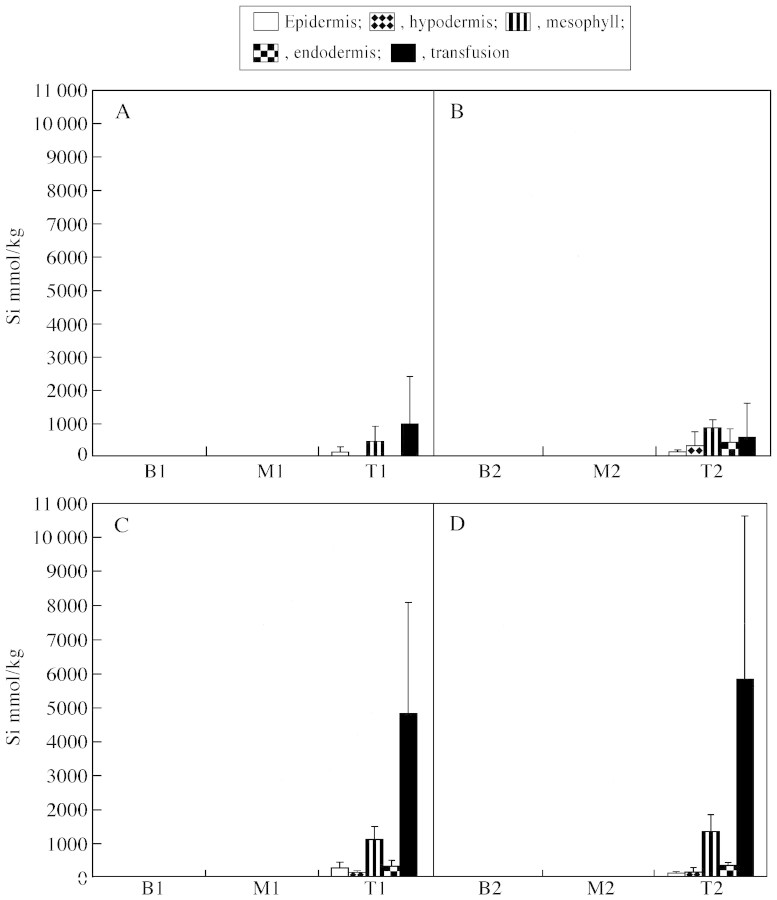
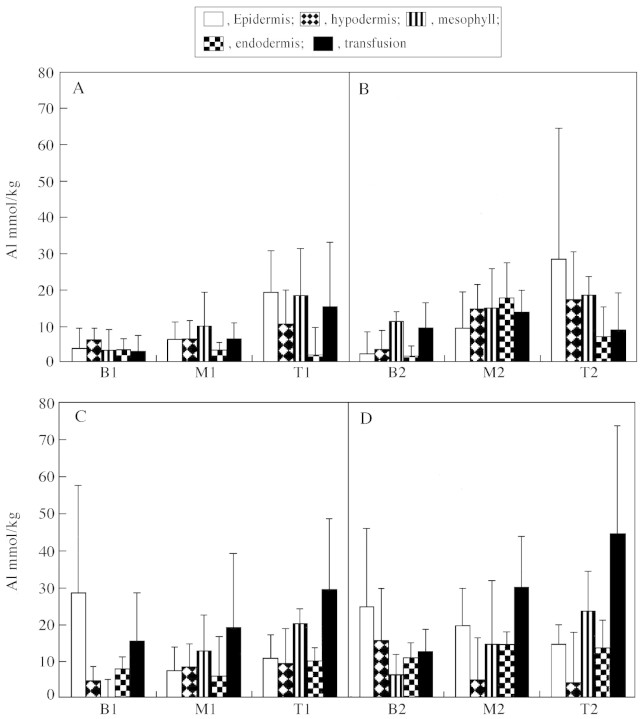
Quantitative microanalytical results for silicon and aluminium are shown in Figs 4 and 5. The error bars indicate that there was substantial variation in the results for particular tissues, especially when these were mineralized. This is to be expected as heavily mineralized cells were commonly observed adjacent to those with little mineralization. However, despite the variability, a number of distinct trends are apparent. It is evident that silicon is predominantly deposited in the needle tips (Fig. 4), that the tips of the Muskoka needles contain more Si than tips of needles from Toronto and that transfusion tissues are the major depositional sites in the Muskoka needles. Figure 5 shows that the Muskoka needles generally had higher aluminium contents than Toronto needles, that the needle tips had higher contents than the rest of the needle and, again, that the transfusion tissues were major sites of accumulation of Al in the Muskoka needles.
Fig. 4. Quantitative microanalytical results for silicon in Eastern white pine needles. All results are expressed as mmol kg–1 and are means of at least five analyses (± s.d.). A, Toronto needles, year 1; B, Toronto needles, year 2; C, Muskoka needles, year 1; D, Muskoka needles, year 2. Material was analysed at three points along the year 1 needles: B1, basal; M1, middle; and T1, tip. Similarly, the year 2 needles were analysed at three points (B2, M2, T2).
Fig. 5. Quantitative microanalytical results for aluminium in Eastern white pine needles. All results are expressed as mmol kg–1 and are means of at least five analyses (± s.d.). A, Toronto needles, year 1; B, Toronto needles, year 2; C, Muskoka needles, year 1; D, Muskoka needles, year 2. Material was analysed at three points along the year 1 needles: B1, basal; M1, middle; and T1, tip. Similarly, the year 2 needles were analysed at three points (B2, M2, T2).
DISCUSSION
In total, 13 elements were detected in this investigation, and those most frequently detected (K, P, S and Cl) were more or less ubiquitous throughout the pine needle tissues (Fig. 3). Magnesium was concentrated in the endodermal cells (Fig. 3E) of white pine, as reported previously for white spruce (Hodson and Sangster, 1998), Norway spruce (Stelzer et al., 1990) and larch (Sangster et al., 2001). Sodium was occasionally detected in the transfusion tissue (data not shown). The four heavy metals, Mn, Fe, Cu and Zn, were most commonly detected at low levels in the transfusion, endodermal and epidermal tissues (Fig. 3A and F; unpubl. res.).
Although Ca was detected in most tissues, it was frequently highest in the epidermal walls and the transfusion tissue (Fig. 2D), and in the endodermis (Fig. 3E). Much of the calcium in conifer needles is deposited as calcium oxalate crystals (Fink, 1991), probably as a means of detoxifying excess calcium. Fink (1991) investigated calcium oxalate distribution in two pine species, Pinus sylvestris and P. cembra, and found the greatest concentrations in the phloem parenchyma and epidermis. Most of the deposition in these Pinus species was intracellular, which differentiated the genus from other conifers studied. In spruce species, Stelzer et al. (1990) briefly reported the presence of Ca in extracellular crystals in the mesophyll, while Godde et al. (1991) and Hodson and Sangster (1998) laid more emphasis on epidermal deposition. The needle endodermis was cited as a major site of Ca localization in larch by Stelzer et al. (1993) and Gierth et al. (1998), but Sangster et al. (2001) also found high concentrations of Ca in the epidermal and hypodermal walls. In conclusion, it appears that pine, spruce and larch species all have high Ca levels in the epidermis and hypodermis, with varying amounts in the mesophyll, endodermis and transfusion tissue.
Several previous publications have reported bulk analyses of silica in the needles of Pinus species (Klein and Geis, 1978; Hodson et al., 1997; Carnelli et al., 2001), and silica content has been found to be in the range 0·01–0·83 % of dry weight. More specifically, Klein and Geis (1978) reported 0·085 % silica in needles of eastern white pine (Pinus strobus L.), while Hodson et al. (1997) found 0·05 % silica in the ‘radiata’ variety of this species growing at Bedgebury Pinetum in Kent, UK. The percentage silica values shown in Table 1 are well within the range reported for pine species, but they are somewhat above those previously reported for Pinus strobus, particularly in the Muskoka samples. These data suggest that environmental factors influence silica uptake and deposition in pine needles, but that silica deposition in this genus is much less than that in spruce or larch where it can reach 2 % of needle dry weight (Hodson and Sangster, 1999).
Silica deposition in P. strobus is relatively light when a bulk sample of needles is analysed, but this hides a very marked concentration of silica in the extreme tips of the needles (Fig. 4). Although all tissues showed this trend to some extent, it was the transfusion tissue that was most prominent in this regard (Figs 1B and 2B, F), particularly in the Muskoka needles. The deposition pattern in P. strobus differed markedly to that found in other conifer species. Silica deposition was heavier in white spruce needles, was greatest in the hypodermis and showed no discernible increase towards the tip (Hodson and Sangster, 1998). In larch (Larix laricina and L. decidua), the epidermal and hypodermal walls were the sites of greatest silica deposition, and although silica deposition increased towards the needle tips the trend was more gradual, and even the basal parts of the mature larch needle contained substantial amounts of silica (Sangster et al., 2001). Concentration of silicon in the needle tip may be a feature of conifer genera, such as pine, which generally do not accumulate high concentrations of Si, whereas heavier Si accumulators such as spruce and larch have less accumulation at the tip.
Coniferous forests on acid soils are vulnerable to potentially toxic levels of aluminium, the solubility of which increases below pH 4·5. The Muskoka needles, sampled from trees growing in a soil of pH 4·2 (at 40 cm), had higher Al contents than the needles from Toronto at soil pH 6·7 (Fig. 5). This difference was most marked in the transfusion tissue, and particularly at the needle tip (Fig. 2C). Aluminium localization has also been investigated in the needles of P. strobus growing in the highly acidic soils (pH 3·5–4·0) near Sudbury, Ontario, and the transfusion tissues of the tips had even higher Al (and Si) contents than those from Muskoka (Hodson and Sangster, 2000). This suggests that needle Al content is related to the acidity of the substrate. Using inductively coupled plasma emission spectrometry, Giertych et al. (1997) also found that Al concentrations increased from the needle base to tip in P. sylvestris samples collected from two field sites in western Poland.
Aluminium and silicon are strongly associated in the needles of P. strobus (Figs 2B, C and 3B, C), and particularly in the transfusion tissue, as found by Godde et al. (1988) for spruce needles from trees suffering from dieback in Germany. In the present study, the foliage of conifers growing at the acidic Muskoka site showed no visible evidence of pollution damage. There is mounting evidence that sequestration of Al by co‐deposition with Si might be an important mechanism for dealing with Al transported into conifer needles, but as yet there are no firm physiological data to support the hypothesis (Hodson and Sangster, 1999; Sangster and Hodson, 2001).
The mechanism of Si and Al accumulation in the needle tips (Figs 4 and 5) of P. strobus is uncertain, but the study by Canny (1993) on the retrieval of solutes from the transpiration stream of black pine (Pinus nigra Arnold) needles may be instructive on this point. The progress of two fluorescent tracers, sulforhodamine G and pyrene trisulfonate, was followed by fluorescence microscopy. The transpiration stream moved outwards in the apoplast after exiting from the central xylem strands of the needle, and was diverted by the transfusion tissue into a symplastic water flow at the boundary with the endodermis. This cell wall interface thus operates as a flume, leaving behind a sump of solutes within the transfusion tracheids. The water continues outwards symplastically through the endodermis and mesophyll until it reaches the hypodermis and epidermis, to evaporate beneath the stomata. Some of the solutes, including Si and Al, would be carried in this stream, eventually to become deposited in cell walls and lumina. Solutes in the sumps that do not enter chutes at the transfusion–endodermis boundary (or flume) have no available exit pathways. Canny (1993) suggested that their fate is uncertain, stating that ‘a simple guess might be that they collect within the tracheids (transfusion) at the tip of the needles’. The present results, which reveal very high concentrations, especially of Si, in the needle tips support Canny’s interpretation.
In conclusion, 13 elements were detected in this study, many of which showed distinct distributions within the needle. Mineral localization, particularly for Al and Si, in the pine needle is very different from that previously observed in other conifers. Environmental factors (e.g. soil type) also influence mineralization. It is hoped that the present study on ion localization will stimulate more physiological studies on ion transport in conifer needles.
ACKNOWLEDGEMENTS
The authors thank Professors M. E. McCully and M. J. Canny (Biology Department, Carleton University, Ottawa, Ontario), and Dr C. Huang, L. Ling and P. Jones (Research Facility for Electron Microscopy, Carleton University). Financial support was received from York University (A.G.S.) and a Staff Development Award from Oxford Brookes University (M.J.H.).
Supplementary Material
Received: 31 August 2001; Returned for revision: 15 October 2001; Accepted: 12 December 2001.
References
- BacicT, Lynch AH, Cutler D.1999. Reactions to cement factory dust by Pinus halepensis needles. Environmental and Experimental Botany 41: 155–166. [Google Scholar]
- CannyMJ.1993. Transfusion tissue of pine needles as a site of retrieval of solutes from the transpiration stream. New Phytologist 123: 227–232. [Google Scholar]
- CarnelliAL, Madella M, Theurillat J‐P.2001. Biogenic silica production in selected alpine plant species and plant communities. Annals of Botany 87: 425–434. [Google Scholar]
- FinkS.1991. Comparative microscopical studies on the patterns of calcium oxalate distribution in the needles of various conifer species. Botanica Acta 104: 306–315. [Google Scholar]
- GierthM, Stelzer R, Lehmann H.1998. Endodermal Ca and Sr partitioning in needles of the European larch (Larix decidua (L.) Mill.). Journal of Plant Physiology 152: 25–30. [Google Scholar]
- GiertychMG, De Temmerman LO, Rachwal L.1997. Distribution of elements along the length of Scots pine needles in a heavily polluted and a control environment. Tree Physiology 17: 697–703. [DOI] [PubMed] [Google Scholar]
- GoddeD, Divoux S, Hofert M, Gonsior B.1991. Quantitative and localized element analysis in cross‐sections of spruce [Picea abies (L.) Karst.] needles with different degrees of damage. Trees 5: 95–100. [Google Scholar]
- GoddeD, Homburg H, Methfessel S, Rosenkranz J.1988. Die Röntgenanalyse hilft beider Aufklärung individueller Waldschäden. LÖLF‐Mitteilungen 4: 23–27. [Google Scholar]
- HibbsDE.1982. White pine in the transition hardwood forest. Canadian Journal of Botany 60: 2046–2053. [Google Scholar]
- HodsonMJ, Sangster AG.1993. The interaction between silicon and aluminium in Sorghum bicolor (L.) Moench: Growth analysis and x‐ray microanalysis. Annals of Botany 72: 389–400. [Google Scholar]
- HodsonMJ, Sangster AG.1998. Mineral deposition in the needles of white spruce [Picea glauca (Moench.) Voss]. Annals of Botany 82: 375–385. [Google Scholar]
- HodsonMJ, Sangster AG.1999. Aluminium/silicon interactions in conifers. Journal of Inorganic Biochemistry 76: 89–98. [Google Scholar]
- HodsonMJ, Sangster AG.2000. Aluminium localization in conifers growing on highly acidic soils in Ontario, Canada. International symposium on impact of potential tolerance of plants on the increased productivity under aluminum stress Research Institute for Bioresources, Okayama University, Kurashiki, Japan. 103–106. Also available at: http://www.hodsons.org/MartinHodson/kurashiki.htm [Google Scholar]
- HodsonMJ, Williams SE, Sangster AG.1997. Silica deposition in the needles of the gymnosperms. I. Chemical analysis and light microscopy. In: Pinilla A, Juan‐Tresserras J, Machado M, eds. The state‐of‐the‐art of phytoliths in soils and plants Madrid, Spain: Monograph 4, Centro de Ciencias Medioambientales, 123–133. [Google Scholar]
- KarleyAJ, Leigh RA, Sanders D.2000. Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends in Plant Science 5: 465–470. [DOI] [PubMed] [Google Scholar]
- KleinRL, Geis JW.1978. Biogenic silica in the Pinaceae. Soil Science 126: 145–156. [Google Scholar]
- PyattFB.1999. Comparison of foliar and stem bioaccumulation of heavy metals by Corsican pines in the Mount Olympus area of Cyprus. Ecotoxicology and Environmental Safety 42: 57–61. [DOI] [PubMed] [Google Scholar]
- SangsterAG, Hodson, MJ.2001. Silicon and aluminium codeposition in the cell wall phytoliths of gymnosperm leaves. In: Meunier JD, Colin F, eds. Phytoliths – applications in earth science and human history Lisse, The Netherlands: A.A. Balkema, 343–355. [Google Scholar]
- SangsterAG, Hodson MJ, Huang CX.2001. X‐ray microanalytical studies of mineral composition in cell walls of needle tissues of American larch [Larix laricina (Du Roi) K. Koch] and European larch [L. decidua (L.) Mill.] In: Labrecque M, ed. L’arbre 2000 The Tree 4th International Symposium on the Tree, Montreal Botanic Garden. Montreal, Canada: Isabelle Quentin Press, 160–167. [Google Scholar]
- SangsterAG, Williams SE, Hodson MJ.1997. Silica deposition in the needles of the gymnosperms. II. Scanning electron microscopy and x‐ray microanalysis. In: Pinilla A, Juan‐Tresserras J, Machado M, eds. The state‐of‐the‐art of phytoliths in soils and plants Madrid, Spain: Monograph 4, Centro de Ciencias Medioambientales, 135–146. [Google Scholar]
- StelzerR, Holste R, Groth M, Schmidt A.1993. X‐ray microanalytical studies on mineral concentrations in vacuoles of needle tissues from Larix decidua (L.) Mill. Botanica Acta 106: 325–330. [Google Scholar]
- StelzerR, Lehmann H, Kramer D, Lüttge U.1990. X‐ray microprobe analyses of vacuoles of spruce needle mesophyll, endodermis and transfusion parenchyma cells at different seasons of the year. Botanica Acta 103: 415–423. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.