Abstract
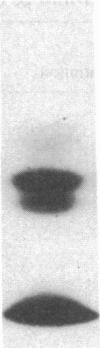
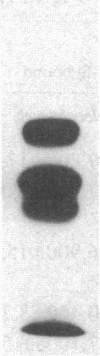
It has been suggested that fibrinogen (fg) or its physiological derivatives influence the motility and growth of endothelial cells (ECs), but direct support for this concept is still lacking. In the present study, the capacity of fg to interact with ECs and induce the migration of ECs was examined. The capacity of fg to induce EC migration was studied by means of a modification of the Boyden chamber technique. fg in the lower compartment of the chamber caused a time- and concentration-dependent migration of ECs across filters. fg present in equal concentrations above and below the filter increased EC migration, but the maximal effect invariably occurred in the presence of a gradient between the lower and the upper compartments. Trypsin or plasmin digestion of fg and preincubation of fg with Fab fragments from specific antibody completely abolished fg-induced EC migration. Dialysis of fg to eliminate small peptides that might contaminate the preparation did not modify fg-induced migration. Plasma obtained from healthy donors induced EC migration, but plasma from an afibrinogenemic patient was completely ineffective. The addition of purified fg to afibrinogenemic plasma restored plasma-induced EC migration. Plasmin degradation fragments D and E, of 100,000 and 50,000 mol wt, respectively, did not induce EC migration. However, fragment E caused dose-related inhibition of fg-induced EC migration Direct interaction of highly purified radioiodinated human fg with cultured human and bovine Ecs was observed. The binding was time dependent and plateaued at 10 min. Nonlabeled fg in a large molar excess inhibited the interaction, but unrelated proteins, including fibronectin, ovalbumin, and myoglobin, did not. Monospecific Fab fragments directed to fg inhibited binding by 38% at a 50 to 1 molar ratio whereas nonimmune Fab caused only 2% inhibition at a similar concentration. The binding of 125I-fg with ECs was saturable, and an apparent dissociation constant of 0.23 x 10(-6) M was estimated from binding isotherms. After 30 min of incubation the interaction between 125I-fg and the cells was completely reversible and displaceable by a large molar excess of unlabeled fg. Autoradiography of the display of EC-bound 125I on polyacrylamide gel showed the constitutive B beta- and gamma-chains of the fg molecule, with a partial loss of the A alpha-chain. Purified fragment E and E were tested for their capacity to inhibit fg binding. At a 1 to 400 125I-fg-to-fragment molar ratio, fragment E, which also inhibited migration, competed for binding by 44%, but fragment D was completely ineffective. These data show that fg may specifically associate with ECs and induce migration of these cells; it also appears that the structural requirement of this activity is located in the N-terminal part of the molecule.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbieri B., Balconi G., Dejana E., Donati M. B. Evidence that vascular endothelial cells can induce the retraction of fibrin clots. Proc Soc Exp Biol Med. 1981 Nov;168(2):204–207. doi: 10.3181/00379727-168-41260. [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Polentarutti N., Acero R., Balsari A., Boraschi D., Ghezzi P., Salmona M., Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983 Apr 8;220(4593):210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- Center D. M., Cruikshank W. Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol. 1982 Jun;128(6):2563–2568. [PubMed] [Google Scholar]
- Colvin R. B., Dvorak H. F. Fibrinogen/fibrin on the surface of macrophages: detection, distribution, binding requirements, and possible role in macrophage adherence phenomena. J Exp Med. 1975 Dec 1;142(6):1377–1390. doi: 10.1084/jem.142.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. B., Gardner P. I., Roblin R. O., Verderber E. L., Lanigan J. M., Mosesson M. W. Cell surface fibrinogen-fibrin receptors on cultured human fibroblasts. Association with fibronectin (cold insoluble globulin, LETS protein) and loss in SV40 transformed cells. Lab Invest. 1979 Nov;41(5):464–473. [PubMed] [Google Scholar]
- Dejana E., Vergara-Dauden M., Balconi G., Pietra A., Cherel G., Donati M. B., Larrieu M. J., Marguerie G. Specific binding of human fibrinogen to cultured human fibroblasts. Evidence for the involvement of the E domain. Eur J Biochem. 1984 Mar 15;139(3):657–662. doi: 10.1111/j.1432-1033.1984.tb08054.x. [DOI] [PubMed] [Google Scholar]
- Dolfini E., Larizza L., Villa P., Fuhrman Conti A. M. Activation of cyclophosphamide by freeze-dried microsomes as an in vitro test of cytogenetic damage. Mutat Res. 1982 Dec;105(6):429–432. doi: 10.1016/0165-7992(82)90189-0. [DOI] [PubMed] [Google Scholar]
- Donati M. B., Poggi A. Malignancy and haemostasis. Br J Haematol. 1980 Feb;44(2):173–182. doi: 10.1111/j.1365-2141.1980.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Dvorak A. M., Manseau E. J., Wiberg L., Churchill W. H. Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis, and fibroplasia in guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. J Natl Cancer Inst. 1979 Jun;62(6):1459–1472. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Glaser B. M., D'Amore P. A., Seppa H., Seppa S., Schiffmann E. Adult tissues contain chemoattractants for vascular endothelial cells. Nature. 1980 Dec 4;288(5790):483–484. doi: 10.1038/288483a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Gregory H. Epidermal growth factor-urogastrone: biological activity and receptor binding of derivatives. Mol Pharmacol. 1980 May;17(3):314–320. [PubMed] [Google Scholar]
- Introna M., Allavena P., Biondi A., Colombo N., Villa A., Mantovani A. Defective natural killer activity within human ovarian tumors: low numbers of morphologically defined effectors present in situ. J Natl Cancer Inst. 1983 Jan;70(1):21–26. [PubMed] [Google Scholar]
- Kadish J. L., Butterfield C. E., Folkman J. The effect of fibrin on cultured vascular endothelial cells. Tissue Cell. 1979;11(1):99–108. doi: 10.1016/0040-8166(79)90010-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landriani G. S., Guardabasso V., Rocchetti M. NL-FIT: a microcomputer program for non-linear fitting. Comput Programs Biomed. 1983 Feb-Apr;16(1-2):35–42. doi: 10.1016/0010-468x(83)90006-5. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Ardaillou N., Cherel G., Plow E. F. The binding of fibrinogen to its platelet receptor. J Biol Chem. 1982 Oct 25;257(20):11872–11875. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Marguerie G. A., Thomas-Maison N., Larrieu M. J., Plow E. F. The interaction of fibrinogen with human platelets in a plasma milieu. Blood. 1982 Jan;59(1):91–95. [PubMed] [Google Scholar]
- Meyer D., Obert B., Pietu G., Lavergne J. M., Zimmerman T. S. Multimeric structure of factor VIII/von Willebrand factor in von Willebrand's disease. J Lab Clin Med. 1980 Apr;95(4):590–602. [PubMed] [Google Scholar]
- Nicosia R. F., Tchao R., Leighton J. Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro. 1982 Jun;18(6):538–549. doi: 10.1007/BF02810077. [DOI] [PubMed] [Google Scholar]
- Niewiarowski S., Budzynski A. Z., Morinelli T. A., Brudzynski T. M., Stewart G. J. Exposure of fibrinogen receptor on human platelets by proteolytic enzymes. J Biol Chem. 1981 Jan 25;256(2):917–925. [PubMed] [Google Scholar]
- Sherman L. A., Lee J. Specific binding of soluble fibrin to macrophages. J Exp Med. 1977 Jan 1;145(1):76–85. doi: 10.1084/jem.145.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte S. The endoendothelial lining as studied by a fluorescent labeling technique in situ. Thromb Res Suppl. 1983;5:93–104. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]