Abstract
Long‐stalked glandular hairs of outer and inner involucral bracts of Sigesbeckia jorullensis, which are important for epizoic fruit propagation, were investigated using light and scanning electron microscopy. The essential oil secreted by the hairs was analysed by chromatographic methods including gas chromatography/mass spectrometry and with a laser microprobe mass analyser. The glandular hairs consisted of a large multicellular stalk and a multicellular secreting head. The apical layer of glandular head cells was characterized by leucoplasts and calcium oxalate crystals. Below the apical cells there were up to six layers of cells containing many chloroplasts around the nucleus and surrounded by vacuoles filled with flavonoids and tannins. The essential oil originating in the head cells was secreted into the subcuticular space and may be liberated by rupture of the cuticle. It was mainly composed of sesqui‐ and diterpenes, with the sesquiterpene hydrocarbon germacrene‐d as the main component. Monoterpenes, n‐alkanes and their derivatives as well as flavonoid aglycones were also detected. The stickiness of the essential oil is probably associated with the high content of oxygenated sesqui‐ and diterpenes. In addition to long‐stalked trichomes, small biseriate trichomes occurred, secreting small quantities of essential oil into a subcuticular space.
Key words: Sigesbeckia jorullensis Kunth, Asteraceae, glandular hairs, fruit propagation, histochemistry, morphology, essential oil, flavonoids, LM, SEM, GC‐MS
INTRODUCTION
The Asteraceae (Compositae) are the largest family of plants, comprising approx. 23 000 known species (Bremer, 1994), some of which, e.g. Bidens and Arctium species, disperse their fruits by epizoochory, which is commonly accomplished by means of hooked hairs or other projecting organs. Instead of hooked hairs, Sigesbeckia and Carpesium species produce sticky substances by which their fruits adhere to animals. This method of epizoic fruit dispersal is unusual within the Asteraceae (Wagenitz, 1979).
Plants of the genus Sigesbeckia are annual herbs of warm areas throughout the world, and several species are cultivated as medicinal plants. Sigesbeckia pubescens is used in Japan as a drug against arthritis. Extracts of its aerial parts contain diterpenes, mostly from the kauran derivative series (Camonica et al., 1969; Murakami et al., 1973; Kim et al., 1979; Liu and Röder, 1991) and other terpenoids, especially sesquiterpene lactones, have also been reported (Baruah et al., 1979, 1980; Zdero et al., 1991). The bitter taste of S. orientalis, which is used as a panacea in Madagascar, is due to darutoside (C26H44O8), a substance that can be split into the diterpene darutigenol and glucose by emulsin and elaterase. Sigesbeckia orientalis and S. jorullensis are not indigenous to Europe, but both species are naturalized (Tutin et al., 1976; Wagenitz, 1979). Sigesbeckia jorullensis, an annual herb growing up to 1·5 m tall and originating from subtropical America, grows in Europe in the undergrowth of bushes, in gardens and on banks. Its outer and inner involucral bracts are covered with glandular hairs whose sticky excretion is important for epizoic fruit dispersal and propagation (Wagenitz, 1979). This paper deals with the morphology, anatomy, histochemistry and biochemistry of the glandular hairs of S. jorullensis.
MATERIALS AND METHODS
Plant material
Plants of Sigesbeckia jorullensis Kunth in Humb., Bonpl. and Kunth (S. cordifolia Kunth), grown from seeds collected in the north‐east of Hamburg (Germany), were cultivated in the Botanical Garden of the University of Graz, Austria. Involucral bracts, sampled before (June/July), during (August) and after (September/October) flowering were investigated.
Light microscopy (LM) and histochemistry
For anatomical and histochemical observations, 2–5 mm long involucral bracts were used. Lipids were localized by Sudan black B, Sudan III and Nile blue A (Cain, 1947). Tannins were detected using ferric chloride and the nitroso reaction (Reeve, 1951; Stafford et al., 1987). For the detection of flavonoids, 5 % aqueous AlCl3 solution and 0·05 % Naturstoffreagenz A (β‐aminodiethylester of diphenylboric acid) dissolved in 10 % methanol were used. Naturstoffreagenz A, a well‐known reagent used in thin layer chromatography to visualize flavonoid compounds (Wollenweber, 1982), reacts with hydroxyl groups causing a yellow‐green fluorescence, depending on the structural features of the flavonoid. Tissues were cleared using chloral hydrate or sodium hypochlorite, and observations were made using a Zeiss Axioplan epifluorescence microscope equipped with the following filter systems: exciter filter 365, dichroic mirror 395 and barrier filter 420. Fujicolor Superia 200 film was used for colour prints and Ilford FP 4, 125, for black and white prints.
Scanning electron microscopy (SEM)
For SEM, bracts were fixed with 2·5 % glutaraldehyde in 50 mm cacodylate buffer at pH 7 for 3 h, dehydrated in a graded series of acetone solutions and critical point dried with solvent‐substituted liquid carbon dioxide. The dried bracts were coated with a thin layer of gold using an AGAR Sputter and examined in a Philips SEM XL 30 ESEM. An SE‐detector (Everhart‐Thornley, Philips Electron Optics, Eindhoven, The Netherlands) was used in the high vacuum mode for the investigation of fixed and dried samples, and a GSED‐detector (gaseous secondary electrons) in the ESEM‐mode (cooling stage 5 °C, 3–5 torr chamber pressure) was used to observe fresh samples without preparation.
Extraction of essential oils and gas chromatography (GC)
Samples of fresh inflorescences (30 g) of S. jorullensis, randomly collected during flowering, were placed in a round‐bottom flask containing 500 ml deionized water. The samples were then steam distilled for 4 h in a Karlsruher‐type apparatus (Stahl, 1953), which is similar to a Clevenger‐type circulatory steam distillation apparatus. The essential oil was trapped in 1 ml analytical grade n‐pentane and analysed by GC and by GC‐mass spectrometry (GC‐MS).
GC was performed using a DANI 8400 Capillary Gas Chromatograph (DANI, Monza, Italy) equipped with a Programmed Temperature Vaporiser (PTV) injection system, a flame ionization detector (FID) and a LDC/Milton Roy CI‐10 B integrator (LDC/Milton Roy, Riviera Beach, FL, USA). The samples were analysed on a fused silica 50 m CP‐SIL 5 CB (dimethylpolysiloxane) capillary column (Chrompack, Middelburg, The Netherlands) with an internal diameter of 0·25 mm and a film thickness of 0·12 µm. The hydrogen carrier gas had an average linear velocity of 40 cm s–1. Oven temperature programming was 40 °C during injection and for an additional 3 min, and was then increased from 40 to 300 °C at the rate of 4 °C min–1. The temperature of the PTV was 40 °C during injection, followed by very rapid heating (approx. 900 °C min–1) to 250 °C. One microlitre of each sample was injected in the split mode (1 : 30). The detector temperature was 300 °C.
GC‐MS was performed on a Hewlett Packard G1800A GCD system (Electron impact voltage, 70 eV; injector temperature, 280 °C; detector temperature, 320 °C; foreline pressure, 4 Pa; mass range, 30–425 amu). Samples were analysed on a DB‐1 (50 m × 0·20 mm i.d.; film thickness 0·33 µm) capillary column. The helium carrier gas had a delivery rate of 1 ml min–1 and the column temperature programming was as follows: 40 °C initial temperature maintained for 5 min, then the temperature was increased from 40 to 300 °C at a rate of 4 °C min–1.
Compounds were identified using both chromatographic and mass spectroscopic criteria. The Wiley275 database was used for automatic identification of GC‐MS peaks; and linear retention indices (van den Dool and Kratz, 1963), obtained on a polar and an apolar column, were compared with published data (Davies, 1990). Whenever possible, mass spectra and retention indices were also compared with data obtained from authentic compounds (Fluka Chemie GmbH, Buchs, Switzerland). Quantitative results were achieved from GC‐FID profiles on a non‐polar column using the area per cent method without consideration of calibration factors (i.e. F = 1·0) for all compounds.
Analysis of flavonoid aglycones
A sample of fresh inflorescences (28 g) of flowering S. jorullensis was dipped for 1–2 s in analytical grade chloroform. After evaporation of the solvent, 0·42 g of a dark green viscous material was obtained (yield 1·5 %). The material was dissolved in warm (50–60 °C) methanol, stored overnight at –25 °C, and wax components were removed by filtration. Five microlitres of the remaining extract was analysed by thin layer chromatography (TLC) according to Wollenweber (1982). The conditions were: stationary phase polyamide 11 F254 (Merck), and the mobile phase toluene/ethyl methyl ketone/methanol, 12/5/3 (v/v/v). The chromatogram was sprayed with 0·5 % methanolic Naturstoffreagenz A.
LAMMA technique
The secretion of the glandular hairs of three outer involucral bracts (covered with a plastic bag against dust) was collected on a copper grid for electron microscopy. Thirty droplets were analysed by means of a laser microprobe mass analyser (Lamma 500; Leybold‐Heraeus, Cologne, Germany). The LAMMA instrument combines a light microscope for observation and exact localization of the sample as well as for focusing the beam of a pulsed laser into the specimen, and an MS (Heinen et al., 1981; Heinrich, 1990). A minute volume of the sample is ionized by the high intensity laser. The resulting ions are analysed by time of flight MS, recording the complete mass spectrum of each laser shot.
RESULTS
Capitulum and involucral bracts
The capitula were small, in lax panicles or, rarely, solitary. The pauciflorous capitulum possessed five outer involucral bracts, which were much longer than the inner bracts and densely covered with long‐stalked gland hairs, smaller glandular hairs and a few non‐glandular hairs. The outer florets with short ligules were female, the inner tubular florets (without pappus) were hermaphroditic and subtended by receptacular scales (Fig. 1A). The outer involucral bracts adhere to animals or humans due to the sticky material secreted by long‐stalked hairs, causing the inner bracts and achenes to be torn out of the capitulum. The fruit dispersion unit is shown in Figs 1B and 3A. The outer involucral bracts were linear to linear–spathulate and possessed a large median vein and two lateral veins linked to smaller veins (Fig. 2A and B). The three main veins fused at the leaf tip leading to an epithem hydathode (Fig. 2B). Small veins, composed mainly of tracheids, terminated near the base of gland cells (Fig. 2D). The epidermis cells of outer involucral bracts contained flavonoids. The mesophyll of outer involucral bracts, composed of loose spongy parenchyma, contained numerous chloroplasts with large starch grains; after flowering the starch content diminished.

Fig. 1. LM micrographs of capitula and long‐stalked hairs of Sigesbeckia jorullensis. Bars = 1 mm (A and B), 50 µm (C–I). A, The pauciflorous capitulum consists of outer female florets with short ligules, inner hermaphrodite florets without a pappus, and five outer involucral bracts, up to 2 cm long, possessing long‐stalked glandular hairs. B, Fruit dispersion unit consisting of a female floret that has been torn out of the receptacle together with the outer and inner involucral bracts. C, Long‐stalked hairs showing a subcuticular space filled mainly with essential oil, yellow coloured cells of the upper layer (UL), green cells of the lower layers (LL) and a long stalk. Lipophilic droplets (arrows) are visible on the cuticle of the subcuticular space. D, Long‐stalked hair with only one row of head cells. E–I, Long‐stalked hairs with damaged cuticle. E, Hairs under UV light (365/395/420). Lower layers of the head and stalk cells show red autofluorescence of chloroplasts. Even in young hairs the apical layer did not contain chlorophyll, and the bright blue autofluorescence indicates lipophilic material. F, Upper head cells stained more intensely with Sudan black B than lower ones. G, Head cells stained with Sudan III. H and I, Reaction with Naturstoffreagenz A. H, Yellow secondary fluorescence indicates the presence of flavonoids in the head cells, and of flavonoid aglycones in the secretion products on the surface of head and stalk cells. I, Higher magnification indicates the presence of flavonoids in vacuoles, whereas the inner part of the cells (asterisks), where the nucleus is surrounded by plastids, is free of flavonoids, as indicated by the lack of secondary fluorescence.
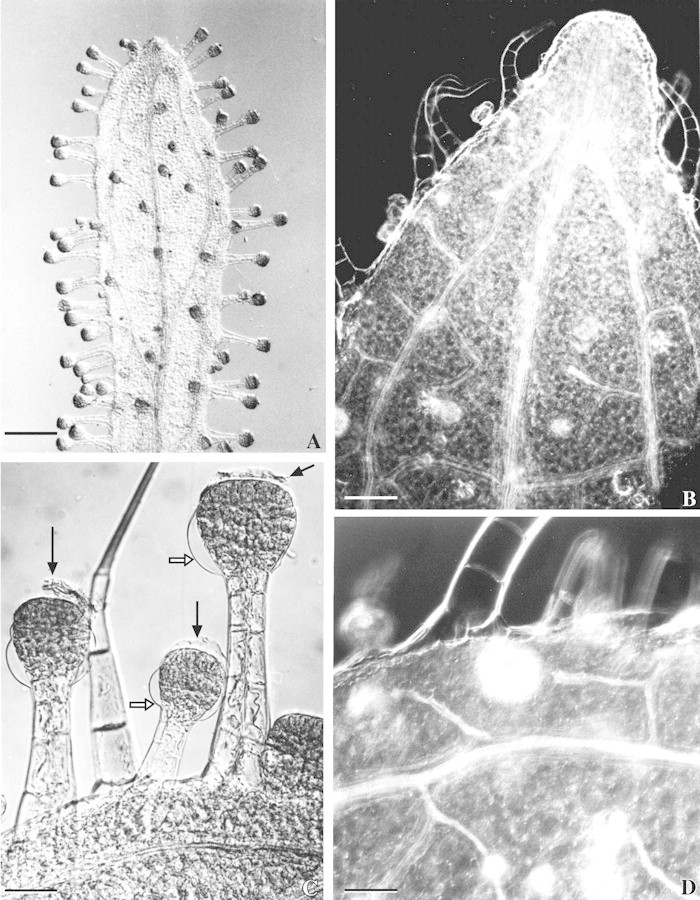
Fig. 2. Vascularization of the outer bracts and long‐stalked glandular hairs of Sigesbeckia jorullensis. A, Outer involucral bract with a large median vein, two lateral veins, smaller veins, and many long‐stalked glands. Bar = 1 mm. B, The three veins fuse to an epithem hydathode. Bar = 200 µm. C, Hairs treated with glycerol‐ethanol and chloral hydrate show the remnants of the cuticle forming the subcuticular space (black arrows) and the girdle between the upper and lower layers of cells, where the cuticle of the lower parts of the head (white arrows) is fixed to the cell wall. Bar = 100 µm. D, Small veins, mainly composed of tracheids, lead to the base of gland cells. Bar = 100 µm.
Long‐stalked glandular hairs
Sigesbeckia jorullensis has two types of glandular hairs. The outer and inner involucral bracts were densely covered with long‐stalked glandular hairs with multicellular heads (Figs 1, 2A, C and 3A, B, D, E). Most, but not all, of these hairs were established at an early stage of leaf differentiation, such that hairs of different developmental stages could be found on young involucral bracts. Some hairs were almost completely differentiated whereas others had passed through only a few cell divisions. Comparing a younger and an older capitulum of the same plant, a 2‐mm long outer involucral bract had 40 ± 6 long‐stalked hairs, whereas a 5 mm bract had 80 ± 15 (data are means ± s.d. of five involucral bracts each). Besides well developed long‐stalked hairs, some apparently mature hairs with fewer cells could be found (Fig. 1D).
Stalk
The stalk of fully developed glandular hairs consisted of 16–40 cells supporting the head of the gland. The cross‐section of the base of the stalk sometimes showed more than 16 cells (Fig. 3C). It was not clear whether the two central cells of the basal region of the stalk derived from the subepidermal layer or not. The upper part of the stalk was composed of one (seldom) or four to six radially arranged cells (bi‐ to triseriate, Fig. 3D). Even in mature gland cells, the chloroplasts of stalk cells contained starch grains (not shown).
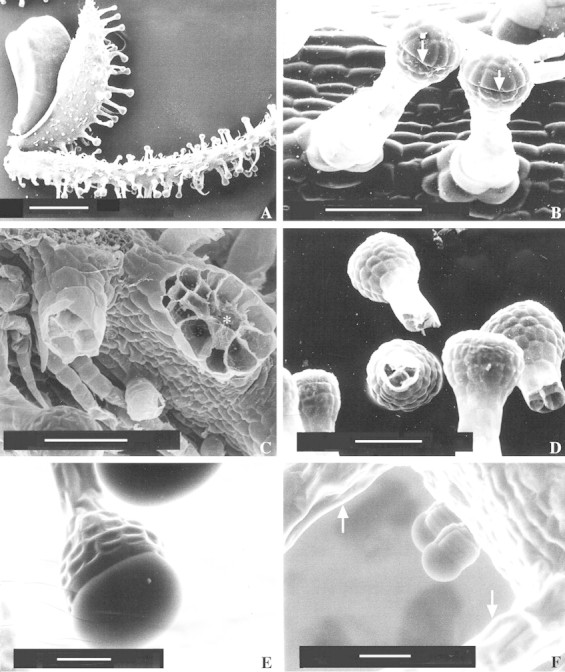
Fig. 3. SEM micrographs of Sigesbeckia jorullensis. A, Fruit dispersion unit consisting of an achene that had been torn out of the receptacle together with the outer and inner involucral bracts. Bar = 1 mm, magnification 22×. B, Young long‐stalked glandular hairs showing the line (arrows) where the cuticle is detached from the wall. Bar = 100 µm, magnification 359×. C, Broken hairs. The base of the hair on the right‐hand side (asterisk) shows 16 cells, whereas the stalk of the hair on the left‐hand side is composed of four cells. Bar = 100 µm, magnification 307×. D, Broken hairs showing four to six stalk cells. Bar = 100 µm, magnification 249×. E and F, ESEM‐mode. E, Long‐stalked hairs without preparation, showing the filled subcuticular space. Bar = 50 µm, magnification 397×. F, Two small ten‐celled hairs and stalks of long hairs (arrows). Bar = 50 µm, magnification 376×.
Head cells
Typically, a long‐stalked hair in an early stage of development had one basal cell, two to four stalk cells and a head, initially composed of two cells, of which the apical head cell divided first. The head of a mature long‐stalked glandular hair was 125 ± 35 µm long, 117 ± 30 µm (mean ± s.d.; n = 50) in diameter and consisted of 60–120 cells arranged in two zones. The apical cell layer consisted of up to 24 cells, which differed fundamentally from the cells of the four to six sub‐apical layers. The cells of the upper layer were yellowish, whereas those of the lower layers were green (Fig. 1C). Secretion started when the hairs were fully developed, and the product accumulated in the subcuticular space of the glandular head formed by detachment of the cuticle from the walls of the apical cells (Fig. 1C). Small droplets of secondary compounds could be observed on the intact cuticle of the subcuticular space (Fig. 1C). In older hairs, or in young hairs treated with reagents, the cuticle was ruptured above the base of the apical cells releasing a lipophilic material. This attachment of the cuticle could be demonstrated in cleared hairs, prepared by incubation for 2 months in glycerol–ethanol, followed by one night in chloral hydrate. The cuticle of the subcuticular chamber was visible as a remnant attached to the apex of the hair, whereas the cuticle of the lower part of the hair was firmly fixed at the border between the upper tier and the chlorophyll‐containing lower head cells (Fig. 2C). SEM micrographs also showed the upper zone of the hair where the cuticle was detached, forming a subcuticular chamber for the secreted material (Fig. 3B and D). The ESEM‐mode facilitated observation of unprepared specimens without significant loss of secretion (Fig. 3E). In LM, the secreted material was first hyaline, later yellowish‐brown and sticky. Calcium oxalate crystals were found exclusively in the upper layer of head cells (Fig. 4).
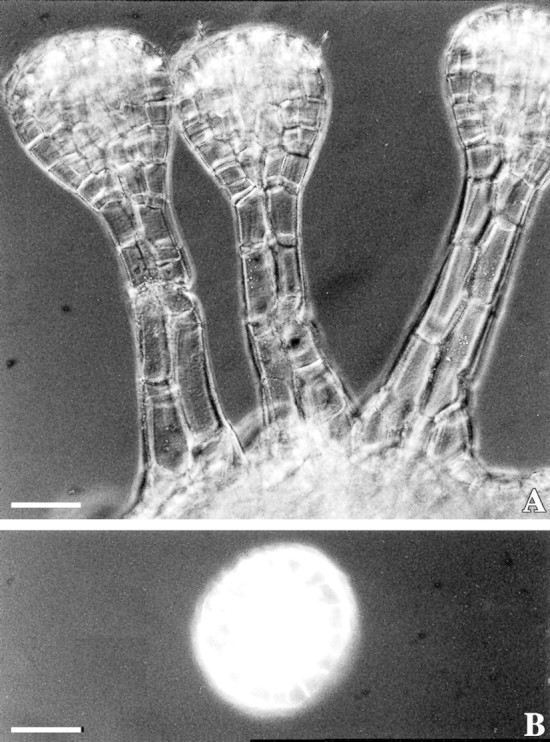
Fig. 4. LM micrographs of cleared tissue under polarized light show calcium oxalate crystals in the apical layer of the head cells of Sigesbeckia jorullensis. Bars = 50 µm.
When subjected to UV light, the apical head cells revealed an intense blue autofluorescence, whereas abundant chloroplasts fluorescing red (chlorophyll autofluorescence) were seen in the lower layers of the head; only a few chloroplasts were found in stalk cells (Fig. 1E). The material secreted into the subcuticular space contained lipophilic substances as tested with Nile blue (not shown), Sudan black B (Fig. 1F) and Sudan III (Fig. 1G). The apical cells stained differently to the cells of the lower layers with Sudan black and Sudan III (Fig. 1F and G).
The fluorochromes for flavonoid detection, aluminium trichloride and Naturstoffreagenz A induced a yellow or green secondary fluorescence in head cells and secreted exudate (Fig. 1H and I). The central part of most cells did not fluoresce because nuclei surrounded by plastids occupy this space, as can be observed under high magnification (Fig. 1I). In addition, head cells containing chloroplasts stained positively for tannins using ferric chloride and the nitroso reaction (not shown).
Short‐stalked glandular trichomes
Beside long‐stalked multicellular hairs, smaller glandular trichomes occurred on the inner involucral bracts (Fig. 3A) and on the outer ones amongst long‐stalked hairs (Fig. 3F); they produced secondary compounds. Comparing the abundance of the two glandular trichome types on three inner and three outer involucral bracts, the ratio between long‐ and short‐stalked hairs was 1 : 1·45 on outer involucral bracts and 1 : 0·16 on inner involucral bracts. The fully developed biseriate, short‐stalked glandular trichome consisted in most cases of ten cells: two basal cells, two stalk cells and six cells forming the glandular head (Fig. 3F). The head cells contained calcium oxalate crystals. The head cells and the material secreted into the subcuticular space stained positively for lipids.
Nature of the secreted material
The secreted material was soluble in methanol (30 s), 2‐propanol, ethyl acetate, chloroform, and only partly soluble in n‐hexane. TLC on polyamide separated four main and three minor bands, which turned yellow or orange after spraying with Naturstoffreagenz A (Table 1).
Table 1.
Flavonoid aglycones in the secretion of Sigesbeckia jorullensis separated by TLC
| Substance zone | RF‐value | Colour after spraying with Naturstoffreagenz A | Abundance |
| 1 | 0·88 | Yellow | +++ |
| 2 | 0·74 | Trace | |
| 3 | 0·62 | Trace | |
| 4 | 0·49 | Orange | ++ |
| 5 | 0·40 | Yellow | ++ |
| 6 | 0·21 | Trace | |
| 7 | 0·12 | Orange | +++ |
Essential oil composition
The essential oil of S. jorullensis was viscous and bright yellow, and its qualitative and quantitative composition did not vary during the vegetative period. One hundred and four volatile components were detected, of which 52 (54·9 %, w/w) could be unambiguously identified (Table 2). A further 33 compounds representing 43·4 % of the sample were partially characterized on the basis of their mass spectral data and their retention indices. Fifty compounds, representing 73·7 % (w/w) of the essential oil, were characterized as terpenes. Main constituents of the 27 sesquiterpenes (35·4 %) were the hydrocarbons germacrene‐d (13·6 %), α‐farnesene (4·9 %), β‐caryophyllene (4·1 %) and trans‐β‐farnesene (4·0 %). Almost all of the 19 diterpenes (together 38·1 %) were oxygenated compounds. The predominant components were epimanoyl oxide (7·4 %) and diterpenes with retention indices of 2480 (12·4 %) and 2031 (8·6 %). Only four monoterpenes (together 0·2 %) were detected. Additional substance classes in the essential oil included n‐alkanes (0·8 %), alkanals (0·5 %), ketones (0·2 %), alcohols (0·2 %), fatty acids (6·6 %) and esters thereof (5·1 %).
Table 2.
Constituents of the essential oil of Sigesbeckia jorullensis
| Components | Retention index | % |
| Monoterpenes | ||
| Sabinene | 970 | tr |
| Myrcene | 991 | 0·1 |
| Limonene | 1026 | tr |
| Thymyl methyl ether | 1234 | 0·1 |
| Sesquiterpenes | ||
| Geijerene (= geyrene) | 1139 | 0·1 |
| Bicycloelemene | 1336 | 0·3 |
| α‐Copaene | 1375 | 0·6 |
| Sesquiterpene hydrocarbon (m/z: 161, 105, 91, 120, 119, 81, 93, . . . 204) | 1388 | 0·1 |
| β‐Elemene | 1391 | 0·2 |
| β‐Caryophyllene | 1418 | 4·1 |
| β‐Cubebene | 1428 | 0·4 |
| trans‐α‐Bergamotene | 1435 | 0·6 |
| Sesquiterpene hydrocarbon (m/z: 161, 91, 105, 79, 93, 81, 41, . . . 204) | 1443 | 0·1 |
| α‐Humulene | 1452 | 1·2 |
| trans‐β‐Farnesene | 1458 | 4·0 |
| Sesquiterpene hydrocarbon (m/z: 161, 105, 119, 93, 91, 79, 81, . . . 204) | 1477 | 0·1 |
| Germacrene‐d | 1482 | 13·6 |
| Sesquiterpene hydrocarbon (m/z: 69, 93, 41, 161, 133, 79, 55, . . . 204) | 1485 | 0·5 |
| Bicyclogermacrene | 1496 | 1·4 |
| α‐Muurolene | 1499 | 0·1 |
| α‐Farnesene | 1509 | 4·9 |
| γ‐Cadinene | 1514 | 0·3 |
| δ‐Cadinene | 1523 | 0·7 |
| 1‐endo‐Bourbonanol | 1574 | 0·1 |
| Oxygenated sesquiterpene (m/z: 117, 43, 99, 70, 83, 85, . . . 187) | 1582 | 0·4 |
| Oxygenated sesquiterpene (m/z: 93, 43, 79, 80, 121, 91, . . . 187) | 1620 | 0·1 |
| Oxygenated sesquiterpene (m/z: 95, 121, 43, 109, 71, 79, 119, . . . 204) | 1642 | 0·4 |
| Oxygenated sesquiterpene (m/z: 161, 119, 121, 105, 95, 79, 43, . . . 204) | 1646 | 0·1 |
| epi‐α‐Muurolol (= T‐Muurolol) | 1654 | 0·6 |
| Oxygenated sesquiterpene (m/z: 67, 81, 79, 41, 95, 55, . . . 189) | 1662 | 0·2 |
| Oxygenated sesquiterpene (m/z: 79, 67, 80, 108, 93, 41, . . . 203) | 1667 | 0·5 |
| Diterpenes | ||
| 8,13‐Epoxy‐15,16‐dinorlab‐12‐ene | 1881 | 0·4 |
| (E,E)‐7,11,15‐Trimethyl‐3‐methylene‐hexadeca‐1,6,10,14‐tetraene | 1918 | 0·1 |
| Epimanoyl oxide | 2014 | 7·4 |
| Oxygenated diterpene (m/z: 69, 81, 93, 41, 107, 95, . . . 290) | 2031 | 8·6 |
| Oxygenated diterpene (m/z: 91, 117, 145, 105, 131, 79, . . . 275) | 2039 | 0·3 |
| Oxygenated diterpene (m/z: 128, 115, 129, 91, 143, 77, . . . 275) | 2054 | 0·1 |
| Phytol | 2112 | 0·2 |
| Oxygenated diterpene (m/z: 120, 43, 93, 138, 121, 81, . . . 275) | 2139 | 0·1 |
| Oxygenated diterpene (m/z: 69, 81, 41, 93, 95, 67, 107, . . . 306 | 2169 | 0·4 |
| Oxygenated diterpene (m/z: 245, 43, 137, 81, 95, 69, . . . 279) | 2177 | 0·2 |
| Oxygenated diterpene (m/z: 69, 109, 41, 81, 137, 43, . . . 276) | 2185 | 0·1 |
| Oxygenated diterpene (m/z: 245, 43, 137, 81, 69, 95, . . . 291) | 2227 | 0·8 |
| Oxygenated diterpene (m/z: 245, 43, 137, 81, 69, 95, . . . 291) | 2242 | 0·8 |
| Oxygenated diterpene (m/z: 91, 81, 79, 105, 93, 123, . . . 286) | 2258 | 0·2 |
| Oxygenated diterpene (m/z: 257, 55, 137, 81, 69, 95, . . . 291) | 2264 | 1·4 |
| Oxygenated diterpene (m/z: 177, 81, 43, 95, 291, 55, . . . 291) | 2318 | 0·1 |
| Oxygenated diterpene (m/z: 245, 137, 43, 81, 69, 95, . . . 309) | 2480 | 12·4 |
| Oxygenated diterpene (m/z: 245, 43, 137, 81, 69, 95, . . . 309) | 2501 | 3·1 |
| Oxygenated diterpene (m/z: 69, 81, 41, 93, 71, 95, 43, . . . 290) | 2406 | 1·6 |
| Alkanes | ||
| Heptacosane | 2699 | 0·6 |
| Nonacosane | 2899 | 0·3 |
| Aldehydes, ketones | ||
| trans‐2‐Hexenal | 846 | 0·1 |
| 2‐Heptanone | 890 | 0·2 |
| n‐Octanal | 1003 | 0·1 |
| n‐Nonanal | 1103 | 0·1 |
| n‐Decanal | 1205 | 0·1 |
| n‐Dodecanal | 1408 | 0·1 |
| Alcohols | ||
| n‐Octanol | 1073 | tr |
| 1‐Dodecanol | 1474 | 0·1 |
| 1‐Tetradecanol | 1675 | 0·1 |
| Acids | ||
| Octanoic acid | 1190 | 4·9 |
| Nonanoic acid | 1276 | 0·7 |
| Decanoic acid | 1370 | 0·8 |
| Dodecanoic acid | 1563 | 0·1 |
| Hexadecanoic acid | 1962 | 0·1 |
| Esters | ||
| Hexanoic acid 2‐methylpropyl ester | 1152 | 0·3 |
| 3‐Methylbutyl hexanoate | 1253 | 0·3 |
| 2‐Methylpropyl octanoate | 1348 | 0·1 |
| Hexanoic acid hexyl ester | 1386 | 0·3 |
| Docosanoic acid methyl ester | 2531 | 1·0 |
| Docosanoic acid ethyl ester | 2596 | 0·5 |
| Tricosanoic acid methyl ester | 2631 | 0·1 |
| Tetracosanoic acid, methyl ester | 2732 | 2·0 |
| Tetracosanoic acid, ethyl ester | 2797 | 0·6 |
| Pentadecanoic acid, methyl ester | 2832 | tr |
| Hexacosanoic acid, methyl ester | 2934 | 0·1 |
| Unknowns | ||
| m/z: 43, 69, 129, . . . 157 | 1161 | 1·2 |
| m/z: 82, 67, 57, 55, 41, 127, . . . 161 | 1578 | 0·1 |
| m/z: 127, 57, 128, 41, 55, 69, . . . 213 | 1703 | 0·1 |
| m/z: 180, 165, 43, 181, 91, 166, . . . 250 | 1707 | 0·2 |
| m/z: 69, 91, 145, 131, 41, 117, . . . 230 | 1916 | 0·1 |
| m/z: 91, 117, 145, 105, 77, 131, . . . 230 | 1977 | 9·0 |
| m/z: 91, 123, 119, 125, 77, 41, . . . 257 | 2036 | 0·2 |
| m/z: 245, 43, 137, 69, 81, 95, . . . 263 | 2496 | 0·3 |
Retention indices were calculated from gas chromatographic separations on a non‐polar stationary phase (column, 50 m CP Sil 5 CB).
tr, trace (< 0·05 %).
Besides the 85 compounds listed above, 19 minor unidentified components (together 1·7 %, w/w) could be detected with GC.
Cations in the lipophilic material
The mean of 30 LAMMA spectra of the lipophilic material of glands located in the outer involucral bracts demonstrated calcium to be the dominant cation (Fig. 5). The second most abundant ion was potassium, followed by sodium, magnesium, manganese and iron.
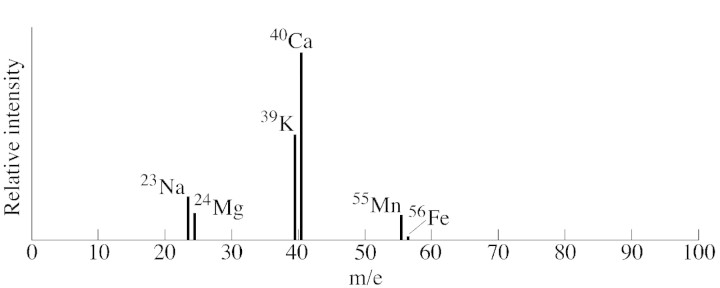
Fig. 5. Mean Lamma spectrum of 30 analyses of the cations found in the secretion of gland hairs from three outer involucral bracts of Sigesbeckiajorullensis.
DISCUSSION
Species of Sigesbeckia and Carpesium are the only members of the Asteraceae known to produce adhesive exudates which assist in epizoic fruit propagation, although mucilaginous glandular trichomes on seeds are found in various plant families (Werker, 2000). In contrast to such trichomes on seeds, the glandular hairs of Sigesbeckia jorullensis produce a sticky lipophilic exudate that adheres to animals even without previous wetting. Whereas the achenes of Carpesium species bear glands producing the exudate, the achenes of S. jorullensis are glabrous and the glands are located in the outer and inner involucral bracts. The diaspores of Sigesbeckia consist of involucral bracts and achenes that are torn out of the capitulum together when dispersed.
The long‐stalked hairs of S. jorullensis are multicellular, bi‐ to triseriate and are trichomes, being derived mostly from epidermal cells. Hairs similar to the short‐stalked trichomes of S. jorullensis have been observed in other members of the Asteraceae, e.g. Artemisia campestris ssp. maritima (Ascensão and Pais, 1987). Some anatomical features of the ten‐celled glandular trichomes of A. campestris ssp. maritima resemble closely those of the long‐stalked hairs of S. jorullensis, e.g. the arrangement of cells with chloro‐ and leucoplasts.
Many essential oil‐producing glandular tissues are non‐photosynthetic (Fahn, 1979). This is the case for the apical row of head cells of Artemisia campestris (Ascensão and Pais, 1987) and S. jorullensis, which contain only leucoplasts, whereas the lower tiers of cells contain many chloroplasts. The chloroplast‐bearing cells of S. jorullensis contain tannins and flavonoids, as demonstrated by histochemical tests and, since there was no evidence for the occurrence of terpenoids in these cells, the apical layer of cells is likely to be the main site of terpenoid production. The photosynthetic cells, comprising the greatest part of the glandular head, might be important for the synthesis of terpene precursors, using photosynthetic products generated locally or delivered from other parts of the plant.
It is noteworthy that small veins only are present close to the stalk of the glandular hairs, and that they are composed mostly of tracheids. The tracheids supply the glands with water and ions, with Ca2+ being the dominant cation. Although the secretion of S. jorullensis is calcium‐rich, as demonstrated by LAMMA, the supply of Ca2+ is so great that excess Ca2+ has to be stored in one vacuole of each head cell. This is the only location in the entire outer involucral bracts and their trichomes where calcium oxalate crystals can be found. Calcium oxalate crystals have also been identified in lipid‐secreting trichomes of Inula viscosa (Werker and Fahn, 1981), Artemisia campestris ssp. maritima (Ascensão and Pais, 1987) and in chlorophyll‐free head cells of long‐stalked hairs of Nicotiana tabacum and N. sylvestris (Meyberg et al., 1991).
Terpenoids have many different functions in plants. Some volatile terpenoids may attract pollinating insects to flowers, while others may protect the plant and its reproductive structures from destruction by herbivores and other pathogens (Kelsey et al., 1984; Harborne, 1993). Monoterpenes dominate the essential oils of conifers (Kubeczka and Schultze, 1987), Rutaceae species (Heinrich and Schultze, 1985) and the Lamiaceae (Werker et al., 1985; Lawrence, 1992); and sesquiterpene lactones, in association with mono‐ and sesquiterpenes, are characteristic constituents of the essential oils and resins of the Asteraceae (Spring, 2000). In S. jorullensis, sesquiterpenes and other terpenes of higher molecular mass are the main components of the sticky oil. In addition to the 27 sesquiterpenes, 18 compounds of the essential oil were found to be oxygenated diterpenes, which probably contribute much of the stickiness.
Because the adhesive strength of the secretion is so high that involucral bracts and achenes are torn out of the capitulum together, one might expect to find small insects trapped by the gland cells of the bracts. However, this was not observed in this study. It is possible that some volatile constituents of the secretion are repellent to insects, as reported for many other terpenoids (Harrewijn et al., 1995). Compounds such as sesquiterpene lactones with antiherbivory properties are common secondary products in members of the Asteraceae, including Sigesbeckia species (Baruah et al., 1979, 1980; Zdero et al., 1991) and closely related genera (Quijano et al., 1997; Bardon et al., 2001). In this study, sesquiterpene lactones were not unambiguously detected, although some of the unidentified compounds listed in Table 2 may have been sesquiterpene lactones. However, these compounds are usually isolated from plant material by extraction with organic solvents rather than by steam distillation, and analysed and identified by methods such as LC‐MS and LC‐NMR which were not applied in this study.
Cheniclet and Carde (1985) proposed that the abundance of leucoplasts correlates with the amount of monoterpenes in the essential oils of various plant species. Although cells of the apical layer of the glandular head contain numerous leucoplasts, the essential oil of Sigesbeckia contains only a small proportion of monoterpenes. Thus, the observations by Cheniclet and Carde (1985) cannot be generalized to all essential oil‐producing plants. In higher plants, the terpenoid precursor isopentenyl diphosposphate (IPP) is synthesized in two different pathways (Lichtenthaler, 1999; Eisenreich et al., 2001), and the site of the recently established 1‐deoxy‐d‐xylulose‐5‐phosphate (DOXP) pathway is in plastids, where IPP is subsequently metabolized to isoprene, mono‐, di‐ and tetraterpenes (carotenoids). The classical acetate/mevalonate pathway of IPP synthesis proceeds in the cytosol and generates sesqui‐, tri‐ and polyterpenes, although partial export of IPP from plastids into the cytoplasm can also occur (Lichtenthaler, 2000). From the present results of light and scanning electron microscopy, it is not possible to identify the mechanism of terpene biosynthesis employed in Sigesbeckia.
Many terpenoid‐synthesizing plants often produce free flavonoid aglycones, and most of these have been reported from the asteraceae (Wollenweber and Dietz, 1981). Light microscopic observations, the chromatographic behaviour at TLC on polyamide and the light absorption properties of the separated compounds indicate the presence of flavonoid aglycones in the secretion of S. jorullensis.
ACKNOWLEDGEMENTS
This paper is dedicated to Professor Dr O. Härtel on the occasion of his 90th birthday. The work was supported by the Jubiläumsfonds of the Österreichische Nationalbank (Project 5324, Heinrich).
Supplementary Material
Received: 3 July 2001 Returned for revision: 14 September 2001; Accepted: 13 December 2001.
References
- AscensãoL, Pais MS.1987. Glandular trichomes of Artemisia campestris (ssp. maritima): ontogeny and histochemistry of the secretory product. Botanical Gazette 148: 221–227. [Google Scholar]
- BardonA, Cardona L, Cartagena E, Catalan CAN, Pedro JR.2001. Melampolides from Enydra anagalis Phytochemistry 57: 125–130. [DOI] [PubMed] [Google Scholar]
- BaruahRN, Sharma RP, Thyagarajan G, Herz W, Govindan SV. 1980. New melampolides and darutigenol from Sigesbeckia orientalis Phytochemistry 19: 323–325. [Google Scholar]
- BaruahRN, Sharma RP, Madhusudanan KP, Thyagarajan G, Herz W, Murari R.1979. A new melampolid from Sigesbeckia orientalis Phytochemistry 18: 991–994. [Google Scholar]
- BremerK.1994. Asteraceae. Cladistics and classification. Portland, Oregon: Timber Press. [Google Scholar]
- CainAJ.1947. The use of Nile blue in the examination of lipids. Quarterly Journal of Microscopy Science 88: 383–392. [Google Scholar]
- CamonicaL, Rindone B, Scolastico C. 1969. A new diterpenoid with pimarane skeleton. Tetrahedron Letters 54: 4801–4804. [Google Scholar]
- ChenicletC, Carde JP. 1985. Presence of leucoplasts in secretory cells and of monoterpenes in the essential oil: a correlative study. Israel Journal of Botany 34: 219–238. [Google Scholar]
- DaviesNW1990. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. Journal of Chromatography 503: 1–24. [Google Scholar]
- EisenreichW, Rohdich F, Bacher A.2001. Deoxyxylulose phosphate pathway to terpenoids. Trends in Plant Science 6: 78–84. [DOI] [PubMed] [Google Scholar]
- FahnA.1979. Secretory tissues in plants. London, New York, San Francisco: Academic Press. [Google Scholar]
- HarborneJB. 1993Introduction to ecological biochemistry 4th edn. London: Academic Press. [Google Scholar]
- HarrewijnP, Minks AK, Mollema C.1995. Evolution of plant volatile production in insect‐plant relationships. Chemoecology 5/6: 55–73. [Google Scholar]
- HeinenHJ, Vogt H, Wechsung R.1981. Laser desorption mass spectrometry with LAMMA. Analytical Chemistry 308: 290–296. [Google Scholar]
- HeinrichG. 1990. Laser physical methods: Laser microprobe mass spectrometry. In: Linskens HF, Jackson JF, eds. Modern methods of plant analysis New series 11. Physical methods in plant sciences Berlin, Heidelberg: Springer‐Verlag, 58–94. [Google Scholar]
- HeinrichG, Schultze W 1985. Composition and site of biosynthesis of the essential oil in fruits of Phellodendron amurense Rupr. (Rutaceae). Israel Journal of Botany 34: 205–217. [Google Scholar]
- KelseyRG, Reynolds GW, Rodriguez E.1984. The chemistry of biologically active constituents secreted and stored in plant glandular trichomes. In: Rodriguez E, Healey PL, Metha J, eds. Biology and chemistry of plant trichomes New York: Plenum Press, 187–241. [Google Scholar]
- KimJH, Han KD, Yamasaki K, Tanaka O.1979. Darutoside, a diterpenoid from Sigesbeckia pubescens and its structure revision. Phytochemistry 18: 894–895. [Google Scholar]
- KubeczkaKH, Schultze W.1987. Biology and chemistry of conifer oils. Flavour and Fragrance Journal 2: 137–148. [Google Scholar]
- LawrenceBM 1992. Chemical components of labiate oils and their exploitation. In: Harley RM, Reynolds T, eds. Advances of Labiatae science Richmond: The Royal Botanic Gardens Kew, 399–436. [Google Scholar]
- LichtenthalerHK.1999. The 1‐deoxy‐d‐xylulose‐5‐phosphate pathway of isoprenoid biosynthesis in plants. Annual Review of Plant Physiology and Plant Molecular Biology 50: 47–65. [DOI] [PubMed] [Google Scholar]
- LichtenthalerHK.2000. Sterols and isoprenoids. Biochemical Society Transactions 28: 785–789. [PubMed] [Google Scholar]
- LiuK, Röder E.1991. Diterpene aus Siegesbeckia glabrescens Planta Medica 57: 395–396. [DOI] [PubMed] [Google Scholar]
- MeybergM, Krohn S, Brümmer B, Kristen U 1991. Ultrastructure and secretion of glandular trichomes of tobacco leaves. Flora 185: 357–363. [Google Scholar]
- MurakamiT, Isa T, Satake T. 1973. Eine Neuuntersuchung der Inhaltsstoffe von Sigesbeckia pubescens M. Tetrahedron Letters 50: 4991–4994. [Google Scholar]
- QuijanoL, Nunez IS, Fronczek FR, Fischer NH.1997. A guaianolide and four melampolides from Melampodium leucanthum Phytochemistry 45: 769–775. [Google Scholar]
- ReeveRM. 1951. Histochemical tests for polyphenols in plant tissues. Stain Technology 26: 91–96. [DOI] [PubMed] [Google Scholar]
- SpringO.2000. Chemotaxonomy based on metabolites from glandular trichomes. Advances in Botanical Research 31: 153–174. [Google Scholar]
- StaffordHA, Lester HH, Weider RM. 1987. Histochemical assay of proanthocyanidin inheterogeneity in cell cultures. Plant Science 52: 99–104. [Google Scholar]
- StahlE. 1953. Eine neue Apparatur zur gravimetrischen Erfassung kleinster Mengen ätherischer Öle. Mikrochemie 40: 367–372. [Google Scholar]
- TutinTG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA 1976Flora europaea 4 London, New York, Melbourne: Cambridge University Press, 140–141. [Google Scholar]
- van den DoolH, Kratz PD.1963. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. Journal of Chromatography 11: 463–471. [DOI] [PubMed] [Google Scholar]
- WagenitzG. 1979. Compositae I: Allgemeiner Teil, Eupatorium – Achillea. In: Conert HJ, Hamann U, Schultze‐Motel W, Wagenitz G, eds. Illustrierte Flora von Mitteleuropa G. Hegi. VI, 3. Hamburg, Berlin: Parey, 237–239. [Google Scholar]
- WerkerE.2000. Trichome diversity and development. Advances in Botanical Research 31: 1–35. [Google Scholar]
- WerkerE, Fahn A. 1981. Secretory hairs of Inula viscosa (L.) Ait.‐ development, ultrastructure, and secretion. Botanical Gazette 142: 461–476. [Google Scholar]
- WerkerE, Ravid U, Putievsky E. 1985. Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae Israel Journal of Botany 34: 31–45. [Google Scholar]
- WollenweberE. 1982. Flavones and flavonols. In: Harborne JB, Mabry TJ, eds. The flavonoids: advances in research London: Chapman and Hall, 189–259. [Google Scholar]
- WollenweberE, Dietz VH.1981. Occurrence and distribution of free flavonoid aglycones in plants. Phytochemistry 20: 869–932. [Google Scholar]
- ZderoC, Bohlmann F, King RM, Robinson H.1991. Sesquiterpene lactones and other constituents from Siegesbeckia orientalis and Guizotia scabra Phytochemistry 30: 1579–1584. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


