Abstract
Nitrogen nutrition can influence cluster root formation in many wild species, but the effect of N form on cluster root formation and root exudation by white lupin is not known. In a solution culture study, we examined the effect of N nutrition (ammonium, nitrate, both or N2 fixation) on cluster root formation and H+ extrusion by white lupin plants under deficient and adequate P supply. The number of cluster roots increased greatly when plants were supplied with 1 μm P compared with 50 μm P, the increase being 7·8‐fold for plants treated with (NH4)2SO4, 3‐fold for plants treated with KNO3 and NH4NO3, and 2·4‐fold for N2‐fixing plants. Under P deficiency, NH4+‐N supply resulted in production of a greater number and biomass of cluster roots than other N sources. Dry weight of cluster roots was 30 % higher than that of non‐cluster roots in P‐deficient plants treated with (NH4)2SO4 and NH4NO3. In plants treated with sufficient P (50 μm), the weight of non‐cluster roots was approx. 90 % greater than that of cluster roots. Both total (μmol per plant h–1) and specific (μmol g–1 root d. wt h–1) H+ extrusions were greatest from roots of plants supplied with (NH4)2SO4, followed by those supplied with NH4NO3 and N2 fixation, whereas plants receiving KNO3 had negative net H+ extrusion between the third and fifth week of growth (indicating uptake of protons or release of OH– ions). The rate of proton extrusion by NH4+‐N‐fed plants was similar under P‐deficient and P‐sufficient conditions. In contrast, proton exudation by N2‐fixing plants and KNO3‐treated plants was ten‐fold greater under P deficiency than under P sufficiency. In comparison with P deficiency, plants treated with 50 μm P had a significantly higher concentration of P in roots, shoots and youngest expanded leaves (YEL). Compared with the N2 fixation and KNO3 treatments, total N concentration was highest in roots, shoots and YEL of plants supplied with (NH4)2SO4 and NH4NO3, regardless of P supply. Under P deficiency, K concentrations in roots decreased at all N supplies, especially in plants treated with (NH4)2SO4 and NH4NO3, which coincided with the greatest H+ extrusion at these P and N supplies. In conclusion, NH4‐N nutrition stimulated cluster root formation and H+ extrusion by roots of P‐deficient white lupin.
Key words: Cluster roots, H+ extrusion, mineral composition, N2 fixation, N nutrition, P deficiency, white lupin
INTRODUCTION
Formation of cluster roots is one adaptation to nutrient acquisition (Gerke et al., 1994; Marschner, 1995; Keerthisinghe et al., 1998; Neumann et al., 1999, 2000; Neumann and Rõmheld, 1999). The release of large amounts of citrate from cluster roots of P‐deficient white lupin is an efficient strategy for chemical mobilization of sparingly soluble P sources in the rhizosphere (Neumann et al., 1999). It has been shown that phosphorus deficiency increases proton extrusion by roots, results in an excess cation concentration in roots and shoots, and enhances the exudation of citrate and malate by white lupin plants (Sas et al., 2001). Localized rhizosphere acidification and organic acid anion extrusion not only mobilize phosphorus, but also iron, manganese and zinc in the rhizosphere and increase their rates of uptake and their contents in plants (Marschner, 1995). Thus, white lupin may be regarded as a model system for plant adaptations related to chemical mobilization of nutrients in the rhizosphere (Neumann et al., 1999, 2000; Neumann and Römheld, 1999).
Many environmental factors may stimulate cluster root formation. Generally, cluster roots are produced under conditions of low phosphate, while their initiation is generally suppressed at higher levels of phosphate supply (Lamont, 1972a, b; Gardner et al., 1983; Marschner et al., 1987). However, it has been demonstrated by Marschner et al. (1987), Johnson et al. (1996a) and Keerthisinghe et al. (1998) that cluster roots may form at moderate or even adequate P levels.
In several species, cluster root formation is also influenced by nitrogen nutrition. Myrica gale seedlings supplied with urea formed root clusters more quickly than seedlings supplied with nitrate (Crocker and Schwintzer, 1993). In Gymnostoma papuanum, formation of root clusters increased with nitrogen supply in the order: NH4+‐N < N2 fixation < nitrate‐N (Racette et al., 1990). At low P supply, cluster root formation was enhanced by low N supply and depressed by high N supply in Hakea spp. (Lamont, 1972a, b). In contrast, cluster root formation in P‐deficient Myrica gale (Crocker and Schwintzer, 1993) and Grevillea robusta (Moore and Kairaitis, 1966) was greater when plants were supplied with adequate N compared with no nitrogen. The form of nitrogen had no effect on cluster root formation in Myrica cerifera (Louis et al., 1990). In white lupin, low levels of N enhanced P deficiency‐induced formation of cluster roots, whereas high N supply had inhibitory effects (Dinkelaker et al., 1995). It is not known, however, how different N forms influence cluster root formation in this species.
The aims of this study were to elucidate the effects of N nutrition on cluster root formation, root morphology and mineral composition of white lupin plants grown under P deficiency or P sufficiency. Special emphasis was placed on characterizing the relationship between P and N supply and root extrusion of H+.
MATERIALS AND METHODS
Plant cultivation
Seeds of white lupin (Lupinus albus L., ‘Kiev Mutant’) were pre‐germinated on a stainless‐steel mesh in a 15 l plastic container containing an aerated solution of 1 mm CaCl2 and 5 µm H3BO3. Plants in the N2‐fixation treatment were inoculated with Bradyrhizobium sp. (Lupinus) WU 425 during the germination stage as well as in the first week of the experiments. Plants were grown in nutrient solution in a glasshouse to facilitate daily pH control. The nutrient solution had the following composition (in µm): 600 K2SO4, 200 MgSO4, 600 CaCl2, 10 FeEDDHA, 0·2 CoSO4, 0·03 Na2MoO4, 5 H3BO3, 0·75 ZnSO4, 1 MnSO4 and 0·2 CuSO4.
N and P treatments
White lupin plants, with their cotyledons removed, were treated with 0·5 mm N as either (NH4)2SO4, KNO3 or NH4NO3 (all treatments non‐inoculated with Bradyrhizobium sp.), while N2‐fixing plants were inoculated with Bradyrhizobium sp. No nitrogen was added to the solution for the initial period before N2 fixation took over. All solutions were supplied with either 1 or 50 µm P as KH2PO4. The lowest P concentration causing intensive formation of cluster roots is 1 µm P, while 50 µm P is twice the amount (25 µm) that suppresses cluster root formation by white lupin plants (Keerthisinghe et al., 1998; Sas et al., 2001). Each experimental combination consisted of triplicate 5·5 l pots, each with five plants. The pH of the nutrient solution was buffered at 5·6 by 0·2 mm MES and was adjusted daily using 0·1 m KOH or 0·1 m HCl. The volume of KOH used was recorded to enable calculations of proton extrusion. Nutrient solutions were aerated continuously and renewed every 2 d. Root temperature was maintained at 20 °C using a water bath.
Plant harvests
Starting from the first week of treatments (7‐d‐old plants), one plant from each pot was harvested weekly. At each harvest, the number of cluster roots was recorded. Cluster roots were defined as those portions of secondary lateral roots bearing bottlebrush‐like clusters with a density of ten or more rootlets per cm (Johnson et al., 1996a). At the final harvest, after 35 d of N and P treatments, total root length, root area, root volume and the number of root tips were determined separately for cluster and non‐cluster roots using a Hewlett Packard scanner (HP ScanJet 6100C) controlled by WinRhizo software (Regent Instruments Inc., Quebec, Canada; Arsenault et al., 1995).
Determination of dry biomass
Dry biomass was determined for roots, shoots and young expanded leaves (YEL) separately, in accordance with the analytical procedure of Ostrowska et al. (1991). Approx. 7 g of air‐dried plant material was dried at 105 °C for 6 h, cooled for 0·5 h in a desiccator filled with silica gel and subsequently weighed. The weighing and drying procedure was repeated until the samples achieved constant weight.
Wet mineralization of plant material to determine total nitrogen (Polish standard PN‐73C‐04576/12)
Plant material for the analysis of mineral composition was dried at 60 °C for 48 h, ground to pass through a 1‐mm sieve, mixed thoroughly, mineralized in Kjeldahl flasks using the wet method with H2SO4 and catalysts H2O2 and CuSO4.5 H2O (Ostrowska et al., 1991), cooled and diluted with deionized water to the specified volume. Total nitrogen was determined using the standard Kjeldahl method based on distillation of ammonia with steam from the alkalized solution, and determining the ammonia content in the distillate by titration.
Wet mineralization of plant material to determine macro‐ and micro‐elements
Ground plant material (see above) was mineralized in teflon‐coated containers in a microwave oven in a 5 : 1 mixture of HNO3 and H2O2 under controlled temperature and pressure. The macro‐ and micro‐element content in diluted digests was determined by an inductively coupled plasma emission spectrometer (ICP) (Cygañski, 1997).
Statistical analysis
Statistical analysis was carried out using single factor ANOVA. Comparisons of means were performed at P < 0·05.
RESULTS
The effect of N and P supply on formation and biomass of cluster roots
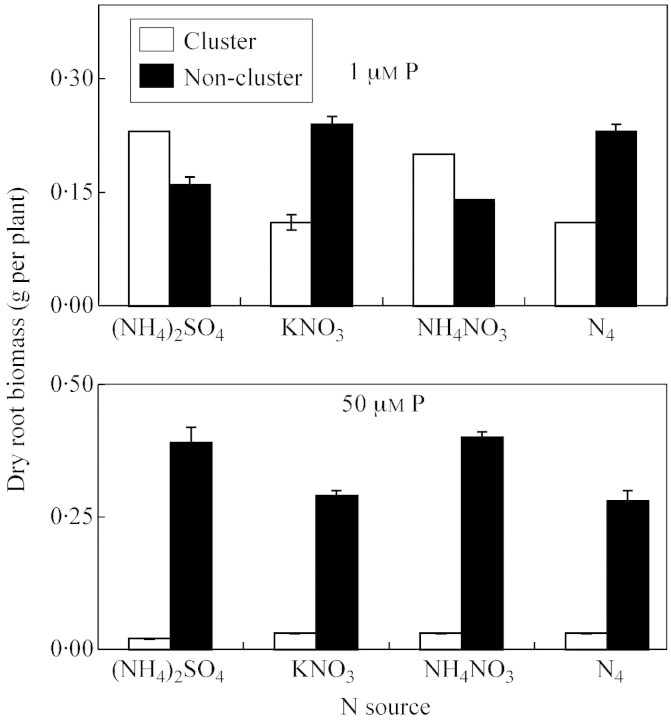
The number of cluster roots increased greatly when plants were supplied with 1 μm P in comparison with 50 μm P, the increase being 7·8‐, 2·9‐, 3·1‐ and 2·4‐fold for (NH4)2SO4, KNO3, NH4NO3 and N2‐fixation treatments, respectively (Fig. 1). These differences were statistically significant. The dry root biomass of cluster roots increased significantly when plants were treated with 1 μm P in comparison with 50 μm P, the increase being 11·5‐, 3·7‐, 6·7‐ and 3·7‐fold for (NH4)2SO4, KNO3, NH4NO3 and N2‐fixation treatments, respectively (Fig. 2). In contrast, under P sufficiency (50 μm P), the dry weight of non‐cluster roots was 1·2–2·9‐times greater than under P deficiency (depending on the N source). At 1 μm P, NH4‐N nutrition increased the dry biomass of cluster roots 1·4‐fold, whereas KNO3 treatment and N2 fixation decreased their biomass by approx. two‐fold in comparison with P sufficiency. Phosphorus supply considerably influenced root length and the ratio of cluster to non‐cluster roots (Fig. 3). Under P deficiency, cluster roots were significantly longer than non‐cluster roots, the increase being greater in the case of (NH4)2SO4 and NH4NO3 nutrition compared with other N sources (Fig. 3). However, for non‐cluster roots, the reverse relationship was observed.
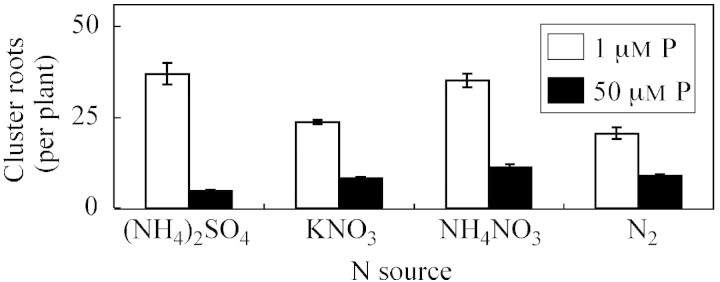
Fig. 1. Number of cluster roots formed per L. albus (‘Kiev Mutant’) plant after 35 d of growth in nutrient solutions containing different N forms and P concentrations. Bars represent ± s.e. (n = 3).
Fig. 2. Dry biomass of cluster and non‐cluster roots of L. albus (‘Kiev Mutant’) after 35 d of growth in nutrient solutions containing different N forms and P concentrations. Bars represent ± s.e. (n = 3).
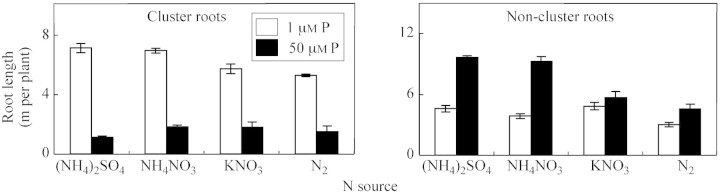
Fig. 3. Total length of cluster and non‐cluster roots of L. albus plants after 35 d of growth in nutrient solutions containing different N forms and P concentrations. Bars represent ± s.e. (n = 3).
Proton extrusion in relation to N and P treatment
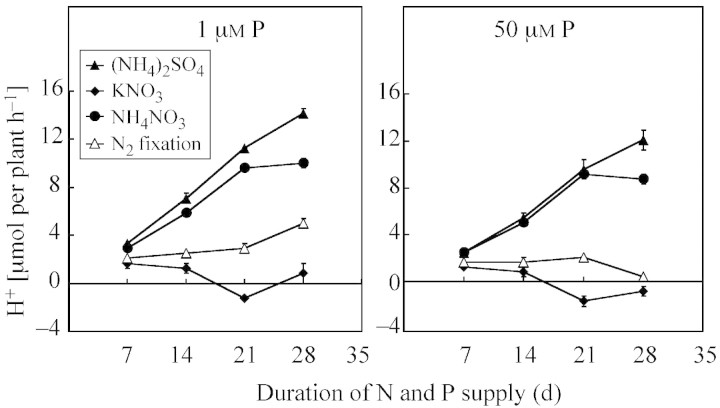
Proton excretion from roots of white lupin was highly dependent on P and N supplies (Figs 4 and 5). Total H+ extrusion (μmol per plant h–1) was highest when plants were supplied with (NH4)2SO4 and decreased under NH4NO3, N2 fixation and KNO3 supply (Fig. 4). Similarly, the highest specific H+ exudation (μmol g–1 root d. wt h–1) (Fig. 5) was observed when plants were supplied with (NH4)2SO4 or NH4NO3. In contrast, KNO3 nutrition caused a release of OH– ions.
Fig. 4. Total H+ extrusion by L. albus plants grown in nutrient solutions under different N forms and at 1 and 50 µm P for 7–35 d. Bars represent ± s.e. (n = 3).
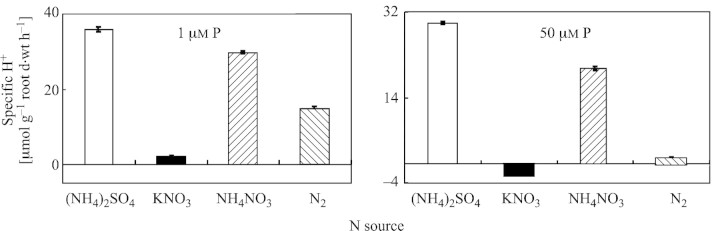
Fig. 5. Specific H+ extrusion by L. albus plants after 35 d of growth in nutrient solutions containing different N forms and either 1or 50 µm P. Bars represent ± s.e. (n = 3).
The effect of P and N supply on mineral composition of white lupin
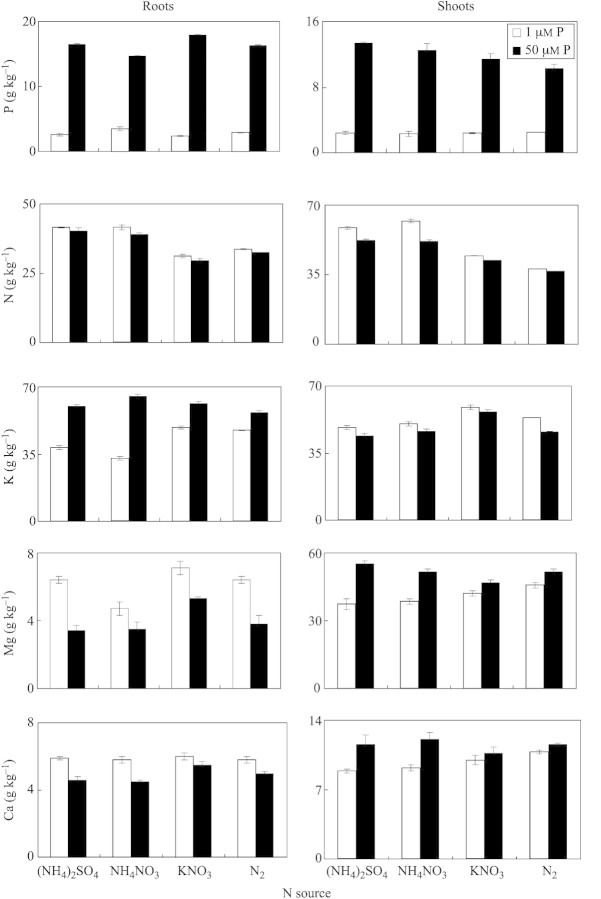
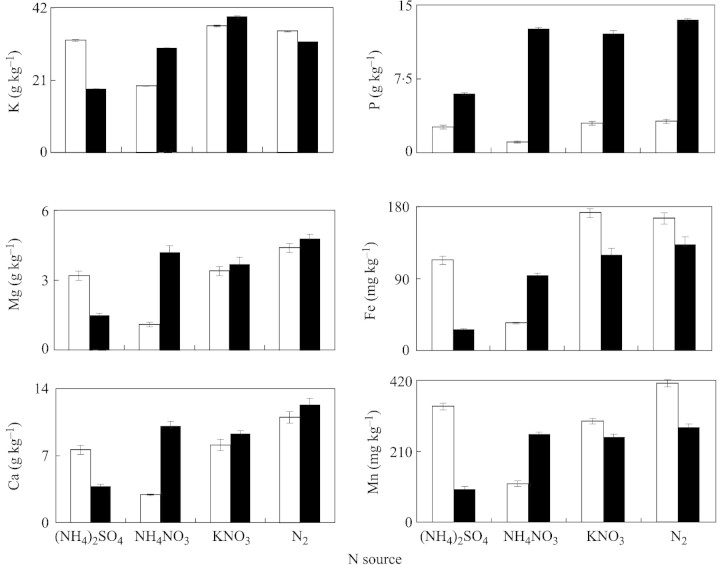
Mineral element concentrations in plants were greatly influenced by P and N nutrition. Treatment of plants with 50 µm P increased the concentration of P in roots and shoots (Fig. 6). Compared with KNO3 or N2 fixation treatments, total nitrogen concentrations in roots and shoots were highest in the case of (NH4)2SO4 and NH4NO3 nutrition (Fig. 6). Root potassium concentration increased significantly when plants were supplied with 50 µm P, the increase being 1·6‐, 2·0‐, 1·3‐ and 1·2‐fold for (NH4)2SO4, NH4NO3, KNO3 and N2‐fixation treatments, respectively (Fig. 6).
Fig. 6. Root and shoot concentrations of P, N, K, Mg and Ca in white lupin after 35 d of growth in nutrient solutions containing different N forms and P concentrations. Bars represent ± s.e. (n = 3).
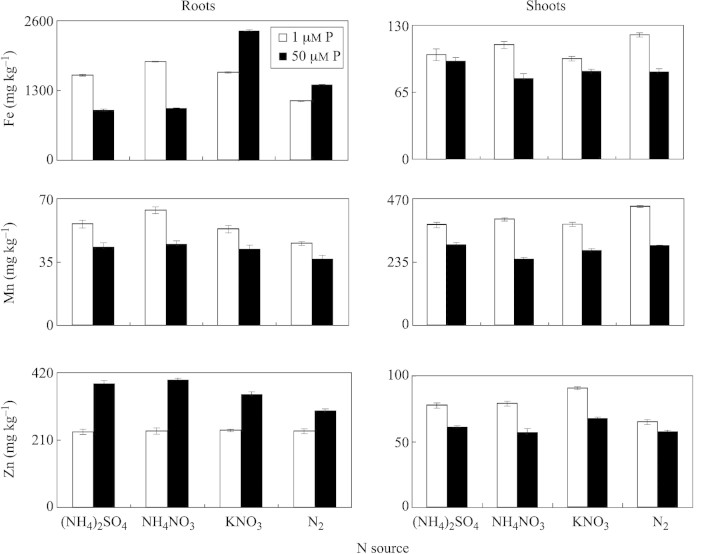
Magnesium and calcium concentrations in roots increased under P deficiency, whereas the reverse was observed in shoots. Regardless of P and N treatment, Fe and Zn concentrations were significantly higher in roots than in shoots, but the reverse was true for Mn (Fig. 7). Compared with phosphorus sufficiency, P deficiency increased the concentration of Mn in roots and shoots at all N supplies. Irrespective of N nutrition, insufficient P supply decreased the Zn concentration in roots and increased it in shoots in comparison with P sufficiency. Generally, P deficiency increased Fe, Mn and Zn in shoots, regardless of N treatment (Fig. 7). Irrespective of N supply, treatment of plants with 1 µm P significantly decreased the P concentration in young expanded leaves, as compared with P sufficiency (Fig. 8). Phosphorus deficiency and (NH4)2SO4 nutrition significantly increased concentrations of K, Mg, Ca, Fe and Mn in YEL, whereas this tendency was not clear for other N treatments.
Fig. 7. Root and shoot concentrations of Fe, Mn and Zn in white lupin after 35 d of growth in nutrient solutions containing different N forms and P concentrations. Bars represent ± s.e. (n = 3).
Fig. 8. Concentrations of P, K, Mg, Ca, Fe and Mn in young expanded leaves of white lupin after 35 d of growth in nutrient solutions containing different N forms and P concentrations. Bars represent ± s.e. (n = 3).
DISCUSSION
The effect of N and P supply on formation and biomass of cluster roots
The large increase in cluster root number under 1 µm P (Fig. 1) confirmed earlier findings that phosphorus deficiency induces cluster root formation in white lupin (e.g. Gardner et al., 1983; Marschner et al., 1987; Johnson et al., 1994, 1996a, b; Keerthisinghe et al., 1998; Neumann et al., 1999, 2000; Neumann and Römheld, 1999; Sas et al., 2001). However, we showed for the first time that under P deficiency the greatest number of cluster roots (37 per plant) was obtained when plants were supplied with (NH4)2SO4, followed by NH4NO3 (35 per plant), KNO3 (24 per plant) and N2 fixation (20 per plant). It is suggested, therefore, that NH4+‐N is a powerful stimulant of cluster root formation in P‐deficient white lupin. Similarly, species in the Proteaceae utilize NH4+ as the predominant N source and also form extensive cluster roots (Stock and Lewis, 1984). Conversely, Diem and Arahou (1996) found that Casuarina glauca only produces cluster roots when grown in nitrate, with no cluster roots being produced in ammonium. Riley and Barber (1971) suggested that the N source might lead to a feedback on cluster root formation in soil via P. If the N source was ammonium, the excess protons released into the rhizosphere could free up P, thus preventing cluster root formation because the threshold level needed for cluster root production would not be crossed due to adequate P being available via the assimilatory consequences of the N source. However, Reddell et al. (1986) found that cluster root production in Casuarinaceae is unaffected by the available forms of N used.
Under 1 µm P, the dry biomass of cluster roots was significantly greater than that of non‐cluster roots only in plants treated with (NH4)2SO4 and NH4NO3, whereas for other P sources the reverse was observed (Fig. 2). Under P deficiency, cluster roots were significantly longer than non‐cluster roots. However, for non‐cluster roots, the reverse relationship was observed (Fig. 3). The number of root tips, root area and root volume followed similar relationships as shown for root length (data not shown). Similarly, in a previous study, Sas et al. (2001) showed that P deficiency (1 μm) significantly increased the total root length and the number of root tips of white lupin as compared with higher levels of P (5 and 25 μm).
Proton extrusion in relation to N and P treatment
The form of nitrogen supplied has a strong impact on the uptake pattern of nutrients, cellular pH regulation and the rhizosphere pH (Raven 1985; Marschner, 1995). Rhizosphere acidification can be caused by excess uptake of cations over anions, and alkalization occurs when anion uptake exceeds cation uptake. Ammonium uptake is generally associated with acidification, while nitrate nutrition induces an increase in rhizosphere pH.
Little is known about the mechanism of citrate exudation from cluster roots. Since P deficiency in white lupin (Dinkelaker et al., 1989; Neumann et al., 1999) and citrate excretion from cluster roots coincide with rhizosphere acidification (Marschner et al., 1987; Dinkelaker et al., 1989), it has been suggested that citrate may be excreted via an anion channel, with a concomitant release of protons to maintain charge balance (Dinkelaker et al., 1989; Johnson et al., 1996b; Neumann et al., 1999; Sas et al., 2001). However, the relationship between N nutrition and proton release in white lupin has not yet been elucidated.
In our study proton excretion from roots of white lupin was highly dependent on P and N supplies (Figs 4 and 5). Both total and specific H+ extrusion increased under P deficiency and (NH4)2SO4 or NH4NO3 nutrition. In contrast, KNO3 nutrition caused a release of OH– ions between days 21 and 35. Interestingly, after 35 d in the NH4+ treatment, the amounts of proton released were similar in P‐deficient and P‐adequate plants, whereas in N2‐fixing plants and those treated with KNO3 phosphorus deficiency increased proton release ten‐fold (Fig. 4). Similarly, in the previous study, P deficiency increased proton extrusion, with the greatest H+ extrusion by N2‐fixing white lupin plants being associated with both organic acid anion exudation and excess uptake of cations over anions (Sas et al., 2001).
The effect of P and N supply on mineral composition of white lupin
In comparison with P deficiency, 35‐d‐old plants treated with 50 µm P had a 6·3‐, 4·2‐, 7·5‐ or 5·6‐fold higher concentration of P in roots and a 5·6‐, 5·4‐, 4·8‐ and 4·1‐fold higher concentration in shoots when supplied with (NH4)2SO4, NH4NO3, KNO3 and N2‐fixation, respectively (Fig. 6). Sas et al. (2001) reported that increasing P supply led to an increased concentration of P and also of K, S, Zn, Fe and Cu in whole roots. At 1 µm P, phosphorus concentrations in roots and shoots were similar (between 2·4 and 3·5 g kg–1 in roots and 2·3 and 2·5 g kg–1 in shoots), regardless of the N form supplied to the plants. The highest N concentrations in roots and shoots under P deficiency and ammonium nutrition (in both (NH4)2SO4 and NH4NO3 treatments) coincided with the greatest number and largest biomass of cluster roots (Figs 1 and 2) and most H+ extrusion (Figs 4 and 5). Conversely, Lamont (1973) reported that increasing N status in Hakea shoots led to a decrease in cluster root formation.
In comparison with the 1 µm P treatment, the root potassium concentration increased significantly when plants were supplied with 50 µm P (Fig. 6). However, in shoots, the reverse was observed. Sas et al. (2001) reported that K concentration in roots was significantly lower at 1 µm P than at 25 µm P, although the K concentration in shoots was not affected by P supply. In the present study, under P deficiency, treatment of plants with (NH4)2SO4 and NH4NO3 decreased K in roots and shoots, which coincided with the greatest H+ extrusion at these N supplies. Since cluster roots of white lupin are the main site for release of H+ and organic anions under P deficiency (Keerthisinghe et al., 1998; Neumann et al., 1999), it is speculated that the release of organic anions is accompanied by K+ efflux (co‐transport). This deserves further investigation. Further work could include a comparison of K levels in cluster and non‐cluster roots under low P. Phosphorus deficiency increased Fe, Mn and Zn in shoots, regardless of N treatment (Fig. 7). We found that phosphorus deficiency and (NH4)2SO4 nutrition significantly increased K, Mg, Ca, Fe and Mn concentrations in young expanded leaves (Fig. 8). Lu and Zhang (1995) also reported that P deficiency led to an increase in Fe, Cu and Zn absorption and transport in lupin plants.
CONCLUSIONS
Phosphorus deficiency increased cluster root formation and the ratio of cluster roots to non‐cluster roots. NH4+‐N stimulated production of a greater number, length and biomass of cluster roots compared with other N sources. Under P deficiency, K concentration in roots decreased at all N supplies, but especially in plants treated with (NH4)2SO4 and NH4NO3, which coincided with the greatest H+ extrusion in these P and N treatments. Total and specific H+ extrusion occurred in the order: (NH4)2SO4 > NH4NO3 > N2 fixation > KNO3 nutrition. The mechanisms of cluster root initiation and formation, and root exudation in relation to N nutrition deserve further investigation.
Supplementary Material
Received: 25 June 2001; Returned for revision: 1 November 2001; Accepted: 6 January 2002.
References
- ArsenaultJL, Poulcur S, Messier C, Guay R.1995. WinRHIZO a root‐measuring system with a unique overlap correction method. HortScience 30: 906. [Google Scholar]
- CrockerLJ, Schwintzer CR.1993. Factors affecting formation of cluster roots in Myrica gale seedlings in water culture. Plant and Soil 152: 287–298. [Google Scholar]
- CygañskiA.1997. Metody spektroskopowe w chemii analitycznej. Warszawa: Wydawnictwa Naukowo‐Techniczne. [Google Scholar]
- DiemHG, Arahou M.1996. A review of cluster root formation: a primary strategy of Casuarinaceae to overcome soil nutrient deficiency. In: Pinyopusarerk K, Turnbull JW, Midgley SJ, eds. Recent Casuarina research and development. Proceedings of the Third International Casuarina Workshop, Da Nang, Vietnam. Forestry and Forest Products. Collingwood, Australia: CSIRO. [Google Scholar]
- DinkelakerB, Hengeler C, Marschner H.1995. Distribution and function of proteoid roots and other root clusters. Botanica Acta 108: 183–200. [Google Scholar]
- DinkelakerB, Römheld V, Marschner H.1989. Citric acid excretion and precipitation of calcium in the rhizosphere of white lupin (Lupinus albus L.). Plant, Cell and Environment 12: 285–292. [Google Scholar]
- GardnerWK, Barber DA, Parberry KG.1983. The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant and Soil 70: 107–124. [Google Scholar]
- GerkeJ, Roemer W, Jungk A.1994. The excretion of citric and malic acid by proteoid roots of Lupinus albus L. Effect on soil solution concentrations of phosphate, iron and aluminium in the proteoid rhizosphere in samples of an oxisol and luvisol. Zeitschrift für Pflanzenernährung and Bodenkunde 157: 289–294. [Google Scholar]
- JohnsonJF, Allan DL, Vance CP.1994. Phosphorus stress‐induced proteoid roots altered metabolism in Lupinus albus Plant Physiology 104: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JohnsonJF, Vance CP, Allan DL.1996a Phosphorus deficiency in Lupinus albus Altered lateral root development and enhanced expression of phosphoenolopyruvate carboxylase. Plant Physiology 112: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JohnsonJF, Allan DL, Vance CP, Veiblen G.1996b Root carbon dioxide fixation by phosphorus deficient Lupinus albus Contribution to organic acid exudation by proteoid roots. Plant Physiology 112: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KeerthisingheG, Hocking PJ, Ryan PR, Delhaize E.1998. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant Cell and Environment 21: 467–478. [Google Scholar]
- LamontBB.1972a The effect of soil nutrients on the production of proteoid roots by Hakea species. Australian Journal of Botany 10: 27–40. [Google Scholar]
- LamontBB.1972b The morphology and anatomy of proteoid roots in the genus Hakea Australian Journal of Botany 20: 155–174. [Google Scholar]
- LamontBB.1973. Factors affecting the distribution of proteoid roots within the root systems of two Hakea species Australian Journal of Botany 24: 691–702. [Google Scholar]
- LouisI, Racette S, Torrey JG.1990. Occurrence of cluster roots on Myrica cerifera L. (Myricaceae) in water culture in relation to phosphorus nutrition. New Phytologist 115: 311–317. [DOI] [PubMed] [Google Scholar]
- LuP, Zhang F.1995. Mechanism of manganese toxicity induced by P‐ or Fe‐deficiency in Lupinus albus L. (Chinese). Acta Phytophysiologica Sinica 21: 289–294. [Google Scholar]
- MarschnerH.1995. Mineral nutrition of higher plants 2nd edn. London: Academic Press. [Google Scholar]
- MarschnerH, Römheld V, Cakmak I.1987. Root‐induced changes of nutrient availability in the rhizosphere. Journal of Plant Nutrition 10: 1175–1184. [Google Scholar]
- MooreCWE, Kairaitis K.1966. Nutrition of Grevilla robusta Australian Journal of Botany 41: 151–163. [Google Scholar]
- NeumannG, Römheld V.1999. Root excretion of carboxylic acids and protons in phosphorus‐deficient plants. Plant and Soil 211: 121–130. [Google Scholar]
- NeumannG, Massonneau A, Martinoia E, Römheld V.1999. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208: 373–382. [Google Scholar]
- NeumannG, Massonneau A, Langlade N, Dinkelaker B, Hengeler Ch, Römheld V, Martinoia E.2000. Physiological aspects of cluster root function and development in phosphorus‐deficient white lupin (Lupinus albus L.). Annals of Botany 85: 909–919. [Google Scholar]
- OstrowskaA, Gawliñski S, Szczubiałka Z.1991. Metody analizy i ocena własciwosci gleb i roslin. Warszawa: Instytut Ochrony Srodowiska. [Google Scholar]
- RacetteS, Louis I, Torrey JG.1990. Cluster root formation by Gymnostoma papuanum (Casuarinaceae) in relation to aeration and mineral nutrient availability in water culture. Canadian Journal of Botany 68: 2564–2570. [Google Scholar]
- RavenJA.1988. Acquisition of nitrogen by the shoots of land plants: its occurrence and implications for acid–base regulation. New Phytologist 109: 1–20. [Google Scholar]
- RavenJA.1985. pH regulation in plants. Scientific Progress, Oxford 69: 495–509. [Google Scholar]
- ReddellP, Bowen GD, Robson AD.1986. Nodulation of Casuarinaceae in relation to host species and soil properties. Australian Journal of Botany 34: 435–444. [Google Scholar]
- RileyD, Barber SA.1971. Effects of ammonium and nitrate fertilizer on phosphorus uptake as related to root‐induced pH changes at the root‐soil interface. Soil Science Society of America Proceedings 35: 301–306. [Google Scholar]
- SasL, Rengel Z, Tang C.2001. Excess cation uptake and extrusion of proton and organic acid anions in Lupinus albus under P deficiency. Plant Science 160: 1191–1198. [DOI] [PubMed] [Google Scholar]
- StockWD, Lewis OAM.1984. Uptake and assimilation of nitrate and ammonium by an evegreen Fynbos shrub species Protea repens L. (Proteaceae). New Phytologist 97: 261–268. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.