Abstract
Aluminium, a potentially phytotoxic metal, is an important constituent of many mine water discharges but has largely been neglected in the literature. The behaviour of this element in the rhizosphere of the wetland plant Phragmites australis was investigated in the laboratory in the presence and absence of Mn and Fe root plaques. Electron microscopy and chemical extraction techniques were utilized to determine the physico‐chemical properties of the plaques and any association of Al. Both Mn and Fe plaques occurred as amorphous coatings on root surfaces with uneven distributions. Al was not adsorbed onto the surface of either plaque type but formed a separate phosphate deposit closely resembling the Fe and Mn plaques. Phosphorus was also found to be adsorbed to the surface of the Fe plaques (but not the Mn plaques). Both mechanisms were found to immobilize P at the root surface but this did not significantly reduce the concentration of P in aerial plant tissues that was sufficient to ensure adequate growth.
Key words: Aluminium, common reed, iron, manganese, metal immobilization, phosphate, Phragmites australis, wetlands
INTRODUCTION
Many coal and ore mines, both abandoned and active, are major sources of metal contamination. Discharges contain elevated concentrations of a range of metals including Fe, Al, Mn and Cu (Banks et al., 1997), together with low levels of nutrients. Passive treatment systems, including constructed wetlands, have been used extensively in the last 10–20 years to treat these discharges of varying pH and composition (e.g. Hedin et al., 1994; Younger, 1997). The plant species used in such systems are able to grow in extremely hostile conditions of low acidity, low nutrient content and elevated metal concentrations. It has been proposed that the presence of iron (oxy‐) hydroxide coatings (iron plaques) on the roots of these wetland species may act as a barrier to the uptake of potentially phytotoxic metals into plant tissues (Otte et al., 1987, 1989; Greipsson and Crowder, 1992; Greipsson, 1994; Wang and Peverly, 1996). To date, a number of research projects have been undertaken to investigate this theory for a range of wetland species and metals: most have suggested that the adsorption onto, and co‐precipitation with, the iron plaques do inhibit metal uptake (Otte et al., 1989; Ye et al., 1998). Iron is not the only metal known to form oxide deposits on the roots of wetland plants. Manganese oxide plaques have been observed on the roots of Oryza sativa grown in the laboratory under elevated Mn (>1 mg l–1) conditions (Bacha and Hossner, 1977; Crowder and Coltman, 1993). Mn oxides have been shown to have a greater capacity for sorptively removing Cu from soils than Fe oxides (McClaren and Crawford, 1973), and thus may be more important than iron plaques in immobilizing copper in the rhizosphere. However, there have been few investigations into the role of Mn plaques and there are no direct reports of the effect on uptake of other metals into plant tissues.
The toxic metal Al is a major constituent of many mine discharges (e.g. Boult, 1996; Banks et al., 1997; Younger et al., 1997), and the more acidic the waters, the greater the mobility (and bioavailability) of the Al species. However, the uptake of Al into plant tissues has largely been neglected for wetland plant species, and there have been no investigations into the effect of root plaques on this process. This is despite the fact that a relationship between root activity and Al behaviour has been demonstrated for Typha latifolia, the roots of which were proven to play a role in the retention and release of Al in laboratory mesocosms (Wieder et al., 1990).
The aim of this study was to determine the effects of Fe and Mn plaques on the behaviour of Al in the rhizosphere of the common wetland plant Phragmites australis, and to identify the processes involved. This was achieved by analysing plaque deposits using scanning electron microscopy (SEM) and electron dispersive spectrometry (EDS), together with conventional chemical extraction techniques.
MATERIALS AND METHODS
Seeds of Phragmites australis Trin. ex Steudel were collected from plants growing at an uncontaminated site at Felixstowe, UK. The seeds were fully imbibed and chilled for 2 weeks in dark conditions at 4 °C before being transferred to a controlled environment growth chamber (8 h, 14 °C night; 16 h, 21 °C d; 80 µmol m–2 s–1 photon flux density). Seedlings were supported on alkathene™ beads for 14 d in 10 % Rorison’s solution (Hewitt, 1966). One hundred and eighty seedlings of a uniform size were selected, and ten were transplanted into each of 18 blackened Perspex vessels (28 × 17 × 9 cm) containing 1·5 l of 10 % Rorison’s solution. The total of 18 units allowed for three replicates of all treatments, arranged in a randomized block design. The seedlings were grown for a further 53 d, with the Rorison’s solution being changed every 3 d to maintain the nutrient supply and to compensate for any chemical changes (e.g. pH) in the solution. At the end of this period, the pH of the solution in all of the containers was reduced gradually, over a period of 7 d, to 3·5 using 0·1 m H2SO4.
The containers were divided into three subsets each consisting of six vessels. In one of these subsets iron plaque formation was induced; in a second, manganese plaques were induced and in the remaining set the roots were left unplaqued. Iron and manganese plaque formation was induced on the roots of seedlings prior to experimentation through the addition of 50 mg l–1 of Fe supplied as ferrous ammonium sulphate [(NH4)2Fe(SO4)2·6H2O] and manganese sulphate (MnSO4·4H2O), respectively, in a nutrient solution that did not contain potassium phosphate (thereby precluding the well‐known interaction of Fe with P). The seedlings were placed in acidified distilled water (pH 3·5) for 12 h before plaquing to prevent a similar interference of P, and following plaquing were left for 7 d in nutrient solution. All seedlings were grown for a further 5 d in 10 % Rorison’s solution prior to experimentation. Where desired, Al was added as aluminium sulfate (Al2SO4)3 at a concentration of 1·0 mg l–1 Al. This was added to the appropriate containers to give the following conditions: (1) no plaque, Al; (2) no plaque, no Al; (3) Fe plaque, Al; (4) Fe plaque, no Al; (5) Mn plaque, Al; and (6) Mn plaque, no Al. During Al exposure, solutions were replaced every 3 d. Roots were re‐plaqued at 21 and 50 d after the start of metal treatment to ensure that the coverage of roots with plaque was maintained. Prior to repeated exposure of roots to iron or manganese, the seedlings were placed in distilled water for 6 h.
At the end of the experiment (150 d), all plants were harvested and rinsed thoroughly in distilled water to remove dust contaminating the surface. To achieve an accurate representation of the root sections as they were harvested, it was necessary to preserve them at the earliest opportunity (within 1 h). To achieve this, sub‐samples of roots from each vessel were cut into 1‐mm sections with a clean razor blade, freeze dried and coated with carbon. The roots were examined using a Jeol scanning electron microscope fitted with an electron dispersive x‐ray spectrometer for the chemical analysis of samples.
The remaining plant material was split into roots and shoots and rinsed thoroughly in distilled water to remove surface contaminants. The root surface deposits were removed using the dithionite–citrate–carbonate (DCB) method of Jackson (1958) as modified by Taylor and Crowder (1983). Following DCB extraction, roots and shoots were dried at 40 °C over 3 d and then acid digested in 5 ml 30 % HNO3 at 90 °C for a minimum of 8 h. Concentrations of Fe, Mn and Al were determined by atomic absorption spectrophotometry (AAS). Concen trations of phosphate were determined using a Tecator 5012 flow injection analysis system.
Statistical analyses were carried out on the metal concentration data using either a one‐way or two‐way ANOVA, followed by a Tukey test. Where necessary, log10 or loge data transformations were carried out.
RESULTS
Iron plaques
Phragmites australis roots showed normal growth with good root development. Plaques were clearly visible on the root surfaces as an orange‐brown colour starting approx. 1 cm behind the root tip and gradually darkening with distance from this region. Under the SEM the deposits were observed to be a non‐crystalline particulate coating on the root surface which followed the contours of the cells (Fig. 1A). This coating was extensive in its coverage but areas were evident where there was no plaque deposit (Fig. 1B). In these regions the epidermal cells were clearly visible, with no evidence of damage. The plaque deposits did not form cell casts or polyhedra and did not penetrate into the cell cavities, but were an external deposit only. There was good development of root hairs over the surface of the sections and these were often coated with the particulate iron plaque (Fig. 1A).
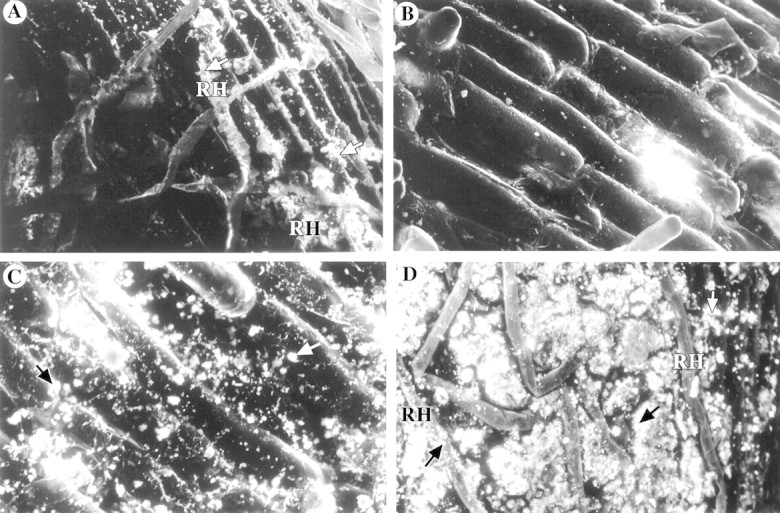
Fig. 1. Scanning electron micrographs of precipitates formed on P. australis roots grown in nutrient solution supplemented with metals. A, 50 mg l–1 Fe (320× backscatter). Arrows indicate iron plaque deposits. B, 50 mg l–1 Fe (480×). Unplaqued area of P. australis root. C, 50 mg l–1 Mn (704× backscatter). Arrows indicate manganese deposits. D, 1·0 mg l–1 Al (352× backscatter). Arrows indicate aluminium deposits. RH, Root hairs.
The chemical signature for deposits formed in the presence of iron only was dominated by Fe and P with no additional metals (Fig. 2A). In the presence of aluminium, however, the deposits were formed of a combination of Fe, P and Al (Fig. 2B), which differed in their ratios with location. There was no clear pattern to the distribution of ratios. No other metals were present in this plaque deposit.
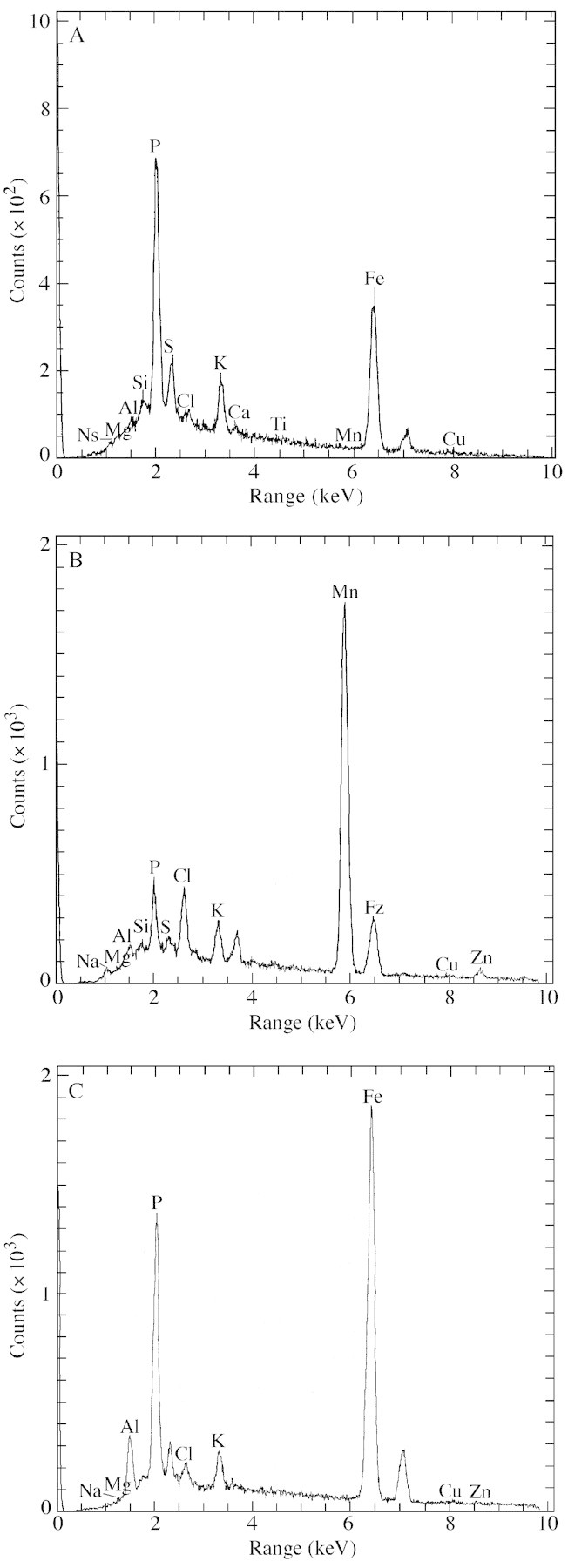
Fig. 2. Electron dispersive analysis of root precipitates formed in 50 mg l–1 Fe (A), 50 mg l–1 Fe and 1·0 mg l–1 Al (B) and 50 mg l–1 Mn (C).
In a few regions of the root there was no evidence of phosphorus in the iron plaque.
Manganese plaques
Plaques were present on the roots as a dark brown staining, but the distribution of the plaques was not as clearly defined as that of iron plaques. Under the SEM the plaque was seen to be an amorphous particulate coating, which was similar in appearance to the iron plaques (Fig. 1C). However, Mn plaques were not as extensive in their coverage as Fe plaques, with large areas of the root being devoid of particles. The Mn deposits did not penetrate into the epidermal cells and were not present around the root hairs. In the unplaqued areas of the root there was evidence of occasional particles that differed from the plaque deposits; these were probably clay particles that originated from dust contamination in the glasshouse.
The plaque deposits were composed of Mn with lesser amounts of K and S. This is a similar signature to that found for iron deposits, with Mn replacing iron. However, they differed from the Fe plaques in that the Mn deposits did not contain P (Fig. 2C). In addition, Fe was always present in association with manganese plaques, suggesting that Fe and Mn co‐precipitate. The small particles found in the unplaqued areas of the root were found to be composed of Si and Al, supporting the hypothesis that they were indeed clay particles.
In the presence of Al, the majority of the granular coating was formed of Al and P, as found for the iron plaques. There was no evidence of any Mn or Fe associated with this material.
No plaques
In the absence of plaque deposits, the chemical signature for the roots represented the residue of the nutrient solution, composed of minor traces of K, S and Cl, together with Si. There was no evidence of Fe, Mn or Al although these were present in trace amounts in the nutrient solution.
The supply of Al to plants grown without Fe and Mn plaques also produced a granular deposit of a similar nature to the other two types. This was also patchy and coated both the root surface and the root hairs (Fig. 1D). EDS revealed that this was composed of Al and P with small traces of K. No Fe or Mn was associated with this material.
The effect of plaque and Al on phosphate uptake
There was a significant effect of the presence of aluminium, plaque type and their interaction on phosphate concentration in all plant extracts (Table 1). In the presence of Al and Fe plaques, higher concentrations of P were found in the DCB extract than in all other treatments. Phosphate concentrations were also lower in the presence of Fe plaque in root extracts compared with the other plaque treatments when Al was present.
Table 1.
Phosphate concentration (mg kg–1 d. wt) within seedlings of Phragmites australis exposed to 0 and 0·1 mg l–1 Al, with iron, manganese or no plaque on roots
| No aluminium | Aluminium | |||||
| No plaque | Fe plaque | Mn plaque | No plaque | Fe plaque | Mn plaque | |
| DCB | 0·64 × 103 ± 0·08 × 103 a | 1·4 × 103 ± 0·20 × 103 c | 0·91 × 103 ± 0·05 × 103 ab | 1·7 × 103 ± 0·19 × 103 c | 4·2 × 103 ± 0·21 × 103 d | 1·3 × 103 ± 0·18 × 103 bc |
| Root | 10·7 × 103 ± 0·76 × 103 abc | 7·9 × 103 ± 0·33 × 103 ab | 7·8 × 103 ± 1·9 × 103 a | 14·0 × 103 ± 0·49 × 103 c | 6·1 × 103 ± 0·93 × 103 a | 16·1 × 103 ± 3·2 × 103 c |
| Shoot | 3·5 × 103 ± 0·02 × 103 a | 4·3 × 103 ± 0·21 × 103 b | 4·3 × 103 ± 0·20 × 103 b | 4·4 × 103 ± 0·15 × 103 b | 4·6 × 103 ± 0·11 × 103 b | 4·3 × 103 ± 0·19 × 103 b |
Data are means ± s.e., n = 3.
Different superscript letters in a row indicate a significant difference at P = 0·05.
Phosphate concentrations in shoot extracts were significantly lower when Al and plaque were both absent. There was no significant difference in shoot concentrations between the remaining treatments.
The effect of plaque on Al uptake
No significant difference was found in the concentration of Al in DCB and shoot extracts between the different plaque treatments (Table 2). Significantly less Al was present in root extracts when Fe plaque was present in comparison with Mn or no plaque.
Table 2.
Aluminium concentration (mg kg–1 d. wt) within seedlings of Phragmites australis exposed to 0·1 mg l–1 Al, with iron, manganese or no plaque on roots
| No plaque | Fe plaque | Mn plaque | |
| DCB | 4·5 × 103 ± 0·71 × 103 | 5·6 × 103 ± 0·76 × 103 | 2·8 × 103 ± 0·56 × 103 |
| Root | 7·8 × 103 ± 0·81 × 103 a | 0·55 × 103 ± 0·14 × 103 b | 6·4 × 103 ± 1·7 × 103 a |
| Shoot | 0·74 × 103 ± 0·12 × 103 | 0·43 × 103 ± 0·07 × 103 | 0·79 × 103 ± 0·09 × 103 |
Data are means ± s.e., n = 3.
Different superscript letters in a row indicate a significant difference at P = 0·05.
DISCUSSION
The composition and structure of iron plaques has been studied extensively (Bacha and Hossner, 1977; Chen et al., 1980a, b; Taylor et al., 1984; Otte et al., 1989; St Cyr et al., 1993; Snowden and Wheeler, 1995). However, there have been few equivalent reports of Mn plaques (Bacha and Hossner, 1977; Levan and Riha, 1986; Crowder and Coltman, 1993) and none of these has included investigations into the structure of the plaque using SEM or EDS. In the present study it has been shown that Mn plaques can be produced on the roots of P. australis when Mn is supplied at elevated concentrations (50 mg l–1). The plaque appeared as a dark brown staining of the root, visible with the naked eye, as previously reported on the roots of O. sativa (Crowder and Coltman, 1993). Iron plaques were also visible with the naked eye but appeared as a distinctive orange staining. In both cases the plaque had a definite zonation over the root starting approx. 1 cm behind the root tip and darkening with distance from the tip; this is in agreement with previous findings (Taylor et al., 1984; Cook, 1990). Under the SEM, Fe and Mn plaques were similar in appearance, both occurring as amorphous layers of precipitate which followed the contours of the cells. However, the Mn plaque was more patchy in its distribution than the Fe plaque and there were large areas of the root where there was no evidence of a plaque deposit. Mn oxides do not rapidly form chemically below a pH of 8·6 and therefore it is likely that most Mn precipitation is biologically mediated. Indeed, in SEM sections of Vallisneria americana roots, microorganisms were found to be surrounded by manganese oxides and it was suggested that these could possibly catalyse the formation of Mn oxides (St Cyr et al., 1993). Fe oxides, however, can form chemically at this low pH (albeit slowly) and so a combination of biotic and abiotic processes may produce a greater quantity of metal oxide than biotic processes alone.
There was no evidence of penetration of either Mn or Fe plaque into the epidermal cells. This contrasts with the report by Levan and Riha (1986) who found that Mn plaque deposits on conifer roots could be detected using an optical microscope in the cell walls of the root epidermis and in the entire cortex. However, these results are for non‐wetland plants and it is inadvisable to compare them directly with P. australis.
In a number of investigations authors have suggested and provided evidence for the adsorption of heavy metals onto the surface of iron plaques (e.g. Otte et al., 1987, 1989; St Cyr and Crowder, 1990; Ye et al., 1997, 1998). However, in this study Al was not found to be present in direct association with the Mn and Fe plaques. This suggests that Al was not adsorbed onto the plaque deposits. However, Al was found to be present on the root surface when supplied at a concentration of 1·0 mg l–1. Al occurred as a precipitate covering the surface of the root and was always present in association with elevated counts of P on the EDS analysis. The Al precipitate formed closely resembled the Fe and Mn plaques, being amorphous but more extensive in coverage than the Mn plaque despite being supplied at a lower concentration. The quantitative data also show that Al concentration in the surface deposits did not differ significantly between the plaque treatments. This suggests that the occurrence and quantity of Al on the roots was not affected, either positively or negatively, by the presence of Fe and Mn deposits.
The demonstration of the presence of Al precipitates on the roots of plants is not new. The formation of Al phosphates around the roots of crop plants has been documented extensively (e.g. McCormick and Yates Borden, 1972, 1974; Wheeler, 1994). However, the Al deposits formed in the present study differ somewhat from those reported previously. McCormick and Yates Borden (1974) found that Al phosphate occurred as scattered globules rather than a continuous layer on the roots of barley (Hordeum vulgare L.). The precipitate formed in this study occurred as a layer around the root covering most of the root surface. This can be attributed to the leakage of oxygen from the roots of wetland plants mediating the precipitation of phosphate. It has been suggested that the formation of these precipitates around roots may be the cause of poor growth in acidic soils, since it would tend to prevent phosphorus being taken up into the plant tissues, thereby promoting P‐deficiency (rather than Al toxicity). It has also been suggested that the presence of Al around roots may remove P from within the roots themselves, thereby exacerbating any deficiency (McCormick and Yates Borden, 1972).
The data presented here show that phosphate concentrations within plaque deposits were indeed greater in those plants supplied with aluminium and/or iron, suggesting that phosphorus may be immobilized at the root surface. However, shoot concentrations of P were not found to be significantly different between the plaque treatments and the presence/absence of Al. In all cases, the amount of phosphate in shoot tissues constituted approx. 4 % of total dry weight, which is sufficient for adequate plant growth. In addition, the presence of Al did not significantly reduce the amount of phosphate present within root tissues, although there was some indication that the presence of Fe plaques may do so.
These results have important implications for the growth of macrophytes in constructed wetlands receiving waters that contain elevated concentrations of Al. In this study, Al was found to sequester phosphate on the root surface through the formation of Al phosphates, although this did not inhibit the uptake of phosphate into the aerial tissues. However, the concentration of P supplied to the plants within the nutrient solution was deemed to be sufficient for adequate plant growth (3·1 mg l–1). One of the characteristics of mine water discharges is that they contain extremely low concentrations of nutrients, including P, often below the limits of detection (<0·1 mg l–1) (Hewitt, 1966). It is possible that at these low concentrations any P available in the water will be removed through the precipitation of Al phosphate complexes either within the sediment/soil or on the surface of plant roots. This in turn may result in P deficiency and poor growth and performance of wetland plants within constructed wetlands. High concentrations of Al have previously been implicated in P deficiency in vascular plants (Andersson, 1988). Plants may overcome this deficiency through the release of root exudates; graminaceous species release phytosiderophores when deficient in iron, resulting in the mobilization of other elements, including nutrients. However, wetland plants growing in wetlands receiving mine drainage are unlikely to suffer from iron deficiency as iron is one of the main contaminants in such discharges. The presence of iron plaques on the surface of roots does not prevent the uptake of metals, including iron, into the plant tissues but merely inhibits it (Batty et al., 2000), thus the species should have an adequate supply of Fe.
Fe plaques produced in this study were shown to be composed of Fe and P, thus they could either be present as an Fe oxide with adsorbed P or as an Fe phosphate. However, in some root samples, Fe plaques were formed only of iron with no evidence of P. This suggests that the iron did not form an iron phosphate complex, but was present as an iron oxide with P adsorbed to the surface. In some areas adsorption appears not to occur. This may be a reflection of the detection limits of the EDS analysis rather than an actual absence of phosphorus. However, it is clear that the distribution of P within the plaque is highly variable, possibly reflecting differences in mineralogy. The presence of Fe plaques apparently does not prevent the formation of Al phosphates by adsorbing available P, since the two precipitates were observed to occur together in the present study. In addition, phosphate concentrations in Al and Fe (without Al) plaques were not significantly different, suggesting that the two metals have a similar ability to immobilize phosphorus. The adsorption of P onto Fe plaques in addition to the formation of Al phosphates could increase P‐deficiency in the plant species. This negative effect of Fe plaques has previously been demonstrated in the submerged aquatic species Lobelia dortmanna where P was assimilated in the Fe plaques resulting in low plant biomass (Christensen and Sand‐Jensen, 1998).
In the present study Mn plaques were found to be composed of Mn with lesser amounts of Fe and no P. This suggests that Mn does not form a phosphate complex, nor does P adsorb to the surface of the resultant Mn oxide. The presence of Fe in Mn plaques demonstrates that Fe can form plaques on root surfaces at very low concentrations, in this case at 0·3 mg l–1. This may also explain the slightly higher phosphate concentrations in DCB extracts of Mn plaques compared with no plaques as the Fe deposits will be forming iron phosphate deposits. When Al and Mn were supplied in combination at low pH, Al phosphate precipitates formed in preference to Mn plaques. This could result in the increased mobility and availability of Mn to the plants.
It has previously been shown that iron plaques formed around the roots of P. australis in the laboratory may act as a physical barrier to the uptake of Cu into plant tissues (Batty et al., 2000). It is possible that the production of Al phosphate plaques could act in a similar way though this would require further investigation.
CONCLUSIONS
(1) Fe and Mn plaques occur as unevenly distributed amorphous precipitates on the surface of roots. (2) Al precipitates as an Al phosphate on the roots of P. australis and physically resembles Fe and Mn plaques. (3) P is adsorbed onto the surface of Fe plaques, but not Mn plaques. (4) Phosphate concentrations in aerial tissues are not affected by the presence of Fe, Mn or Al plaques.
ACKNOWLEDGEMENTS
We thank Professor C. D. Curtis and D. Plant for the provision of SEM facilities at the University of Manchester. We would also like to thank Dr Paul Younger for helpful comments on the text. This work was funded by a Hossein Farmy Scholarship from the University of Sheffield.
Supplementary Material
Received: 11 September 2001; Returned for revision: 18 November 2001; Accepted: 6 January 2002.
References
- AnderssonM.1988. Toxicity and tolerance of aluminium in vascular plants: A literature review. Water, Air and Soil Pollution 39: 439–462. [Google Scholar]
- BachaRE, Hossner LR.1977. Characteristics of coatings formed on rice roots as affected by iron and manganese additions. Soil Science Society of America Journal 41: 931–935. [Google Scholar]
- BanksD, Younger PL, Arnesen RT, Iversen ER, Banks SB. 1997. Mine‐water chemistry: the good the bad and the ugly. Environmental Geology 32: 157–174. [Google Scholar]
- BattyLC, Baker AJM, Curtis CD, Wheeler BD.2000. The effect of pH and plaque on the uptake of Cu and Mn in P. australis (Cav.) Trin ex. Steudel. Annals of Botany 86: 647–653. [Google Scholar]
- BoultS.1996. Fluvial metal transport near sources of acid mine‐ drainage: relationships of soluble, suspended and deposited metal. Mineralogical Magazine 60: 325–335. [Google Scholar]
- ChenCC, Dixon JB, Turner FT.1980a Iron coatings on roots: mineralogy and quantity influencing factors. Soil Science Society of America Journal 44: 635–639. [Google Scholar]
- ChenCC, Dixon JB, Turner FT.1980b Iron coatings on rice roots: morphology and models of development. Soil Science Society of America Journal 44: 1113–1119. [Google Scholar]
- ChristensenKK, Sand‐Jensen K.1998. Precipitated iron and manganese plaques restrict root uptake of phosphorus in Lobelia dortmanna Canadian Journal of Botany 76: 2158–2163. [Google Scholar]
- CookRED.1990. Iron toxicity to wetland plants PhD Thesis, University of Sheffield, UK. [Google Scholar]
- CrowderAA, Coltman DW.1993. Formation of manganese oxide plaque on rice roots in solution culture under varying pH and manganese (Mn2+) concentration conditions. Journal of Plant Nutrition 16: 589–599. [Google Scholar]
- Dodds‐SmithME, Payne CA, Gusek JJ.1995. Reedbeds at Wheal Jane. Mining Environmental Management 1995: 22–24. [Google Scholar]
- GreipssonS.1994. Effects of iron plaque on roots of rice on growth and metal concentration of seeds and plant tissues when cultivated in excess copper. Communications in Soil Science and Plant Analysis 25: 2761–2769. [Google Scholar]
- GreipssonS, Crowder AA.1992. Amelioration of copper and nickel toxicity by iron plaque on roots of rice (Oryza sativa). Canadian Journal of Botany 70: 824–830. [Google Scholar]
- HedinRS, Watzlaf GR, Vairn RW. 1994. Passive treatment of acid mine drainage with limestone. Journal of Environmental Quality 23: 1338–1345. [Google Scholar]
- HewittEJ.1966. Sand and water culture methods used in the study of plant nutrition. Technical Communication No. 22. Farnham Royal, Bucks, UK: Commonwealth Agricultural Bureaux. [Google Scholar]
- JacksonML.1958. Soil chemical analysis‐advanced course Madison, Wisconsin: University of Wisconsin, 44–51. [Google Scholar]
- LevanMA, Riha SJ.1986. The precipitation of black oxide coatings on flooded conifer roots of low internal porosity. Plant and Soil 95: 33–42. [Google Scholar]
- McCormickLH, Yates Borden F.1972. Phosphate fixation by aluminium in plant roots. Soil Society of America Proceedings 36: 799–802. [Google Scholar]
- McCormickLH, Yates Borden F.1974. The occurrence of aluminium‐phosphate precipitate in plant roots. Soils Society of America Proceedings 38: 931. [Google Scholar]
- McClarenRG, Crawford DV.1973. Studies on soil copper: 1. The fractionation of copper in soils. Journal of Soil Science 24: 172–181. [Google Scholar]
- OtteML, Buijs EP, Riemer L, Rozema J, Broekman RA.1987. The iron‐plaque on the roots of saltmarsh plants: a barrier to heavy metal uptake? In: Lindberg SE, Hutchinson, TC. Proceedings of the International Conference Heavy Metals in the Environment New Orleans (USA), Edinburgh: CEP Consultants, 407–409. [Google Scholar]
- OtteML, Rozema J, Koster L, Haarsma MS, Broekman RA.1989. Iron plaque on roots of Aster tripolium L.: interaction with zinc uptake. New Phytologist 111: 309–317. [DOI] [PubMed] [Google Scholar]
- SnowdenRED, Wheeler BD.1995. Chemical changes in selected wetland plant species with increasing Fe supply, with specific reference to root precipitates and Fe tolerance. New Phytologist 131: 503–520. [DOI] [PubMed] [Google Scholar]
- St CyrL, Crowder AA.1990. Manganese and copper in the root plaque of Phragmites australis (Cav.) Trin. ex Steudel. Soil Science 149: 191–198. [Google Scholar]
- St CyrL, Fortin D, Campbell PGC.1993. Microscopic observations of the iron plaque of a submerged aquatic plant (Vallisneria americana Michx). Aquatic Botany 46: 155–167. [Google Scholar]
- TaylorGJ, Crowder AA.1983. Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. American Journal of Botany 70: 1254–1257. [Google Scholar]
- TaylorGJ, Crowder AA, Rodden R.1984. Formation and morphology of an iron plaque on the roots of Typha latifolia L. grown in solution culture. American Journal of Botany 71: 666–675. [Google Scholar]
- WangT, Peverly JH.1996. Oxidation states and fractionation of plaque iron on roots of common reeds. Soil Science Society of America Journal 60: 323–329. [Google Scholar]
- WheelerDM.1994. Effects of growth period, plant age and changes in solution aluminium toxicity in wheat. Plant and Soil 166: 21–30. [Google Scholar]
- WiederRK, Linton MN, Heston KP.1990. Laboratory mesocosm studies of Fe, Al, Mn, Ca, and Mg dynamics in wetlands exposed to synthetic acid coal mine drainage. Water, Air and Soil Pollution 51: 181–196. [Google Scholar]
- YeZH, Baker AJM, Wong MH, Willis AJ.1997. Copper and nickel uptake, accumulation and tolerance in Typha latifolia with and without iron plaque on the root surface. New Phytologist 136: 481–488. [DOI] [PubMed] [Google Scholar]
- YeZH, Baker AJM, Wong MH, Willis AJ.1998. Zinc, lead and cadmium accumulation and tolerance in Typha latifolia as affected by iron plaque on the root surface. Aquatic Botany 61: 55–67. [Google Scholar]
- YoungerPL. 1997. Minewater treatment using wetlands. Proceedings of a National Conference, University of Newcastle, UK London: Chartered Institution of Water and Environmental Management, 65–81. [Google Scholar]
- YoungerPL, Curtis TP, Jarvis A, Pennell R.1997. Effective passive treatment of aluminium‐rich, acidic colliery spoil drainage using a compost wetland at Quaking Houses, County Durham. Journal of the Chartered Institution of Water and Environmental Management 11: 200–208. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


