Abstract
The structure resulting from branching on 1‐year‐old apple tree trunks was analysed in a set of apple cultivars with diverse branching and fruiting habits. Four different lateral types borne on successive nodes were observed when vegetative and flowering fates, as well as sylleptic and proleptic branching, were taken into account. The location and grouping of lateral types along the trunk were analysed for all cultivars, but are detailed for one cultivar only. This cultivar showed a succession of zones, each zone being characterized by its composition of lateral types. Statistical models—hidden semi‐Markov chains—were built to take this structure into account and to characterize the cultivar’s specific branching pattern. The models showed that most of the branching zones had a similar location in the different cultivars, even though zone composition and zone length differed among cultivars. On a more detailed scale, the nodes bearing a lateral, regardless of its type, were frequently followed by latent buds. The validity of the models and their biological interpretation are discussed with respect to parent shoot dynamics, hormonal gradients and competition between neighbouring buds.
Key words: Malus × domestica Borkh., plant architecture, branching, hidden semi‐Markov chain, stochastic modelling
INTRODUCTION
Branching is a complex process with a physiological basis that is inadequately understood (Wilson, 2000). Several studies have demonstrated relationships between the development and fate of axillary buds (vegetative or floral, long or short shoots) and the growth characteristics of their bearing shoot (Crabbé, 1984; Kervella et al., 1995). In particular, sylleptic branching, which occurs in mesotonic locations (Tomlinson and Gill, 1973; Crabbé, 1984) has been shown to result from a fast growth rate (Champagnat, 1954, 1965; Génard et al., 1994). Acrotony, i.e. the decrease in the length of the laterals observed from the top of the bearing shoot (Bell, 1991), is another example of a relationship between the location and fate of axillary buds. This gradient is usually interpreted as resulting from apical dominance and control, both exerted via hormonal gradients (Cline, 1997; Wilson, 2000; Cook et al., 2001).
The branching process in apple trees (Malus domestica Borkh.) has been studied by counting the number of spurs per metre and determining their characteristics (Ketchie, 1984; Warrington et al., 1990). Other studies have demonstrated the existence of an acrotonic gradient (Crabbé, 1987; Cook, 1998) and have shown that the location of axillary buds along shoots determines whether they develop into short (≤5 cm) or long (>5 cm) laterals (Kaini et al., 1984; Ouellette and Young, 1995). The mesotonic location of sylleptic shoots has been demonstrated in this species by several authors (Crabbé, 1984; Costes and Guédon, 1997). All these studies suggest that branching is organized along annual shoots in apple according to particular lateral type locations.
Over several growth periods it has been shown that growth—and consequently the number of laterals—decreases rapidly with ageing (Ouellette and Young, 1995). Such a decrease in the growth and branching characteristics with plant development and ageing has been described by Gatsuk et al. (1980) and Barthélémy et al. (1997) and has been demonstrated in many woody species, e.g. Prunus armeniaca L. (Costes, 1993), Cedrus atlantica Endl. (Sabatier and Barthélémy, 1999) and Fagus sylvatica L. (Nicolini, 1998). As a consequence, when growing conditions are appropriate, the first annual growth of the stem developing from the grafted bud is the longest in the tree and bears the limbs which will later make up the tree structure. This led us to believe that it might be possible to evaluate tree growth and branching habits by analysing the branching pattern of the first annual shoot of the trunk.
By using appropriate models, we aimed to test the assumption that a specific branching pattern exists in young apple tree trunks. In addition, we also addressed the question of whether such a pattern, if it exists, differs between cultivars with different branching habits. Several classifications are used to categorize individual apple trees. Two main growth habits i.e. spurs and standards, can be considered (Dennis et al., 1996; Lane et al., 1996). Other classifications rely on a qualitative and global evaluation of growth and branching, e.g. UPOV classification of tree forms into columnar, upright, spreading, drooping and weeping classes (UPOV, 1982) or Lespinasse’s (1977) classification. In the present paper we refer to the latter classification which uses branching and fruiting habits as well as tree geometry to define four types, I to IV. Trees belonging to types I and II exhibit mainly short laterals and are characterized by an axillary fruiting location, whereas trees belonging to types III and IV exhibit more medium and long laterals and are characterized by a terminal fruiting location.
A previous study brought to light remarkable patterns resulting from sylleptic branching (Costes and Guédon, 1997) using statistical models built from samples of sequences measured on 1‐year‐old trunks (Guédon and Costes, 1999; Guédon et al., 2001). This study extends the previous research by taking account of the proleptic branching that occurs after winter rest, and which is thus delayed with respect to bud development. [Sylleptic laterals were called immediate laterals in other studies by Costes and Guédon (1997) and proleptic shoots are sometimes called delayed shoots.] These two lateral types differ both in their location and in their contribution to tree function. Since these laterals are not leafy during the same year of growth, their contribution to carbon acquisition is shifted in time. In addition, proleptic branching is organized acrotonically while sylleptic branching develops over the previous growth year, from the bottom to the top. Thus, branching along 1‐year‐old trunks results from two branching processes, one organized from the bottom (sylleptic laterals) and the other from the top (proleptic laterals). These different development times leading to sylleptic or proleptic lateral types and the vegetative or floral fate were taken into account in the present study.
In what follows, our aim is to analyse branching structures to gain insight into the underlying biological functions. Since measurements were taken at a macroscopic scale, the structure does not express directly the overall complexity of the functions (and interactions) involved. According to Baldi and Brunak (1998) who discuss this issue in the framework of computational molecular biology, the models used for analysing such data should be both structural and probabilistic (see also Durbin et al., 1998). The generic problem of analysing successions of homogeneous zones in discrete sequences is currently addressed in both computational molecular biology and computational plant architecture. Hidden semi‐Markov chains emerged independently in these two scientific communities as the reference class of statistical models [see Burge and Karlin (1997) and Lukashin and Borodovsky (1998) for application in gene finding; Guédon and Costes (1999) and Guédon et al. (1999, 2001) for application in the pattern analysis of branching and axillary flowering sequences]. Hidden semi‐Markov chains were thus used to characterize the branching patterns and to capture embedded structures in our data sets.
MATERIALS AND METHODS
Plant material
The study was carried out on six apple cultivars (Malus domestica Borkh.) corresponding to three basic growth and fruiting habits, described as types II–IV by Lespinasse (1977). The six cultivars were: ‘Belrène’ and ‘Reinette Blanche du Canada’ (Type II), ‘Imperial Gala’, ‘Elstar’ and ‘Fuji’ (Type III), and ‘Granny Smith’ (Type IV) (Ctifl, 1993). Twenty trees per cultivar, bench‐grafted on M.7 rootstock, were planted at the Arboriculture Laboratory of the Institut National de la Recherche Agronomique (INRA), Montpellier, France. After the first year of growth, the shoot that developed from the grafted bud was cut back to one bud and the trees were transplanted in a single block. Spacing was sufficiently large (6 × 4·5 m) to allow each tree to develop without constraints. The trees were then allowed to develop without pruning.
At the end of the first year of growth, the number of metamers (sensu White, 1979) and the location and the length of the sylleptic shoots were recorded for the shoot that had developed from the retained bud. Sylleptic shoots were considered as a unique class of laterals, but the distinction between short and long shoots was drawn from the previous study (Costes and Guédon, 1997) and is mentioned when necessary in this paper.
At the end of the second year of growth, three other types of axillary bud fate which led to proleptic development were recorded: (1) Spur or short shoot: the axillary bud develops into a shoot consisting of preformed organs only (i.e. contained in the resting bud; Rivals, 1965), with no or little elongation of the internodes. The terminal meristem remains vegetative. (2) Long shoot: laterals continue to grow after the establishment of the preformed organs. The part of the shoot formed this way is termed ‘neoformed’ according to Rivals (1965, 1966). The corresponding internodes are elongated. This shoot type is vegetative. (3) Bourse: a bourse results from the differentiation of the meristem into an inflorescence, after the development of a few preformed leaves. It is thus a preformed and mixed unit, composed of vegetative organs in its proximal part and floral organs in its distal part. The bourse can also bear a sylleptic axillary shoot called a ‘bourse shoot’ whose first preformed metamers are contained in the resting bud (Crabbé and Escobedo, 1991). Like other shoots, the bourse shoot can develop into a short or a long shoot, after the establishment of its preformed organs. In the present study, we considered the fate of the axillary buds directly located along the trunks and their floral differentiation into a bourse without considering the fate of the bourse shoot.
Data analysis and model building
The first annual shoots on the trunks were described as a succession of metamers and were represented by a sequence of symbols, indexed with the node rank. Each lateral type borne on a node was represented by a symbol: 0 for a latent bud, 1 for a spur, 2 for a long shoot, 3 for a bourse and 4 for a sylleptic shoot. Data were analysed using AMAPmod software (freely available at http://www.cirad.fr/presentation/programmes/amap.shtml) (Godin et al., 1997, 1999), in three main steps: (1) exploratory data analysis and model specification; (2) model building; (3) model evaluation and interpretation.
Exploratory analysis and model specification require a hypothesis concerning the causality of the phenomenon studied in order to determine the direction in which the sequences must be described. We chose to describe shoots from the top to bottom to focus on the apical effect. The sequences were thus analysed according to three points of view, ‘intensity’, ‘counting’ and ‘interval’, previously described in Guédon and Costes (1999) and Guédon et al. (2001). Briefly, the ‘intensity’ point of view consists of extracting from the sample of sequences the empirical distribution of the different symbols for each node rank. The ‘interval’ point of view consists of extracting three different types of intervals: (1) time up to the first occurrence of a symbol, i.e. the number of nodes before the first occurrence of this symbol; (2) recurrence time, i.e. the number of internodes between a given symbol occurring and re‐occurring; and (3) sojourn time in a symbol (or run length of a symbol), i.e. the number of successive occurrences of a given symbol (in these definitions, ‘time’ is used by convention and refers to the node rank that is used as an index). The ‘counting’ point of view consists of counting the number of occurrences of a given ‘pattern’ for each sequence. The two patterns of interest correspond to the occurrences of a given symbol and its run in a given sequence.
The families of characteristic distributions play different roles in the exploratory analysis. The intensity point of view gives an overview of process dynamics. For instance, the change in the symbol distribution with node rank makes it possible to identify the branching zones and locate them along the stem. This overview is complemented by three other types of characteristic distributions which help to highlight the scattered or aggregated distribution of a given symbol and also to express local dependencies: (1) recurrence time distributions; (2) sojourn time distributions; and (3) the distribution of the number of runs of a symbol per sequence. The characteristic distributions were analysed paying particular attention to the long laterals since these are the most important laterals for evaluating the perennial structure of the trees. The distributions of the number of occurrences of the different symbols per sequence were compared among cultivars using a Kolmogorov–Smirnov non‐parametric test, because the variables were not normally distributed and the number of sequences was less than 20. The tests were performed with the non‐parametric module of Statistica® software.
The second step consisted of specifying a parametric model to account for the observed sequences. As in a previous study (Costes and Guédon, 1997), a hierarchical model with two levels of representation was chosen. At the first level, a semi‐Markov chain represents both the succession of zones and the length of each zone. Each zone is represented by a mathematical object called a state with an associated zone length distribution called a state occupancy distribution. The different states are connected by transitions with associated probabilities summing to one for the transitions leaving a given state. Initial probabilities are also needed to select the initial zones. A semi‐Markov chain is defined by the following parameters: initial probabilities (probabilities of being in a given state at the beginning of the sequence); transition probabilities (probabilities of moving from state i at rank n–1 to state j at rank n); and occupancy distributions. The occupancy distribution represents the length of the corresponding zone in terms of number of metamers and is associated with each state, except the final one.
The second level consists of associating within each zone a discrete distribution representing the composition of the laterals within that zone. For each state, the observation probabilities constitute a non‐parametric distribution defined on the set of possible symbols. The whole model is a hidden semi‐Markov chain.
Model parameters were estimated by an iterative algorithm, which maximizes the likelihood of the observed sequences (Guédon and Cocozza‐Thivent, 1990; Guédon, 1998), within the STAT module of AMAPmod software. The theoretical distributions corresponding to the intensity, interval and counting points of view defined above were computed from the estimated model and fitted to the corresponding empirical distributions extracted from the data (Guédon, 1999). This enabled us to evaluate model accuracy.
The last step consisted of analysing the homogeneity of cultivar behaviour and comparing the cultivars. This was performed by examining model parameters, particularly transition probabilities and observation distributions.
RESULTS
The mean length of the sequences varied from 66 to 90 nodes, depending on the cultivar (Table 1). The proportion of lateral types was consistent with the classification used as reference since the mean number of total long shoots increased from type II to type IV cultivars (Table 1). Conversely, the mean number of lateral spurs, either sylleptic or proleptic, and the mean number of bourses decreased globally, although some exceptions were noted to this general rule: the mean number of lateral bourses was higher for Gala than for type II cultivars and the mean number of Fuji spurs was relatively high when compared with other type III and IV cultivars.
Table 1.
Mean (s.d.) number of nodes and lateral types (latent buds and bourses, proleptic and sylleptic shoots) on 1‐year‐old trunks of six different apple cultivars
| Proleptic shoots | Sylleptic shoots | ||||||||||
| Cultivar | Node number | Latent bud | Bourse | Long | Spurs | Total | Long | Spurs | Total | Total long shoots | Total spurs |
| Reinette | 73·0 (3·78)a | 36·50 (4·42)c | 7·13 (0·9)ab | 7·44 (2·8)bc | 11·0 (0·9)a | 18·4 (1·6)a | 3·3 (0·4)c | 7·69 (0·7)b | 10·9 (0·9)bc | 10·69 (4·42)a | 18·69 (6·63)a |
| Belrène | 89·53 (4·41)a | 47·73 (8·18)a | 5·87 (2·85)b | 7·07 (2·25)bc | 10·2 (4·72)a | 17·3 (6·94)ab | 4·7 (2·63)bc | 13·93 (6·16)a | 18·7 (7·06)a | 11·80 (4·84)a | 24·13 (10·72)a |
| Gala | 70·0 (4·14)a | 40·05 (5·65)bc | 9·53 (3·77)a | 4·17 (2·13)c | 1·5 (2·12)b | 5·7 (4·15)c | 7·3 (2·99)ab | 7·47 (3·59)bc | 14·7 (3·08)ab | 11·48 (5·09)a | 9·00 (5·41)b |
| Fuji | 73·93 (4·56)a | 45·93 (7·86)ab | 4·06 (3·58)b | 11·87 (7·89)a | 5·2 (6·01)b | 17·07 (13·84)ab | 2·1 (1·67)c | 5·27 (3·24)bc | 7·3 (3·73)c | 13·93 (9·40)a | 10·47 (9·14)b |
| Elstar | 66·13 (9·02)a | 39·56 (12·31)bc | 5·38 (3·30)b | 7·56 (3·65)abc | 2·6 (3·44)b | 10·12 (6·85)bc | 7·3 (3·63)ab | 3·73 (2·54)c | 11·1 (4·87)bc | 14·38 (5·98)a | 6·18 (5·01)b |
| Granny | 75·06 (5·62)a | 45·50 (4·35)ab | 6·63 (3·48)ab | 8·63 (2·90)ab | 1·12 (2·92)b | 9·76 (5·32)bc | 8·1 (3·22)a | 5·06 (2·49)bc | 13·2 (3·87)b | 16·75 (6·50)a | 6·06 (5·08)b |
For proleptic and sylleptic shoots, long shoots and spurs were distinguished. The total number of long shoots and spurs is the sum of proleptic and sylleptic shoots for these two categories. Within a column, different superscripts indicate significant differences according to the Kolmogorov–Smirnov test (P < 5 %).
In what follows, the exploratory analysis and model building are detailed for ‘Reinette Blanche du Canada’, which was then used as a reference for comparing cultivars.
Exploratory analysis of ‘Reinette Blanche du Canada’ sequences
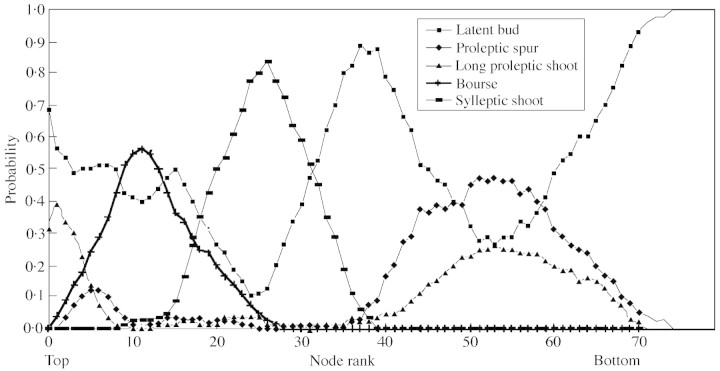
The analysis of the sequence sample with the three points of view previously described provided an intuitive idea of the structure of the 1‐year‐old trunks. The ‘intensity’ point of view highlighted a succession of differentiated zones, each characterized by homogeneous composition properties in terms of laterals (Fig. 1). The succession of zones in ‘Reinette’ may be summarized as follows (Figs 1 and 2). Long shoots were located on the first five nodes from the top and were mixed with latent buds and a few spurs. The following nodes were occupied mainly by lateral bourses, mixed with latent buds. The third zone corresponded to sylleptic shoots. It is noteworthy that no other laterals developed within this zone which did not contain any proleptic shoots. These first three zones spread over the distal half of the shoots. The basal half of the shoots corresponded to three other zones: two unbranched zones on both sides of a large zone where proleptic long shoots and spurs were mixed with latent buds.
Fig. 1. Probability of the different types of laterals according to the node rank observed on 1‐year‐old trunks in ‘Reinette Blance du Canada’.
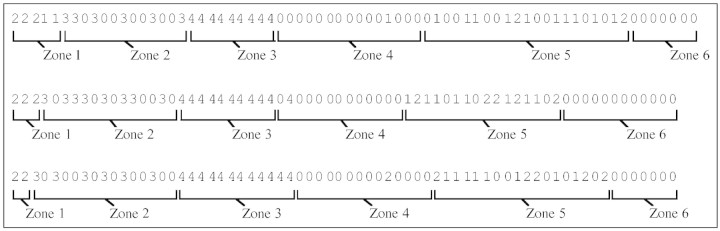
Fig. 2. Example of differentiated zones on three sequences corresponding to three 1‐year‐old trunks of ‘Reinette Blanche du Canada’. 0, latent bud; 1, proleptic spur; 2, long proleptic shoot; 3, bourse; 4, sylleptic shoot.
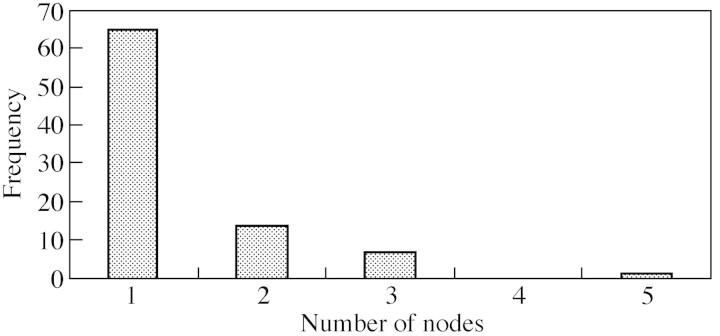
The characteristics of the long laterals were extracted for the three zones able to bear this lateral type: the first zone, near the top, with proleptic shoots; the median zone with sylleptic shoots—those being long were counted using the data collected during the previous study on the same trees (Costes and Guédon, 1997); and the last zone near the base, again with proleptic shoots. The total number of long shoots per sequence, including both sylleptic and proleptic long shoots, was considered first (Table 1). The balance between the three zones showed that the long shoots were more numerous in the basal zone (Table 2). In addition, the density of the long laterals within a given zone was evaluated by their frequency in this zone and their aggregation was examined by both ‘interval’ (run length of long laterals) and ‘counting’ characteristics (number of long axillary shoot runs). These points of view highlighted the isolation of long shoots along the 1‐year‐old trunk since the most frequent run length was one (Fig. 3).
Table 2.
Mean (± s.d.) number of long shoots per zone along 1‐year‐old trunks of six apple cultivars
| Cultivar | Distal zone | Median zone | Basal zone | Total |
| Reinette | 1·9 ± 1·4 | 3·3 ± 1·7 | 5·1 ± 3·3 | 10·7 |
| Belrène | 3·5 ± 1·1 | 4·7 ± 2·6 | 4·7 ± 2·4 | 11·8 |
| Gala | 1·9 ± 1·2 | 7·3 ± 3·0 | 2·7 ± 2·0 | 11·5 |
| Fuji | 3·9 ± 1·9 | 2·1 ± 1·7 | 8·8 ± 7·2 | 14·0 |
| Elstar | 3·6 ± 3·0 | 7·3 ± 3·6 | 3·4 ± 2·4 | 14·9 |
| Granny | 6·7 ± 2·8 | 8·1 ± 3·2 | 0·7 ± 1·6 | 16·7 |
Three zones with long shoots were observed: the distal and the basal zones contain proleptic long shoots while the median zone contains sylleptic long shoots.
Fig. 3. Number of successive nodes bearing a long shoot on 1‐year‐old trunks of ‘Reinette Blanche du Canada’.
A similar analysis was performed for the other types of laterals. When all the shoot types in ‘Reinette’ were considered, 50 % of axillary buds developed along the trunks. Of the developing buds, approx. 17 % differentiated into a bourse. This corresponds to 0–13 axillary bourses per sequence located in a single zone, just above sylleptic shoots. The two other types of vegetative laterals were represented in approximately equal numbers: the mean number of proleptic spurs and the mean total of sylleptic shoots per sequence was approx. 11 in both cases. Proleptic spurs were located in the same zones as the proleptic long shoots, and were relatively more numerous in the basal zone. The other laterals types were isolated in the same manner as the long shoots: the most frequent run length was one for all the lateral types (data not shown).
Specification, building and evaluation of a hidden semi‐Markov chain for modelling ‘Reinette Blanche du Canada’ sequences
After the exploratory analysis, the branching sequences were viewed as a succession of zones in which the composition (in terms of lateral types) did not change substantially within each zone, but changed between zones. The composition may be defined by a single lateral type but is more generally defined by a given mixture of types. Moreover, the successive zones were transient, i.e. the branching zones of a given type occurred only once along a given sequence.
To represent this structure, an oriented ‘left–right’ hidden semi‐Markov chain was built (Fig. 4). In this model, the transient states were obtained by placing constraints on the transition probabilities of the underlying Markov chain with transition from a given state to the previous states being forbidden. The model is composed of six successive transient states representing the successive zones of the shoot, and a final absorbing state defined such that after entering it, it is impossible to leave. The time spent in this final absorbing state is thus not included in the model definition.
Fig. 4. Modelling branching on 1‐year‐old trunks of ‘Reinette Blanche du Canada’ using a hidden semi‐Markov chain. Each state, corresponding to a branching zone, is represented by a circle. Transient states are edged by a single line while the final state is edged by a double line. The possible transitions between states are represented by arcs with the attached probabilities noted nearby (for legibility, only transitions with probabilities exceeding 0·02 are shown). Thick arcs entering states indicate initial states. The attached initial probabilities are noted nearby. The occupancy distributions are shown above the corresponding states, as are the possible lateral types observed in each state. Each lateral type is represented by a symbol: 0, latent bud; 1, proleptic spur; 2, proleptic long shoot; 3, bourse; 4, sylleptic shoot.
Constraints were also expressed on the observation probabilities, forbidding the observation of a given symbol in a given state. This helped to differentiate the states with regard to observations. Therefore, only symbol 0 (latent bud) can be observed in the first and last states, which represent, respectively, the apical unbranched zone and the basal unbranched zone.
Six of the states, corresponding to the six well‐differentiated successive zones, were hypothesized during the exploratory analysis. The seventh is a small zone containing latent buds and located just below the top of the sequences. This zone is expressed in approx. 60 % of the sequences. The mixture of laterals observed in the zones was reflected in the observation distributions (Fig. 4). Four zones contained almost a unique lateral type: the median zone which contained only sylleptic laterals and three zones which contained only latent buds (the first zone, the last zone and that located just below the sylleptic zone). The three other zones contained mixtures of proleptic shoots, previously described. The zone lengths were reflected in the corresponding state occupancies, which were all bell‐shaped distributions (Fig. 4). This justifies a posteriori the choice of a hidden semi‐Markov chain, since the possibility of fitting such occupancy distributions—which are far from being geometric distributions—is a property of this model, compared with a hidden Markov chain.
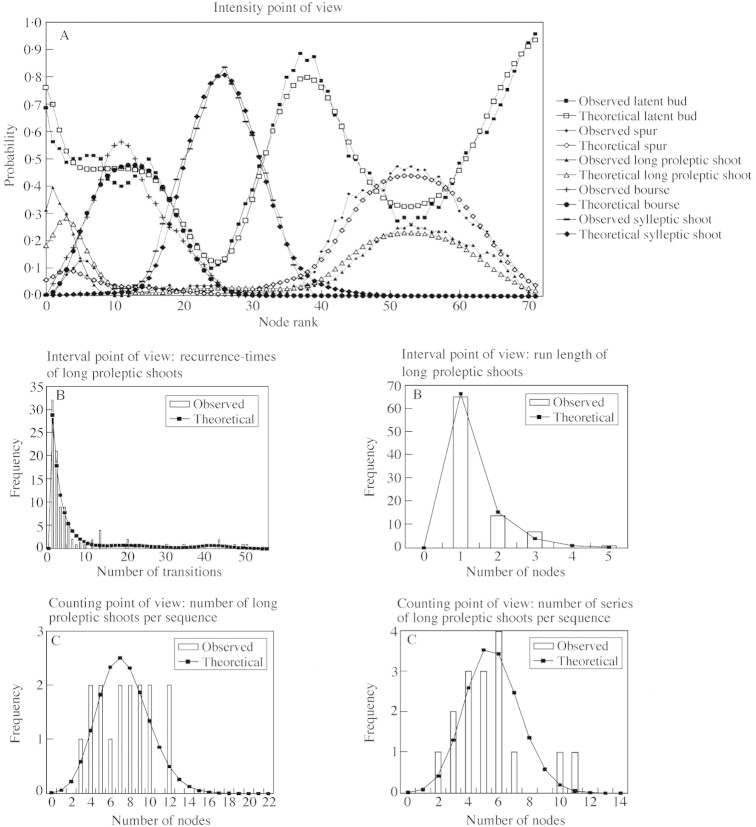
The accuracy of the entire model was evaluated by comparing the theoretical characteristics, organized according to the ‘intensity’, ‘interval’ and ‘counting’ points of view with the corresponding characteristics extracted from the observed sequences (Fig. 5).
Fig. 5. Model assessment: comparison of the characteristics extracted from the observed sequences with those computed from model parameters, i.e. intensity (A), interval (B) and counting (C) points of view.
The isolation of laterals, previously hypothesized on the basis of the run length histograms was confirmed by the theoretical distributions. In the case of independent events occurring at the successive nodes, the theoretical run length distributions should be geometric. In the present results, the deviation from the geometric distribution was of the same magnitude for all lateral types. The run length distributions took the form of a high frequency for value 1 followed by a geometric tail. Moreover, the most frequent event following the development of a lateral, whatever its type, was a latent bud (data not shown). These results are discussed more thoroughly using mathematical arguments in Guédon et al. (2001).
The homogeneity of the sequence sample is expressed in the model structure in particular by initial probabilities and transition probabilities that make it possible to jump states or to obtain states in parallel, and by the variability of the state occupancy. Except in the upper part where the latent bud zone was absent in 40 % of the trees (Fig. 4), the sequences exhibit a remarkable homogeneity in their structure since all the transition probabilities are 1 or close to 1. Thus, except for the upper zone, all the zones were present and located in the same order in all the observed sequences. In addition, the state occupancy distributions exhibit low dispersions. This means that the number of successive nodes corresponding to a given zone is relatively stable since the standard deviations were less than 3 nodes, whatever the zone. Given these results, the ‘Reinette’ branching pattern may be considered very homogeneous between sequences.
Comparison among cultivars
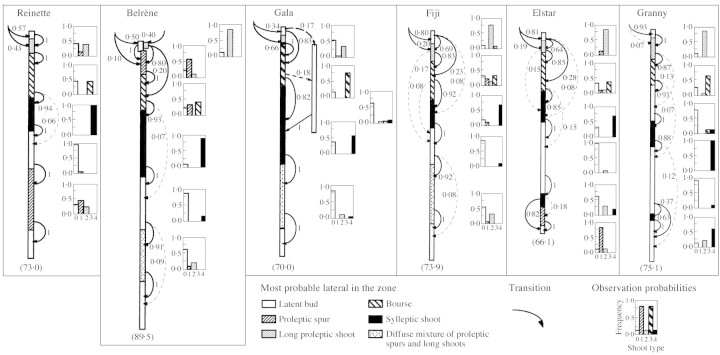
The exploratory analysis highlighted a succession of homogeneous transient zones for all the other cultivars. This prompted us to build a model based on similar assumptions to those previously described, for each remaining cultivar. Figure 6 shows a simplified representation of the models corresponding to each cultivar. It shows that most of the branching zones were located in the same order as previously described for ‘Reinette’. In particular, five zones were always in the same position in all cultivars: the unbranching zone at the top, long shoots just below followed by a floral zone, the sylleptic shoots in a median position and the latent buds at the base. Like ‘Reinette’, all cultivars exhibited high transition probabilities, especially in the median and basal parts of the sequences.
Fig. 6. Schematic representations of the hidden semi‐Markov chains estimated for each cultivar. The branching zones are represented by different motifs and their length corresponds to the mean value of the occupancy distribution. The observed lateral types are represented by histograms (except for the top and bottom zones where only latent buds are observed). The oriented edges represent the possible transitions between the zones and their respective probabilities are noted nearby. The mean total number of nodes is indicated in parentheses at the bottom of each diagram.
Despite the existence of this remarkable homogeneity in their common branching pattern, each cultivar exhibited some specific differences. These were investigated by highlighting the main differences in terms of branching zones and by a comparison of the characteristic distributions.
The number of zones differed among the cultivars, ranging from six (‘Gala’) to eight (‘Elstar’ and ‘Granny’). The two type II cultivars, ‘Reinette’ and ‘Belrène’, differed in spur location, which was mainly basal in ‘Reinette’ and apical in ‘Belrène’. ‘Gala’ was characterized by the absence of a zone defined by a high frequency of latent buds in more than 80 % of the sequences. The latent buds were as numerous as in other cultivars (Table 1) but were generally scattered throughout all the zones, mixed with other lateral types. ‘Fuji’ exhibited a similar succession of branching zones to that of ‘Reinette’ and differed only by more frequent long shoots and fewer spurs. ‘Granny’ and ‘Elstar’ were characterized by a large number of latent buds in the basal half of the trunk and by additional zones. ‘Granny’ showed an additional latent bud zone between the floral and the sylleptic lateral zones. In contrast to the other cultivars, the top of the sequences in ‘Granny’ was homogeneous while the basal part was more heterogeneous: the basal branching zone was present in only 37 % of the sequences. ‘Elstar’ exhibited a particularly long median zone with latent buds. As a consequence, the basal branching zone was close to the base of the trunk. An additional branching zone with spurs was located in this basal part but was present in only 18 % of the sequences.
The balance between the three zones of long laterals was roughly consistent with the classification into types since the long shoots were more numerous at the base for ‘Reinette’, in the median zone for ‘Gala’ and ‘Elstar’, and at the top for ‘Granny’. However, it should be underlined that this balance was not always as expected: ‘Belrène’ showed a balanced distribution of the long shoots between the three zones; ‘Fuji’ was characterized by a large number of long shoots in the basal zone; and ‘Granny’, despite a strong acrotony, exhibited numerous long shoots in the median zone.
DISCUSSION
Analysing the succession of lateral types along 1‐year‐old trunks as discrete sequences highlighted the existence of successive zones within which the lateral type composition was homogeneous, but changed between zones. This structure was ‘hidden’ in that it could not be predicted from usual qualitative observations, in the absence of quantitative investigations. It also confirmed that the fate of buds depends on their location along the bearing shoot and, therefore, on the time of their appearance. The five zones that were common to and located in the same position in all the cultivars studied suggest that successive developmental stages occur in the same order over a growing season and can be used to explain bud fate.
This is particularly obvious for the sylleptic branching zone and for the floral zone. The development of sylleptic laterals is assumed to result from a rapid growth period (Champagnat, 1954; Génard et al., 1994). In the results presented here, this phase was noted for all the cultivars but occurred more or less rapidly during the growing season (or closer to the roots) depending on the cultivar. The location of the floral zone between the sylleptic branching zone and growth cessation suggests that floral differentiation occurs when the growth rate of the bearing shoot is decreasing. This location is consistent with results reported by Crabbé and Escobedo (1991), who considered that floral differentiation in axillary buds results from an intermediate plastochron rate.
To interpret the location of the other lateral types, two different events must be distinguished, namely bud burst and the ability to pursue growth in order to develop into long shoots. The presence of latent buds within branching zones indicates that not all buds can burst. This fact was mentioned by Cline (2000) as a drawback in studying hormonal effects. Budburst has been interpreted to be the result of hormonal equilibrium, with auxin and cytokinins playing a major role. In particular, apical dominance (i.e. the apex and the long laterals inhibiting the axillary buds located below them) is usually considered as the result of a basipetal gradient of auxin from the apex (Wilson, 2000). The implication of other hormones such as cytokinins, which are probably shoot‐derived, has recently been demonstrated in apple shoots (Cook et al., 2001). These hormonal gradients may be responsible for inhibition of the axillary buds located just below the end of the annual shoots that constitute the unbranched zone present in 60 % of the trunks.
When located at a given distance from the apex, axillary buds are able to burst. The presence of latent buds mixed within the branching zones may be interpreted as local antagonism between buds, and possibly resulting from competition for assimilates prior to burst. This hypothesis is supported by studies showing that the intensity of budburst depends on stored carbohydrates and their access to the meristematic zone (Oliveira and Priestley, 1988; Bonhomme et al., 1999). In all cultivars, buds very rarely showed proleptic budburst within the median sylleptic branching zone. This suggests that their inhibition remains stable once a hierarchy between buds has been established. This inhibited state is maintained until the local position or the growth conditions of the buds are changed. This can be achieved by pruning, bending (Wareing and Nasr, 1961) or ageing (Drenou, 1994).
The percentage of latent buds increased from type II to type IV cultivars. This observation, made on 1‐year‐old trunks, is consistent with observations made on older branches. Working with fruiting branches, Lauri et al. (1995, 1997) observed that the percentage of latent buds—which decreases proportionally with the number of developing sites—may be related to the fruiting behaviour and to the regular bearing of the cultivars. This suggests that the percentage of latent buds could provide an early evaluation of fruiting habit, which is relevant for breeders.
A given number of axillary buds stop growing just after development of the preformed organs within the winter bud to form a spur, while a few shoots continue their development to form a long shoot. Competition for assimilates may explain the mixture of spurs and long shoots within the branching zones. However, another assumption recently tested in apricot trees implies that competition between primary and secondary growth could be responsible for the early primary growth cessation of most of the developing shoots (Costes et al., 2000).
The presence of a distal zone with long proleptic shoots is consistent with the description of an acrotonic gradient in apple shoots (Crabbé, 1987; Cook et al., 1998). However, proximal long proleptic laterals were also observed in all cultivars. This may reflect a basitonic tendency, first observed in apple and pear trees, and then in many other angiosperm species (for a review, see Barnola and Crabbé, 1991). This tendency is expressed temporarily in early winter; it has sometimes been observed at the end of summer (Mullins, 1965) or following suboptimal winter chilling (Cook and Jacobs, 1999).
Inhibition of the basal axillary buds can be interpreted, like acrotony, as the result of hormonal equilibrium. Indeed, low cytokinin concentrations were observed in proximal compared with distal parts of shoots in ‘Braeburn’ and ‘Granny’ cultivars (Cook et al., 2001). The ability of certain buds to develop closer to the roots differed between the cultivars, with ‘Elstar’ being the cultivar with the most basal laterals. The development of basal buds into long laterals is a drawback for tree management. Indeed, long branches close to the base of the trunk, whether sylleptic or proleptic, are usually removed by pruning. Thus, the determination of their number and location along the trunk can help to develop more effective selection strategies to ease tree training and to reduce pruning.
More generally, the difference between the cultivars in both the number of zones and their composition in terms of lateral types is of interest since it improves our knowledge of genetic differences and underlying physiological mechanisms. The statistical modelling led us to capture the embedded structure and contributed to a more precise definition of ‘branching pattern’ for apple tree in comparison with previous studies on genetic variations in growth and branching habits (Zagada and Faust, 1983). Moreover, the model outputs provide new insights into biological mechanisms, which can generate new assumptions or new questions. For example, the present results could be used to improve bud or tissue sampling strategies in order to better account for bud fate when studying the molecular mechanisms involved in budburst and lateral development. They could also be used to evaluate the outputs of other models, in particular structure–function models which generate three‐dimensional tree structures (e.g. de Reffye et al., 1997; Prusinkiewicz, 1998).
The results of this study show that trees of a given cultivar show a homogeneous behaviour regarding both the length of the 1‐year‐old trunks and their branching pattern. The fact that the trees were cut back to one node after transplantation probably induced a very rapid and homogeneous growth as well as a large, possibly maximal, number of laterals. But branching patterns are also likely to vary with agronomic practices such as propagation method and rootstock, or with climatic conditions. Thus, the modifications possibly induced by agronomic or climatic conditions remain to be analysed and interpreted as a natural continuation of this work.
ACKNOWLEDGEMENTS
We are grateful to P. E. Lauri and G. Garcia for their contribution to field observations and data input, and to C. Godin and Y. Caraglio for helpful comments on previous versions of this paper.
Supplementary Material
Received 15 October 2001; Returned for revision: 4 December 2001; Accepted 16 January 2002.
References
- BaldiP, Brunak S.1998. Bioinformatics: The machine learning approach. Cambridge MA: MIT Press. [Google Scholar]
- BarnolaP, Crabbé J.1991. La basitonie chez les végétaux ligneux. Déterminismes et variabilité d’expression. ‘L’arbre—Biologie et développement’. Naturalia Monspeliensia 7: 381–396. [Google Scholar]
- BarthélémyD, Caraglio Y, Costes E.1997. Architecture, gradients morphogénétiques et âge physiologique chez les végétaux. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architecture des végétaux. Science Update. Paris: INRA Editions, 89–136. [Google Scholar]
- BellAD.1991. Plant form. An illustrated guide to flowering plant morphology. Oxford: Oxford University Press. [Google Scholar]
- BonhommeM, Rageau R, Richard JP, Erez A, Gendraud M.1999. Influence of three contrasted climatic conditions on endodormant vegetative and floral peach buds: analyses of their intrinsic growth capacity and their potential sink strength compared with adjacent tissues. Scientia Horticulturaee 80: 157–171. [Google Scholar]
- BurgeC, Karlin S.1997. Prediction of complete gene structures in human genomic DNA. Journal of Molecular Biology 268: 78–94. [DOI] [PubMed] [Google Scholar]
- ChampagnatP.1954. Recherches sur les ‘rameaux anticipés’ des végétaux ligneux. Revue de Cytologie et de Biologie Végétale 15: 1–51. [Google Scholar]
- ChampagnatP.1965. Physiologie de la croissance et de l’inhibition des bourgeons: dominance apicale et phénomènes analogues. Encyclopedia of plant physiology, Vol 15. Berlin: Springer Verlag, 1106–1164. [Google Scholar]
- ClineMG.1997. Concepts and terminology of apical dominance. American Journal of Botany 84: 1064–1069. [PubMed] [Google Scholar]
- ClineMG.2000. Execution of the auxin replacement apical dominance experiment in temperate woody species. American Journal of Botany 87: 182–190. [PubMed] [Google Scholar]
- CookNC, Jacobs G.1999. Suboptimal winter chilling impedes development of acrotony in apple shoots. HortScience 34: 1213–1216. [Google Scholar]
- CookNC, Bellstedt DU, Jacobs G.2001. Endogeneous cytokinin distribution patterns at budburst in Granny Smith and Braeburn apple shoots in relation to bud growth. Scientia Horticulturaee 87: 53–63. [Google Scholar]
- CookNG, Rabe E, Keulemans J, Jacobs G.1998. The expression of acrotony in deciduous fruit trees: a study of the apple rootstock M.9. Journal of American Society for Horticultural Science 123: 30–34. [Google Scholar]
- CostesE.1993. Architecture aérienne de l’abricotier en conditions naturelles. Acta Botanica Gallica 140: 249–261. [Google Scholar]
- CostesE, Guédon Y.1997. Modelling the sylleptic branching on one year‐old trunks of apple cultivars. Journal of American Society for Horticultural Science 122: 53–62. [Google Scholar]
- CostesE, Fournier D, Salles JC.2000. Changes in primary and secondary growth as influenced by crop load effects in ‘Fantasme®’ apricot trees. The Journal of Horticultural Science & Biotechnology 75: 510–519. [Google Scholar]
- CrabbéJ.1984. Vegetative vigour control over location and fate of flower buds in fruit trees. Acta Horticulturae 149: 55–63. [Google Scholar]
- CrabbéJ.1987. Aspects particuliers de la morphogenèse caulinaire des végétaux ligneux et introduction à leur étude quantitative. Bruxelles: I.R.S.I.A. Presses University. [Google Scholar]
- CrabbéJ, Escobedo Alvarez J.1991. Activités méristématiques et cadre temporel assurant la transformation florale des bourgeons chez le pommier (Malus domestica Borkh. cv. Golden Delicious). ‘L’arbre—Biologie et développement’ Naturalia Monspeliensia 7: 369–380. [Google Scholar]
- CTIFL. 1993Pomme, les variétés. Paris: CTIFL. [Google Scholar]
- DennisFG, Masabni JG, Ketchie DO.1996. Evaluating twenty‐eight strains of ‘Delicious’ apple in Michigan. Journal of American Society for Horticultural Science 121: 988–995. [Google Scholar]
- deReffyePH, Fourcaud T, Blaise F, Barthélémy D, Houllier F.1997. A functional model of tree growth and architecture. Silva Fennica 31: 297–311. [Google Scholar]
- DrenouC.1994. Approche architecturale de la sénescence des arbres. Le cas de quelques angiospermes tempérées et tropicales. Thesis, Univ. Montpellier II, France. [Google Scholar]
- DurbinR, Eddy SR, Krogh A, Mitchison GJ.1998. Biological sequence analysis: probabilistic models of proteins and nucleic acids. Cambridge: Cambridge University Press. [Google Scholar]
- GatsukLE, Smirnova OV, Vorontzova LI, Zaugolnova LB, Zhukova LA.1980. Age states of plants of various growth forms: a review. Journal of Ecology 68: 675–696. [Google Scholar]
- GénardM, Pagés L, Kervella, J.1994. Relationship between sylleptic branching and components of parent shoot development in the peach tree. Annals of Botany 74: 465–470. [Google Scholar]
- GuédonY.1998. Hidden semi‐Markov chains: a new tool for analysing nonstationary discrete sequences. In: Janssen J, Limnios N, eds. Proceedings of the 2nd international symposium on semi‐Markov models: theory and applications. Compiègne. [Google Scholar]
- GuédonY.1999. Computational methods for discrete hidden semi‐Markov chains. Applied Stochastic Models in Business and Industry 15: 195–224. [Google Scholar]
- GuédonY, Cocozza‐Thivent C.1990. Explicit state occupancy modelling by hidden semi‐Markov models: application of Derin’s scheme. Computer Speech and Language 4: 167–192. [Google Scholar]
- GuédonY, Costes E.1999. A statistical approach for analyzing sequences in fruit tree architecture. Acta Horticulturae 499: 281–288. [Google Scholar]
- GuédonY, Barthélémy D, Caraglio Y.1999. Analyzing spatial structures in forest tree architectures. In: Amaro A, Tomé M, eds. Empirical and process‐based models for forest tree and stand growth simulation Lisbon: Edições Salamandra, 23–42. [Google Scholar]
- GuédonY, Barthélémy D, Caraglio Y, Costes E.2001. Pattern analysis in branching and axillary flowering sequences. Journal of Theoretical Biology 212: 481–520. [DOI] [PubMed] [Google Scholar]
- GodinC, Guédon Y, Costes E.1999. Exploration of a plant architecture database with the AMAPmod software illustrated on an apple tree hybrid family. Agronomie 19: 163–184. [Google Scholar]
- GodinC, Guédon Y, Costes E, Caraglio Y.1997. Measuring and analyzing plants with the AMAPmod software. In: Michalewicz MT, ed. Plants to ecosystems – Advances in computational life sciences, Vol. I Collingwood: CSIRO Publishing, 53–84. [Google Scholar]
- KainiBR, Jackson DI, Rowe RN.1984. Studies on shoot growth pattern in Lincoln canopy apple. Journal of Horticultural Science 59: 141–149. [Google Scholar]
- KetchieDO.1984. Flowering, spur formation and limb angles of delicious apple strains. Fruit Varieties Journal 38: 150–153. [Google Scholar]
- KervellaJ, Pagés, L, Génard M.1995. Growth context and fate of axillary meristems of young peach trees. Influence of parent shoots growth characteristics and of emergence date. Annals of Botany 76: 559–567. [Google Scholar]
- LaneWD, MacDonalds Ketchie DO.1996. Evaluation of quality traits and yield of 28 strains of ‘Delicious’ apple. Fruit Varieties Journal 50: 167–174. [Google Scholar]
- LauriPE, Lespinasse JM, Térouanne E.1997. Relationship between the early development of apple fruiting branches and the regularity of bearing. An approach to the strategies of various cultivars. Journal of Horticultural Science 72: 519–530. [Google Scholar]
- LauriPE, Térouanne E, Lespinasse JM, Regnard JL, Kelner JJ.1995. Genotypic differences in the axillary bud growth and fruiting pattern of apple fruiting branches over several years – An approach to regulation of fruit bearing. Scientia Horticulturaee 64: 265–281. [Google Scholar]
- LespinasseJM.1977. La conduite du pommier I. Types de fructification. Incidence sur la conduite de l’arbre. Paris: INVUFLEC. [Google Scholar]
- LukashinAV, Borodovsky M.1998. GeneMark.hmm: new solutions for gene finding, Nucleic Acids Research 26: 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MullinsMG.1965. The gravitational responses of young apple trees. Journal of Horticultural Science 40: 237–247. [Google Scholar]
- NicoliniE.1998. Architecture and morphological gradients in young beech trees (Fagus sylvatica L. Fagaceae) in a forest environment Canadian Journal of Botany 76: 1232–1244. [Google Scholar]
- OliveiraCM, Priestley CA.1988. Carbohydrate reserves in deciduous fruit trees. Horticultural Reviews 10: 403–430. [Google Scholar]
- OuelletteDV, Young E.1995. Lateral shoot development in six diverse seedling populations of apple. Fruit Varieties Journal 49: 248–254. [Google Scholar]
- PrusinkiewiczP.1998. Modelling of spatial structure and development of plants: a review. Scientia Horticulturaee 74: 113–149. [Google Scholar]
- RivalsP.1965. Essai sur la croissance des arbres et leur système de floraison (Application aux espèces fruitières). Journal d’Agriculture Tropicale et de Botanique Appliquée 12: 655–686. [Google Scholar]
- RivalsP.1966. Essai sur la croissance des arbres et leur système de floraison (Application aux espèces fruitières). Journal d’Agriculture Tropicale et de Botanique Appliquée 13: 91–122. [Google Scholar]
- RivalsP.1967. Essai sur la croissance des arbres et leur système de floraison (Application aux espèces fruitières). Journal d’Agriculture Tropicale et de Botanique Appliquée 14: 67–102. [Google Scholar]
- SabatierS, Barthelemy D.1999. Growth dynamics and morphology of annual shoots, according to their architectural position, in young Cedrus atlantica (Endl.) Manetti ex Carriere (Pinaceae). Annals of Botany 84: 387–392. [Google Scholar]
- TomlinsonPB, Gill AM.1973. Growth habits of tropical trees: some guiding principles. In: Meggers B. Tropical forest ecosystems in Africa and South America: a comparative review Washington: Smithsonian Institution Press, 129–143. [Google Scholar]
- UPOV.1982. Apple descriptors. In: Watkins, Smith. Rome: IBPGR. [Google Scholar]
- WareingPF, Nasr TAA.1961. Gravimorphism in trees. 1. Effects of gravity on growth and apical dominance in fruit trees. Annals of Botany 25: 321–340. [Google Scholar]
- WarringtonIJ, Ferree DC, Schupp JR, Ketchie DO.1990. Strain and rootstock effect on spur characteristics and yield of ‘Delicious’ apple strains. Journal of American Society for Horticultural Science 115: 348–356. [Google Scholar]
- WhiteJ.1979. The plant as a metapopulation. Annual Review Ecological Systems 10: 109–145. [Google Scholar]
- WilsonBF.2000. Apical control of branch growth and angle in woody plants. American Journal of Botany 87: 601–607. [PubMed] [Google Scholar]
- ZagadaSW, Faust M.1983. Population analysis of vigor and growth pattern of apple seedlings with short internode parentage. Journal of American Society for Horticultural Science 108: 939–944. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.