Abstract
While the importance of cortical aerenchyma in flood tolerance is well established, this pathway for gaseous exchange is often destroyed during secondary growth. For woody species, therefore, an additional pathway must develop for oxygen to reach submerged tissues. In this paper we examine the potential for the aerenchymatous phellem (cork) of Lythrum salicaria L. to provide a pathway for gas transport from shoots to roots and assess its importance in flood tolerance. Plants in which the continuity of the aerenchymatous phellem between shoots and roots was broken showed a significant reduction in oxygen levels in roots, but no difference in carbon dioxide levels compared with controls that retained an intact phellem. These plants also had a greater total shoot height and shoot dry weight, and an increase in shoot/root dry mass ratios compared with controls. Total dry weight was not significantly affected by this treatment. This study is the first to show that the aerenchymatous phellem can provide a pathway for gaseous exchange between roots and shoots and can influence plant morphology and patterns of resource allocation. This suggests that this tissue may play a significant role in the flood tolerance of a woody plant.
Key words: Lythrum salicaria, purple loosestrife, flooding, aerenchyma, oxygen transport, gas chromatography, phellem, roots, shoots, secondary growth, flood tolerance, wetlands
INTRODUCTION
Flooding is a common occurrence in wetland areas and plants in such environments must either cope with the stresses associated with submergence or perish (Blom et al., 1994). Strategies such as delayed germination or a reduced life cycle allow avoidance of exposure to flooding events, while, in the short term, physiological adaptations may overcome these stresses (Blom, 1999). When flood events are not cyclic and predictable, or last for substantially longer periods of time than can be compensated for by physiological means, other compensatory mechanisms are necessary. Several morphological and anatomical adaptations may play a role in flood tolerance of many wetland species; the development of aerenchyma in roots and shoots being one of the most common characteristics associated with flood tolerance (Blom, 1999; Jackson and Armstrong, 1999; Drew et al., 2000). The importance of aerenchyma formation has, however, only been discussed in relation to its presence in primary tissues, particularly in cortical tissues of stems and roots, although the presence of an aerenchymatous phellem (cork) has been documented for over a century in Decodon verticillatus L. (Schrenk, 1889, in Arber, 1920), Lythrum salicaria L. and Heimia myrtifolia Cham.&Schlecht (Schenck, 1889). In fact, the term aerenchyma was coined by Schenck (1889) to describe the lacunose tissue derived from the phellogen (cork cambium) of Jussiaea sp. More recently this tissue has been noted in Epilobium hirsutum L. (Etherington, 1984) and several members of the Lythraceae (Lempe et al., 2001). In a recent review, Jackson and Armstrong (1999) state that ‘the process of secondary thickening in plants and its implications for flood tolerance. . . is an area which desperately needs further study’.
In roots, with few exceptions, the cambia involved in secondary growth are initiated internal to the endodermal layer. With the increased girth associated with secondary growth, the tissues external to this (cortical tissue and epidermal tissue) often perish and are shed. In shoots, the phellogen (cork cambium) may arise in any cell layer external to the xylem, but generally with increased secondary growth the cortical tissue in stems is also destroyed. For a plant relying on cortical aerenchyma to provide a source of oxygen to the submerged tissues this presents a considerable problem. Unless a secondary pathway for oxygen transport is established, any tissue below the point at which secondary growth initiates could be cut off from an oxygen source. The development of an aerenchymatous phellem may provide the link between the cortical aerenchyma of the shoots and roots, thereby restoring a continuous pathway for gaseous exchange between these systems. However, there is currently no experimental support for this.
Lythrum salicaria L. (Lythraceae) is an amphibious species that produces a thick aerenchymatous phellem in submerged roots and stems that have initiated secondary growth (Stevens et al., 1997; Fig. 1A and B). This tissue forms the outermost region of stems and roots and, due to its loosely adhering layers, it can be removed without damaging the tissues internal to it (phellogen, phloem, vascular cambium and xylem). This provides a unique opportunity to manipulatively examine the importance of this tissue in gas transport and its influence on plant performance under partially flooded conditions. The objective of this paper is to determine the role played by aerenchymatous phellem in the flood tolerance of L. salicaria.
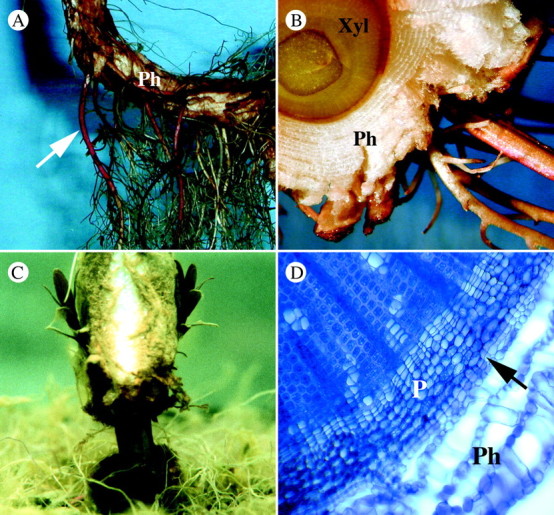
Fig. 1. Characteristics of Lythrum salicaria grown under flooded conditions and an illustration of phellem removal. A, Adventitious roots (arrow) and aerenchymatous phellem (Ph) along the submerged portion of a stem. B, Transverse section of a submerged stem showing adventitious roots and considerable aerenchymatous phellem (Ph) in relation to secondary xylem (Xyl). C, Submerged section of a stem in which the continuity of the aerenchymatous phellem between shoots and roots was disrupted. D, Transverse section of a submerged stem that experienced bi‐weekly phellem removal, showing intact phloem (P), and phellogen (arrow) and displaying the capacity for continued phellem (Ph) development. Stem was hand‐sectioned, stained with Toludine Blue O, viewed with a Leitz Biomed microscope at 200× magnification and images captured with a Nikon Coolpix 800 digital camera.
MATERIALS AND METHODS
Pre‐treatment
Seeds of Lythrum salicaria were collected from several locations in Guelph, Canada, in autumn 1997. They were refrigerated until required. On 18 June 1998, seeds were scattered onto the surface of filter paper, moistened with distilled water in 15 cm Petri dishes and placed in a glasshouse. One week after germination, two or three germinated seedlings were transferred to the surface of sand‐filled 15 cm pots, and 5 weeks after transplanting all plants were thinned to one plant per pot. Pot locations were randomized in the glasshouse at 2‐week intervals, until treatments were initiated.
Flooding
Plants were flooded 10 weeks after transplanting. To impose partially flooded conditions, pots containing plants were placed in laundry tubs (40 × 40 × 40 cm) and water levels raised in the tubs. Each of the ten tubs used constituted a block in the data analysis with ten replicates per treatment. Immediately prior to flooding, plant heights were measured and these heights were used to assign plants to tubs. All tubs contained plants of similar height so that all plants in a tub would experience similar levels of flooding. Since there was considerable variation in plant height among tubs, water levels varied among tubs. In each tub, the initial water level was such that water reached midway up the stems of all plants. Thus, water levels ranged from 3·75 to 8·5 cm above the soil surface for blocks containing the shortest and tallest plants, respectively. These levels were maintained for the duration of the experiment by adding water when required. One week after flooding, phellem removal was initiated. Plants were removed separately from the tubs and a ring of phellem was removed from the stem base to break the continuity between the aerenchymatous phellem in stems and roots. To do this, two cuts were initially made: around the stem base and 1·5 cm above this. The aerenchymatous phellem was then peeled away from this area (Fig. 1C). This method removed only the aerenchymatous phellem, while retaining the phellogen and the immediate derivatives of this layer (Fig. 1D). Twice weekly, any subsequent aerenchymatous phellem that developed was gently scraped away using the curved edge of a bent metal probe. Plants that retained an intact phellem (controls) were also lifted out of the tubs each week for the same duration as those that experienced phellem removal.
Assessment
Main stem height and the height of all branches greater than 1 cm were obtained immediately prior to flooding and at approx. 10‐d intervals following flooding. Seven weeks following flooding, extensive branching made height assessment difficult and plant height measurement was suspended until harvesting. Harvesting began 10 weeks after flooding and, due to constraints on equipment availability, continued for a 2‐week period. However, all plants within a block were harvested within an 8‐h period. For processing, plants were removed from the tubs and transferred to a water‐filled aquarium (62 × 32 × 32 cm). Stems were removed at the soil surface and roots were freed of the substrate by gentle agitation of the root system while maintaining the root system under water. Gas samples were obtained from stem and root pieces by immersing these in a large tray of water, squeezing the tissue and collecting the resulting gas bubbles in an inverted funnel with a rubber sleeve serum stopper placed over the neck‐end. This gas was extracted from the funnel using a 2·5 ml Hamilton gas‐tight syringe and injected into a Shimadzu Model 8A gas chromatograph equipped with an external 0·5 ml injection port, a Haye Sept D column (30 ft × 1/8 inch diameter, 100/120 mesh) with a Mandel thermal conductivity detector and a Shimadzu CR3A integrator. The system was run at 25 °C with 30 ml min–1 of helium. Concentrations of N2, O2 and CO2 were expressed as a percentage of the total gas volume (N2 + O2 + CO2). Only samples consisting of 0·5 ml of gas or more were used for gas analysis. For each set of analyses, a sample of ambient air was injected into the chromatograph to monitor calibration. Main stem height and lateral branch heights were measured to the nearest centimetre. Dry weights of the shoot and root system were obtained following drying at 80 °C for a minimum of 48 h.
Data analysis
All data were analysed using Proc Mixed in SAS (SAS Institute Inc., Cary, NC, USA); blocks were considered random effects. Shoot height was analysed using Proc Mixed with Repeated Measures and the covariance matrix was modelled using the Guassian algorithm with sampling date as the correction factor. To meet the requirements of equal variance and normality, data were transformed as follows.
Gas analysis: shoot O2 levels, y = y2; root O2 and CO2 levels, y = y; shoot CO2 levels, shoot/root O2 and CO2 ratio, y = log (y).
Dry weights: shoot dry weight, y = y; root dry weight and shoot/root dry weight ratio, y = log (y); total dry weight, y = y–2.
Repeated measures analysis: shoot height, y = log (y).
Comparisons were conducted using the contrast statement in SAS.
RESULTS
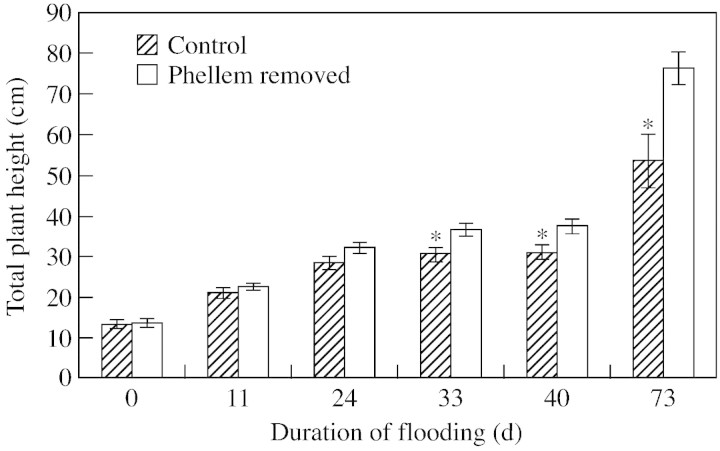
Differences in mean total shoot heights became evident 33 d after flooding. Plants that experienced phellem removal had a significantly greater total shoot height than the controls; this trend persisted throughout the duration of the experiment (Fig. 2). By harvest, total stem height in the controls was approx. 30 % shorter than that of plants that experienced phellem removal. A similar reduction was noted for shoot dry weight, which was also significantly greater in plants whose phellem had been removed (Table 1). There were no detectable differences in root or total dry weight between treatments (Table 1). Shoot/root dry weight ratios were approx. 50 % lower in the controls compared with plants from which the phellem was removed (Table 1).
Fig. 2. Repeated measures analysis of the effects of phellem removal on the total shoot height of L. salicaria plants grown under partially flooded conditions. Data presented are raw means ± 1 s. e. To satisfy statistical requirements, analysis was conducted on log‐transformed data. Asterisk indicates a significant difference from the controls (P < 0·05).
Table 1.
Effects of phellem removal on shoot, root and total dry weight, and shoot/root dry weight ratio of Lythrum salicaria plants grown under partially flooded conditions
| Control | Phellem removed | |
| Shoot dry weight* | 1·40 ± 0·25 g | 2·16 ± 0·39 g |
| Root dry weight | 3·35 ± 0·55 g | 2·48 ± 0·18 g |
| Total dry weight | 4·85 ± 0·77 g | 4·59 ± 0·33 g |
| Shoot/root ratio* | 0·47 ± 0·06 g | 0·97 ± 0·31 g |
Data presented are raw means ± 1 s. e. When necessary, to satisfy statistical requirements, analysis was conducted on transformed data. Asterisk indicates significant difference from the controls (P < 0·05).
While phellem removal had no detectable effect on shoot O2 levels, O2 levels in the roots were significantly reduced (Table 2). Shoot/root O2 levels were significantly higher in plants from which the phellem was removed, while in plants retaining the phellem these ratios approached one (Table 2). There was no detectable difference in shoot or root CO2 levels or shoot/root CO2 ratios (Table 2).
Table 2.
Effects of phellem removal on percentage by volume of O2 and CO2 in the shoot and root of Lythrum salicaria plants grown under partially flooded conditions
| Control | Phellem removed | |
| Shoot O2 | 17·18 ± 1·70 | 18·89 ± 0·45 |
| Root O2* | 17·23 ± 0·83 | 13·66 ± 0·63 |
| Shoot CO2 | 1·21 ± 0·17 | 0·98 ± 0·12 |
| Root CO2 | 1·40 ± 0·15 | 1·39 ± 0·14 |
| Shoot/root O2* | 0·99 ± 0·09 | 1·41 ± 0·08 |
| Shoot/root CO2 | 0·87 ± 0·08 | 0·73 ± 0·06 |
Data presented are raw means ± 1 s.e. When necessary, to satisfy statistical requirements, analysis was conducted on transformed data. Asterisk indicates significant difference from the controls (P < 0·05).
DISCUSSION
The reduction in root O2 levels associated with phellem removal indicates that this tissue is involved in gas transport from shoots to roots. We were, however, unable to establish a direct relationship between phellem removal and changes in CO2 levels between roots and shoots, but it is unlikely that gaseous exchange was limited to oxygen. While O2 levels remained just below atmospheric levels (18 %) in the roots of plants that retained the phellem, there was a significant reduction in O2 levels in the roots of all plants from which the phellem was removed. Oxygen levels were still approx. 13 % in the roots of plants from which the phellem was removed, which suggests that either the aerenchymatous phellem was not completely removed by the treatments, or that an additional pathway for O2 transport exists, independent of the aerenchymatous phellem. Although our technique for phellem removal removed the majority of the phellem, the immediate derivatives of the phellogen were left intact and it is likely that the smaller intercellular spaces found in these layers were sufficient to account for some exchange of gases between the shoot and root systems. Additionally, the act of removing the plants from the tubs immediately prior to gas extraction may have allowed some gaseous exchange to occur.
It is well established that submerged plants will undergo petiole, peduncle or internode elongation to re‐establish atmospheric contact for gas exchange (Voesenek et al., 1992; Armstrong et al., 1994; Blom, 1999). This process has been described as ‘depth accommodation’ (Ridge, 1987) since the elongation ceases once the leaves or stems reach the surface of the water. In our study, however, atmospheric contact was not breached, yet plants that experienced a break in continuity between the aerenchymatous phellem of the shoots and roots were consistently taller than the controls. This result indicates that a lowered level of oxygen availability in roots plays a role in influencing shoot extension. The increase in plant height coincides with an increase in shoot biomass and an increased shoot/root dry mass ratio. However, since total dry mass did not differ significantly between treatments, this suggests that a change in resource partitioning between roots and shoots occurred rather than a decreased capacity for resource uptake, and in particular for carbon fixation. But this may not be adaptive. If the production and maintenance of the below‐ground root system were limited by O2 availability, this would make resources available to the shoot which otherwise would be utilized for root growth and maintenance. The adaptive value, if any, of an increase in resource allocation to shoots when atmospheric continuity is sustained needs to be assessed.
The role of cortical aerenchyma in gaseous exchange between below‐ground root systems and the above‐ground shoot system is well documented, and its physiological basis is being elucidated (Blom, 1999; Jackson and Armstrong, 1999; Drew et al., 2000). However, the fate of this system once secondary growth is initiated is often overlooked. This oversight may be due to the fact that most species investigated to date do not undergo secondary growth, or that plants studied may have been too young to initiate it. Since a considerable number of wetland plants do undergo secondary growth, this represents a large gap in our understanding of the potential mechanisms of flood tolerance in these species. Justin and Armstrong (1987) suggested that a limited degree or suppression of secondary growth may be advantageous under flooded conditions, but this response is not a feasible mechanism for flood tolerance if considerable secondary growth has occurred prior to the flooding event. The current study is the first to show that the aerenchymatous phellem offers an additional pathway for gaseous exchange, and one that may have the potential to restore the continuity of the cortical aerenchyma between shoots and roots following disruption by the onset of secondary growth. Furthermore, while the impact on respiration rates of the observed reduction in O2 levels following phellem removal is unclear, it is clear that phellem removal can affect plant morphology and patterns of resource allocation between shoots and roots.
ACKNOWLEDGEMENTS
We thank Chris Quarrie, Chris Burns and George Bassel for help with harvesting, Dr R. Yada for use of the gas chromatograph, and the Ontario Graduate Scholarship Program and Natural Sciences and Engineering Research Council of Canada for partial funding of this project.
Supplementary Material
Received: 12 November 2001; Returned for revision: 4 January 2002; Accepted: 29 January 2002.
References
- ArberA 1920Water plants; a study of aquatic angiosperms. Cambridge: Cambridge University Press. [Google Scholar]
- ArmstrongW, Brandle R, Jackson MB 1994. Mechanisms of flood tolerance in plants. Acta Botanica Neerlandica 43: 307–358. [Google Scholar]
- BlomCWPM 1999. Adaptations to flooding stress: from plant community to molecule. Plant Biology 1: 261–273. [Google Scholar]
- BlomCWPM, Voesenek LACJ, Visser EJW 1994. Physiological ecology of river species: adaptive responses of plants to submergence. Annals of Botany 74: 253–263. [Google Scholar]
- DrewMC, He CJ, Morgan PW 2000. Programmed cell death and aerenchyma formation in roots. Trends in Plant Science 5: 123–127. [DOI] [PubMed] [Google Scholar]
- EtheringtonJR 1984. Comparative studies of plant growth and distribution in relation to waterlogging: 10. Differential formation of adventitious roots and their experimental excision in Epilobium hirsutum and Chamerion angustifolium Journal of Ecology 72: 389–404. [Google Scholar]
- JacksonMB, Armstrong W 1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1: 274–287. [Google Scholar]
- JustinSHFW, Armstrong W 1987. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106: 465–495. [Google Scholar]
- LempeJ, Stevens KJ, Peterson RL 2001. Shoot responses of six Lythraceae species to flooding. Plant Biology 3: 186–193. [Google Scholar]
- RidgeI 1987. Ethylene and growth control in amphibious plants. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats Oxford: Blackwell Scientific Publications, 53–77. [Google Scholar]
- SchenckH 1889. Über das Aërenchym, ein dem Kork homologes Gewebe bei Sumpflanzen. Jahrbûcher fûr wissenshaftliche Botanik 20: 526–574. [Google Scholar]
- StevensKJ, Peterson RL, Stephenson GR 1997. Morphological and anatomical responses of Lythrum salicaria L. (purple loosestrife) to an imposed water gradient. International Journal of Plant Sciences 158: 172–183. [Google Scholar]
- VoesenekLACJ, Van der Sman AJM, Harren FJM, Blom CWPM 1992. An amalgamation between hormone physiology and plant ecology: a review on flooding resistance and ethylene. Journal of Plant Growth Regulation 11: 171–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.