Abstract
The study was conducted to identify the self‐incompatibility mechanism in Eucalyptus globulus ssp. globulus. Controlled self‐ and cross‐pollinations were conducted on individual flowers from three mature trees that had self‐incompatibility levels of 76, 99·6 and 100 %. Flowers were harvested at 4, 6 and 8 weeks after pollination. Embryology was investigated by bright field microscopy on material harvested at 4 and 6 weeks after pollination. Fertilization had taken place at 4 weeks after pollination with zygotes and free nuclear endosperm visible. There was a greater proportion of healthy, fertilized ovules in the cross‐ compared with the self‐pollination treatment, and approx. half the ovules examined from both pollen treatments were not fertilized or were degenerating. By 6 weeks after pollination a few zygotes were starting to divide. The number of healthy, fertilized ovules was still greater in the cross‐pollination treatment, but the number of healthy fertilized ovules was lower in both treatments compared with 4 weeks after pollination, and many ovules were degenerating. Fertilized ovules were significantly larger than non‐fertilized or degenerating ovules and this difference was detectable by eye at 6 and 8 weeks after pollination. The mechanism of self‐incompatibility appears to have both late pre‐ and post‐zygotic components.
Key words: Eucalyptus globulus ssp. globulus, Tasmanian blue gum, self‐incompatibility, controlled pollination, bright field microscopy, embryology, fertilization, ovule, zygote
INTRODUCTION
Eucalyptus globulus ssp. globulus (hereafter referred to as E. globulus) is widely planted around the world for pulpwood production (Eldridge et al., 1994). E. globulus plantations are often established by planting out seedlings grown from seed sourced from seed orchards. These orchards have been established with the aim of producing genetically superior seed in large quantities. However, self‐compatible individuals occur in E. globulus seed orchards, for example Moncur et al. (1995) studied seed quality in a Tasmanian seed orchard and reported the outcrossing rate to be 77 %. The production of selfed seed in eucalypt species leads to inbreeding depression in the next generation at several growth stages (Eldridge and Griffin, 1983; Potts et al., 1987; Hardner and Potts, 1995, 1997;Hardner and Tibbits, 1998; Hardner et al., 1998), so it is therefore undesirable to include selfed seedlings in plantations (Moncur et al., 1995).
To improve seed orchard management practices it is important to gain an understanding of the breeding system of the species concerned (Moncur et al., 1995). The genus Eucalyptus has a breeding system that is preferentially outcrossing; however, self seed production commonly occurs (Griffin et al., 1987, Eldridge et al., 1994). Within eucalypt species individual trees can range from self‐compatible to completely self‐incompatible (Potts and Savva, 1988; Ellis and Sedgley, 1992). Ideally seed orchards should contain only self‐incompatible trees to eliminate production of selfed seed. Otherwise, seed should only be harvested from trees within a seed orchard that have been identified as self‐incompatible. Identifying the mechanism of self‐incompatibility within a species may enable development of a relatively quick test for self‐incompatibility in individual trees.
Self‐incompatibility mechanisms have traditionally been found to operate in styles with either a sporophytic or gametophytic incompatibility system (de Nettancourt, 1977, 1984; Lewis, 1979). More recently, evidence has been accumulating for late pre‐ and post‐zygotic self‐incompatibility systems (Seavey and Bawa, 1986). At present the self‐incompatibility mechanism has only been determined for a few eucalypt species, and these range from late pre‐zygotic control in E. woodwardii (Sedgley, 1989; Sedgley and Smith, 1989), to post‐zygotic control in E. regnans (Sedgley et al., 1989), E. cladocalyx and E. leptophylla (Ellis and Sedgley, 1992). Both pre‐ and post‐zygotic barriers have been reported in E. spathulata and E. platypus: a reduction in pollen tube penetration of ovules and ovule fertilization was found following self‐pollination compared with cross‐pollination, and few of the ovules fertilized following selfing developed past zygote division (Sedgley and Granger, 1996). Pound et al. (2002) found reduced ovule penetration following self‐pollination compared with cross‐pollination in E. globulus, but this difference did not completely account for the level of self‐incompatibility known to exist in the trees studied. The present study aimed to determine the self‐incompatibility mechanism in E. globulus by investigating embryological processes. A further aim was to attempt to develop a test for screening individual E. globulus trees for self‐incompatibility.
MATERIALS AND METHODS
Plants and pollinations
This experiment was conducted on three of the same trees used as females in Pound et al. (2002) (trees 319, 503 and 537), which were wild, mature trees located south of Hobart, Tasmania. The level of self‐incompatibility of trees 319, 503 and 537 was found to be 76, 99·6 and 100 %, respectively, using the formula:
SI = 100 [(Vcp – Vsp)/Vcp](1)
where SI is self‐incompatibility, Vcp is viable seed per flower cross‐pollinated and Vsp is viable seed per flower self‐pollinated.
Flowers were emasculated just prior to anthesis, and as eucalypt flowers are protandrous, flowers were isolated individually from pollen contamination in wax‐coated paper bags. Seven days after emasculation, stigmas had secreted a sticky exudate indicating receptivity to pollen, so isolation bags were opened, flowers pollinated with either self or cross pollen, and the bags re‐closed and left over the flowers. Pollinations were conducted using the same pollen source used in Pound et al. (2002), either self pollen collected from the individual experimental tree, or a cross pollen mixture from ten E. globulus trees unrelated to trees 319, 503 and 537. Flowers used were spread throughout all accessible lower branches and the pollen treatments were randomly applied. Pollinations were conducted on all three trees simultaneously throughout September and early October 1999, the same time as pollinations were conducted in Pound et al. (2002).
Field harvests and microscopy
Five flowers were harvested per treatment per tree at 4, 6 and 8 weeks after pollination. One locule was dissected from each flower and the number of locules present in each flower was recorded. The tissue processing method used was similar to that described by Feder and O’Brien (1968). The dissected locules were fixed in 3 % glutaraldehyde in 0·025 m phosphate buffer, pH 7·2, for a minimum of 48 h at 0–4 °C. Once fixed, one locule per treatment from trees 319, 503 and 537 from the 4‐week harvest, and one locule per treatment from tree 319 from the 6‐week harvest was dehydrated through an alcohol series: methoxy‐ethanol, ethanol, propanol and butanol. Whilst in ethanol, individual ovules were dissected from locules. Ovules were left in each alcohol for a minimum of 2 h, then infiltrated overnight in a 1 : 1 mixture of butanol : glycol methacrylate (GMA). Samples were then infiltrated with two changes of 100 % GMA over 4 d. Ovules were embedded in GMA in gelatine capsules and polymerized at 60 °C. The remaining locules were dehydrated through methoxy‐ethanol to ethanol and stored for observation.
Serial, longitudinal sections (LS) 3 µm thick were cut through every ovule per locule embedded for tree 319, but only 35 ovules per locule for tree 503 and 25 ovules per locule for tree 537 due to the large number of ovules present per locule. Sections were stained with Periodic acid‐Schiff’s reagent and Toluidine blue O (PAS/TBO) and observed using a Zeiss microscope in bright field mode. The state of the nucellus cells was observed and recorded, along with the contents of each embryo sac. From embryo sac observations each ovule was classified as fertilized or not fertilized.
The length and diameter of every ovule and its embryo sac was measured using a calibrated microscope eyepiece. Data analysis was performed using Genstat 5 release 4·1, 4th edition. Two‐sample t‐tests were performed on these parameters between ovules that were fertilized and those that were not, for both self and cross pollen treatments from each tree. Data from the self pollen treatment of tree 537 were excluded from the analysis due to the small sample size of fertilized ovules. Data on ovule length and width, and embryo sac length and width were pooled separately from the observations collected 4 weeks after pollination. Due to the unbalanced nature of the pooled data sets, the data were analysed using Restricted Maximum Likelihood (REML). The statistical model included terms for the fixed effects of pollen treatment, fertilization status of ovules (fertilized or not) and the interaction between pollen treatment and fertilization status, and the random effects of tree and the interaction between tree and flowers pollinated. The significance of the fixed effects was tested using Wald tests.
RESULTS
Ovule structure
Ovules consisted of an outer integument that surrounded the inner integument to form the micropyle, with the inner integument surrounding the nucellus and embryo sac. By 4 weeks after pollination, all three trees had some ovules with nucellus cells that were degenerating (darkly stained), and embryo sacs that were collapsing (embryo sac lumen still present) (Fig. 1A) or that had collapsed completely (no embryo sac lumen present). By 6 weeks after pollination, the number of ovules with collapsed embryo sacs and nucellus degeneration had increased from that observed at 4 weeks (Table 1). When an embryo sac was present, ovules were clearly unfertilized if the embryo sac contained egg apparatus (egg cell with two synergid cells with filiform apparatus) and a central cell with two polar nuclei either fused (Fig. 1B) or separate (Fig. 1C). Ovules were also categorized as unfertilized if the egg apparatus or the central cell was degenerating or had completely disappeared, or if the embryo sac had collapsed. This was done to discriminate between healthy, fertilized ovules that had a chance of developing to seed maturity, and those ovules that did not.
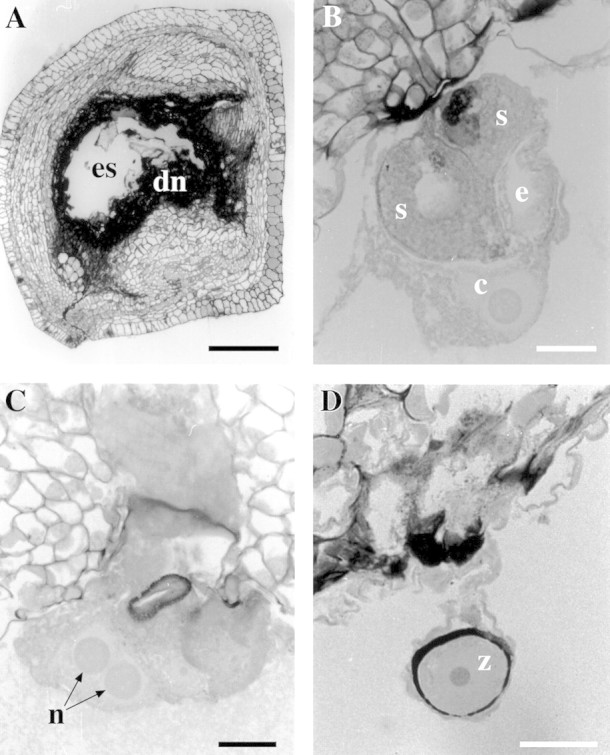
Fig. 1. Longitudinal sections of Eucalyptus globulus ovules, stained with PAS/TBO. A, Degenerating ovule at 6 weeks after self‐pollination showing the embryo sac (es) and degenerating nucellus (dn). Bar = 200 μm. B, Micropylar end of the embryo sac at 4 weeks after cross‐pollination showing synergids (s), egg cell (e) and central cell (c). Bar = 25 μm. C, Micropylar end of the embryo sac at 4 weeks after cross‐pollination showing polar nuclei (n). Bar = 25 μm. D, Micropylar end of the embryo sac at 6 weeks after cross‐pollination showing zygote (z). Bar = 25 μm.
Table 1.
Anatomical details of ovules from both self‐ and cross‐pollinations, at 4 and 6 weeks after pollination
| n (%) | |||||||||
| Flowers harvested | Tree | Pollination treatment | No. of ovules observed (total ovules in locule) | Embryo sac still present | Nuclear endosperm | Zygote | Persistent synergid | Fertilization | Nucellus degeneration |
| 4 weeks after pollination | 319 | Self | 42 (42) | 38 (90) | 10 (24) | 10 (24) | 12 (29) | 13 (31) | 21 (50) |
| Cross | 38 (38) | 38 (100) | 19 (50) | 11 (29) | 16 (42) | 20 (53) | 1 (3) | ||
| 503 | Self | 35 (63) | 33 (94) | 15 (43) | 12 (34) | 25 (71) | 15 (43) | 8 (23) | |
| Cross | 35 (94) | 29 (83) | 15 (43) | 14 (40) | 22 (63) | 15 (43) | 15 (43) | ||
| 537 | Self | 25 (60) | 14 (56) | 1 (4) | 1 (4) | 2 (8) | 1 (4) | 16 (62) | |
| Cross | 25 (67) | 23 (92) | 6 (24) | 4 (16) | 7 (28) | 6 (24) | 3 (12) | ||
| 6 weeks after pollination | 319 | Self | 29 (29) | 22 (76) | 5 (17) | 6 (21) | 6 (21) | 6 (21) | 25 (86) |
| Cross | 35 (35) | 19 (54) | 12 (34) | 12 (34) | 10 (29) | 12 (34) | 23 (66) | ||
n, number of ovules for which a particular feature was present, with percentage in parentheses.
Fertilization had occurred by 4 weeks after pollination in all three trees for both treatments. Zygotes were observed at the micropylar end of the ovule as thick‐walled single cells with a distinct nucleus (Fig. 1D) or they were beginning to form and take shape. By 6 weeks after pollination, zygotes were obvious with a single nucleus; however, three zygotes had developed to the extent that the nucleus had divided to give two distinct nuclei (Fig. 2A). Many zygotes were adjacent to a persistent synergid with filiform apparatus. Fertilized ovules had free nuclear endosperm present. This was seen as either two large nuclei resulting from the first division following fusion of one sperm nucleus with the two fused polar nuclei (polar fusion nucleus), or as several smaller nuclei, joined by cytoplasm (Fig. 2B). The nuclear endosperm formed a ring in longitudinal sections within the embryo sac lumen (Fig. 2C). Ovules were considered fertilized and healthy if a zygote and free nuclear endosperm were present, and there was no cellular degeneration. At 4 weeks after pollination some ovules had free nuclear endosperm but no distinguishable zygote. Free nuclear endosperm was often quite extensive when some zygotes were present with cell wall thickening incomplete. It was assumed that ovules that had free nuclear endosperm and no observable zygote had been fixed before zygote formation had taken place and they were therefore classified as fertilized. In some cases the path of a pollen tube could be seen through the nucellus to a synergid cell (Fig. 2D).
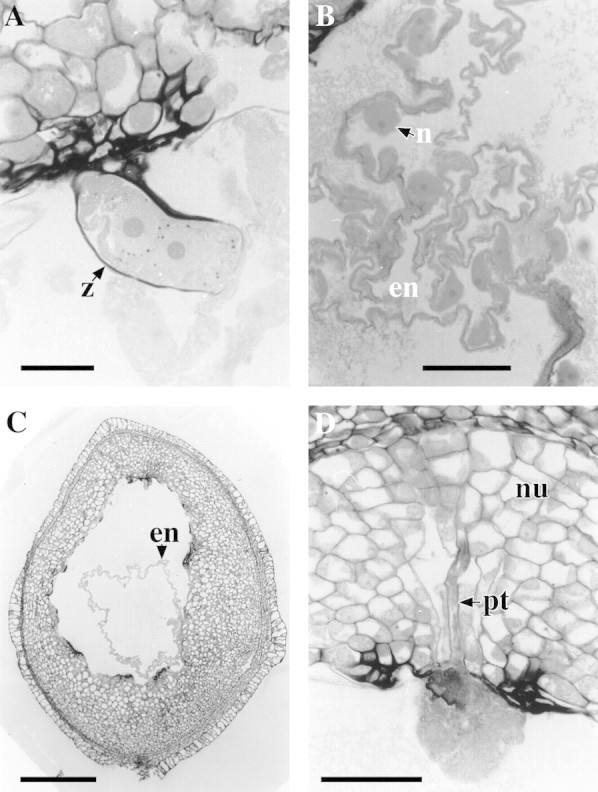
Fig. 2. Longitudinal sections of Eucalyptus globulus ovules, stained with PAS/TBO. A, Micropylar end of the embryo sac at 6 weeks after cross‐pollination showing zygote (z) with divided nucleus. Bar = 25 μm. B, Embryo sac at 6 weeks after cross‐pollination showing nuclei (n) of free nuclear endosperm (en). Bar = 50 μm. C, Ovule at 6 weeks after cross‐pollination showing free nuclear endosperm (en) forming a ring of cytoplasm in the embryo sac lumen. Bar = 500 μm. D, Micropylar end of ovule 6 weeks after cross‐pollination showing a pollen tube (pt) in the nucellus tissue (nu). Bar = 50 μm.
Ovule fertilization
At 4 weeks after pollination the proportion of ovules fertilized following self‐pollination ranged from 4 % in tree 537 to 43 % in tree 503. Following cross‐pollination, ovule fertilization ranged from 24 % in tree 537 to 53 % in tree 319 (Table 1). In trees 319 and 537, a greater proportion of ovules were fertilized following cross‐pollination than self‐pollination. There was no difference in the proportion of ovules fertilized between treatments in tree 503. Tree 319 had a smaller proportion of fertilized ovules in both treatments at 6 weeks compared with 4 weeks after pollination, indicating that some fertilized ovules had ceased to develop and had degenerated. There was still a greater proportion of fertilized ovules in the cross‐pollination treatment compared with the self‐pollination treatment.
E. globulus flowers have either four or five locules, and the number of ovules per locule varies (Pound et al., 2002). As the ovules sectioned were from a whole or part of a single locule, the proportions of ovules from both fertilized and unfertilized categories were extrapolated to a whole flower, and total numbers per flower were compared (Table 2). These data reveal that whilst tree 503 may have had a similar proportion of fertilized ovules, there was a greater number of fertilized ovules in the cross‐pollination treatment compared with the self‐pollination treatment. Furthermore, in all three trees a greater number of ovules were fertilized in both pollen treatments at 4 weeks after pollination, and in tree 319 at 6 weeks after pollination, compared with the mean number of ovules that developed into mature seeds (Table 2) (mean mature seed numbers presented in from Pound et al., 2002).
Table 2.
Number of ovules fertilized at 4 and 6 weeks after pollination, and mean number of seeds per flower, following self‐ and cross‐pollination
| Number of fertilized ovules per flower | Mean number of seeds per capsule | |||||
| 4 weeks after pollination following: | 6 weeks after pollination following: | 12 months after pollination following: | ||||
| Selfing | Crossing | Selfing | Crossing | Selfing | Crossing | |
| Tree 319 | 52·0 | 100·0 | 30·0 | 48·0 | 7·6 | 31·7 |
| Tree 503 | 125·1 | 201·4 | – | – | 3·0 | 73·5 |
| Tree 537 | 9·6 | 64·3 | – | – | 0·0 | 35·9 |
Fertilization data extrapolated from that collected from a single locule or part locule.
–, Variables for which no data were collected.
Ovule dimensions
In all trees, fertilized ovules from cross‐pollination treatments were longer and wider than non‐fertilized ovules (Table 3). This pattern was not consistent following self‐pollination. Tree 319 showed no significant difference in ovule lengths and widths at both 4 and 6 weeks after pollination. Whilst not significant, there was a trend for increased ovule lengths and widths in fertilized ovules compared with non‐fertilized ovules in tree 319.
Table 3.
Mean ovule lengths and widths
| Ovule length (μm) | Ovule width (μm) | |||||||||||
| Flowers harvested | Tree | Treatment | Fertilized | s.d. | Not fertilized | s.d. | P | Fertilized | s.d. | Not fertilized | s.d. | P |
| 4 weeks after | 319 | Self | 1054 | 171 | 1023 | 189 | 0·620 | 759 | 150 | 672 | 209 | 0·060 |
| pollination | Cross | 1027 | 145 | 907 | 117 | 0·010 | 744 | 147 | 619 | 90 | 0·004 | |
| 503 | Self | 953 | 116 | 878 | 75 | 0·028 | 704 | 97 | 639 | 76 | 0·033 | |
| Cross | 926 | 103 | 852 | 76 | 0·019 | 654 | 71 | 581 | 86 | 0·011 | ||
| 537 | Self | 1110 | – | 889 | 148 | – | 770 | – | 652 | 131 | – | |
| Cross | 1097 | 187 | 933 | 130 | 0·023 | 813 | 112 | 681 | 118 | 0·024 | ||
| 6 weeks after | 319 | Self | 1580 | 452 | 1105 | 189 | 0·050 | 1080 | 408 | 726 | 149 | 0·090 |
| pollination | Cross | 1973 | 235 | 1032 | 169 | <0·001 | 1357 | 276 | 714 | 191 | <0·001 | |
–, Variables for which data are unavailable.
In all trees from both pollen treatments at both 4 and 6 weeks after pollination, embryo sacs were longer in fertilized ovules compared with non‐fertilized ovules, except for the self pollen treatment in tree 319, 4 weeks after pollination. Fertilized ovules also had wider embryo sacs compared with non‐fertilized ovules for both pollen treatments over all trees, but no difference was found in the self pollen treatment from tree 319, 6 weeks after pollination (Table 4). Again, although measurements were not significant in tree 319, the trend was for fertilized ovules to be larger.
Table 4.
Mean embryo sac lengths and widths
| Embryo sac length (μm) | Embryo sac width (μm) | |||||||||||
| Flowers harvested | Tree | Treatment | Fertilized | s.d. | Not fertilized | s.d. | P | Fertilized | s.d. | Not fertilized | s.d. | P |
| 4 weeks after pollination | 319 | Self | 465 | 91 | 395 | 181 | 0·120 | 375 | 130 | 212 | 121 | <0·001 |
| Cross | 532 | 58 | 407 | 147 | 0·002 | 387 | 57 | 298 | 146 | 0·020 | ||
| 503 | Self | 423 | 83 | 338 | 94 | 0·011 | 257 | 51 | 187 | 84 | 0·009 | |
| Cross | 391 | 56 | 321 | 66 | 0·004 | 261 | 47 | 213 | 60 | 0·022 | ||
| 537 | Self | 480 | – | 415 | 150 | – | 340 | – | 210 | 114 | – | |
| Cross | 547 | 155 | 268 | 129 | <0·001 | 377 | 81 | 139 | 87 | <0·001 | ||
| 6 weeks after pollination | 319 | Self | 820 | 373 | 377 | 89 | 0·030 | 420 | 346 | 162 | 86 | 0·130 |
| Cross | 1195 | 181 | 334 | 218 | <0·001 | 770 | 127 | 90 | 120 | <0·001 | ||
–, Variables for which data are unavailable.
Combining data collected 4 weeks after pollination from all trees showed that the interaction between pollen treatment and fertilization status was not statistically significant. There was no effect of pollen treatment on ovule size, but all four ovule measurements were statistically larger in fertilized ovules compared with non‐fertilized ovules (Table 5).
Table 5.
Average ovule length and width, and embryo sac length and width for fertilized and non‐fertilized ovules at 4 weeks after pollination among all three trees
| Ovule length (μm) | Ovule width (μm) | Embryo sac length (μm) | Embryo sac width (μm) | |
| Fertilized ovules | 1003·4 | 735·0 | 463·7 | 314·5 |
| Non‐fertilized ovules | 915·8 | 640·7 | 381·8 | 210·1 |
| P‐value | <0·001 | <0·001 | <0·001 | <0·001 |
Visual observation of locules harvested at 6 and 8 weeks showed that several ovules in the cross‐pollinated flowers had increased greatly in size (Fig. 3A), whereas self‐pollinated flowers had small, relatively uniform ovules (Fig. 3B). Tree 319, the most self‐compatible tree, was occasionally observed to have some large ovules following self‐pollination, but fewer than following cross‐pollination.
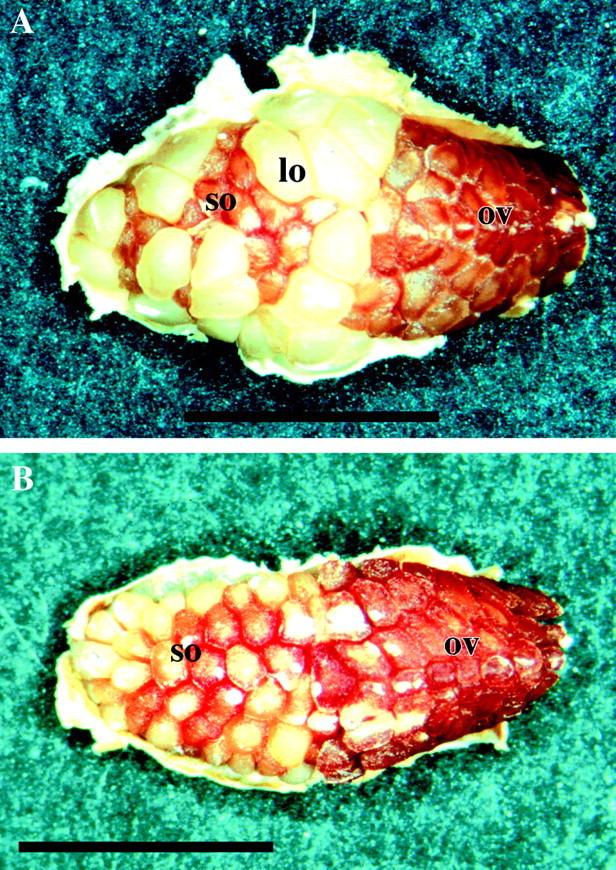
Fig. 3. Dissected Eucalyptus globulus locules 6 weeks after pollination viewed with a dissecting microscope. A, Locule from a cross‐pollinated flower showing large ovules (lo), small ovules (so) and ovulodes (ov). Bar = 500 μm. B, Locule from a self‐pollinated flower showing only small ovules (so) and ovulodes (ov). Bar = 450 μm.
DISCUSSION
The three trees used in this study were found to have self‐incompatibility levels of 76 % (tree 319), 99·4 % (tree 503) and 100 % (tree 537) (Pound et al., 2002). Pound et al., (2002) also found no difference in pollen tube numbers in styles of self‐ and cross‐pollinated flowers, and a small but statistically significant decrease in ovule penetration by self pollen tubes (9·5 %) compared with cross pollen tubes (13·6 %) over five trees collectively. As fertilization had already occurred at 4 weeks after pollination, the exact timing of fertilization events remains unclear. It is possible that fertilization occurred before 4 weeks and a resting zygote was seen at this first sampling time. Furthermore, it is possible that some ovules with little or no embryo sac left at 4 weeks may have been fertilized before degeneration took place. Earlier harvest times of both control pollinated and unpollinated flowers would help determine a more precise time sequence of events. The present study found a greater total number of healthy, fertilized ovules for all three trees following cross‐pollination compared with self‐pollination at 4 weeks after pollination, and in tree 319 at 6 weeks after pollination. At 6 weeks after pollination, there were fewer healthy, fertilized ovules than at 4 weeks for both pollen treatments, and a further reduction in developing ovules must have occurred after this time to produce the final seed set numbers. This information suggests that the self‐incompatibility mechanism in E. globulus does not occur in the style, but is evident in a slight reduction in pollen tube penetration of ovules and post‐zygotic abortion. In short, the self‐incompatibility mechanism appears to be both late pre‐ and post‐zygotic. The difference in the level of self‐incompatibility between the three trees appears to be determined by the number of selfed ovules successfully fertilized that increase in size and develop on until seed maturity.
One mechanism that may account for late‐acting, pre‐zygotic self‐incompatibility before ovule penetration is a reduction in the availability of fertile ovules due to the absence of a required stimulus for normal ovule development following self‐pollination (Sage et al., 1999). It is unclear whether this is occurring in E. globulus as embryo sacs were investigated from 4 weeks after pollination.
Late‐acting self‐incompatibility mechanisms occurring after pollen tube penetration have been reported to occur in 35 plant species from diverse families (Gibbs and Bianchi, 1999). Within Eucalyptus, post‐zygotic self‐incompatibility mechanisms have been reported in E. regnans (Sedgley et al., 1989), E. cladocalyx and E. leptophylla (Ellis and Sedgley, 1992). However, embryological development was not studied for the latter two species, so late pre‐zygotic barriers, after pollen tube penetration of ovules, cannot be ruled out. Other species found to have post‐zygotic self‐incompatibility mechanisms following examination of embryological development include Capparis retusa (Bianchi and Gibbs, 2000), Chorisia chodatii, C. speciosa, Tabebuia caraiba and T. ochracea (Gibbs and Bianchi, 1993). The above examples clearly show that as the breeding systems of more species are studied in detail at an embryological level, the self‐incompatibility mechanisms operating in these species will be better understood.
There was a reduction in healthy, fertilized ovules over time in both cross‐ and self‐pollinated flowers. Late‐acting self‐incompatibility mechanisms can explain the reduction in apparently healthy self‐fertilized ovules, but the reason for the reduction in healthy cross‐fertilized ovules is not as clear. Angiosperms are known to invest maternal resources into seed development after fertilization has taken place (Westoby and Rice, 1982). Perhaps the reproductive strategy employed in E. globulus is to allocate resources into producing fewer, larger, more competitive seeds than many smaller seeds. Following controlled cross‐pollinations on E. globulus, it was observed that when capsules produced few seeds, the seeds were much larger compared with those produced when capsules contained many seeds (pers. obs.). The number of fertilized ovules following both self‐ and cross‐pollinations at 16 weeks after pollination in E. regnans was much higher than mature seed numbers (Sedgley et al., 1989). These authors suggested that the reproductive strategy was for fewer, more competitive offspring. Trees may select against fertilized ovules whose zygotes or embryos are of an inferior genotype due to pollen source variability (Wiens, 1984; Wiens et al., 1987) by directing available resources to potentially superior offspring (Westoby and Rice, 1982). Both pollen source and resource allocation were considered to be important factors in the production of fewer seeds compared with fertilized ovules following both self‐ and cross‐pollination in E. spathulata and E. platypus (Sedgley and Granger, 1996). The level of selection within E. camaldulensis fruits of a self‐pollinated tree was dependent on the number of seeds and ovules per capsule (James and Kennington, 1993), which also suggests the involvement of pollen source and resource allocation. Whilst the cross‐pollen mixture used in this experiment was presumed to be genetically unrelated to the pollen recipients, some gene combinations may have resulted in less vigorous progeny which were subsequently aborted. Within cross‐pollinated, fertilized E. globulus ovules there appears to be a slight lack of synchrony in zygote development at 6 weeks after pollination, and not all large ovules observed at 6 and 8 weeks were of a similar size. Perhaps the ovules that develop faster and are larger at an early age are the ones that survive to seed maturity.
The percentage of ovules fertilized in both pollen treatments in tree 503 at 4 weeks after pollination was 43 %. This figure is much greater than the mean ovule penetration in tree 503 4 weeks after self‐pollination (14·3 %) or cross‐pollination (14·8 %) (Pound et al., 2002). These figures were analysed separately for within‐tree differences but were not statistically significant. The discrepancy between fertilization and ovule penetration suggests that the pollen tube observations at the ovule level were an underestimate of the true value. Dissection of ovules from the placenta may have resulted in some pollen tubes being removed from some of the ovules. It is also possible that uneven fluorescence of pollen tubes occurred, thereby preventing observation. The phenomenon of uneven fluorescence of pollen tubes has been reported previously in styles of Tecona grandis (Tangmitcharoen and Owens, 1997), and at the ovule level in Dolichandra cynanchoides (Gibbs and Bianchi, 1999). As both self‐ and cross‐pollen tubes were visually similar in E. globulus, it would seem unlikely that genetic differentiation for patchiness of staining between the two pollen types existed. Therefore, although pollen tube penetration data are an underestimate, comparisons between the two pollen types can still be made. It is possible that staining sections to show pollen tubes prior to staining with PAS/TBO may provide a better estimate of pollen tube penetration. The difference between pollen tube penetration and ovule fertilization highlights the importance of examining individual embryo sacs for evidence of fertilization to gain a complete understanding of the breeding system of a species.
This study has shown that fertilized ovules appear larger, on average, than non‐fertilized ovules. Furthermore, there appear to be clear differences in the size of ovules following cross‐pollination at 6 and 8 weeks after pollination, with no such trend following self‐pollination in the most self‐incompatible trees. These size differences suggest that the degeneration of fertilized selfed ovules occurs uniformly and very early in the 12 month development to seed maturity. This also suggests that the degeneration of fertilized selfed ovules is indeed a self‐incompatibility response, as opposed to early inbreeding depression which occurs at many developmental stages following fertilization to seed maturity (Charlesworth, 1985; Seavey and Bawa, 1986).
The differences in size observed in ovules at 6 and 8 weeks after pollination may be used to determine whether or not an individual tree is 100 % self‐incompatible. Whilst larger‐scale verification is required, it is proposed that a tree may be considered 100 % self‐incompatible if, at 6–8 weeks following controlled self‐ and cross‐pollinations, selfed flowers consistently reveal no observable differences in ovule size compared with cross‐pollinated flowers. It presently takes about a year, using seed set numbers following controlled pollinations, to determine the level of self‐incompatibility in a tree. This new method would greatly reduce the time required for ascertaining the level of self‐incompatibility in E. globulus.
ACKNOWLEDGEMENTS
Thanks to M. Lorimer from BiometricsSA for statistical assistance, and to J. Foster for field assistance. This research was funded by Gunns Ltd, Southern Tree Breeding Association, Silvagene, and WACAP Tree Farms Ltd through an Australian Research Council Australian Postgraduate Award (Industry) to L.M.P.
Supplementary Material
Received: 5 November 2001; Returned for revision: 19 December 2001; Accepted: 28 January 2002.
References
- BianchiMB, Gibbs PE.2000. Late‐acting self‐incompatibility in Capparis retusa (Capparaceae), a species of Chaco woodland in NE Argentina. Revista Brasileira de Botânica 23: 395–400. [Google Scholar]
- CharlesworthD.1985. Distribution of dioecy and self‐incompatibility in angiosperms. In: Greenwood PJ, Harvey PH, Slatkin M, eds. Evolution – essays in honour of John Maynard Smith Cambridge: Cambridge University Press, 237–268. [Google Scholar]
- deNettancourtD.1977. Incompatibility in angiosperms. Heidelberg: Springer‐Verlag. [Google Scholar]
- deNettancourtD.1984. Incompatibility In: Linskens HF, Heslop‐Harrison J, eds. Cellular interactions Vol. 17. Berlin: Springer‐Verlag. [Google Scholar]
- EldridgeKG, Griffin AR.1983. Selfing effects in Eucalyptus regnans Silvae Genetica 32: 216–221. [Google Scholar]
- EldridgeK, Davidson J, Harwood C, van Wyk G.1994. Eucalypt domestication and breeding. Oxford: Clarendon Press. [Google Scholar]
- EllisMF, Sedgley M.1992. Floral morphology and breeding system of three species of Eucalyptus, section Bisectaria (Myrtaceae). Australian Journal of Botany 40: 249–262. [Google Scholar]
- FederM, O’Brien TP.1968. Plant microtechnique: some principles and new methods. American Journal of Botany 55: 123–142. [Google Scholar]
- GibbsPE, Bianchi M.1993. Post‐pollination events in species of Chorisia (Bombacaceae) and Tabebuia (Bignoniaceae) with late‐acting self‐incompatibility. Botanica Acta 106: 64–71. [Google Scholar]
- GibbsPE, Bianchi MB.1999. Does late‐acting self‐incompatibility (LSI) show family clustering? Two more species of Bignoniaceae with LSI: Dolichandra cynanchoides and Tabebuia nodosa Annals of Botany 84: 449–457. [Google Scholar]
- GriffinAR, Moran GF, Fripp YJ.1987. Preferential outcrossing in Eucalyptus regnans F. Muell. Australian Journal of Botany 35: 465–475. [Google Scholar]
- HardnerCM, Potts BM.1995. Inbreeding depression and changes in variation after selfing in Eucalyptus globulus spp. globulus Silvae Genetica 44: 46–54. [Google Scholar]
- HardnerCM, Potts BM.1997. Postdispersal selection following mixed mating in Eucalyptus regnans Evolution 51: 103–111. [DOI] [PubMed] [Google Scholar]
- HardnerC, Tibbits W.1998. Inbreeding depression for growth, wood and fecundity traits in Eucalyptus nitens Forest Genetics 51: 11–20. [Google Scholar]
- HardnerCM, Potts BM, Gore PL.1998. The relationship between cross success and spatial proximity of Eucalyptus globulus ssp. globulus parents. Evolution 52: 614–618. [DOI] [PubMed] [Google Scholar]
- JamesSH, Kennington, WJ.1993. Selection against homozygotes and resource allocation in the mating system of Eucalyptus camaldulensis Dehnh. Australian Journal of Botany 41: 381–391. [Google Scholar]
- LewisD.1979. Sexual incompatibility in plants. London: Edward Arnold. [Google Scholar]
- MoncurMW, Mitchell A, Fripp Y, Kleinschmidt GJ.1995. The role of honey bees (Apis mellifera) in eucalypt and acacia seed production areas. Commonwealth Forestry Review 74: 350–354. [Google Scholar]
- PottsBM, Savva M.1988. Self‐incompatibility in eucalypts In: Knox RB, Singh MB, Troiani LF, eds. Pollination ’88 Victoria: University of Melbourne, 165–170. [Google Scholar]
- PottsBM, Potts WC, Cauvin B.1987. Inbreeding and interspecific hybridization in Eucalyptus gunnii Silvae Genetica 36: 194–199. [Google Scholar]
- PoundLM, Wallwork MAB, Potts BM, Sedgley M.2002. Self‐incompatibility in Eucalyptus globulus ssp. globulus (Myrtaceae). Australian Journal of Botany (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- SageTL, Strumas F, Cole W, Barrett SPH.1999. Differential ovule development following self‐ and cross‐pollination: the basis of self‐sterility in Narcissus triandrus (Amaryllidaceae). American Journal of Botany 86: 855–870. [PubMed] [Google Scholar]
- SeaveySR, Bawa KS.1986. Late‐acting self‐incompatibility in angiosperms. The Botanical Review 52: 195–219. [Google Scholar]
- SedgleyM.1989. Ovule and seed development in Eucalyptus woodwardii Maiden (Symphyomyrtus). Botanical Gazette 150: 271–280. [Google Scholar]
- SedgleyM, Granger L.1996. Embryology of Eucalyptus spathulata and E. platypus (Myrtaceae) following selfing, crossing and reciprocal interspecific pollination. Australian Journal of Botany 44: 661–671. [Google Scholar]
- SedgleyM, Smith RM.1989. Pistil receptivity and pollen tube growth in relation to the breeding system of Eucalyptus woodwardii (Symphyomyrtus: Myrtaceae). Annals of Botany 64: 21–31. [Google Scholar]
- SedgleyM, Hand FC, Smith RM, Griffin AR.1989. Pollen tube growth and early seed development in Eucalyptus regnans F. Muell. (Myrtaceae) in relation to ovule structure and preferential outcrossing. Australian Journal of Botany 37: 397–411. [Google Scholar]
- TangmitcharoenS, Owens JN.1997. Pollen viability and pollen‐tube growth following controlled pollination and their relation to low fruit production in teak (Tectona grandis Linn. F.). Annals of Botany 80: 401–410. [Google Scholar]
- WestobyM, Rice B.1982. Evolution of the seed plants and inclusive fitness of plant tissues. Evolution 36: 713–724. [DOI] [PubMed] [Google Scholar]
- WiensD.1984. Ovule survivorship, brood size, life history, breeding systems, and reproductive success in plants. Oecologia 64: 47–53. [DOI] [PubMed] [Google Scholar]
- WiensD, Calvin CL, Wilson CA, Davern CI, Frank D, Seavey SR.1987. Reproductive success, spontaneous embryo abortion, and genetic load in flowering plants. Oecologia 71: 501–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


