Abstract
The aim of this work was to establish the role of factors that may trigger elongation growth in the dehardening response, namely temperature during daylight, photoperiod and vernalization. Fully cold‐acclimated seedlings of winter (with incomplete vernalization) and spring oilseed rape were subjected to deacclimation under temperatures of 2/12, 12/2, 12/12, 12/20, 20/12 and 20/20 °C (day/night) and a 12 h photoperiod. Plants were also deacclimated under photoperiods of 8 and 16 h at constant temperatures of 12 and 20 °C. After deacclimation, plants were subjected to reacclimation. Results suggest that the level of growth activity induced during deacclimation affects both the deacclimation rate and the capacity for reacclimation. Deacclimation is fully reversible if it is not accompanied by induction of elongation growth. In such cases the rate of the decrease in freezing tolerance depends on the mean temperature of deacclimation. Deacclimation becomes partially or completely irreversible when it is connected with promotion of elongation growth. The stimuli triggering elongation growth during deacclimation may be the growth‐promoting temperature (20 °C) during the day and the lack of vernalization blockage of elongation growth. When elongation growth was stimulated by other factors such as long‐day treatments, rehardening was also disturbed.
Key words: Brassica napus var. oleifera, cold deacclimation, elongation growth, oilseed rape, photoperiod, temperature, vernalization, water status
INTRODUCTION
Gay and Eagles (1991) provided quantitative data on the effects of a range of temperatures on deacclimation in Lolium. The time course of dehardening changed exponentially to an asymptote that was logistically related to temperature. Svenning et al. (1997) showed a similar relationship in white clover. Eagles and Williams (1992) studied the effects of fluctuating temperatures on dehardening in Lolium. They showed that low night‐time temperatures may, in some cases, reduce the dehardening response to higher daytime temperature.
There is little information available in the literature on the effect of environmental factors during deacclimation on the capacity for reacclimation, which appears essential for overwintering. Recent studies on the temperature dependence of the deacclimation rate have shown that not only temperature per se but also the temperature of the light phase is crucial for the reversibility of the process (unpubl. res.). Increasing daytime temperatures accelerated the rate of elongation growth and in such cases deacclimation was non‐reversible. This suggests involvement of the redox state of photosystem II, which is proposed to be one of the possible sensors of temperature change and which also affects the rate of elongation growth (Huner et al, 1998; Rapacz, 1998a, b). The elongation growth rate in oilseed rape is also controlled by vernalization requirements (Rapacz et al., 2001) and photoperiod (Dahanayake and Galwey, 1999).
The present experiment was designed to establish the role of factors that may promote elongation growth during deacclimation. To this end, the effect of fluctuating daytime temperatures which promote (20 °C) and halt (12 and 2 °C) elongation growth were investigated. The effect of photoperiod during deacclimation at constant temperatures (12 and 20 °C) was studied to evaluate the role of photoperiodic control in the dehardening response to growth‐promoting and non‐growth‐promoting temperatures. The experiment was performed on spring (no vernalization requirement) and winter (incomplete vernalization) cultivars of oilseed rape. (The winter cultivar ‘Górczanski’ used in the experiment requires 8–10 weeks at 2 °C to complete vernalization; Filek and Dubert, 1994.)
MATERIALS AND METHODS
Plant material and growth conditions
The experiments were performed on spring (Brassica napus L. var. oleifera f. annua ‘Star’; DLF Trifolium, Denmark) and winter oilseed rape (Brassica napus L. var. oleifera f. bienae ‘Górczanski’; HBP, Poland). Seeds were sown in 20 cm diameter pots containing loam : sand : peat (1 : 1 : 1; v/v/v), with nine seeds per pot. Seeds were germinated under controlled‐environment conditions with a photosynthetic photon flux density (PPFD) of 300 µmol m–2 s–1 (sodium light; ‘Agro’, Philips), a temperature of 20 °C and a photoperiod of 12 h. When approx. 50 % of the plants had emerged from the soil the temperature was reduced to 12 °C and seedlings were thinned to eight per pot (if necessary). After 5 weeks, plants were subjected to cold acclimation at 2 °C with a 12 h photoperiod and a PPFD as above. After 4 weeks, plants were subjected to deacclimation in various day/night temperature regimes: 20/20, 20/12, 12/20, 12/12, 12/2, 2/12 and 2/2 °C (as the control) for 2 weeks. Plants subjected to fluctuating temperatures were transferred on trolleys between growth chambers at the appropriate temperature. The transfer time was less than 2 min and is highly unlikely to confound the results. In all conditions a 12 h photoperiod was maintained. In the constant 20 and 12 °C treatments, plants were also deacclimated for 2 weeks under 8 and 16 h photoperiods. The correct photoperiod was maintained using rigid light‐proof walls that divided 12 and 20 °C chambers into compartments with their own lamps and air flow from side walls. Light source and PPFD was as in the previous stages of the experiment. After deacclimation, the plants were transferred back to the cold‐acclimating conditions (as above) in order to reacclimate. Water was supplied during the day as required and plants were fertilized once a week with half‐strength Hoagland’s solution.
All data presented are means of three independent experiments. Each experiment involved five biological replications (pots) for each experimental treatment.
Frost resistance
Estimation of LT50 (the temperature at which 50 % of samples are injured) for leaf discs (area approx. 1·75 cm2) was performed on the basis of electrolyte leakage tests following the method described by Rapacz (1999). Discs cut from the two youngest, but fully expanded leaves were placed individually on ice in plastic vessels. They were frozen at a rate of 0·2 K min–1 to the desired testing temperature (–3, –6, –9, –12, –15 and –18 °C), which was maintained for 1 h. Thawing was performed for 24 h at +2 °C on a laboratory shaker. The index of injury was calculated according to Flint et al. (1967). LT50 was calculated from the linear regression fitted within the linear range of the relationship between freezing temperature and index of injury (using at least three temperatures). Determinations were performed on ten replicates (discs) for each freezing temperature.
Other analyses
In each of the three experiments, stem length of five plants was measured every week during deacclimation and at the end of reacclimation (i.e. for 15 plants in total for each temperature and photoperiod combination). Leaf area expansion rate was measured on the same plants as stem length. Leaf area was measured every 2 d during deacclimation or every week during reacclimation using a hand scanner and Delta‐T‐Scan software (Delta T, London, UK).
The osmotic potential of cell sap in leaves was measured in 15 replicates (five plants from each experiment) with a dewpoint microvoltometer (HR 33T; Wescor, Logan, UT, USA) supplied with a C‐52 sample chamber. In each replicate, cell sap was squeezed out with a fixed amount of force from two leaf discs (7 mm in diameter) taken from the youngest fully expanded leaf and frozen in liquid nitrogen.
RESULTS
Deacclimation rate and reacclimation ability
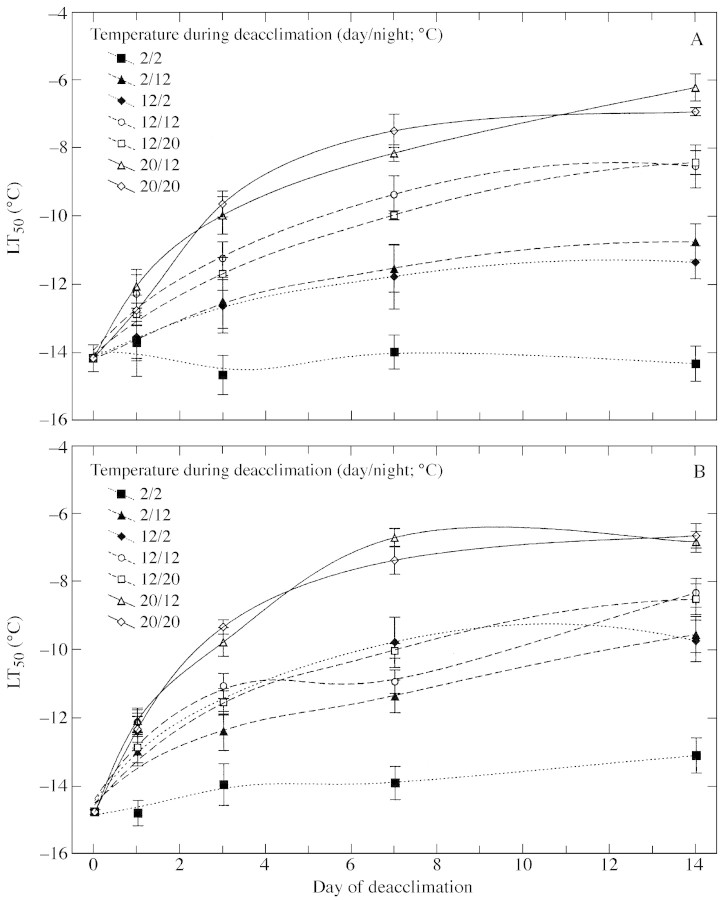
Frost resistance of both cultivars studied in the present experiment was strongly related to the temperature of deacclimation (Fig. 1). The rate of deacclimation was faster at 20 °C than at 12 °C in both winter and spring cultivars. The response for fluctuating temperatures was almost the same in both cultivars. The deacclimation rate was similar in the 12/12, 12/20, 20/12 and 20/20 °C (day/night) treatments. However, when lower temperatures were applied (12 and 2 °C), the dehardening rate appeared to depend on the sum of day and night temperatures. Deacclimation rates observed at 12/2 and at 2/12 °C were similar, and were lower than those observed in continuous 12 °C conditions. This effect was stronger in the winter cultivar ‘Górczanski’.
Fig. 1. The effect of fluctuating temperature during deacclimation (12 h photoperiod) on frost resistance of winter ‘Górczanski’ (A) and spring ‘Star’ (B) cultivars of oilseed rape. Plants were initially acclimated for 4 weeks at 2 °C under a 12 h photoperiod. Data points indicate LT50 (based on electrolyte leakage tests from frozen leaf discs) ± confidence intervals for P = 0·05.
Manipulation of photoperiod also affected deacclimation (Fig. 2). When long‐day (LD) conditions (16 h day/8 h night) were applied during the 2 weeks of deacclimation, hardiness of the spring oilseed rape decreased significantly regardless of the deacclimating temperature (Fig. 2B). In the winter cultivar no effect of photoperiod on deacclimation at 12 °C was recorded, whereas a small decrease in resistance was observed under LD conditions at 20 °C.
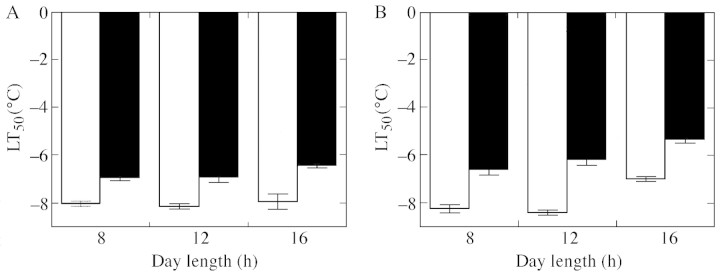
Fig. 2. The effect of photoperiod during 2 weeks of deacclimation at +12 (open bars) and +20 °C (closed bars) on frost resistance of winter ‘Górczanski’ (A) and spring ‘Star’ (B) cultivars of oilseed rape. Plants were initially acclimated for 4 weeks at 2 °C under 12 h photoperiod. Data points indicate LT50 (based on electrolyte leakage tests from frozen leaf discs) ± confidence intervals for P = 0·05.
The persistence of deacclimation as affected by thermo‐ and photoperiod was studied during reacclimation at 2 °C and a 12 h photoperiod. After 4 weeks of reacclimation, plants of the winter cultivar recovered resistance to a similar level or higher than that obtained prior to deacclimation (–14·2 °C) at 2/12, 12/2, 12/12 and 12/20 °C, irrespective of the photoperiod (Table 1). In plants deacclimated at 20/12 and 20/20 °C reacclimation ability was reduced by approx. 2 °C. The effect of photoperiod was statistically insignificant. In the spring cultivar, the decrease in frost resistance was observed even in control plants which were characterized by an LT50 of –14·7 °C after initial cold acclimation (Table 1). As in the winter cultivar, plants deacclimated at 2/12, 12/2, 12/12 and 12/20 °C were as resistant as the control. However the effect of photoperiod was remarkable, and LD‐12/12 °C‐deacclimated plants were less hardy. Plants deacclimated under warm days (20/12 and 20/20 °C) were unable to reacclimate to a great extent. As a consequence, frost resistance of plants deacclimated at 20/20 °C and under LD was similar to that observed after deacclimation (Fig. 2B, Table 1).
Table 1.
The effect of temperature and photoperiod during 2 weeks of deacclimation on the reacclimation ability of oilseed rape
| Temperature of | Photoperiod during | LT50 | |
| deacclimation(°C; day/night) | deacclimation(day length; h) | Winter cultivar‘Górczanski’ | Spring cultivar‘Star’ |
| 2/2 (control) | 12 | –14·8 ± 0·25 | –13·8 ± 0·24 |
| 2/12 | 12 | –14·5 ± 0·34 | –13·9 ± 0·35 |
| 12/2 | 12 | –14·7 ± 0·23 | –13·5 ± 0·31 |
| 12/12 | 12 | –14·3 ± 0·36 | –13·8 ± 0·32 |
| 12/12 | 8 | –14·5 ± 0·34 | –13·8 ± 0·21 |
| 12/12 | 16 | –14·2 ± 0·23 | –12·5 ± 0·27 |
| 12/20 | 12 | –14·6 ± 0·45 | –13·5 ± 0·21 |
| 20/12 | 12 | –12·9 ± 0·34 | –9·7 ± 0·38 |
| 20/20 | 12 | –12·1 ± 0·23 | –8·3 ± 0·51 |
| 20/20 | 8 | –12·2 ± 0·37 | –7·8 ± 0·38 |
| 20/20 | 16 | –11·8 ± 0·45 | –6·8 ± 0·24 |
LT50 was measured after 4 weeks of reacclimation at 2 °C by means of electrolyte leakage tests from frozen leaf discs.Indicated confidence intervals for P = 0·05.
Growth rate and water relations during deacclimation and reacclimation
The effects of various temperature/day length treatments on frost resistance during dehardening and reacclimation can be explained by differences in growth rate during deacclimation and reacclimation (Table 2). More rapid elongation of stems and higher rates of leaf area expansion were recorded in ‘Górczanski’ plants deacclimated under warm day conditions (20/12 and 20/20 °C), irrespective of the photoperiod. No differences (stems) or only small differences (leaves) were recorded in elongation growth rate between plants deacclimated at 2/12, 12/2, 12/12 and 12/20 °C. Nor was any significant effect of photoperiod recorded. The results correspond well with differences in frost resistance recorded on leaves during deacclimation—the more rapid the elongation growth the more rapid the decrease in frost resistance.
Table 2.
Elongation growth of stem and leaf area expansion rate during 2 weeks of deacclimation of winter and spring oilseed rape as affected by temperature and photoperiod
| Temperature of | Photoperiod during | Stem elongation (cm) | Leaf area expansion rate (cm2/14 d) | ||
| deacclimation(°C; day/night) | deacclimation(day length; h) | Winter cultivar‘Górczanski’ | Spring cultivar‘Star’ | Winter cultivar‘Górczanski’ | Springcultivar ‘Star’ |
| 2/2 (control) | 12 | 0·2 ± 0·11 | 0·8 ± 0·21 | 1·4 ± 0·9 | 7·4 ± 0·9 |
| 2/12 | 12 | 0·4 ± 0·09 | 1·8 ± 0·39 | 2·6 ± 0·8 | 9·9 ± 1·2 |
| 12/2 | 12 | 0·3 ± 0·12 | 1·5 ± 0·37 | 2·7 ± 0·7 | 10·8 ± 0·8 |
| 12/12 | 12 | 0·5 ± 0·19 | 2·7 ± 0·46 | 5·6 ± 0·8 | 13·5 ± 1·6 |
| 12/12 | 8 | 0·4 ± 0·21 | 2·1 ± 0·34 | 5·3 ± 0·6 | 12·8 ± 1·2 |
| 12/12 | 16 | 0·6 ± 0·28 | 3·2 ± 0·31 | 5·1 ± 0·8 | 13·4 ± 1·3 |
| 12/20 | 12 | 0·7 ± 0·26 | 2·5 ± 0·28 | 5·8 ± 0·9 | 14·2 ± 0·9 |
| 20/12 | 12 | 1·5 ± 0·38 | 10·8 ± 0·38 | 21·0 ± 1·7 | 25·2 ± 1·1 |
| 20/20 | 12 | 1·3 ± 0·29 | 11·8 ± 0·54 | 23·2 ± 1·3 | 24·3 ± 0·7 |
| 20/20 | 8 | 1·2 ± 0·28 | 10·2 ± 0·65 | 22·5 ± 1·2 | 24·7 ± 1·2 |
| 20/20 | 16 | 1·8 ± 0·36 | 14·1 ± 0·78 | 23·9 ± 1·1 | 25·3 ± 1·5 |
Mean of 15 replications (plants) ± s. e.
In the spring cultivar ‘Star’, the fastest elongation growth rate was noticed under warm‐day conditions of deacclimation (20/12, 20/20 °C) (Table 2). Elongation growth rates for stems and leaves did not differ for plants deacclimated at 12/20 and 12/12 °C, or for plants deacclimated at 12/2 and 2/12 °C. However, rates were faster in the former than in the latter group of plants. The same was observed for frost resistance (Fig. 1B). A 16 h photoperiod enhanced stem elongation at both 12 and 20 °C without affecting leaf area expansion rate (Table 2).
Increases in osmotic potential and water content in leaves were recorded in both cultivars during deacclimation (Table 2). For both cultivars the highest increase in osmotic potential was recorded in 12/20 °C‐deacclimated plants whereas the highest increase in water content was in 20/12 and 20/20 °C‐deacclimated plants. Temperatures of 12/20 °C may favour dark respiration over photosynthesis. The high water content of leaves in the 20/20 and 20/12 °C treatments may be connected with the rapid elongation growth recorded for these plants (Table 2).
Plants that started to elongate rapidly during deacclimation also continued to elongate rapidly during reacclimation (Table 4). The spring cultivar was characterized by increased stem elongation compared with the winter cultivar. In ‘Górczanski’ and ‘Star’, the fastest elongation of stems was recorded in plants deacclimated at 20/12 and 20/20 °C. Additionally, when ‘Star’ was deacclimated under LD (at both 12 and 20 °C), the elongation rate of stems was greater than that of plants dehardened under 12 and 8 h photoperiods. These results are in good agreement with findings concerning reacclimation ability and suggest that fast elongation growth may disrupt reacclimation.
Table 4.
The effect of temperature and photoperiod during deacclimation of oilseed rape on stem elongation during reacclimation and the osmotic potential after 4 weeks of reacclimation at +2 °C
| Temperature during | Photoperiod during | Stem elongation (cm) | Osmotic potential (MPa) | ||
| deacclimation(°C; day/night) | deacclimation(day length; h) | Winter cultivar‘Górczanski’ | Spring cultivar‘Star’ | Winter cultivar‘Górczanski’ | Spring cultivar‘Star’ |
| 2/2 (control) | 12 | 0·03 ± 0·01 | 1·8 ± 0·23 | –1·14 ± 0·08 | –0·90 ± 0·07 |
| 2/12 | 12 | 0·04 ± 0·02 | 1·6 ± 0·18 | –1·25 ± 0·17 | –1·07 ± 0·11 |
| 12/2 | 12 | 0·02 ± 0·01 | 1·9 ± 0·31 | –1·19 ± 0·09 | –0·88 ± 0·07 |
| 12/12 | 12 | 0·06 ± 0·03 | 1·7 ± 0·28 | –1·02 ± 0·06 | –0·89 ± 0·06 |
| 12/12 | 8 | 0·03 ± 0·01 | 1·6 ± 0·34 | –1·00 ± 0·08 | –0·87 ± 0·04 |
| 12/12 | 16 | 0·02 ± 0·01 | 2·2 ± 0·25 | –1·05 ± 0·11 | –0·81 ± 0·09 |
| 12/20 | 12 | 0·02 ± 0·02 | 1·8 ± 0·35 | –1·09 ± 0·04 | –0·73 ± 0·06 |
| 20/12 | 12 | 0·15 ± 0·03 | 3·6 ± 0·41 | –1·00 ± 0·06 | –0·85 ± 0·03 |
| 20/20 | 12 | 0·17 ± 0·05 | 3·2 ± 0·34 | –0·96 ± 0·02 | –0·67 ± 0·04 |
| 20/20 | 8 | 0·16 ± 0·06 | 2·9 ± 0·44 | –0·91 ± 0·05 | –0·71 ± 0·05 |
| 20/20 | 16 | 0·18 ± 0·04 | 3·8 ± 0·27 | –0·89 ± 0·07 | –0·69 ± 0·03 |
Mean of 15 replications ± s. e.
Although differences in osmotic potential recorded after reacclimation did not reflect differences in frost resistance, plants less able to reacclimate were generally characterized by a reduced ability to decrease osmotic potential during reacclimation (Tables 1 and 4). This was apparent for ‘Górczanski’ plants deacclimated at 20/20 °C and for almost all ‘Star’ plants. ‘Star’ plants deacclimated at 20/20 °C, which were almost completely unable to reacclimate, were also unable to lower their osmotic potential during reacclimation.
DISCUSSION
Effects of temperature and photoperiod during dehardening
The present results suggest that the level of growth activity induced during deacclimation affects both the deacclimation rate and the capacity for reacclimation. Deacclimation is fully reversible if it is not accompanied by induction of elongation growth. In such cases the rate of the decrease in freezing tolerance depends on the mean temperature of deacclimation. Deacclimation becomes partially or completely irreversible when it is associated with promotion of elongation growth during deacclimation and subsequent reacclimation. Both reversible and irreversible deacclimation are primarily regulated by temperature (i.e. temperature rise is an absolute requirement for full deacclimation). If elongation growth is not promoted during deacclimation, then the rate of deacclimation depends on the average temperature, irrespective of fluctuations. Similar results were obtained by Gay and Eagles (1991), Eagles and Williams (1992), and Svenning et al. (1997).
Irreversibility of deacclimation seems to be associated with promotion of elongation growth during deacclimation which, according to the present results, may be triggered either by (1) high temperatures during the day; (2) LD conditions; or (3) full vernalization.
The first factor that may trigger elongation growth during deacclimation is a rise in daytime temperature. Both winter and spring cultivars of oilseed rape commenced elongation when daytime temperatures reached 20 °C, regardless of the night‐time temperature. This suggests that it is not temperature per se but the interaction of low light and high temperature (PSII excitation pressure; Huner et al., 1998) that is required for growth promotion during deacclimation. Thus, under low irradiance (for instance in northern latitudes during winter), elongation growth may be promoted at relatively lower temperatures and consequently the most efficient deacclimation may be expected under conditions of low light or in darkness. Modulation of the relative reduction state of PSII by a combination of light and temperature may modulate the rate of elongation growth synergistically with phytochrome (Bowler and Chua, 1994) or sugar sensing (Koch, 1996). The range of temperatures that trigger elongation seems to depend on species and even cultivar. For oilseed rape, any temperature over approx. 15 °C (depending on irradiance) should be considered to promote elongation growth (Rapacz, 1998b). During dehardening of two white clover cultivars, stolon elongation was observed at 12 or at 18 °C, respectively (Svenning et al., 1997).
Irreversible deacclimation depends not only on temperature but also on photoperiod and vernalization response. LD conditions accelerate stem elongation and flowering in both winter and spring cultivars of oilseed rape (Hillman, 1969; Dahanayake and Galwey, 1999). In the present experiment, LD conditions favour irreversible deacclimation in the spring cultivar deacclimated at 12 and 20 °C. This indicates that LD can affect the deacclimation and reacclimation of spring plants, with no vernalization requirement, but cannot affect deacclimation and reacclimation in non‐fully‐vernalized winter rape. This suggests that LD conditions have an effect on the stimulation of generative development. In addition, LD conditions affected stem elongation only, while other factors triggered both stem elongation and leaf expansion. Other studies have shown that photoperiod may influence dehardening if it promotes bud break and initiation of growth (Junttila, 1997). Photoperiodic effects on deacclimation have been demonstrated, e.g. in timothy and white clover (Eagles, 1994). Two cultivars of white clover both produced at the Institute of Grassland and Environmental Research in the UK were characterized by different responses to photoperiod during dehardening. The cultivar ‘AberHerald’ was equally susceptible to dehardening at temperatures from 4 to 12 °C both in long‐ and short‐days (SD), whereas the cultivar ‘AberCrest’ dehardened in LD, but retained much of its acquired hardiness in SD conditions.
In spring oilseed rape, vernalization is not required for flowering but nevertheless cold markedly accelerates formation of flower shoots and buds (Dahanayake and Galwey, 1999). The present results showed that spring oilseed rape was more susceptible than winter oilseed rape to any conditions of deacclimation and that it may lose its acquired resistance even during prolonged low temperature. On the other hand, the dehardening response to high temperature was similar in both spring and winter cultivars. This suggests that there may be an additive response of plants to vernalization and high daytime temperatures.
Vernalization may trigger loss of frost resistance even under cold‐acclimating conditions. This has been demonstrated for wheat and rye (Fowler et al., 1996), for various Cruciferae species excluding winter oilseed rape (Laroche et al., 1992) and for spring oilseed rape (Laroche et al., 1992; Rapacz et al. 2001).
The role of growth cessation during reacclimation
We have demonstrated that further reacclimation ability is limited if elongation growth has begun during deacclimation. According to Levitt (1972), a cessation of elongation growth is a prerequisite for cold acclimation. The effect of growth cessation or dormancy on acclimation ability has been noted in many experiments involving both woody and herbaceous plants. In oilseed rape, growth cessation during cold acclimation may enhance frost resistance almost two‐fold (Rapacz, 1998b). Growth cessation and hardening were clearly correlated in Scots pine (Pinus silvestris L.), indicating that growth must cease prior to hardening, and that earlier cessation of growth leads to earlier frost hardening of stems and needles (Repo et al., 2000). In alfalfa (Medicago sativa L.), winter hardiness and late summer and autumn growth (autumn dormancy) are strongly associated (Brummer et al., 2000).
According to Levitt (1972), rapid elongation growth may interfere with cold acclimation mainly as a result of competition for photoassimilates between growth and acclimation (when assimilates accumulate to reduce osmotic potential). The water content is also higher in growing parts of the seedling and thus they can be more susceptible to freezing. The present results showed that reacclimation was accompanied by accumulation of osmotically active particles but that changes in water relations during deacclimation were independent of both frost resistance and elongation growth rate.
It can be assumed that acceleration of elongation growth interferes with cold acclimation not only through energy competition and water balance of the seedling, but also through reprogramming of the whole development process, which may disturb signal transduction pathways and gene expression involved in cold acclimation. Evidence for such reprogramming was reported recently in the case of winter wheat and winter barley plants moving from the vegetative to reproductive stage (Mahfoozi et al., 2000, 2001a, b). These authors suggested that vernalization and photoperiod responses down‐regulate the expression of low‐temperature tolerance genes through their influence on the rate of plant development. The present results confirm that deacclimation was more effective and non‐reversible in oilseed rape plants that had entered the reproductive stage during deacclimation (spring plants, especially under LD treatment). On the other hand, the decrease in cold reacclimation ability was also observed in plants that remained in the vegetative stage but that lost the prehardening‐induced cessation of elongation growth (winter rape plants). Thus, it appears that not only genes controlling the stage of phenological development (including genes controlling elongation growth rate), but also genes controlling elongation growth rate per se can regulate the expression of low‐temperature tolerance genes.
ACKNOWLEDGEMENTS
This research was supported by the Polish State Committee for Scientific Research (KBN) grant 5 P06A 032 18.
Table 3.
Osmotic potential of cell sap in leaves and water content in leaves during cold deacclimation of winter and spring oilseed rape as affected by temperature
| Temperature during | Osmotic potential (MPa) | Water content (% of f. wt) | ||
| deacclimation(°C; day/night) | Winter cultivar‘Górczanski’ | Spring cultivar‘Star’ | Winter cultivar‘Górczanski’ | Spring cultivar‘Star’ |
| 2/2 (control) | –1·06 ± 0·05 | –0·94 ± 0·02 | 73·5 ± 1·2 | 76·9 ± 1·4 |
| 2/12 | –0·81 ± 0·01 | –0·82 ± 0·01 | 72·8 ± 1·7 | 78·9 ± 1·1 |
| 12/2 | –0·90 ± 0·07 | –0·89 ± 0·05 | 76·2 ± 1·1 | 79·2 ± 0·6 |
| 12/12 | –0·81 ± 0·05 | –0·72 ± 0·02 | 76·3 ± 0·6 | 78·7 ± 0·7 |
| 12/20 | –0·68 ± 0·09 | –0·69 ± 0·02 | 75·8 ± 1·5 | 77·4 ± 2·5 |
| 20/12 | –0·90 ± 0·02 | –0·72 ± 0·02 | 81·4 ± 2·6 | 82·6 ± 0·7 |
| 20/20 | –0·87 ± 0·04 | –0·85 ± 0·04 | 79·2 ± 2·1 | 80·3 ± 1·6 |
Mean of 15 replications (plants) ± s. e.
Supplementary Material
Received: 11 September 2001; Returned for revision: 5 December 2001; Accepted: 4 February 2002.
References
- BowlerC, Chua N‐H.1994. Emerging themes of plant signal transduction. Plant Cell 6: 1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BrummerEC, Shah MM, Luth D.2000. Reexamining the relationship between fall dormancy and winter hardiness in alfalfa. Crop Science 40: 971–977. [Google Scholar]
- DahanayakeSR, Galway NW.1999. Effects of interactions between low‐temperature treatments, gibberellin (GA3) and photoperiod on flowering and stem height of spring rape (Brassica napus var. annua). Annals of Botany 84: 321–327. [Google Scholar]
- EaglesCF.1994. Temperature, photoperiod and dehardening of forage grasses and legumes. In: Dörffling K, ed. Crop adaptation to cool climates. COST 814 workshop. University of Hamburg, Germany, October12–14, 1994 Brussels: European Commission, 75–82. [Google Scholar]
- EaglesCF, Williams J.1992. Hardening and dehardening of Lolium perenne in response to fluctuating temperatures. Annals of Botany 70: 333–338. [Google Scholar]
- FilekW, Dubert F.1994. Effect of completion of vernalization on generative development of winter rape (Brassica napus var. oleifera) plants. Journal of Agronomy and Crop Sciences 172: 29–37. [Google Scholar]
- FlintHJ, Boyce BR, Brattie DJ.1967. Index of injury, a useful expression of freezing injuries to plant tissues as determinated by the electric method. Canadian Journal of Plant Sciences 47: 229–239. [Google Scholar]
- FowlerDB,Limin AE, Wang SY, Ward RW.1996. Relationship between low‐temperature tolerance and vernalization response in wheat and rye. Canadian Journal of Plant Sciences 76: 37–42. [Google Scholar]
- GayAP, Eagles CF.1991. Quantitative analysis of cold hardening and dehardening in Lolium Annals of Botany 67: 339–345. [Google Scholar]
- HillmanWS.1969. Photoperiodism and vernalization. In: Wilkins MB, ed. Physiology of plant growth and development London: McGraw‐Hill, 557–601. [Google Scholar]
- HunerNPA, Oquist G, Sarhan F.1998. Energy balance and acclimation to light and cold. Trends in Plant Sciences 3: 224–230. [Google Scholar]
- JangJC, Sheen J.1994. Sugar sensing in higher plants. Plant Cell 6: 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JunttilaO.1997. Environmental and hormonal control of acclimation and deacclimation. In: Molecular and physiological aspects of cold and chilling tolerance of northern crops. Proceedings of the Finnish–Japanese Workshop, Jokioinen, March 17–20, 1997, 78–87. [Google Scholar]
- KochKE.1996. Carbohydrate–modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 509–540. [DOI] [PubMed] [Google Scholar]
- LarocheA, Geng XM, Singh J.1992. Differentiation of freezing tolerance and vernalization responses in Cruciferae exposed to a low temperature. Plant Cell and Environment 15: 439–445. [Google Scholar]
- LevittJ.1972. Responses of plants to environmental stresses. New York: Academic Press. [Google Scholar]
- MahfooziS, Limin AE, Fowler DB.2001a Influence of vernalization and photoperiod responses on cold hardiness in winter cereals. Crop Science 41: 1006–1011. [Google Scholar]
- MahfooziS, Limin AE, Fowler DB.2001b Developmental regulation of low‐temperature tolerance in winter wheat. Annals of Botany 87: 751–757. [Google Scholar]
- MahfooziS, Limin AE, Hayes PM, Hucl P, Fowler DB.2000. Influence of photoperiod response on the expression of cold hardiness in wheat and barley. Canadian Journal of Plant Science 80: 721–724. [Google Scholar]
- RapaczM.1998a The effects of day and night temperatures during early growth of winter oilseed rape (Brassica napus L var. oleifera cv Gorczanski) seedlings on their morphology and cold acclimation responses. Acta Physiologiae Plantarum 20: 67–72. [Google Scholar]
- RapaczM.1998b The after‐effects of temperature and irradiance during early growth of winter oilseed rape (Brassica napus L var. oleifera cv Gorczanski) seedlings on the progress of their cold acclimation. Acta Physiologiae Plantarum 20: 73–78. [Google Scholar]
- RapaczM.1999. Frost resistance and cold acclimation abilities of spring‐type oilseed rape. Plant Science 147: 55–64. [Google Scholar]
- RapaczM, Tokarz K, Janowiak F.2001. The initiation of elongation growth during long‐term low‐temperature stay of spring‐type oilseed rape may trigger loss of frost resistance and changes in photosynthetic apparatus. Plant Science 161: 221–230. [DOI] [PubMed] [Google Scholar]
- RepoT, Zhang G, Ryyppo A, Rikala R, Vuorinen M.2000. The relation between growth cessation and frost hardening in Scots pines of different origins. Trees – Structure and Function 14: 456–464. [Google Scholar]
- SvenningMM, Rosnes K, Junttila O.1997. Frost tolerance and biochemical changes during hardening and dehardening in contrasting white clover populations. Physiologia Planarum 101: 31–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.