Abstract
Amplified fragment length polymorphism (AFLP) and inter‐simple sequence repeat markers were employed to characterize a genetic resource collection of Miscanthus, a grass under trial in Europe as a biomass crop. The 26 polymorphic markers produced by two ISSR fingerprinting primers were able to discriminate taxa and identify putative clones. AFLP fingerprints were fully reproducible and produced a larger number of markers for the three primer pairs tested, of which 998 were polymorphic (representing 79·3 % of all bands). AFLP markers distinguished species, infra‐specific taxa (varieties and cultivars) and putatively clonal material. They were also used to assess the inter‐relationships of the taxa, to investigate the origin of important hybrid plants and to estimate the overall level of genetic variation in the collection. They were useful for assessing the species status of certain taxa such as M. transmorrisonensis, an endemic from Taiwan that was clearly distinct from M. sinensis; whereas other taxa of disputed species status, such as M. condensatus and M. yakushimanum were not genetically distinct from M. sinensis. The AFLP markers detected a high degree of infra‐specific variation and allowed subdivisions of the genetic resource collection to be made, particularly within M. sinensis.
Key words: Miscanthus, AFLP, ISSR, inter‐microsatellites, biomass, genetic resources, taxonomy, hybrids, molecular phylogenetics
INTRODUCTION
Reform of the European Common Agriculture Policy led to the introduction of the set‐aside scheme, in which land must be taken out of food production or used for growing non‐food crops (Kilpatrick et al., 1994). A strong candidate for a new crop suited to this style of agriculture is the grass genus Miscanthus Anderss., which has received considerable attention in northern Europe as a biomass source for renewable energy production and as a raw material for the cellulose and paper industries. Currently, biomass accounts for approx. 14 % of the world’s non‐solar energy (Kilpatrick et al., 1994). Evaluation trials assessing biomass production of these perennial species have produced annual yields of 20–44 t d. wt ha–1 and crop heights in excess of 3 m (Bullard et al., 1995, 1997). In the United Kingdom the prospects for Miscanthus have been improved by the introduction of the non‐fossil fuel obligation scheme (NFFO) and the development of short‐rotation coppicing of willow and poplar trees, which is now near the point of commercial exploitation (Kilpatrick et al., 1994). The first commercial plantings of Miscanthus in the UK have been made recently (Bullard, pers. comm.) since there is a market for a herbaceous alternative to short‐rotation coppice of willow and poplar. Miscanthus is also used for thatching, forage, wind breaks, erosion protection, silk dyeing and in breeding programmes with sugar cane (Hodkinson, data gathered from labels on herbarium specimens at Kew; Wikberg, 1990), and it is a popular hardy garden plant.
Most research investigating the productivity and economic potential of Miscanthus has centred on two taxa: M. sacchariflorus (Maxim.) Benth. & Hook., a species from northern China and Japan, and M. × giganteus Greef & Deuter ex Hodkinson & Renvoize (Hodkinson and Renvoize, 2001), a putative hybrid between M. sacchariflorus and M. sinensis Anderss. (Adati and Shiotini, 1962; Linde‐Laursen, 1993). Miscanthus × giganteus Greef & Deuter ex Hodkinson & Renvoize is also known as M. × giganteus Greef & Deuter (Greef and Deuter, 1993) but the latter name is invalid under the rules of the International Code of Botanical Nomenclature because the diagnosis and description were in English and no type specimen was designated (Greuter et al., 2000). Miscanthus × giganteus is also incorrectly known as M. giganteus or M. sinensis ‘Giganteus’ and is often mistaken for M. sacchariflorus. See Hodkinson and Renvoize (2001) and Hodkinson et al. (2002) for further discussion of the nomenclature of M. × giganteus. The agricultural community is fully aware of the hazards and limitations of relying on single clones for high yields because these are at high risk from pests and diseases and are not likely to be adapted to local climatic or edaphic conditions. However, few attempts have been made to broaden the genetic base of the crop, and little effort has been given to evaluating and characterizing the genetic diversity available within the genus (Renvoize et al., 1997).
Studies using DNA sequences from the internal transcribed spacer of nuclear ribosomal DNA (nrITS) have elucidated species inter‐relationships in the genus but were unable to resolve the differences between cultivars or varieties of Miscanthus (Hodkinson et al., 1997). Little variation was detected for the variants of M. sinensis, and it is at this taxonomic level that further information is required. This is in contrast to some other studies that have successfully used DNA sequence data to assess infra‐specific variation in plants (Jorgensen and Cluster, 1988; Cox et al., 1992; Ramakrisha et al., 1995). Two further DNA regions, 5S nuclear ribosomal DNA spacers (Baum and Appels, 1992; Cox et al., 1992; Sastri et al., 1992) and the trnL‐F intron and intergenic spacer (Taberlet et al., 1991) were also sequenced by Hodkinson et al. (1997) but did not discriminate between the infra‐specific taxa.
Isozyme analysis has been used to assess genetic diversity within Miscanthus spp. (Chou et al., 1987; Chou and Chang, 1992; Von Wuhlish et al., 1994), and DNA markers were first used by Greef et al. (1997), who applied amplified fragment length polymorphism (AFLP™) fingerprinting (Vos et al., 1995) to assess variation in three Miscanthus species. The study was limited in terms of species, infra‐specific taxa and cultivars sampled, but did prove that the markers were suitable for assessing genetic infra‐specific variation in Miscanthus.
Two DNA fingerprinting methods, amplified fragment length polymorphisms (AFLPs) and inter‐simple sequence repeat (ISSR, also known as inter‐microsatellites; Weising et al., 1995) were used to characterize genetic diversity in the collections held at the Royal Botanic Gardens, Kew, UK, and ADAS Arthur Rickwood Research Station, Cambs, UK. The collections include a number of different species but are composed mainly of M. sinensis cultivars which represent an important resource for both biomass production and horticulture. We provided a preliminary overview of our AFLP work on Miscanthus in Hodkinson et al. (1997), but present the empirical data here for the first time.
ISSR PCR (Zietkiewicz et al., 1994; Weising et al., 1995) uses a single primer and is experimentally similar to random amplified polymorphic DNA (RAPD; Williams et al., 1990), but differs in a number of important details. It uses primers based on simple sequence repeats (microsatellites) such as (GACA)4 or (CA)8. Such sequences are common in the genome and are therefore good targets for such a PCR‐based fingerprinting technique. The selectivity of the primers can also be modified by adding an anchor such as RG to one end (anchored ISSR PCR; Zietkiewicz et al., 1994). Regions between these microsatellites are amplified, separated typically by agarose gel electrophoresis and then detected by ethidium bromide/UV light. Primers are usually longer (16–20 bp) than those employed in RAPD (10 bp), and this allows increased stringency. Such an approach should theoretically improve reliability and reproducibility in comparison with RAPD (Weising et al., 1995).
The AFLP technique has several advantages over other marker systems currently in use (Vos et al., 1995; Reeves et al., 1998; Ridout and Donini, 1999). It produces a high number of polymorphic informative markers per primer pair, is highly sensitive, requires small amounts of DNA, and has proved to be robust, reliable and reproducible (Mueller and Wolfenbarger, 1999; Ridout and Donini, 1999; Hodkinson et al., 2000), unlike some other PCR‐based techniques, such as RAPD (Karp et al., 1996). AFLP fingerprinting is based on the selective amplification of restriction fragments from a digest of total genomic DNA and has been adapted for use on an automated DNA sequencer.
The complex multi‐locus fingerprints produced by AFLP provide a large number of informative markers derived from loci widely dispersed throughout the nuclear genome (Ridout and Donini, 1999). For example, in barley (Hordeum vulgare L.), AFLP markers are located on the long and short arms of all seven chromosomes, with a strong correlation between the number of markers per chromosome and the length of the chromosome (Waugh et al., 1997). In rice, Oryza sativa L., Mackill et al. (1996) used AFLP to map an F2 population and found that the 50 AFLP markers were spread across nearly all chromosomes. However, clustering of AFLP markers within certain regions has been reported in other taxa (Qi et al., 1998).
MATERIALS AND METHODS
Plant material
Only fully vouchered material from collections at RBG Kew and the ADAS Arthur Rickwood Research Station (Mepal, Ely, Cambridgeshire, UK; a site for Miscanthus biomass trials) were included in the molecular analysis so that results could be compared with morphology of the plants in question. In total, 75 accessions were included in the study and are listed in Table 1.
Table 1.
Grasses and associated voucher specimens held at the Royal Botanic Gardens Kew, UK and ADAS Arthur Rickwood Research Station used in the study
| Taxon | ID | Voucher and/or Kew accession number |
| M. floridulus (Labill.) Warb. ex K. Schum. & Lauterb. | 72 | Hodkinson 30. 1978–1387 |
| M. × giganteus Greef & Deuter ex Hodkinson & Renvoize | 2 | Renvoize s.n. 1990 381 |
| M. × giganteus | 8 | Gilbert s.n. 13/11/90. 1969–19097 |
| M. × giganteus | 22 | Hodkinson s.n. 1993–1779 |
| M. × giganteus | 23 | Kew living 1993–1780 |
| M. × giganteus | 26 | Living ADAS MB93/01 |
| M. × giganteus | 60 | ADAS MB95/30 |
| M. × giganteus | 187 | ADAS PN96/19 |
| M. × giganteus | 188 | ADAS PN96/20 |
| M. × giganteus | 189 | ADAS PN96/21 |
| M. × giganteus | 190 | ADAS PN96/22 |
| M. × giganteus | 180 | ADAS PN96/05 |
| M. nepalensis (Trin.) Hack. | 25 | Hodkinson 1 |
| M. nepalensis | 66 | Hodkinson 22 |
| M. oligostachyus Stapf. | 16 | Hodkinson 13 |
| M. oligostachyus ‘Nanus Variegatus’ | 161 | Hodkinson s.n. 1996–1065 |
| M. sacchariflorus (Maxim.) Benth. & Hook. ‘Purpurascens’ | 61 | Hodkinson s.n. 1987–2727 |
| M. sinensis Anderss. var. variegatus Beal | 1 | Hodkinson 33 |
| M. sinensis ‘Silver Feather’ | 3 | Hodkinson 24. 1975–930 |
| M. sinensis ‘Silberspinne’ | 4 | Hodkinson s.n. 1988–2519 |
| M. sinensis | 5 | Hodkinson 40. 1978–1389 |
| M. sinensis ssp. condensatus (Hackel) T. Koyama | 7 | Renvoize s.n. 1969 19091 |
| M. sinensis var. zebrinus Beal | 10 | Hodkinson 35 |
| M. sinensis ‘Poseidon’ | 11 | Hodkinson s.n. 1995–1866 |
| M. sinensis ‘Undine’ | 12 | Hodkinson 26 |
| M. sinensis ‘Silver Feather’ | 13 | Hodkinson s.n. 1975–915 |
| M. sinensis var. zebrinus | 14 | Hodkinson 34 |
| M. sinensis var. variegatus | 17 | Hodkinson s.n. 1973–10370 |
| M. sinensis ‘Graziella’ | 18 | Hodkinson 29 |
| M. sinensis ‘Kleine Silberspinne’ | 19 | Hodkinson s.n. 1995–1865 |
| M. sinensis sp. | 20 | Hodkinson 36 |
| M. sinensis | 21 | Hodkinson 3 |
| M. sinensis | 24 | Hodkinson 28 |
| M. sinensis ‘Goliath’ | 27 | ADAS MB93/02 |
| M. sinensis ‘Gracillimus’ | 28 | Hodkinson s.n. MB94/05 |
| M. sinensis ‘Roland’ | 29 | Hodkinson s.n. ADAS MB94/06 |
| M. sinensis | 30 | ADAS MB94/07 |
| M. sinensis ‘Grosse Fontäne’ | 31 | Renvoize s.n. PN95/01 |
| M. sinensis ‘Malepartus’ | 33 | ADAS PN95/03 |
| M. sinensis ‘Ferner Osten’ | 36 | ADAS PN95/06 |
| M. sinensis ‘Kleine Fontäne’ | 38 | ADAS PN95/08 |
| M. sinensis ‘Kleine Silberspinne’ | 40 | ADAS PN95/10 |
| M. sinensis ‘Vorläufer’ | 42 | ADAS PN95/12 |
| M. sinensis ‘Kaskade’ | 43 | ADAS PN95/13 |
| M. sinensis ‘Roland’ | 46 | ADAS PN95/16 |
| M. sinensis ‘Poseidon’ | 47 | ADAS PN95/17 |
| M. sinensis ‘Wetterfahne’ | 48 | ADAS PN95/18 |
| M. sinensis ‘Gewitterwaike’ | 49 | ADAS PN95/19 |
| M. sinensis ‘Sirene’ | 50 | ADAS PN95/19 |
| M. sinensis ‘Nippon’ | 51 | ADAS PN95/21 |
| M. sinensis ‘Afrika’ | 56 | ADAS PN95/26 |
| M. sinensis ‘Zwergelephant’ | 57 | ADAS PN95/27 |
| M. sinensis var. variegatus Beal | 62 | Hodkinson s.n. 1973–2834 |
| M. sinensis ‘Yakushimanum’ | 63 | Hodkinson 21. 1987–1148 |
| M. sinensis | 64 | Hodkinson 23 |
| M. sinensis ‘Variegatus’ | 67 | Hodkinson s.n. 1969–34750 |
| M. sinensis | 68 | Hodkinson 19 |
| M. sinensis ‘Silver Feather’ | 69 | Hodkinson 18 |
| M. sinensis var. gracillimus | 71 | Hodkinson s.n. 1969–19098 |
| M. sinensis ‘Sirene’ | 113 | Hodkinson s.n. |
| M. sinensis ‘Nippon’ | 143 | Hodkinson s.n. 1996–823 |
| M. sinensis ‘Grosse Fontäne’ | 144 | Hodkinson s.n. 1996–1294 |
| M. sinensis ‘Pünktchen’ | 145 | Hodkinson s.n. |
| M. sinensis ‘Ferne Osten’ | 147 | Hodkinson s.n. 1996–1303 |
| M. sinensis | 48 | Hodkinson s.n. |
| M. sinensis ‘Malepartus’ | 149 | Hodkinson s.n. 1996–1301 |
| M. sinensis ‘Sarabande’ | 150 | Hodkinson s.n. |
| M. sinensis ‘Yakushima Dwarf’ | 151 | Hodkinson s.n. 1996–822 |
| M. sinensis ‘Kleine Silberspinne’ | 152 | Hodkinson s.n. 1996–820 |
| M. sinensis ‘Strictus’ | 153 | Hodkinson s.n. 1996–1297 |
| M. sinensis ‘Kaskade’ | 154 | Hodkinson s.n. 1996–1064 |
| M. sinensis ‘Yakushima’ | 178 | Hodkinson s.n. ADAS 96/03 |
| M. sinensis | 184 | ADAS PN96/14 |
| M. sinensis ‘Goliath’ | 194 | ADAS PN96/30 |
| M. transmorrisonensis Hayata | 65 | Hodkinson 20. 1990–2748 |
| Saccharum officinarum L. | 104 | RBG Kew 1973–12242 |
DNA extraction
DNA was extracted from 0·5–1·0 g of fresh leaf material using the modified 2 × CTAB procedure of Doyle and Doyle (1987) and precipitated using 100 % ethanol for at least 48 h at –20 °C. The DNA was then pelleted, washed with 70 % ethanol and purified via caesium chloride/ethidium bromide (1·55 g ml–1) gradient centrifugation with subsequent dialysis. DNA was then stored in TE buffer (10 mM Tris‐HCl; 1 mM EDTA; pH 8·0) at –20 °C until use. Total genomic DNA was quantified by measuring light absorption using a Philips PV 8820 UV/VIS spectrophotometer.
ISSR PCR
The ISSR primers screened for PCR fingerprinting of Miscanthus were (GACA)4, (CA)8RY, (CA)8RG, (GATA)4 and (CA)7NNN. PCR was performed using 100 ng template DNA, 100 µm of each dNTP, 2 mm MgCl2 in a 100 µl reaction using 0·5 units of Taq polymerase (Promega Ltd, Southampton, UK). The thermal cycling for all PCR reactions comprised 40 cycles, each with 20 s denaturation at 93 °C, 1 min annealing at 50 °C and an extension of 20 s at 72 °C. A final extension of 6 min at 72 °C was also included. Amplification products (10 µl) were separated using standard 2 % agarose or 2 % NuSieve 3 : 1 agarose (FMC Bioproducts; Flowgen, Lichfield, UK), a gel suited for the separation of DNA fragments of less than 1 kb in length.
AFLP
Reactions were performed using the AFLP Plant Mapping Kit of PE Applied Biosystems Inc. (ABI, Warrington, UK), and DNA fragments were detected on an ABI 377 automated DNA sequencer with ABI GeneScan 2·02 and Genotyper version 1·1 software. Thirty‐two primer pairs with various three‐base anchors were used initially to screen six accessions and to establish the most informative combinations for Miscanthus fingerprinting (Table 2). Suitable primer pairs were defined as those that produced a large number of well defined, polymorphic bands. DNA fragments ranging from 50 to 500 bp in size from the AFLP analysis were scored. Three primer pairs (indicated in Table 2) were used to fingerprint 75 accessions of Miscanthus, including a large number of M. sinensis clones, M. floridulus (Labill.) Warb., M. sacchariflorus, M. × giganteus, M. oligostachyus Stapf., M. nepalensis (Trin.) Hack. and M. transmorrisonensis Hayata. Saccharum officinarum L., being the closest known relative of Miscanthus (Clayton and Renvoize, 1986), was also included for comparison. Replicate samples and samples of known clonal origin were used to evaluate reproducibility of the fingerprints and to estimate scoring error in the analysis.
Table 2.
Selective amplification primers tested for AFLP analysis
| EcoRI primer anchor | Fluorescent label | MseI primer anchor | EcoRI primer anchor | Fluorescent label | MseI primer anchor |
| 01 AAC | TAMRA | CAG | 17 AAC | TAMRA | CTA |
| 02 AAC | TAMRA | CAC | 18 AAC* | TAMRA | CAT |
| 03 AAG | JOE | CAG | 19 AAG | JOE | CTC |
| 04 AAG | JOE | CAC | 20 AAG | JOE | CAT |
| 05 ACA | FAM | CAG | 21 ACA | FAM | CTA |
| 06 ACA* | FAM | CAA | 22 ACA | FAM | CAT |
| 07 ACC | TAMRA | CAG | 23 ACC | TAMRA | CTA |
| 08 ACC | TAMRA | CAC | 24 ACC* | TAMRA | CAT |
| 09 ACG | JOE | CAG | 25 ACG | JOE | CTA |
| 10 ACG | JOE | CAC | 26 ACG | JOE | CAT |
| 11 ACT | FAM | CAG | 27 ACT | FAM | CTA |
| 12 ACT† | FAM | CAA | 28 ACT | FAM | CTC |
| 13 AGC | TAMRA | CAG | 29 AGC | TAMRA | CTA |
| 14 AGC | TAMRA | CAC | 30 AGC | TAMRA | CAT |
| 15 AGG | JOE | CAG | 31 AGG | JOE | CTA |
| 16 AGG | JOE | CAC | 32 AGG | JOE | CAT |
See ABI kit for further details on primer composition.
* The most successful primers that were used to screen a further 75 Miscanthus accessions.
† A successful primer combination used in Hodkinson et al. (2002) but not in this study.
Data analysis
Neighbour‐joining (NJ) analysis was applied to a distance matrix based on mean character difference (PAUP 4·0; Swofford, 1998) for both the ISSR and AFLP data. An NJ analysis was also applied using Nei and Li distances (Nei and Li, 1985), and the results (not presented) were not significantly different from the NJ tree based on mean character difference. Internal support for groupings was assessed using 1000 bootstrap replicates (Felsenstein, 1985). Principal coordinates analysis (PCO) was performed with Le Progiciel R v4·0d (Casgrain, 1999) using Dice distances (Dice, 1945).
RESULTS
ISSR PCR
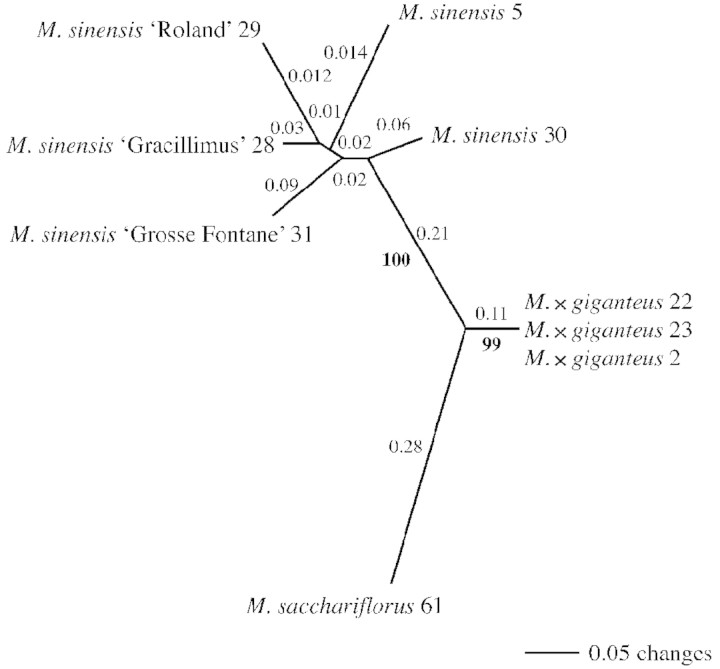
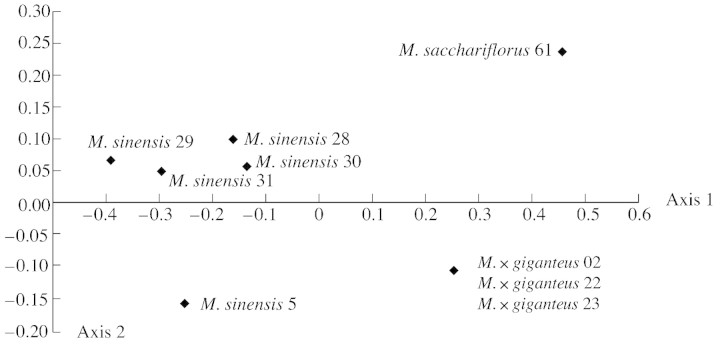
Two of the five ISSR primers, (GACA)4 and (CA)8RG, gave clear reproducible banding patterns with some levels of polymorphism. Three of the primers, (CA)8RY, (GATA)4 and (CA)7NNN, did not produce suitable molecular markers. The fingerprints produced with the two good primers were difficult to score when a large number of accessions were included, and therefore only a few individuals could be handled reliably using this method. A total of 26 markers (all polymorphic) was produced from the two ISSR primers, of which 14 were shared by more than one individual. In the NJ analysis (Fig. 1), strong support was found for the grouping of M. sinensis with 100 % support (bootstrap percentage, BP) and M. × giganteus clones (99 BP). Miscanthus × giganteus was approximately equidistant from M. sinensis and M. sacchariflorus, its putative parental species. Considerable variation was detected between the M. sinensis accessions (varieties and cultivars), but no genetic variation was detected between M. × giganteus accessions. Similar groupings were evident in the PCO analysis (Fig. 2). The accessions can be separated using the first two axes of the PCO, and these cumulatively account for 87·2 % (73·7 % and 13·5 %, respectively) of the data variance, the third axis (not shown) representing 7·4 %.
Fig. 1. Unrooted neighbour joining tree for ISSR data. The NJ tree shows the results of two primers, (GACA)4 and (CA)8RG. Values above the branches are genetic distances. Values below the branches are bootstrap percentages.
Fig. 2. Principal coordinates analysis (PCO) for ISSR data using Dice distances. The accessions can be separated using the first two axes of the PCO and these cumulatively account for 87·2 % (73·7 % and 13·5 %, respectively) of the data variance.
AFLP
Three primer pairs produced a total of 1259 DNA markers, of which 998 were polymorphic (representing 79·3 % of all bands). The AFLP fingerprints distinguished Miscanthus species and infra‐specific taxa. Representative fingerprints of Miscanthus accessions are given in Fig. 3, which also illustrates the power of the technique to detect clonal material (i.e. the traces from two plants are remarkably consistent). Two plants initially labelled as different taxa, M. sacchariflorus and M. × giganteus, were morphologically indistinguishable and gave identical fingerprints in all AFLP primer combinations tested (and were therefore invariant at otherwise highly polymorphic loci). Other cultivars of Miscanthus were also accurately assessed, and they separated out as distinct groups in the NJ tree (Fig. 4). Many of the taxa in our collection had not been allocated species names, and the AFLP data, combined with morphological examination, allowed an accurate identification to be made. The taxa presented in Table 3 were identified in this way.
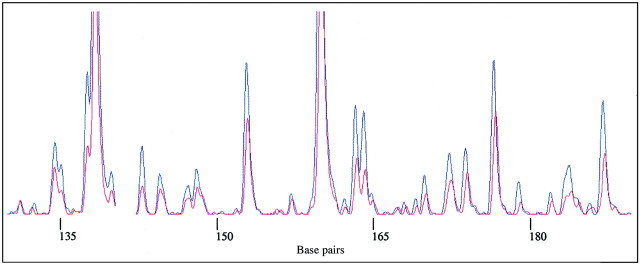
Fig. 3. AFLP traces of two Miscanthus accessions: M. × giganteus, originally called M. sacchariflorus 23 (red) and M. × giganteus 22 (blue). Note the two are identical in all fragments in the analysis.
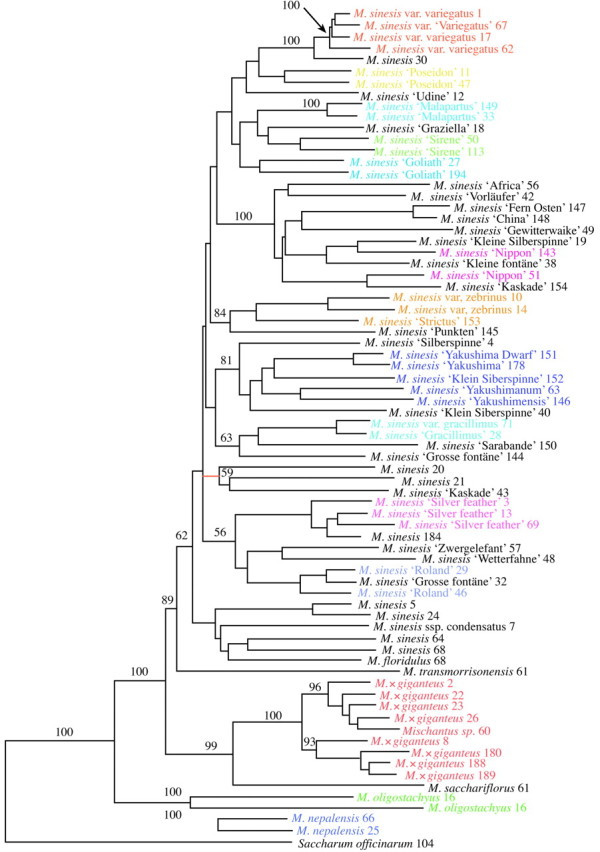
Fig. 4. Neighbour joining (NJ) tree of AFLP data. Branch length is proportional to genetic distance. Values above branches are bootstrap percentages. Species are unambiguously separated using the AFLP markers. Furthermore, cultivars are also accurately grouped on the basis of genetic distance and have been highlighted with different colours.
Table 3.
Miscanthus taxa growing in the living collection at ADAS Arthur Rickwood or RBG Kew identified using AFLP and morphological data
| ID | Previous identity | New identity based on AFLP data |
| 8 | M. sacchariflorus | M. × giganteus |
| 20 | Miscanthus sp. | M. sinensis |
| 23 | M. sacchariflorus | M. × giganteus |
| 26 | M. sinensis ‘Giganteus’ | M. × giganteus |
| 60 | Miscanthus sp. | M. × giganteus |
| 61 | M. purpurascens | M. sacchariflorus |
| 64 | M. chinensis | M. sinensis |
| 148 | Miscanthus sp. ‘China’ | M. sinensis ‘China’ |
| 150 | Miscanthus sp. ‘Sarabande’ | M. sinensis ‘Sarabande’ |
| 161 | M. tinctorius ‘Nanus Variegatus’ | M. oligostachyus ‘Nanus Variegatus’ |
| 180 | M. sinensis ‘Giganteus’ | M. × giganteus |
| 194 | M. × giganteus ‘Goliath’ | M.sinensis ‘Goliath’ |
Note: some taxa were previously unnamed or misnamed.
Miscanthus transmorrisonensis is the closest relative of M. sinensis (89 BP). A group consisting of M. sacchariflorus, M. × giganteus and M. oligostachyus represents the next most genetically similar species to this major group. Accessions of M. sinensis and a number of subgroups are genetically distinct. For example, a group containing a number of taxa with name Yakushima or similar (such as M. sinensis ‘Yakushimanum’ and M. sinensis ‘Yakushima’) has strong bootstrap support.
DISCUSSION
Measuring genetic diversity using ISSR PCR
The ISSR PCR and anchored ISSR methods provided evidence for the clonal nature of M. × giganteus accessions and indicated that incorrectly labelled material, such as one M. sacchariflorus accession, was actually M. × giganteus. From the NJ tree (Fig. 1) and the PCO (Fig. 2), it can be seen that considerable infra‐specific variation is detected in the M. sinensis accessions, but no variation was detected at these otherwise polymorphic loci in the M. × giganteus accessions.
We considered ISSR methods less efficient than AFLPs for screening large numbers of Miscanthus accessions. One problem is the manual scoring of bands on agarose gels in which the sizing of fragments is not as accurate as automated genotyping methods. It is also difficult to compare results from samples run on different agarose gels, which increases scoring error. Standard 2 % agarose gels cannot accurately differentiate between fragments of similar length, whereas 2 % NuSieve 3 : 1 gel, used in this study, improved the resolution but was still limited in its ability to separate fragments differing by less than 10 bp in length. Detection of ISSRs could be automated by fluorescently labelling the primers, but many of the markers would be lost because they are too long for detection using standard acrylamide gels. This method would not provide any added benefit over that of AFLP analysis, which is generally more reliable and informative (Karp et al., 1996). Far fewer polymorphic markers were produced per primer using ISSR PCR (mean of 13) than a corresponding primer pair in an AFLP analysis (mean of 332).
ISSR PCR and anchored ISSR PCR have proved useful for assessing genetic diversity within various plant groups (Weising et al., 1995; Wilkinson et al., 2000), including grasses (Li and Ge, 2001), but we believe that they are better suited to screening of small numbers of plants and testing specific hypotheses regarding inter‐relationships rather than large‐scale screening. For example, Wilkinson et al. (2000) used them effectively to discover the parental species of a Brassica hybrid found in sympatric populations of B. napus (oilseed rape) and B. rapa (wild turnip). ISSRs are, however, cheaper than AFLP and require less technology to collect and analyse.
Measuring genetic diversity and infra‐specific variation using AFLP
The AFLP technique, in contrast to ISSR analysis, proved to be a suitable method for characterizing large numbers of Miscanthus species and infra‐specific taxa. One of the main strengths of the multi‐locus AFLP technique is its ability to produce a larger number of reliable molecular markers, in this case 998 polymorphic markers from just three primer pairs.
AFLPs helped to detect clonal or nearly clonal material. For example, two morphologically indistinguishable plants growing under different species names, M. sacchariflorus and M. × giganteus, had identical fingerprints (Fig. 3) in all AFLP primer combinations tested and have since been shown to have the triploid chromosome complement characteristic of the latter species (2n = 3x = 57; Hodkinson, unpubl. res.). In the NJ analysis (based on the initial scoring of bands without subsequent detailed examination), some variation was evident among M. × giganteus accessions but this was shown to be due to scoring error (see below). Greef et al. (1997) assessed genetic variation in three Miscanthus species (M. sinensis, M. sacchariflorus and M. × giganteus) using radioactively detected AFLPs and, in agreement with our findings, also discovered little genetic variation in their M. ×giganteus accessions. There may be only two or three closely related clones of M. × giganteus in cultivation (outside of research institutes, where new hybrids are being produced; J. Clifton‐Brown, pers. comm.).
The fluorescently labelled automated AFLP method utilized in this study was efficient at separating large numbers of cultivars and varieties of M. sinensis (Figs 3 and 4). The different cultivars are labelled with different colours, and it can be seen that these group together in most cases. For example, four accessions with the name M. sinensis var. variegatus (or similar; at the top of the tree) group closely together with 100 % bootstrap support. Many Miscanthus cultivars are now available, and accurate cultivar identification is becoming important. Cultivar names are not governed by the International Code of Botanical Nomenclature (Greuter et al., 2000) and can often proliferate out of control. In our collection there are numerous cultivars with similar but not identical cultivar names, which when examined with AFLP can be accurately clustered. Deviation from expected groupings of taxa is rare and can be easily explained by misnaming or duplicate naming for the same taxon or group. Alternatively, slight deviations from the expected groupings may be expected because of scoring error in the AFLP analysis (see below). Known clonal material or duplicate material should be identical in AFLP profile, unless somatic variation has occurred, and no variation should be found in experimental replicates. By comparing AFLP profiles of these identical clones and replicate samples it has been estimated that the scoring error lies approximately in the range of 1–2 %, which is consistent with that estimated in other studies (Mueller and Wolfenbarger, 1999).
There is clearly a high level of variation within M. sinensis, and subgroups can be identified. A group containing a number of taxa with name Yakushima or similar (such as M. sinensis ‘Yakushimanum’ and M. sinensis ‘Yakushima’; 81 BP) has been considered as a separate species but is clearly a member of the M. sinensis group.
Establishing the inter‐relationships of the taxa
In the NJ tree (Fig. 4), the various species are well separated and this system closely matches identification based on gross morphology. Most taxa in the living genetic resource collections are identified as M. sinensis, some as M. × giganteus, one as M. sacchariflorus, two as M. nepalensis, two as M. oligostachyus and one as M. transmorrisonensis. Miscanthus transmorrisonensis is morphologically similar to M. sinensis, and we considered it as an infra‐specific taxon of M. sinensis until both DNA sequence data (Hodkinson et al., 1997) and AFLP highlighted its uniqueness. It is endemic to Taiwan and may have diverged sufficiently on a molecular level to merit species recognition. A group consisting of M. sacchariflorus and M. × giganteus accessions is closely related to the major M. sinensis group and M. transmorrisonensis. The results also support the hypothesis that M. × giganteus is a hybrid between M. sinensis and M. sacchariflorus. A hybrid would be expected to inherit approximately equal numbers of AFLP markers from its two parent species. Miscanthus × giganteus has an intermediate position between M. sinensis and M. sacchariflorus on the first axis of the PCO analysis (Fig. 2) but is displaced on the second axis, which may indicate that Miscanthus × giganteus has some unique variation not present in the sampled representatives of its parental species. We do not, therefore, have the exact two parental genotypes of our M. × giganteus accessions.
Miscanthus oligostachyus (Japan) and M. nepalensis (Himalayas) are separated from the rest of the Miscanthus species, a result consistent with DNA sequence data (Hodkinson et al., 1997). Hodkinson et al. (1997) found that M. oligostachyus is sister to a core Miscanthus group including M. sinensis, M. floridulus and M. sacchariflorus, and should therefore be included in a strictly defined Miscanthus group. Miscanthus nepalensis, in contrast, should not be classified as Miscanthus sensu stricto since it groups more closely with other genera of Saccharinae. The results of the AFLP analysis are broadly congruent with the taxonomic treatment of Lee (1964b, c, d). However, Miscanthus sinensis ssp. condensatus and M. floridulus are embedded within M. sinensis (Fig. 4), and their species status is questionable. Nevertheless, on morphological examination, they were found to be correctly identified. Miscanthus floridulus is primarily Pacific in its distribution and overlaps with M. sinensis in a limited area; M. sinensis is distributed mainly within continental southern and eastern Asia as well as Japan, Taiwan and Malaysia. It is morphologically distinguished from M. sinensis by its smaller spikelets and longer inflorescence axis in relation to its racemes. Intermediates are evident, and the two species are difficult to separate using quantitative morphometric data (Renvoize et al., 1997). The data presented here do not support any obvious division. More M. floridulus individuals need to be added to this analysis to reach a firm conclusion about its species status. Miscanthus sinensis ssp. condensatus, a taxon endemic to Japan, was separated from M. sinensis on the basis of leaf anatomy by Lee (1964a) but was considered a subspecies by Koyama (1987). The AFLP analysis presented here also finds no evidence in support of its distinct species status.
Providing a reliable marker system for future plant breeding and utilization
The living collections at ADAS Arthur Rickwood Research Station and RBG Kew represent important resources for future exploitation. The AFLP marker system used in this study has proved to be efficient for screening a large genetic resource collection. If Miscanthus is to become a viable biomass crop in northwestern Europe, it must be bred for tolerance of cold weather, particularly in the early part of the growing season. A number of cold‐tolerant varieties exist, such as M. sinensis ssp. condensatus, which appear to be less influenced than other accessions of Miscanthus by late spring or early autumn cold weather. The AFLP fingerprints are held in a database and can be assessed further. Using breeding experiments, the markers can be utilized for marker‐aided selection and quantitative trait loci analysis (Godwin, 1997; Kearsey, 1997). The AFLP method also proved efficient at identifying horticultural varieties and cultivars of M. sinensis and would therefore be useful as a method to demonstrate the uniqueness of newly developed horticultural varieties for patenting purposes. AFLP will undoubtedly have potential in the future to help distinguish cultivars for commercial purposes.
ACKNOWLEDGEMENTS
We thank Mike Bullard and Peter Nixon, our sub‐contractors, at ADAS Arthur Rickwood Research Station, UK, for the collection and maintenance of a large living collection of Miscanthus. We also thank Mary Thorpe of the Living Collections Department, Royal Botanic Garden, Kew, UK for her assistance throughout this project. Thanks are due to Mike Fay at Kew for his assistance with the AFLP analysis. This work was supported by the Ministry of Agriculture Fisheries and Food, UK (MAFF project code QA3580).
Supplementary Material
Received: 27 October 2000; Returned for revision: 13 April 2001; Accepted: 4 February 2002.
References
- AdatiS, Shiotani I.1962. The cytotaxonomy of the genus Miscanthus and its phylogenic status. The Bulletin of the Faculty of Agriculture, Mie University 25: 1–14. [Google Scholar]
- BaumBR, Appels R.1992. Evolutionary change at the 5S DNA loci of species in the Triticeae. Plant Systematics and Evolution 183: 195–208. [Google Scholar]
- BullardMJ, Heath MC, Nixon PM.1995. Shoot growth, radiation interception and dry matter production and partitioning during the establishment phase of Miscanthus sinensis ‘Giganteus’ grown at two densities in the UK. Annals of Applied Biology 126: 94–102. [Google Scholar]
- BullardMJ, Nixon PM, Heath MC.1997. Quantifying the yield of Miscanthus × giganteus in the UK. Aspects of Applied Biology 49: 199–206. [Google Scholar]
- CasgrainP.1999. Le Progiciel Rv4·0d1. Development Release. [Google Scholar]
- ChouCH, Chang FC.1992. Phylogenetic relationship among species of Miscanthus populations in Taiwan. Botanic Bulletin Academia Sinica 33: 63–73. [Google Scholar]
- ChouCH, Hwang SY, Chang FC.1987. Population study of Miscanthus floridulus (Labill.) Warb. Botanic Bulletin Academia Sinica 28: 247–281. [Google Scholar]
- ClaytonWD, Renvoize SA.1986. Genera Graminum, grasses of the world. Kew Bulletin Additional Series XIII. [Google Scholar]
- CoxAV, Bennett MD, Dyer TA.1992. Use of the polymerase chain reaction to detect spacer size heterogeneity in plant 5S‐rRNA gene clusters and to locate such clusters in wheat (Triticum aestivum L.). Theoretical and Applied Genetics 83: 684–690. [DOI] [PubMed] [Google Scholar]
- DiceIR.1945. Measures of the amount of ecological association between species. Ecology 26: 295–302. [Google Scholar]
- DoyleJJ, Doyle JL.1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin of the Botanical Society of America 19: 11–15. [Google Scholar]
- FelsensteinJ.1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- GodwinID.1997. Gene identification, isolation and transfer. In: Callow JA, Ford‐Lloyd BV, Newbury HJ, eds. Biotechnology and plant genetic resources Wallingford: CAB International, 203–233. [Google Scholar]
- GreefJM, Deuter M.1993. Syntaxonomy of Miscanthus × giganteus Greef et Deu. Angewandte Botanik 67: 87–90. [Google Scholar]
- GreefJM, Schondelmaier J, Deuter M.1997. Genetic diversity of European Miscanthus species revealed by AFLP fingerprinting. Aspects of Applied Biology 49: 231–235. [Google Scholar]
- GreuterW, McNeill J, Barrie FR, Burdet HM, Demoulin V, Figueiras S, Nicolson DH, Silva PC, Skog JE, Trehane P, Turland J, Hawksworth DL.2000. International code of botanical nomenclature (Saint Louis Code). Regnum Vegetabile 138. Königstein: Koeltz Scientific Books. [Google Scholar]
- HodkinsonTR, Renvoize SA.2001. Nomenclature of Miscanthus × giganteus (Poaceae). Kew Bulletin 56: 757–758. [Google Scholar]
- HodkinsonTR, Renvoize SA, Chase MW.1997. Systematics of Miscanthus Aspects of Applied Biology 49: 189–198. [Google Scholar]
- HodkinsonTR, Renvoize SA, Ní Chonghaile G, Stapleton C, Chase MW.2000. A comparison of ITS nuclear rDNA sequence data and AFLP markers for phylogenetic studies in Phyllostachys (Bambusoideae, Poaceae). Journal of Plant Research 113: 259–269. [Google Scholar]
- HodkinsonTR, Chase MW, Takahashi C, Leitch IJ, Bennett MD, Renvoize SA. 2002 The use of DNA sequencing (ITS and trnL‐F), AFLP and fluorescent in‐situ hybridisation to study allopolyploid Miscanthus (Poaceae). American Journal of Botany 89: 279–286. [DOI] [PubMed] [Google Scholar]
- JorgensenRA, Cluster PD.1988. Modes and tempos in the evolution of nuclear ribosomal DNA: new characters for evolutionary studies and new markers for genetic and population studies. Annals of the Missouri Botanical Garden 75: 1238–1247. [Google Scholar]
- KarpA, Seberg O, Buiatti M.1996. Molecular techniques in the assessment of botanical diversity. Annals of Botany 78: 143–149. [Google Scholar]
- KearseyMJ.1997. Genetic resources and plant breeding. In: Callow JA, Ford‐Lloyd BV, Newbury HJ. eds. Biotechnology and plant genetic resources Wallingford: CAB International, 233–253. [Google Scholar]
- KilpatrickJB, Heath MC, Speller CS, Nixon PMI, Bullard MJ, Spink JG, Cromack HTH.1994. Establishment, growth, and dry matter yield of Miscanthus sacchariflorus over two years under UK conditions. In: Alternative oilseed and fibre crops for cool and wet regions of Europe Workshop organized by the Dutch delegation of the management committee of COST 814, Centre for Plant Breeding and Reproduction Research, 181–183. [Google Scholar]
- KoyamaT.1987. Grasses of Japan and its neighboring regions: an identification manual. Tokyo: Kodansha Ltd. [Google Scholar]
- LeeYN.1964a Taxonomic studies on the genus Miscanthus: anatomical patterns of leaves. Botanic Magazine of Tokyo 77: 122–130. [Google Scholar]
- LeeYN.1964b Taxonomic studies on the genus Miscanthus: relationships among the section, subsection and species, part 1. Journal of Japanese Botany 39: 196–205. [Google Scholar]
- LeeYN.1964c Taxonomic studies on the genus Miscanthus: relationships among the section, subsection and species, part 2, enumeration of species and varieties. Journal of Japanese Botany 39: 257–265. [Google Scholar]
- LeeYN.1964d Taxonomic studies on the genus Miscanthus: relationships among the section, subsection and species, part 3, enumeration of species and varieties. Journal of Japanese Botany 39: 289–298. [Google Scholar]
- LiA, Ge S.2001. Genetic variation and clonal diversity of Psammochloa villosa (Poaceae) detected by ISSR markers. Annals of Botany 87: 585–590. [Google Scholar]
- Linde‐LaursenIB.1993. Cytogenetic analysis of Miscanthus ‘Giganteus’, an interspecific hybrid. Hereditas 119: 297–300. [Google Scholar]
- MackillDJ, Zhang Z, Redona ED, Colowit PM.1996. Level of polymorphism and genetic mapping of AFLP markers in rice. Genome 39: 969–977. [DOI] [PubMed] [Google Scholar]
- MuellerUG, Wolfenbarger L.1999. AFLP genotyping and fingerprinting. Trends in Ecology and Evolution 14: 389–394. [DOI] [PubMed] [Google Scholar]
- NeiM, WHI Li.1985. Mathematical model for studying genetic variation in terms of restriction nucleases. Proceedings of the National Academy of Sciences of the USA 76: 5269–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QiX, Stam P, Lindhout P.1998. Use of locus specific markers to construct a high density map of barley. Theoretical and Applied Genetics 96: 376–384. [DOI] [PubMed] [Google Scholar]
- RamakrishaW, Chowdari KV, Lagu MD, Gupta VS, Ranjekar, PK.1995. DNA fingerprinting to detect genetic variation in rice using hypervariable DNA sequences. Theoretical and Applied Genetics 90: 1000–1006. [DOI] [PubMed] [Google Scholar]
- ReevesG, Francis D, Davies MS, Rogers HJ, Hodkinson TR.1998. Genome size is negatively correlated with altitude in natural populations of Dactylis glomerata Annals of Botany 82 (supplement A): 99–105. [Google Scholar]
- RenvoizeSA, Hodkinson TR, Chase MW.1997. Miscanthus in Britain: a molecular based review of diversity in the living resources held in the UK and available in Europe. Ministry of Agriculture Fisheries, and Food, Research Development MAFF QA3580. [Google Scholar]
- RidoutCJ, Donini P.1999. Use of AFLP in cereals research. Trends in Plant Science 4: 76–79. [DOI] [PubMed] [Google Scholar]
- SastriDC, Hilu K, Appels R, Lagudah S, Playford J, Baum BR.1992. An overview of evolution in plant 5S DNA. Plant Systematics and Evolution 183: 169–181. [Google Scholar]
- SwoffordDL.1998. Phylogenetic analysis using Parsimony (PAUP) version 4·0. Sunderland MA: Sinauer Associates. [Google Scholar]
- TaberletP, Gielly L, Pautou G, Bouvet J.1991. Universal primers for amplification of three non‐coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- VonWuhlischG, Deuter M, Muhs HJ.1994. Identifizierung verschiedener Miscanthus Sorten mittels Isoenzymen. Journal of Agronomy and Crop Science 172: 247–254. [Google Scholar]
- VosPR, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Kuiper M, Zabeau M.1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research 23: 4407–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WaughR, Bonar N, Baird E, Thomas B, Gramer A, Hayes P, Powell W.1997. Homology of AFLP products in three mapping populations of barley. Molecular and General Genetics 255: 311–321. [DOI] [PubMed] [Google Scholar]
- WeisingK, Nybom H, Wolff K, Meyer W.1995. DNA fingerprints in plants and fungi. CRC Press. [Google Scholar]
- WikbergS.1990. The genus Miscanthus: a summary of available literature. Project Agro‐fibre. Thesis, University of Lund, Sweden. [Google Scholar]
- WilkinsonMJ, Davenport IJ, Charters YM, Jones AE, Allainguillaume J, Butler HT, Mason DC, Raybould AF.2000. A direct regional scale estimate of transgene movement from genetically modified oilseed rape to its wild progenitors. Molecular Ecology 9: 983–991. [DOI] [PubMed] [Google Scholar]
- WilliamsJGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV.1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research 18: 6531–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZietkiewiczE, Rafalski A, Labuda D.1994. Genome fingerprinting by simple sequence repeat (SSR)‐anchored polymerase chain reaction. Genomics 20: 176–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.