Abstract
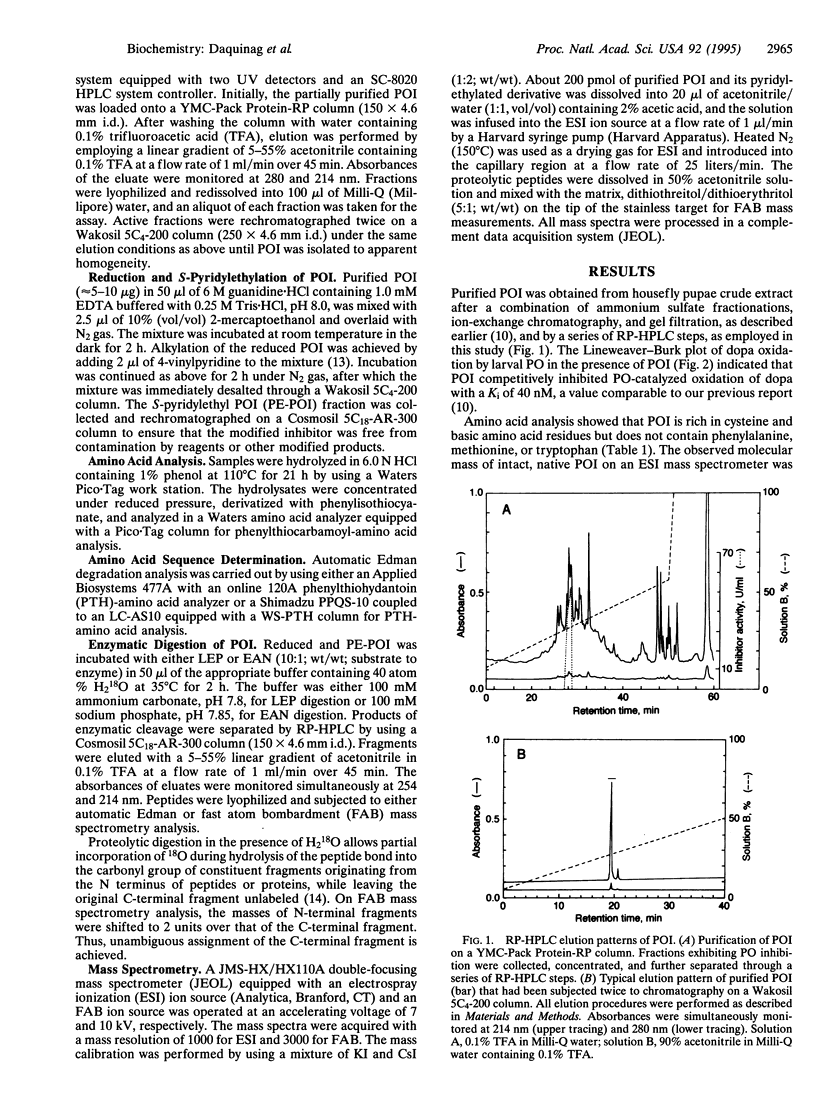
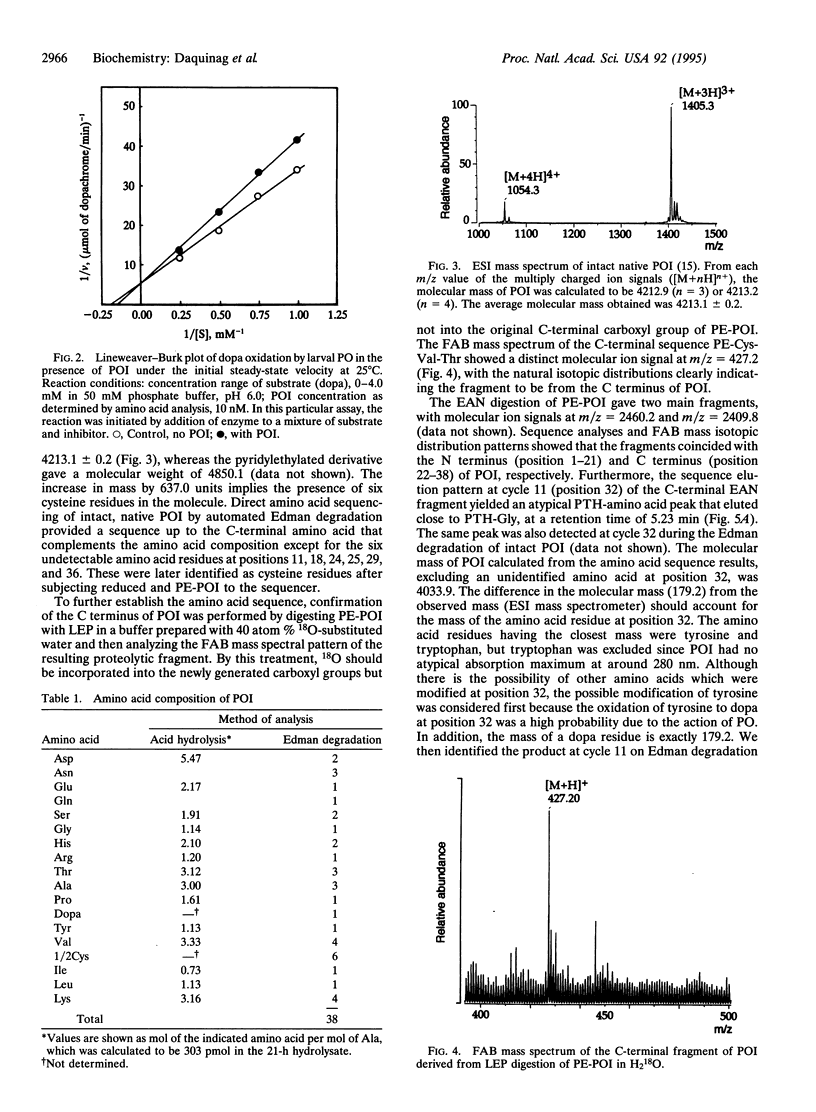
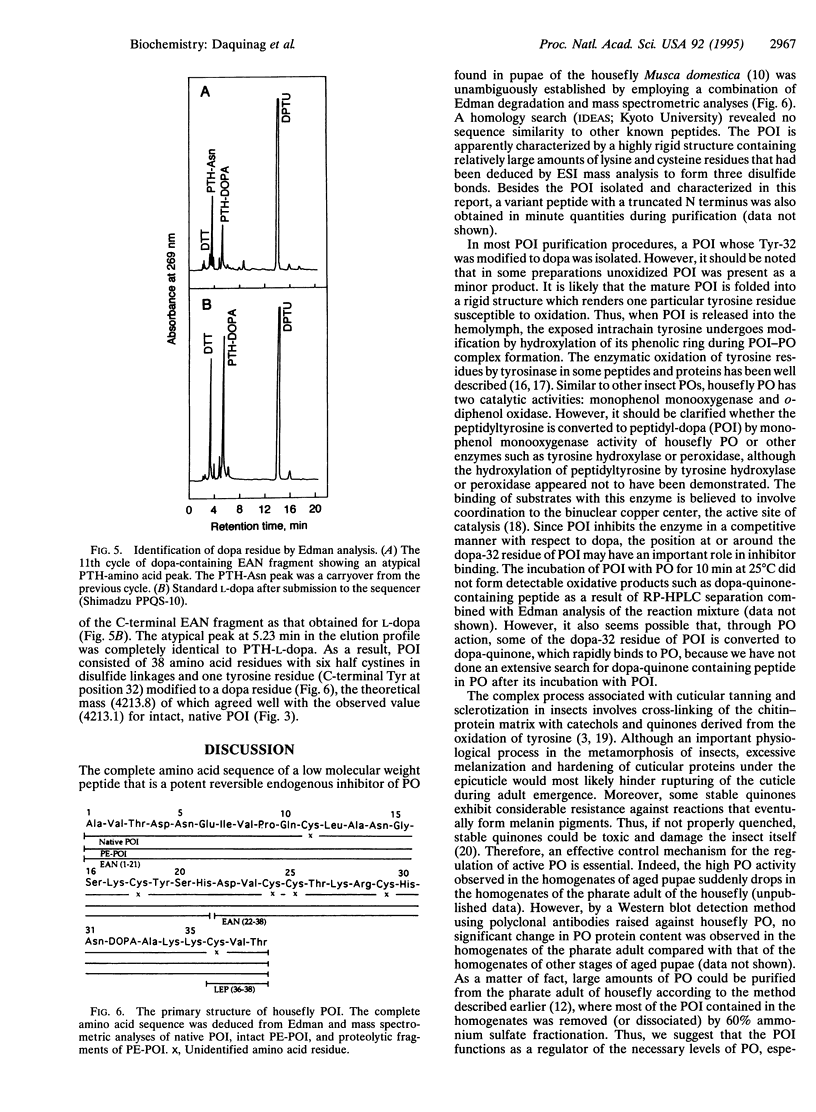
The complete amino acid sequence of a low molecular weight peptide from the hemolymph of the housefly Musca domestica L., which had been determined to competitively inhibit phenol oxidase (PO; monophenol, dihydroxy-phenylalanine:oxygen oxidoreductase; EC 1.14.18.1) in the nM range, was unambiguously established by employing both automatic Edman degradation and mass spectrometry. The physiologically active peptide, which was designated phenol oxidase inhibitor (POI), has an observed molecular weight of 4213.1 +/- 0.2 by electrospray ionization mass spectrometry. The relatively short and structurally dense peptide contained 38 amino acid residues rich in cysteine and lysine. Comparison of the observed and calculated molecular mass indicates that apparently all six cysteine residues form disulfide bridges. Interestingly, sequence analyses of both the intact and protease-digested S-pyridylethylated POI showed that one of the two tyrosine residues (Tyr-32) is hydroxylated to a 3,4-dihydroxyphenylalanine (dopa) residue. This agreed with the increase of 16 mass units observed in mass spectrometric measurements. This was further verified by submission of free L-dopa to the sequencer, which gave a retention time consistent with the atypical peak observed at the Edman cycle of the peptide containing dopa. This study demonstrates the existence of a biologically active, dopa-containing peptide among the insects. Since the POI activity was most prominent in aged pupae, especially pharate adults, the POI may play an important role in smoothing the way of adult emergence through hindering excessive melanization, as well as hardening, of cuticular proteins under the epicuticle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashida M., Doke K., Onishi E. Activation of prephenoloxidase. 3. Release of a peptide from prephenoloxidase by the activating enzyme. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1089–1095. doi: 10.1016/0006-291x(74)90808-0. [DOI] [PubMed] [Google Scholar]
- Azumi K., Yokosawa H., Ishii S. Halocyamines: novel antimicrobial tetrapeptide-like substances isolated from the hemocytes of the solitary ascidian Halocynthia roretzi. Biochemistry. 1990 Jan 9;29(1):159–165. doi: 10.1021/bi00453a021. [DOI] [PubMed] [Google Scholar]
- Cory J. G., Frieden E. Differential reactivities of tyrosine residues of proteins to tyrosinase. Biochemistry. 1967 Jan;6(1):121–126. doi: 10.1021/bi00853a021. [DOI] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- HOROWITZ N. H., SHEN S. C. Neurospora tyrosinase. J Biol Chem. 1952 May;197(2):513–520. [PubMed] [Google Scholar]
- Hopkins T. L., Morgan T. D., Aso Y., Kramer K. J. N-beta-Alanyldopamine: Major Role in Insect Cuticle Tanning. Science. 1982 Jul 23;217(4557):364–366. doi: 10.1126/science.217.4557.364. [DOI] [PubMed] [Google Scholar]
- Lerch K. Neurospora tyrosinase: structural, spectroscopic and catalytic properties. Mol Cell Biochem. 1983;52(2):125–138. doi: 10.1007/BF00224921. [DOI] [PubMed] [Google Scholar]
- Rose K., Savoy L. A., Simona M. G., Offord R. E., Wingfield P. C-terminal peptide identification by fast atom bombardment mass spectrometry. Biochem J. 1988 Feb 15;250(1):253–259. doi: 10.1042/bj2500253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T., Ichimaru Y., Kanegae N., Watanabe K., Yamaura I., Katsura Y., Funatsu M. Identification and isolation of endogenous insect phenoloxidase inhibitors. Biochem Biophys Res Commun. 1992 Apr 15;184(1):86–92. doi: 10.1016/0006-291x(92)91161-i. [DOI] [PubMed] [Google Scholar]
- Waite J. H., Rice-Ficht A. C. A histidine-rich protein from the vitellaria of the liver fluke Fasciola hepatica. Biochemistry. 1989 Jul 11;28(14):6104–6110. doi: 10.1021/bi00440a056. [DOI] [PubMed] [Google Scholar]
- Waite J. H., Tanzer M. L. Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science. 1981 May 29;212(4498):1038–1040. doi: 10.1126/science.212.4498.1038. [DOI] [PubMed] [Google Scholar]
- YASUNOBU K. T., PETERSON E. W., MASON H. S. The oxidation of tyrosine-containin peptides by tyrosinase. J Biol Chem. 1959 Dec;234:3291–3295. [PubMed] [Google Scholar]


