Abstract
Microsporangium dehiscence, pollen production and dispersal were studied in Himalayan cedar (Cedrus deodara) during 1998 and 1999. Microsporangium dehiscence showed diurnal periodicity and was found to be related to air temperature and relative air humidity, with a strobilus taking 2 d to dehisce completely in warmer conditions and 3 d in cooler ones. The frequency of flowering in C. deodara was highly variable during the two successive years; however, cyclical production of pollen grains was observed in 50 % of the trees. The maximum concentration of pollen grains in the air was found between 1200 and 1600 h, and this period was also noted to be the best time for pollination. Studying migration of pollen grains from isolated single trees in three directions showed that migration was not uniform in all directions. Long‐distance transport of pollen grains was observed in the downhill direction. However, in the uphill and horizontal directions grains could travel only up to 97·5 and 195·1 m, respectively, and the frequency of pollen grains to the source frequency at these distances was only 1·9 and 2·5 %, respectively. The results suggest that an isolation barrier of 190 m may be considered as a minimum for the management of deodar seed orchards.
Key words: Anthesis, dehiscence, pollination, pollen dispersion, microsporangium, Cedrus deodara, strobili
INTRODUCTION
Pollen grains are reduced male gametophytes which, upon pollination, produce pollen tubes that grow through the tissues of the pistil to effect fertilization and seed set. The production and the dispersal of pollen have both biological and genetic implications for the quantity and genetic value of the seed produced. Hence pollen biology is of immense significance in tree improvement programmes as it determines gene flow and heterozygosity of the population, and these in turn determine genetic variability.
Pollen migration provides a mechanism for maintenance of genetic variability, particularly in sessile wind‐pollinated, long‐lived forest trees. The amount of gene flow (i.e. pollen contamination) from surrounding unselected trees into the seed orchards affects the genetic quality of seed lots by reducing the expected genetic gain, reducing within‐orchard inter‐clonal mating, producing maladapted stock, and changing the allelic and genotypic frequencies between the seed orchard population and its seed lots. Pollen contamination can be eliminated or reduced in several ways: (1) by geographic isolation, i.e. establishing orchards outside the species range (El‐Kassaby and Davidson, 1990); (2) reducing the frequency of outside pollen by the use of supplemental‐mass‐pollination (SMP) (Bridgwater and Trew, 1981; El‐Kassaby and Ritland, 1986); (3) establishing larger orchards (El‐Kassaby et al., 1984); and (4) physiological manipulation by the use of overhead water spray cooling treatments, i.e. bloom delay (Fashler and El‐Kassaby, 1987). The level of pollen contamination is also affected by the proximity and the size of the source of contamination, by reproductive synchrony between the orchard and the outside source, by the size of the breeding population (neighbourhood size) and by the management practices used (Bridgwater and Trew, 1981; El‐Kassaby et al., 1984; El‐Kassaby and Ritland, 1986; El‐Kassaby and Davidson, 1990).
Himalayan cedar (Cedrus deodara Roxb. ex D. Don) is a precious timber resource of western Himalaya which grows in pure stands or in association with other Himalayan conifers at an altitude of 1800–2600 m asl (occasionally between 1200–3000 m asl). The objective of the present investigation was to gain a better understanding of pollen biology by studying: (1) anthesis and microsporangium dehiscence in two different localities in relation to the time of the day; (2) changes in pollen productivity in two successive years; (3) distribution of air‐borne pollen grains at different time intervals; and (4) spatial dispersion of pollen from isolated single trees and estimation of parameters using the dispersal model of Bateman (1947).
MATERIALS AND METHODS
The study was conducted during two successive flowering seasons, October 1998 and October 1999, in the deodar forests of the Pauri Garhwal district, India (29°20′–30°15′N, 78°10′–79°20′E) at two different locations, the warmer Khirsu (1800 m asl), and the cooler Teka (1900 m asl), both situated in the central part of western Himalaya.
Anthesis and microsporangium dehiscence
Observations of microsporangium dehiscence began at the time of anthesis by sampling ten strobili of C. deodara (which had just begun to flower) on five different trees at each location. Samplings continued until all the anthers in a strobilus had dehisced. Each strobilus was examined every 2 h to identify the patterns of anthesis and dehiscence. Observations were made using a hand lens (20×) by scoring and removing to avoid duplication. The prevailing air temperature and relative humidity were also recorded close to the strobili studied during each observation using a thermohygrometer.
Pollen productivity analysis
Cedrus deodara has a large number of strobili arranged mostly on the main branches of the crown. First, the main branches were counted, and then a sample of five representative branches was selected at random and their strobili were counted. In total, 20 strobili were sampled from each tree, and the number of microsporophylls was counted manually. Microsporophylls were obtained from closed strobili, kept in 70 % ethanol, washed in distilled water, measured and placed in test tubes. The microsporophylls were crushed using a glass rod and the pollen grains were suspended in 1 ml distilled water. From this concentrate, five 10 µl droplets were removed and the pollen grains were counted using a binocular microscope. Pollen grains were counted on five microsporophylls from different strobili of each tree. The method used for pollen productivity analysis was modified after Tormo Molina et al. (1996). To estimate the total production of pollen grains per tree, the total number of microsporophylls per tree was calculated by multiplying the total number of strobili by the average number of microsporophylls per strobilus. The result was then multiplied by the average number of pollen grains produced per microsporophyll.
Sampling of air‐borne pollen grains
The amount of pollen in the air was determined by sampling pollen on microscope slides coated with petroleum jelly (Vaseline) at 2, 6 and 12 h intervals for up to 6 d. The slides were mounted vertically on iron rods and were either placed perpendicular to the prevailing wind direction, or were positioned all around the source tree. The rods were mounted on trees at heights corresponding to the heights of the strobili, and slides were replaced at suitable intervals. The number of pollen grains per slide was counted in a 1 cm2 area under a binocular microscope. The wind speed was recorded by a digital anemometer.
Observations on the dispersion of pollen grains
A representative deodar tree, situated on the agricultural fields of Premnagar village (altitude 1200 m) was selected as the pollen source. It was 21 m tall with a crown diameter of 8·0 m, and was isolated from possible contamination from foreign pollen by more than 1·60 km. Pollen samples were collected at increasing distances from the source tree: (1) parallel to the average wind direction and up to 390·1 m (at distances 0, 1·5, 3·1, 6·1, 12·2, 24·4, 48·8, 97·5, 195·1 and 390·1 m) horizontally from the source tree, with an average slope of ±5°; (2) up to 195·1 m away from the source tree in an uphill direction (average slope 23°); and (3) up to 780·3 m from the source tree in a downhill direction (average slope 32°). Pollen frequencies could not be measured beyond these specified distances because they became negligible and also because geographical barriers were present in the form of forests of other species, particularly banj oak (Quercus leucotrichophora). Samples were collected for 4 d during 1998 and 1999. Thirty pollen slides were fixed daily at geometrically increasing intervals in three possible directions during the peak flowering periods of both the years.
Ordinary microscopic slides, covered with a thin coat of petroleum jelly were used as pollen traps. The slides were fixed in a horizontal position on a 2·5 m long pole. The slides were unprotected and exposed to the open air. New slides were mounted every day between 1600 and 1700 h and were collected after 24 h. Slides were covered with a cover slip when they were collected to protect the samples. Pollen counts were made in the laboratory using a binocular microscope. The area in which grains were counted was fixed at 1 cm2 per slide, so that the frequency near the source tree ranged from 200 to 500 grains per slide, which gave a base for the estimates of frequencies at more distant positions. Analysis of one complete set of slides taken from a series of transects around a single source tree gives the slope of one pollen dispersion curve. Replications were made by duplication of complete transects on other days and in another year.
Mathematical treatment of pollen dispersion data
Bateman (1947) found that the function F = F0e–kD fitted dispersion data well. In this formula F and F0 are pollen frequencies at distances D and 0, respectively, and e is the base of natural logarithms. If the curve is converted to a straight line, the quantity k is the slope.
RESULTS
Anthesis and microsporangium dehiscence
In C. deodara, the opening of strobili and dehiscence of microsporangia were observed to take place at the same time. The dehiscence of microsporophylls proceeds, as a rule, from the broader apical to the narrower basal end, with two longitudinal lines gradually widening towards the basal end (i.e. lateral dehiscence). Field observations have also revealed that, in the majority of strobili, anthers do not dehisce simultaneously but take, on average, 2–3 d, depending on the site and weather conditions. Even two lobes of the same microsporangium do not dehisce simultaneously.
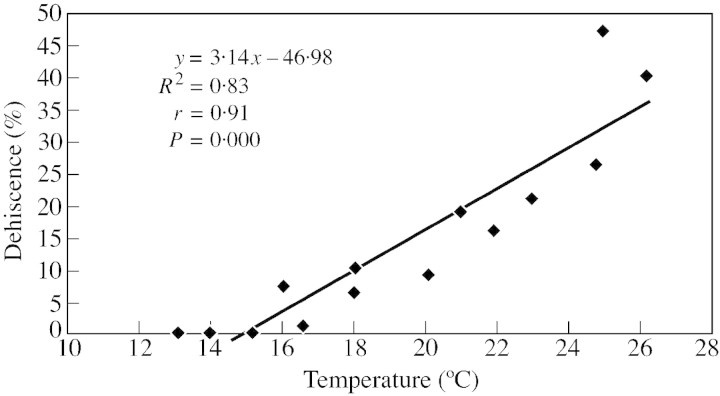
Table 1 presents data on microsporangium dehiscence at two sites differing in altitude and, therefore, in temperature. The data revealed that in natural conditions microsporangium dehiscence occurred between 0800 and 1800 h at both sites. However, a strobilus took 2·5 d to dehisce completely at the warmer location (Khirsu), and 3 d at the cooler location (Teka), probably due to variation in prevalent air temperature and relative humidity. Maximum dehiscence (6·6–21·3 % at the warmer site, and 6·1–16·3 % at the cooler site, respectively) was recorded between 1200 and 1400 h, which was the peak period of microsporangium dehiscence in both the cases. This corresponded to the time of maximum air temperature and minimum atmospheric humidity (Table 1).
Table 1.
Microsporangium dehiscence in Cedrus deodara at two sites
| Khirsu (warmer location; 1800 m asl) | Teka (cooler location; 1900 m asl) | |||||||||
| Microsporangium dehiscence (%) (n = 10) | Microsporangium dehiscence (%) (n = 10) | |||||||||
| Time (h) | Temp. (°C) | RH (%) | 5 Oct. 1998 | 6 Oct. 1998 | 7 Oct. 1998 | Temp. (°C) | RH (%) | 12 Oct. 1998 | 13 Oct. 1998 | 15 Oct. 1998 |
| 0600 | 14·0 | 90 | – | – | – | 13·1 | 92 | – | – | – |
| 0800 | 16·6 | 86 | – | 0·64 ± 0·15 | – | 15·2 | 87 | – | – | 0·65 ± 0·14 |
| 1000 | 20·1 | 72 | 1·36 ± 0·21 | 4·32 ± 0·29 | 3·17 ± 0·21 | 18·0 | 78 | 2·15 ± 0·26 | 1·66 ± 0·21 | 2·49 ± 0·32 |
| 1200 | 24·8 | 62 | 8·73 ± 0·64 | 10·56 ± 0·42 | 6·58 ± 0·42 | 23·0 | 68 | 7·96 ± 0·51 | 6·63 ± 0·62 | 6·13 ± 0·54 |
| 1400 | 26·2 | 58 | 18·23 ± 0·89 | 21·32 ± 0·93 | – | 25·0 | 62 | 16·25 ± 0·76 | 14·26 ± 0·67 | 16·10 ± 0·84 |
| 1600 | 21·9 | 75 | 7·35 ± 0·56 | 8·36 ± 0·67 | – | 21·0 | 73 | 6·96 ± 0·50 | 6·47 ± 0·54 | 5·14 ± 0·41 |
| 1800 | 18·0 | 82 | 4·13 ± 0·37 | 5·23 ± 0·37 | – | 16·5 | 80 | 3·15 ± 0·32 | 2·32 ± 0·43 | 1·66 ± 0·37 |
Pollen production
In C. deodara, the number of strobili per branch, microsporophylls per strobilus and the number of branches which produced male strobili per tree varied considerably in 1998 and 1999 (Table 2). Similarly, the production of pollen grains per microsporophyll, per strobilus, per branch and per tree in both the years was highly variable (Table 3). The average rates of pollen production per branch were 393·7 ± 109·9 × 106 and 279·7 ± 130·6 × 106 in 1998 and 1999, respectively, at the warmer location (Khirsu), and 528·7 ± 134·0 × 106 and 157·7 ± 129·4 × 106 in 1998 and 1999, respectively, at the cooler location (Teka). Average rates of pollen production per tree were 11·3 ± 2·9 × 109 and 2·76 ± 1·3 × 109 at Khirsu in 1998 and 1999, respectively, and 14·7 ± 3·2 × 109 and 1·7 ± 1·2 × 109 grains per tree at Teka in 1998 and 1999, respectively (Tables 2 and 3).
Table 2.
Pollen production data for each tree studied
| 1998 | 1999 | |||||||||||
| Tree no. | cbh (cm) | Height (m) | Total flowering branches | Strobili/branch | Microsporophylls/strobilus | Strobili/tree | Microsporophylls/tree | Total number of flowering branches | Strobili/branch | Microsporophylls/strobilus | Strobili/tree | Microsporophylls/tree |
| Khirsu (warmer location; 1800 m asl) | ||||||||||||
| 1 | 74 | 15 | 24 | 56·4 ± 21·2 | 449·3 ± 21·1 | 1353·6 | 608·2 | 12 | 67·4 ± 15·2 | 410·6 ± 25·5 | 808·8 | 332·1 |
| 2 | 110 | 19 | 33 | 104·6 ± 37·5 | 402·5 ± 37·2 | 3451·8 | 1389·4 | 7 | 142·6 ± 45·1 | 472·4 ± 32·2 | 998·2 | 471·6 |
| 3 | 87 | 16 | 30 | 132·4 ± 52·6 | 383·6 ± 40·6 | 3972·0 | 1523·7 | 12 | 110·2 ± 24·4 | 417·2 ± 36·5 | 1322·4 | 551·7 |
| 4 | 132 | 25 | 25 | 172·8 ± 74·2 | 249·4 ± 27·5 | 4320·0 | 1077·4 | Flowering totally absent | ||||
| 5 | 105 | 17 | 37 | 32·4 ± 7·4 | 506·2 ± 52·2 | 1198·8 | 606·8 | Flowering totally absent | ||||
| Teka (cooler location; 1900 m asl) | ||||||||||||
| 1 | 56 | 11 | 21 | 44·6 ± 16·4 | 492·2 ± 43·6 | 936·6 | 461·0 | 12 | 38·4 ± 12·9 | 254·7 ± 23·1 | 3419·2 | 119·0 |
| 2 | 80 | 16 | 47 | 134·6 ± 64·9 | 402·6 ± 13·4 | 6326·2 | 254·7 | Flowering totally absent | ||||
| 3 | 125 | 24 | 20 | 164·8 ± 65·8 | 294·2 ± 29·8 | 3296·0 | 969·7 | Flowering totally absent | ||||
| 4 | 91 | 17 | 51 | 38·2 ± 5·1 | 484·2 ± 43·4 | 1948·2 | 943·3 | Flowering totally absent | ||||
| 5 | 175 | 31 | 23 | 164·6 ± 45·9 | 381·5 ± 140·1 | 3785·8 | 1444·3 | 10 | 156·7 ± 42·2 | 403·7 ± 36·2 | 1567·0 | 632·6 |
cbh, circumference at breast height.
Table 3.
Variation in pollen production in two successive years
| 1998 | 1999 | |||||||||
| Tree no. | Microsporophyll length | Pollen grains/microsporophyll | Pollen grains/strobilus (×103) | Pollen grains/branch (×106) | Pollen grains/tree (×106) | Microsporophylls length | Pollen grains/microsporophyll | Pollen grains/strobilus (×103) | Pollen grains/branch (×106) | Pollen grains/tree (×106) |
| Khirsu (warmer location; 1800 m asl) | ||||||||||
| 1 | 3·0 ± 0·17 | 6954·6 ± 632·1 | 3124·7 | 176·2 | 4·2 | 3·4 ± 0·13 | 8517·2 ± 553·6 | 3497·2 | 235·7 | 6·76 |
| 2 | 3·8 ± 0·21 | 9148·2 ± 754·3 | 3682·2 | 385·2 | 12·7 | 3·8 ± 0·17 | 8905·3 ± 698·4 | 4206·9 | 599·9 | 4·20 |
| 3 | 4·1 ± 0·23 | 11 540·5 ± 863·4 | 4442·3 | 588·2 | 17·6 | 4·2 ± 0·19 | 12 245·4 ± 1045·6 | 5108·8 | 563·0 | 6·76 |
| 4 | 4·4 ± 0·27 | 15 978·8 ± 1215·3 | 3985·1 | 688·6 | 17·2 | Flowering totally absent | ||||
| 5 | 3·1 ± 0·15 | 7953·6 ± 625·2 | 4024·5 | 130·5 | 4·8 | Flowering totally absent | ||||
| Teka (cooler location; 1900 m asl) | ||||||||||
| 1 | 4·0 ± 0·30 | 20 450·8 ± 2968·9 | 10065·9 | 448·9 | 9·4 | 3·5 ± 0·21 | 13 425·5 ± 1312·2 | 3419·5 | 119·0 | 1·58 |
| 2 | 3·1 ± 0·38 | 8752·0 ± 1074·2 | 3523·6 | 474·3 | 22·3 | Flowering totally absent | ||||
| 3 | 4·1 ± 0·32 | 12 537·2 ± 755·3 | 3688·4 | 607·9 | 12·2 | Flowering totally absent | ||||
| 4 | 3·8 ± 0·17 | 7719·2 ± 788·4 | 3737·6 | 142·8 | 7·3 | Flowering totally absent | ||||
| 5 | 4·1 ± 0·27 | 15 443·2 ± 1536·1 | 5891·6 | 969·8 | 22·3 | 3·1 ± 0·19 | 10 533·2 ± 1029·3 | 4252·3 | 666·3 | 6·66 |
Ratios of pollen produced by different developmental units
There was a significant (P < 0·05) positive correlation between the number of pollen grains per strobilus and microsporophylls per strobilus; grains per branch and strobili per branch; grains per tree and microsporophylls per tree; grains per tree and strobili per tree; and strobili per tree and strobili per branch.
By using logarithmic values these correlations were maintained, and other significant correlations, although negative, also became apparent (Table 4). The number of pollen grains per microsporophyll decreased significantly when the number of microsporophylls per strobilus increased and when the number of microsporophylls per tree increased. Furthermore, the number of grains per strobilus decreased significantly when the number of strobili per tree increased. The number of microsporophylls per strobilus decreased significantly when the number of strobili per tree or the number of strobili per branch increased.
Table 4.
Correlation coefficients between the production rates of pollen grains (gr.), microsporophylls (mcrl.) and strobili (str.) per superior production unity
| Variables | log (gr./mcrl.) | log (gr./strobilus) | log (gr./branch) | log (gr./tree) | log (mcrl./strobilus) | log (mcrl./tree) | log (str./branch) |
| log (gr./strobilus) | 0·117 | – | – | – | – | – | – |
| <0·0005 | |||||||
| log (gr./branch) | –0·136 | 0·427 | – | – | – | – | – |
| 0·001 | 0·002 | ||||||
| log (gr./tree) | 0·131 | 0·265 | 0·684 | – | – | – | – |
| 0·002 | 0·001 | 0·826 | |||||
| log (mcrl./strobilus) | –0·129 | 0·287 | –0·175 | –0·063 | – | – | – |
| <0·0005 | 0·303 | 0·001 | <0·0005 | ||||
| log (mcrl./tree) | –0·0001 | 0·170 | 0·461 | 0·632 | 0·114 | – | – |
| 0·001 | 0·002 | 0·969 | 0·856 | <0·0005 | |||
| log (str./branch) | –0·203 | 0·006 | 0·906 | 0·628 | –0·352 | 0·419 | – |
| <0·0005 | 0·007 | 0·688 | 0·535 | <0·0005 | 0·660 | ||
| log (str./tree) | 0·163 | –0·242 | 0·288 | 0·541 | –0·625 | 0·207 | 0·446 |
| 0·001 | 0·004 | 0·864 | 0·696 | <0·0005 | 0·834 | 0·818 |
Each pair of data represents the correlation coefficient, r, and probability P.
Sampling of air‐borne pollen grains
The time as well as the rate of pollen deposition on microscope slides exposed at intervals of 2 h, 6 h and 12 h from 1800 h onwards on 9 Oct. 1999 until 0600 h on 14 Oct. 1999 (Table 5) revealed that the concentration of pollen grains deposited between 0600 and 1800 h was 215 grains cm–2 (on first day), 264 grains cm–2 (on the third day) and 136 grains cm–2 (on the sixth day), whereas the concentration of pollen grains deposited between 1800 and 0600 h was 27 grains cm–2 (on the first day), 36 grains cm–2 (on the third day) and 19 grains cm–2 (on the fifth day). About 88 % of the total pollen output of all 6 d was released into the air during the day, and only 12 % during the following night. This confirms that the rates of pollen deposition were higher during the daytime than at night. The census of air‐borne pollen grains on a single day on a 2‐hourly basis showed that the maximum concentration of pollen grains in the air was 74 and 106 grains cm–2 (on the first day), 84 and 116 grains cm–2 (on the second day), and 123 and 87 grains cm–2 (on the third day), between 1200 and 1400 h, and 1400 and 1600 h, respectively (Table 5). Therefore, the period between 1200 and 1600 h was considered the best time for pollination. Ingold (1971) stated that ‘wind pollinated plants shed pollen almost entirely during daytime and pollen are (sic) set free under conditions suitable for effective dispersal’. This is verified by the present results.
Table 5.
Temporal variation of air–borne pollen grains trapped on slides (from 9–14 Oct. 1999)
| Every 12 h | Every 6 h | Every 2 h | ||||||
| Time (h) | Pollen grains deposited cm–2 (n = 5) | Time (h) | Pollen grains deposited cm–2 (n = 5) | Time (h) | Day 1 Pollen grains deposited cm–2 (n = 5) | Day 2 Pollen grains deposited cm–2 (n = 5) | Day 3 Pollen grains deposited cm–2 (n = 5) | Wind speed (m s–1) |
| 0600 | – | 0600 | – | 0600 | – | 16 ± 0·3 | 20 ± 0·5 | 0·06 ± 0·02 |
| 1800 | 215 ± 12·1 | 1200 | 87 ± 3·1 | 0800 | 15 ± 0·2 | 12 ± 0·4 | 12 ± 0·2 | 0·27 ± 0·09 |
| 0600 | 27 ± 4·2 | 1800 | 164 ± 12·3 | 1000 | 28 ± 0·7 | 34 ± 0·4 | 23 ± 0·3 | 0·33 ± 0·09 |
| 1800 | 189 ± 14·3 | 2400 | 18 ± 1·4 | 1200 | 54 ± 4·3 | 63 ± 2·4 | 60 ± 1·5 | 0·45 ± 0·09 |
| 0600 | 21 ± 2·3 | 0600 | 10 ± 0·7 | 1400 | 74 ± 4·4 | 84 ± 7·9 | 123 ± 12·1 | 1·27 ± 0·15 |
| 1800 | 264 ± 20·1 | 1200 | 69 ± 6·4 | 1600 | 106 ± 13·1 | 116 ± 10·8 | 87 ± 7·2 | 1·40 ± 0·38 |
| 0600 | 36 ± 6·5 | 1800 | 129 ± 10·1 | 1800 | 37 ± 1·1 | 31 ± 1·3 | 26 ± 0·4 | 0·36 ± 0·11 |
| 1800 | 249 ± 17·5 | 2400 | 17 ± 0·9 | 2000 | 17 ± 0·1 | 12 ± 0·4 | 10 ± 0·1 | 0·21 ± 0·08 |
| 0600 | 32 ± 5·1 | 0600 | 9 ± 0·3 | |||||
| 1800 | 164 ± 12·3 | 1200 | 104 ± 11·6 | |||||
| 0600 | 19 ± 2·2 | 1800 | 194 ± 14·8 | |||||
| 1800 | 136 ± 12·1 | 2400 | 22 ± 3·4 | |||||
| 0600 | 25 ± 1·6 | 0600 | 12 ± 0·9 | |||||
Dispersion of pollen grains from an isolated single tree
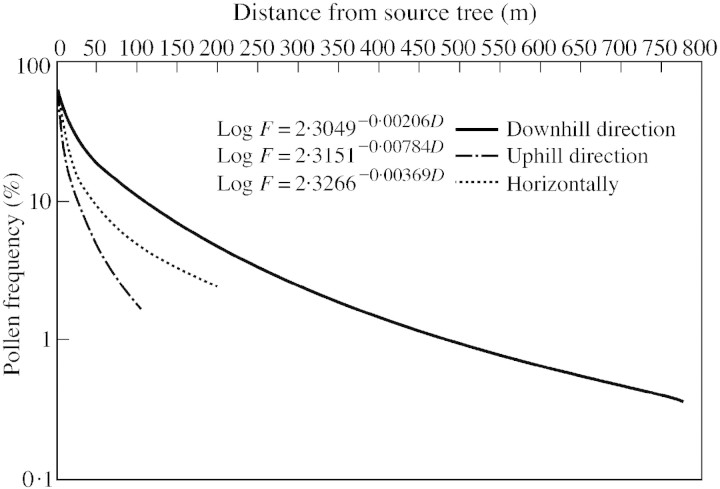
Pollen dispersion in C. deodara from the source tree in three possible directions at geometrically increasing intervals was recorded during the peak flowering periods in two successive years. The data show that migration of pollen grains was not uniform in all directions. Although dispersion of the majority of pollen grains was observed up to 24·4 m in the uphill direction and 48·8 m in the horizontal direction, long‐distance transport of pollen grains was observed only in the downhill direction (up to 97·5 m). The mean pollen frequencies in this direction relative to the source frequency were 11·8, 5·2 and 1·5 %, at 97·5, 195·1, and 390·1 m, respectively (Fig. 1). The total pollen output per unit area (i.e. 1 cm2) on a series of 11 pollen slides in this direction was averaged at 1150 pollen grains. In the uphill and horizontal directions pollen grains travelled up to 97·5 and 195·1 m only, and the average frequency of pollen grains relative to the source frequency at these distances was 0·7 and 0·85 %, respectively (Fig. 1). It was postulated by Wright (1962) that pollen dispersion distances are best expressed as the slope of the dispersion curve. Therefore, the respective regression equations calculated for three different directions were as follows: log F = 2·3049 – 0·00206D in the downhill direction; log F = 2·3151 – 0·00784D in the uphill direction; and log F = 2·3266 – 0·00368D in the horizontal direction.
Fig. 1. Pollen frequency curve showing pollen grains trapped at varying distances from isolated source tree.
DISCUSSION
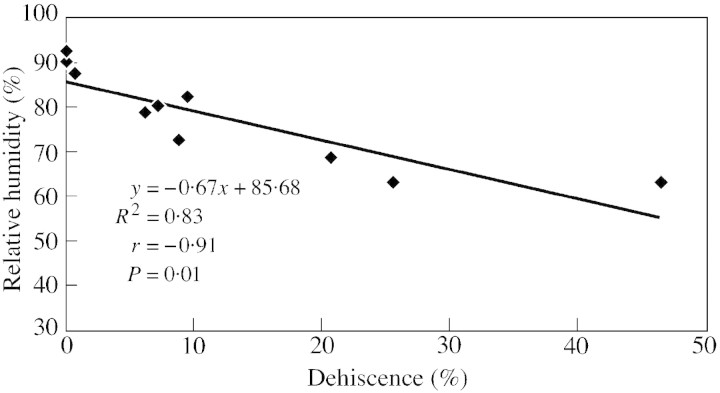
It is clear from the observations that climatic factors influence microsporangium dehiscence and pollen dispersal in C. deodara. Air temperature must exceed, and relative air humidity must remain below, certain critical levels to start the anthesis process: it took 2 d for a strobilus to dehisce completely in the warmer location (Khirsu), and 3 d in the comparatively cooler location (Teka). A significant positive correlation (r = 0·91, P < 0·0005) was achieved between temperature and percentage microsporangium dehiscence. Similarly, a negative correlation (r = – 0·91, P < 0·01) was obtained between relative humidity and percentage microsporangium dehiscence (Figs 2 and 3). Temperature seems to have a major effect on anthesis, as has been reported previously (Davidson, 1941; Emecz, 1962; Liem and Groot, 1973; Sharma et al., 1998; Khanduri et al., 1999; Khanduri and Sharma, 2000). Gregor (1928) was of the opinion that humidity promotes anthesis in Lolium, while Cocks (1958) suggested that it hinders the process. Kramer (1932) observed that wind is an important factor promoting anthesis, in addition to temperature.
Fig. 2. Regression between temperature and microsporangium dehiscence.
Fig. 3. Regression between humidity and microsporangium dehiscence.
The quantity of pollen produced by an individual plant is influenced by various factors (Stanley and Linskens, 1974), which vary from year to year (Rogers, 1993). It is most important to have an estimate of the total pollen production per plant in order to record the number of pollen grains that could be in the air during a given season, once the density of plants is known. This can also be used to estimate seed production (Campbell and Halama, 1993; Allison, 1990), as the efficiency of anemophilous pollination decreases as the amount of air‐borne pollen falls (Whitehead, 1983).
In C. deodara, the frequency of flowering was highly variable in two successive years at two different localities. It was also observed that flowering was totally absent in about 50 % of the trees at both sites in 1999 (Tables 2 and 3). This may be due mainly to variation in the frequency of flowering years, and partly due to morphological (sometimes the previous year’s flowers or inflorescences are situated in such a position that no flower can be formed at that place in the following year) and physiological factors (a period of ‘rest’ being necessary before the next profuse flowering). Hyde (1952, 1953) indicated that cyclical production was not correlated with climatic conditions, and that different species had different intervals between bumper years of production; for example, a cycle of 5 years for Quercus, 2 years for Pinus and Fagus, and 3 years for Fraxinus and Ulmus. Furthermore, the maximum for different species with the same cycle interval did not always coincide. Allison (1990) pointed out that pollination success in Taxus canadensis is positively correlated with the frequency of flowering years. He also confirmed that pollen production decreased in a population in 1 year, which was reflected in reduced pollination success in that population.
The existence of an inverse relationship between different production units leads us to believe that there is a constant value for pollen production in C. deodara. As the number of pollen grains per microsporophyll, microsporophylls per strobilus, strobili per branch and strobili per tree varies considerably, there is a tendency to compensate by increasing one or reducing the other so that the net product is generally within some defined interval.
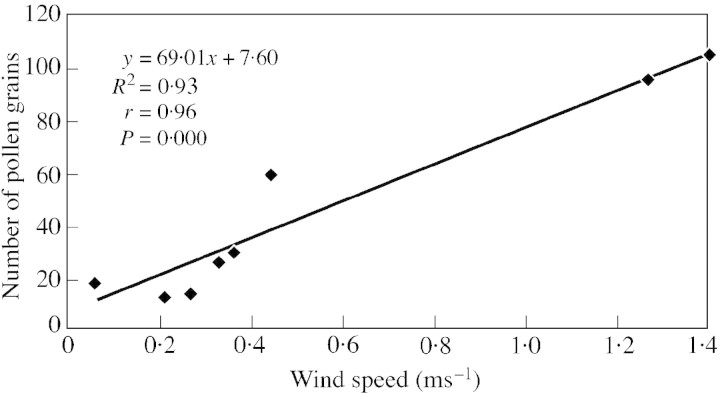
The dispersal rate of the liberated/discharged pollen grains in the ambient atmosphere depends on the extent of air movement and/or the magnitude of atmospheric turbulence, which vary according to changes in air temperature throughout the day. Pollen grains disperse if the prevailing winds are strong and sufficiently turbulent. Evidence for the realizations of these expectations can be drawn from the data obtained in the present study (Table 5). The circadian periodicity of air‐borne pollen grains was maximum when the wind velocity was comparatively high, which is supported by the positive (r = 0·96, P < 0·0005) correlation obtained between the wind speed and amount of pollen grains in the air (Fig. 4). Similar results have been recorded by Sarvas (1962) and Kapyla (1984) for Pinus sylvestris, Subba Reddi et al. (1980) for Xanthium, Subba Reddi and Reddi (1984) for Emblica and Cicca, and Khanduri (1999) for Pinus roxburghii.
Fig. 4. Regression between wind speed and amount of pollen in the air.
The necessary isolation of a species against being contaminated by foreign pollen is dictated by pollen dispersion distances, particularly in anemophilous taxa. Knowledge of the average distance that pollen is dispersed is essential for estimating the extent to which isolation is important in race formation, and for successful seed set. The amount of pollen transported over longer distances is small as compared with the total amount of pollen produced by an individual. The decrease in pollen frequency with increasing distances from a pollen source is of great practical value in planning and managing a seed orchard. A rapid decrease in pollen deposition with increasing distances (1 % of the source frequency at 91·4 m in Pinus edulis Engelm. and 2–5 % of the source frequency at 152·4 m in Pinus elliottii Engelm.) was recorded by Wright (1952) and Wang et al. (1960). However, in the present study, a pollen frequency of 2·5–5 % relative to the source frequency was recorded in the horizontal and downhill directions at 190 m, which suggests that an isolation strip of 190 m may be considered minimal for managing a C. deodara seed orchard.
ACKNOWLEDGEMENT
The authors thank Dr Richard W. Weber for valuable suggestions and constructive criticism.
Supplementary Material
Received: 17 May 2001; Returned for revision: 9 October 2001; Accepted: 8 February 2002.
References
- AllisonTD.1990. Pollen production and plant density affect pollination and seed production in Taxus canadensis Ecology 71: 516–522. [Google Scholar]
- BatemanAJ.1947. Contamination in seed crops. II. Wind pollination. Heredity 1: 235–246. [Google Scholar]
- BridgwaterFE, Trew IF.1981. Supplemental mass pollination. In: Franklin EC, ed. Pollen management handbook Washington DC: U.S.D.A. Agriculture Handbook 587, 15–19. [Google Scholar]
- CampbellDR, Halama KJ.1993. Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology 74: 1043–1051. [Google Scholar]
- CocksB.1958. Welsh Plant Breeding Station Report. 1950–1956, 32. [Google Scholar]
- DavidsonR.1941. A note on anthesis in some common grasses near Johannesburg and the relation of anthesis to collection of pollen for medical purposes. Journal of South African Botany 7: 145–152. [Google Scholar]
- EmeczTI.1962. The effect of meteorological conditions on anthesis in agricultural grasses. Annals of Botany 26: 159–172. [Google Scholar]
- El‐KassabyYA, Davidson R.1990. Impact of crop management practices on the seed crop genetic quality in a Douglas‐fir seed orchard. Heredity 66: 49–55. [Google Scholar]
- El‐KassabyYA, Ritland K.1986. The relation of outcrossing and contamination to reproductive phenology and supplemental mass pollination in a Douglas fir seed orchard. Silvae Genetica 35: 240–244. [Google Scholar]
- El‐KassabyYA, Fashler AM, Sziklai O.1984. Reproductive phenology and its impact on genetically improved seed production in a Douglas fir seed orchard. Silvae Genetica 33: 240–244. [Google Scholar]
- FashlerAMK, El‐Kassaby YA.1987. The effect of water spray cooling treatment on reproductive phenology in a Douglas‐fir seed orchard. Silvae Genetica 36: 254–259. [Google Scholar]
- GregorJW.1928. Pollination and seed production of the rye grass. Transactions of the Royal Society Edinburgh 55: 773–794. [Google Scholar]
- HydeHA.1952. Studies in atmospheric pollen. V. A daily census of pollen at Cardiff for the six years 1943–48. New Phytologist 51: 281–293. [Google Scholar]
- HydeHA.1953. Atmospheric pollen studies in Great Britain and their relation to the pollen analysis of post‐glacial deposits. Proceedings of the International Botanical Congress 7: 882–883. [Google Scholar]
- IngoldCT.1971. Mechanisms of liberation of spores and pollen. DFG‐Milleilung VI, Kommission zur Erforschung der Luftverunreinigung S, 7–24. [Google Scholar]
- KapylaM.1984. Diurnal variation of tree pollen in the air in Finland. Grana 23: 167–176. [Google Scholar]
- KhanduriVP.1999. The ecology of anther dehiscence, pollen productivity, release and multilocational dispersal in Pinus roxburghii Sargent. PhD Thesis, Department of Forestry, HNB Garhwal University, Srinagar Garhwal. [Google Scholar]
- KhanduriVP, Sharma CM.2000. Development of groups of male strobili, anthesis and microsporangium dehiscence in Pinus roxburghii Grana 39: 169–174. [Google Scholar]
- KhanduriVP, Sharma CM, Gaur RD.1999. Anthesis, pollen production and dispersal in Grevillea robusta Journal of the Indian Botanical Society 78: 179–180. [Google Scholar]
- KramerM.1932. De bloeiwijzen en het bloiien der weidegrassen (Dutch). Landbouwkerndig. Tijdschrift 44: 287–342. [Google Scholar]
- LiemASN, Groot J.1973. Anthesis and pollen dispersal of Holcus lanatus L. and Festuca rubra L. in relation to climate factors. Review of Palaeobotany and Palynology 15: 3–16. [Google Scholar]
- RogersCA.1993. Application of aerobiological principles in palaeoecology. Review of Palaeobotany and Palynology 79: 1113–1140. [Google Scholar]
- SarvasR.1962. Investigations on the flowering and seed crop of Pinus sylvestris Communications Instituti Forestalis Fenniae 53,54: 1–198. [Google Scholar]
- SharmaCM, Khanduri VP, Chamoli N.1998. The influence of air temperature and humidity on the anthesis and anther dehiscence in P. roxburghii at three different altitudes. Current Science 75: 761–763. [Google Scholar]
- StanleyRG, Linskens HF.1974. Pollen, biology, biochemistry, management. Berlin: Springer. [Google Scholar]
- SubbaReddiC, Reddi EUB.1984. Wind‐pollination in two tropical tree species of Euphorbiaceae. Proceeding of Indian National Science Academy B50: 66–80. [Google Scholar]
- SubbaReddiC, Reddi EUB, Bai JA, Raju KVR, Reddi MS.1980. The ecology of anther dehiscence, pollen liberation and dispersal in Xanthium strumarium L. Indian Journal of Ecology 7: 171–181. [Google Scholar]
- TormoMolinaR, Munoz Rodriguez A, Silva Palacios I, Lopez FG.1996. Pollen production in anemophilous trees. Grana 35: 38–46. [Google Scholar]
- WangCW, Perry TO, Johnson AG.1960. Pollen dispersion of slash pine (Pinus elliottii Engelm.) with special reference to seed orchard management. Silvae Genetica 6: 78–86. [Google Scholar]
- WhiteheadDR.1983. Wind pollination: Some ecological and evolutionary perspectives. In: Real L, ed. Pollination biology New York: Academic Press, 97–108. [Google Scholar]
- WrightJW.1952. Pollen dispersion of some forest trees. Northeast Forest Experiment Station, Station Paper 46. [Google Scholar]
- WrightJW.1962. Genetics of forest tree improvement. Rome: FAO. [Google Scholar]
- WrightS.1943. Isolation by distance. Genetics 28: 114–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.