Abstract
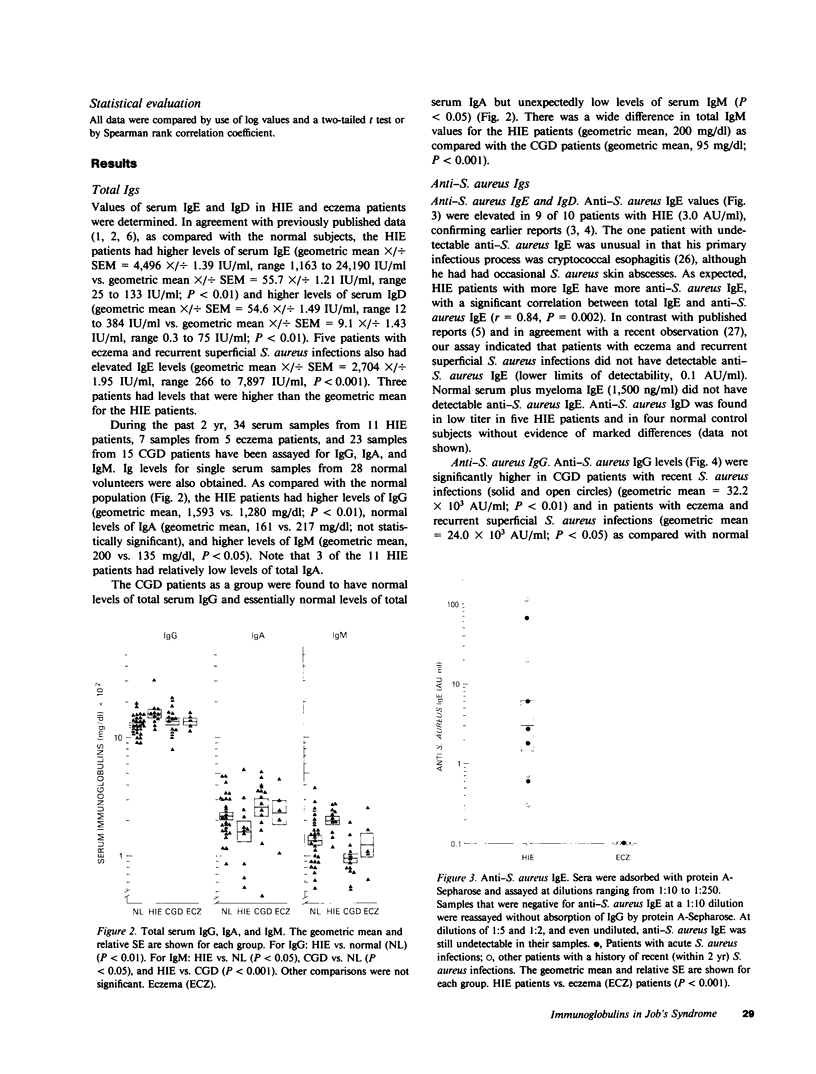
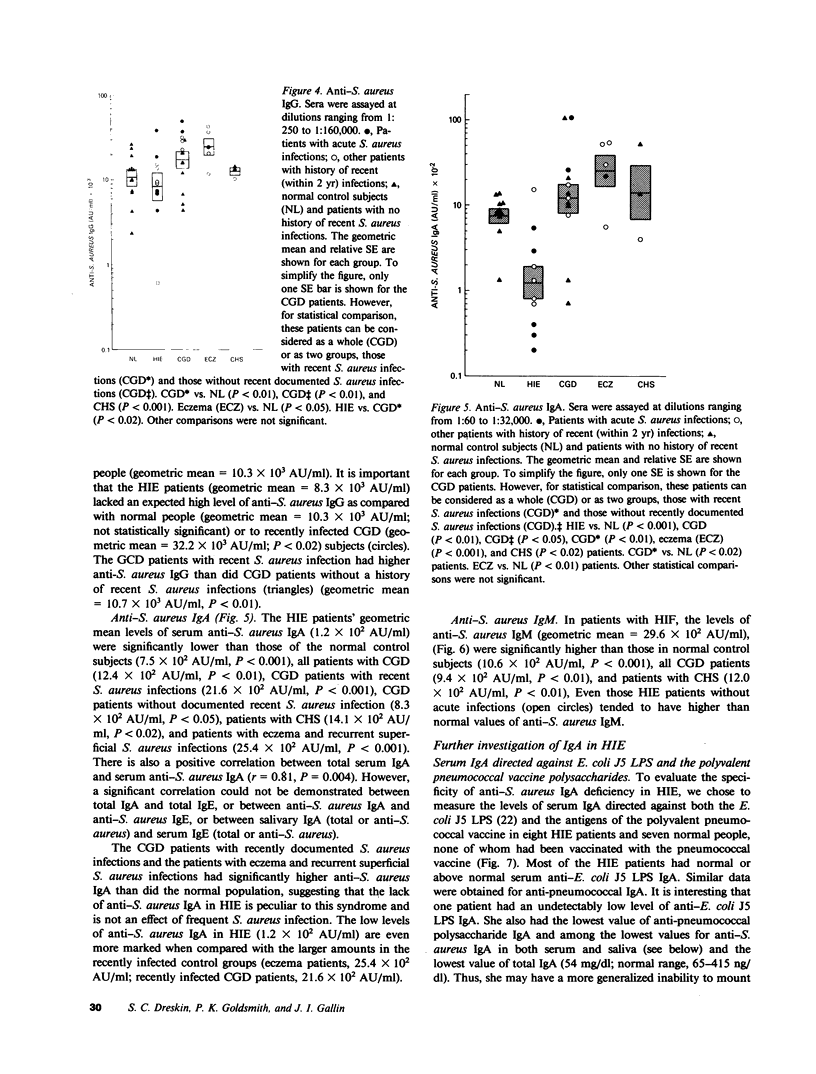
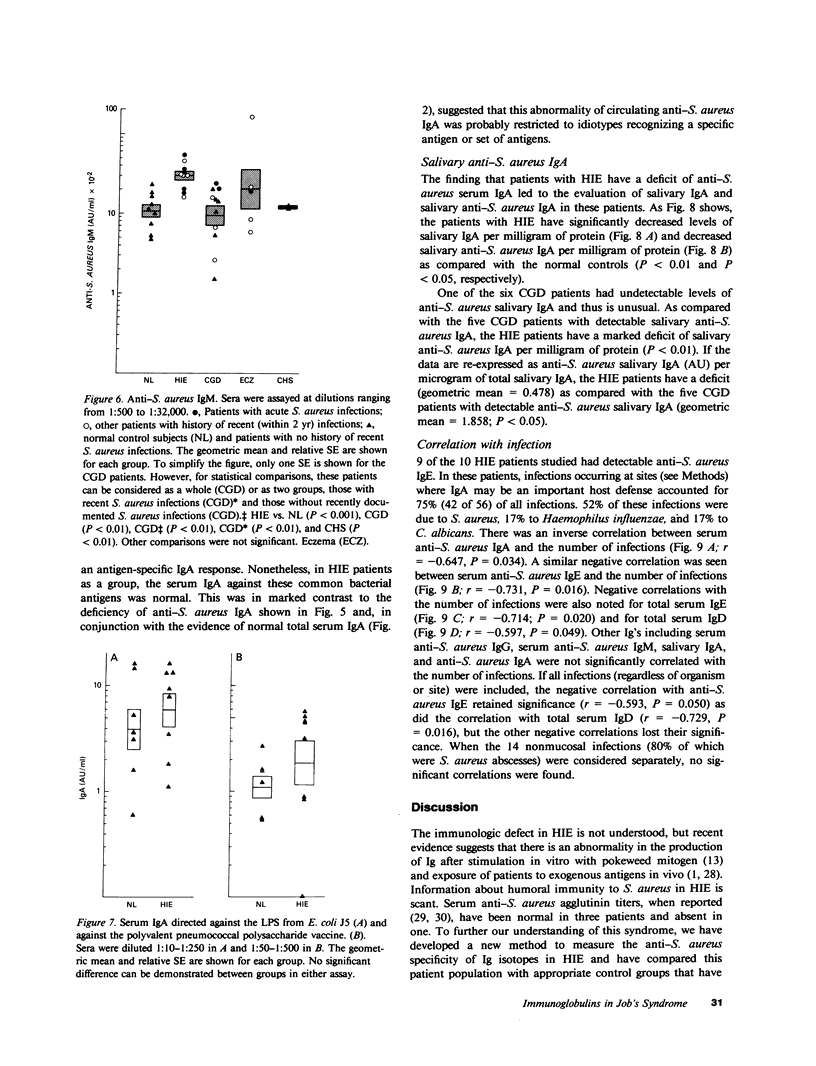
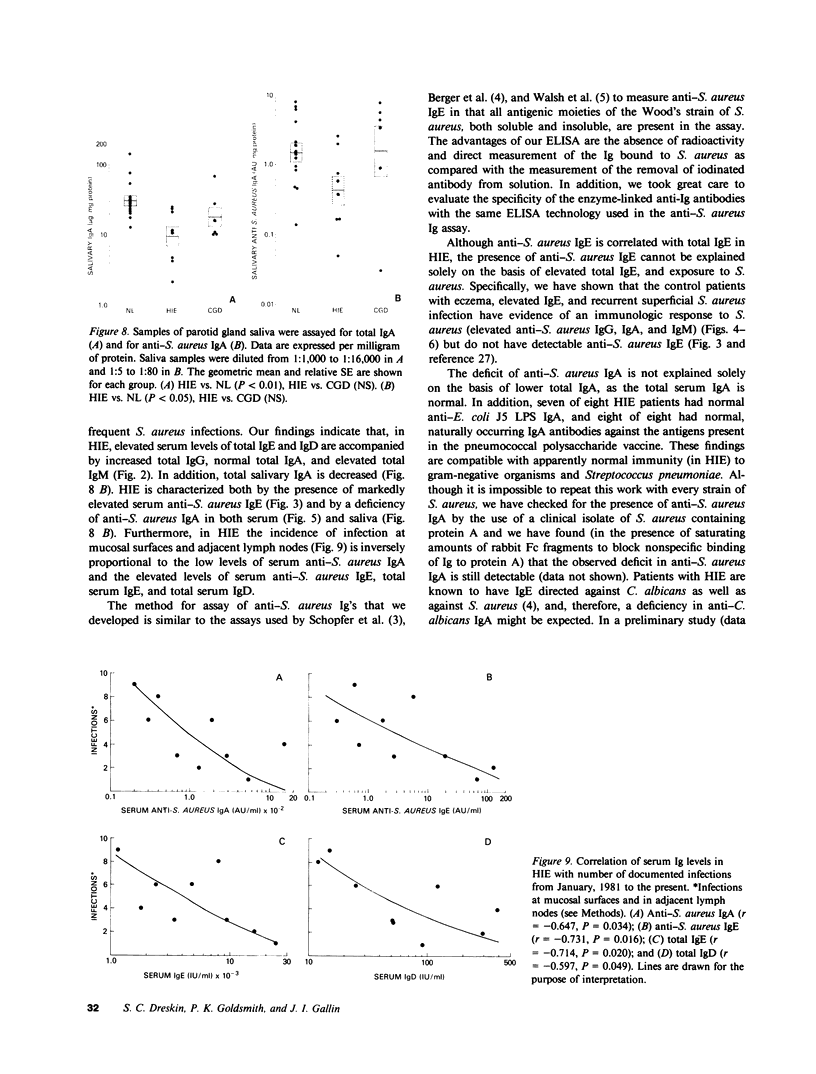
Patients with the hyperimmunoglobulin E and recurrent infection syndrome (HIE) characteristically have frequent skin and respiratory infections caused by Staphylococcus aureus. We have developed a set of enzyme-linked immunosorbent assays that use whole S. aureus (Wood's strain) immobilized on 0.22-micrometers filters and highly specific, affinity-purified enzyme conjugates of goat anti-human IgE, anti-human IgD, anti-human IgG, anti-human IgA, and anti-human IgM. These reagents were used to determine S. aureus-specific immunoglobulin (Ig) levels. As previously published, 10 patients with HIE had markedly higher levels of anti-S. aureus IgE than did 5 patients with eczema and recurrent superficial S. aureus infections (P less than 0.001). The HIE patients were also found to have a deficit of anti-S. aureus serum IgA as compared with 12 normal subjects, 12 patients with chronic granulomatous disease, 5 patients with chronic eczema and recurrent superficial S. aureus infections, and 3 patients with the Chediak-Higashi syndrome (P less than 0.01 for each comparison). In addition the HIE patients had an excess of anti-S. aureus IgM as compared with normal subjects (P less than 0.01). An expected excess of anti-S. aureus IgG was absent. These abnormalities cannot be explained by variations of total serum Ig levels or by a general inability to produce antigen-specific IgA because levels of naturally occurring IgA antibody against Escherichia coli lipopolysaccharide and the antigens of the pneumococcal vaccine are normal. Parotid saliva from patients with HIE contained less salivary IgA per milligram of protein (P less than 0.01) and less salivary anti-S. aureus IgA per milligram of protein (P less than 0.05) than did normal controls. The incidence of infection at mucosal surfaces and adjacent lymph nodes correlated inversely with serum anti-S. aureus IgA (r = -0.647, P = 0.034), serum anti-S. aureus IgE (r = -0.731, P = 0.016), total serum IgE (r = -0.714, P = 0.020), and total serum IgD (r = -0.597, P = 0.049). These findings are evidence of a previously undescribed immunoregulatory defect in patients with HIE, which may contribute to the increased susceptibility to infection in this syndrome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Befus D., Bienenstock J. Factors involved in symbiosis and host resistance at the mucosa-parasite interface. Prog Allergy. 1982;31:76–177. [PubMed] [Google Scholar]
- Berger M., Kirkpatrick C. H., Goldsmith P. K., Gallin J. I. IgE antibodies to Staphylococcus aureus and Candida albicans in patients with the syndrome of hyperimmunoglobulin E and recurrent infections. J Immunol. 1980 Dec;125(6):2437–2443. [PubMed] [Google Scholar]
- Bringel H., Vela C., Ureña V., Gurbindo D., Garcia R., Lahoz C. IgG antibodies: in vitro blocking activity of IgE mediated reactions. Clin Allergy. 1982 Jan;12(1):37–46. doi: 10.1111/j.1365-2222.1982.tb03124.x. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Wray B. B., Belmaker E. Z. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972 Jan;49(1):59–70. [PubMed] [Google Scholar]
- Capron M., Bazin H., Joseph M., Capron A. Evidence for IgE-dependent cytotoxicity by rat eosinophils. J Immunol. 1981 May;126(5):1764–1768. [PubMed] [Google Scholar]
- Clark R. A., Root R. K., Kimball H. R., Kirkpatrick C. H. Defective neutrophil chemotaxis and cellular immunity in a child with recurrent infections. Ann Intern Med. 1973 Apr;78(4):515–519. doi: 10.7326/0003-4819-78-4-515. [DOI] [PubMed] [Google Scholar]
- Davis S. D., Schaller J., Wedgwood R. J. Job's Syndrome. Recurrent, "cold", staphylococcal abscesses. Lancet. 1966 May 7;1(7445):1013–1015. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Donabedian H., Gallin J. I. Mononuclear cells from patients with the hyperimmunoglobulin E-recurrent infection syndrome produce an inhibitor of leukocyte chemotaxis. J Clin Invest. 1982 May;69(5):1155–1163. doi: 10.1172/JCI110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian H., Gallin J. I. The hyperimmunoglobulin E recurrent-infection (Job's) syndrome. A review of the NIH experience and the literature. Medicine (Baltimore) 1983 Jul;62(4):195–208. doi: 10.1097/00005792-198307000-00001. [DOI] [PubMed] [Google Scholar]
- Donabedian H., Gallin J. I. Two inhibitors of neutrophil chemotaxis are produced by hyperimmunoglobulin E recurrent infection syndrome mononuclear cells exposed to heat-killed staphylococci. Infect Immun. 1983 Jun;40(3):1030–1037. doi: 10.1128/iai.40.3.1030-1037.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Goldstine S. N., Shen L. Cytofluorographic analysis of receptors for IgA on human polymorphonuclear cells and monocytes and the correlation of receptor expression with phagocytosis. Mol Immunol. 1983 Sep;20(9):1019–1027. doi: 10.1016/0161-5890(83)90043-3. [DOI] [PubMed] [Google Scholar]
- Friedman S. J., Schroeter A. L., Homburger H. A. Whole organisms and purified cell walls compared as immunosorbents for the detection of IgE antibodies to Staphylococcus aureus. J Immunol Methods. 1984 Feb 10;66(2):369–375. doi: 10.1016/0022-1759(84)90350-8. [DOI] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Gallin J. I. Abnormal phagocyte chemotaxis: pathophysiology, clinical manifestations, and management of patients. Rev Infect Dis. 1981 Nov-Dec;3(6):1196–1220. doi: 10.1093/clinids/3.6.1196. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Buescher E. S., Seligmann B. E., Nath J., Gaither T., Katz P. NIH conference. Recent advances in chronic granulomatous disease. Ann Intern Med. 1983 Nov;99(5):657–674. doi: 10.7326/0003-4819-99-5-657. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Malech H. L., Davis J. M., Klempner M. S., Kirkpatrick C. H. Disorders of phagocyte chemotaxis. Ann Intern Med. 1980 Apr;92(4):520–538. doi: 10.7326/0003-4819-92-4-520. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Reinherz E., Leung D., McKee K. T., Jr, Schlossman S., Rosen F. S. Deficiency of suppressor T cells in the hyperimmunoglobulin E syndrome. J Clin Invest. 1981 Sep;68(3):783–791. doi: 10.1172/JCI110315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith P. K. A highly sensitive enzyme-linked immunosorbent assay for human immunoglobulin E: comparison of microtiter plate and disk methodologies. Anal Biochem. 1981 Oct;117(1):53–60. doi: 10.1016/0003-2697(81)90690-4. [DOI] [PubMed] [Google Scholar]
- Ito J. I., Jr, Wunderlich A. C., Lyons J., Davis C. E., Guiney D. G., Braude A. I. Role of magnesium in the enzyme-linked immunosorbent assay for lipopolysaccharides of rough Escherichia coli strain J5 and Neisseria gonorrhoeae. J Infect Dis. 1980 Oct;142(4):532–537. doi: 10.1093/infdis/142.4.532. [DOI] [PubMed] [Google Scholar]
- Jacobs D. H., Macher A. M., Handler R., Bennett J. E., Collen M. J., Gallin J. I. Esophageal cryptococcosis in a patient with the hyperimmunoglobulin E-recurrent infection (Job's) syndrome. Gastroenterology. 1984 Jul;87(1):201–203. [PubMed] [Google Scholar]
- Johnson R. B., Jr, Liu J. The application of enzyme immunoassay to the study of salivary IGA. J Immunoassay. 1982;3(1):73–89. doi: 10.1080/15321818208056987. [DOI] [PubMed] [Google Scholar]
- Josephs S. H., Buckley R. H. Serum IgD concentrations in normal infants, children, and adults and in patients with elevated IgE. J Pediatr. 1980 Mar;96(3 Pt 1):417–420. doi: 10.1016/s0022-3476(80)80684-6. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Fauci A. S. Activation of human B lymphocytes after immunization with pneumococcal polysaccharides. J Clin Invest. 1983 Apr;71(4):1032–1040. doi: 10.1172/JCI110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Griffiss J. M., Brandt B. L., MacDermott R. P. Antibody-dependent mononuclear cell-mediated antimeningococcal activity. Comparison of the effects of convalescent and postimmunization immunoglobulins G, M, and A. J Clin Invest. 1980 Aug;66(2):260–267. doi: 10.1172/JCI109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melewicz F. M., Zeiger R. S., Mellon M. H., O'Connor R. D., Spiegelberg H. L. Increased IgE-dependent cytotoxicity by blood mononuclear cells of allergic patients. Clin Exp Immunol. 1981 Mar;43(3):526–533. [PMC free article] [PubMed] [Google Scholar]
- Polmar S. H., Waldmann T. A., Balestra S. T., Jost M. C., Terry W. D. Immunoglobulin E in immunologic deficiency diseases. I. Relation of IgE and IgA to respiratory tract disease in isolated IgE deficiency, IgA deficiency, and ataxia telangiectasia. J Clin Invest. 1972 Feb;51(2):326–330. doi: 10.1172/JCI106817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C., Ballet J. J. Serum IgE and IgG antibodies to tetanus toxoid and candidin in immunodeficient children with the hyper-IgE syndrome. J Clin Immunol. 1983 Apr;3(2):178–183. doi: 10.1007/BF00915490. [DOI] [PubMed] [Google Scholar]
- Schopfer K., Baerlocher K., Price P., Krech U., Quie P. G., Douglas S. D. Staphylococcal IgE antibodies, hyperimmunoglobulinemia E and Staphylococcus aureus infections. N Engl J Med. 1979 Apr 12;300(15):835–838. doi: 10.1056/NEJM197904123001506. [DOI] [PubMed] [Google Scholar]
- Sternberg J. C. A rate nephelometer for measuring specific proteins by immunoprecipitin reactions. Clin Chem. 1977 Aug;23(8):1456–1464. [PubMed] [Google Scholar]
- Svanborg Edén C., Andersson B., Hagberg L., Hanson L. A., Leffler H., Magnusson G., Noori G., Dahmén J., Söderström T. Receptor analogues and anti-pili antibodies as inhibitors of bacterial attachment in vivo and in vitro. Ann N Y Acad Sci. 1983 Jun 30;409:580–592. doi: 10.1111/j.1749-6632.1983.tb26900.x. [DOI] [PubMed] [Google Scholar]
- Walsh G. A., Richards K. L., Douglas S. D., Blumenthal M. N. Immunoglobulin E anti-Staphylococcus aureus antibodies in atopic patients. J Clin Microbiol. 1981 Jun;13(6):1046–1048. doi: 10.1128/jcm.13.6.1046-1048.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]