Abstract
This population-based descriptive epidemiology study demonstrates that rates of conjoined twins, teratomas, neural tube defects, microcephaly, and microphthalmia in the Rivne province of Ukraine are among the highest in Europe. The province is 200 km distant from the Chornobyl site and its northern half, a region known as Polissia, is significantly polluted by ionizing radiation. The rates of neural tube defects, microcephaly and microphthalmia in Polissia are statistically significantly higher than in the rest of the province. A survey of at-birth head size showed that values were statistically smaller in males and females born in one Polissia county than among neonates born in the capital city. These observations provide clues for confirmatory and cause-effect prospective investigations. The strength of this study stems from a reliance on international standards prevalent in Europe and a decade-long population-based surveillance of congenital malformations in two distinct large populations. The limitations of this study, as those of other descriptive epidemiology investigations, is that identified cause-effect associations require further assessment by specific prospective investigations designed to address specific teratogenic factors.
Keywords: blastopathies, Chornobyl, congenital malformations, ionizing radiation, sex ratio
Introduction
The 1986 Chornobyl disaster in Ukraine (Chernobyl in Russian) is among the largest man-caused disasters and has impacted and continues to impact human health, ecologic integrity, and the social welfare of multiple generations of culturally and ethnically diverse large populations. Some have called the Chornobyl tragedy a “natural experiment” and as pointed out by Garruto et al. (1999) such events represent unique opportunities for studying biomedical processes, disease etiology, and pathogenesis in populations with diverse ethnic and genetic structures, living under special circumstances. This report summarizes the results of an investigation aimed at determining population-based rates and patterns of congenital malformations (CM) in the Rivne province of Ukraine during the 2000–2009 decade. Although two concurrent identical CM population-based surveillance programs are ongoing in Khmelnytsky and Volyn provinces, which adjoin Rivne, this report is focused on observations in the latter province. The term “blastopathies” appears in the title of this report to stress that the CM reported are present prior to the embryonic implantation and organogenesis, a notion elaborated upon later. To our knowledge, there are no other population-based long term investigations of CM rates and patterns relying on international methods, focusing on an area relatively proximal and heavily impacted by ionizing radiation (IR) from the 1986 Chornobyl disaster (Fig. 1). The northern half of the Rivne province is a region of forested wetlands known as Polissia, which is inhabited by a native population known as Polishchuks. Coincidentally, the fallout of Chornobyl IR impacted mostly the Rivne-Polissia zone henceforth referred to as Rivne-P or simply Polissia (note that there are also Polissia regions in Volyn, Zhytomyr and Kyiv provinces). The non-Polissia regions henceforth referred to as Rivne-nP were less impacted by Chornobyl IR. Noteworthy is that reports on the subject rarely point out contrasts between Rivne-P and Rivne-nP regions (Likhtarev et al. 1996, 2000; Zamostian et al. 2002). Polissia may be referred to as the Prypiat Marshlands or in older medical literature as Polisie, Poliesia, Polessky, and Polesie.
Figure 1.
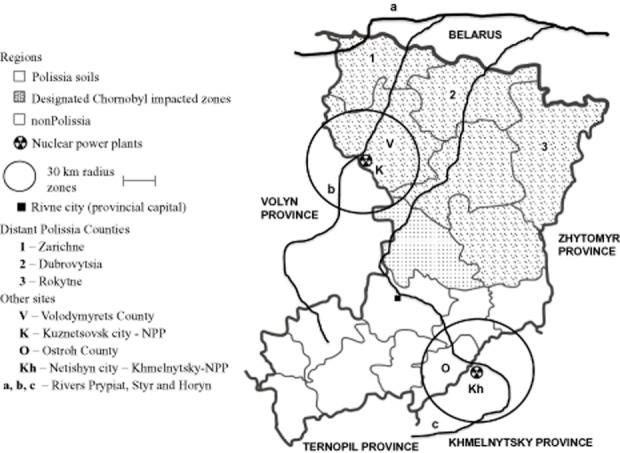
Schematic representation of Rivne province counties indicating those with soils characteristics of a region called Polissia and those counties designated as impacted by Chornobyl ionizing radiation. Also shown is the area of “distant” Polissia, the location sites of nuclear power plants and the trajectory of major rivers of the region.
In this report, unless indicated otherwise, Polissia solely refers to Rivne-P, which is described in some detail in the Data Supplement. It is sufficient to underscore here that the native people of Rivne-P are known as Polishchuks and that they represent a population isolate surviving mostly by consumption of locally grown products, foods and fuels inherently contaminated by nuclides. Polishchuks continue to inhale and ingest nuclides and among whom, a growing proportion of individuals are exposed since birth. Furthermore, a growing proportion of pregnant Polishchuk women have themselves incorporated nuclides to which all of their conceived children are exposed prenatally. The large size and well defined nature of the Polishchuk population facilitates long term studies of the health and teratogenic impacts of protracted exposures to low levels of IR.
In this report, we confirm and expand previous studies in Rivne that demonstrated elevated population-based rates of CM and include initial results of a series of surveys that may reflect impacts of IR among other causes (Yuskiv et al. 2004; Wertelecki 2010). To sustain in Rivne and two adjoining provinces (Volyn and Khmelnytsky) ongoing population-based CM monitoring systems, which uphold international standards and international partnership, we established OMNI-Net, a not for profit international organization registered in Kyiv, Ukraine (Wertelecki 2006). This report primarily concerns observations in Rivne, which are occasionally expanded by observations in the two adjoining provinces. Among the goals of the OMNI-Net is to promote and maintain international research and humanitarian partnerships intended to define causes, and to promote treatments to minimize and prevent CM. In Rivne, the OMNI-Net center is co-located with the Clinical Genetics and Prenatal Fetal Ultrasonography Services of the Provincial Diagnostic Center of the Rivne province, henceforth referred to as OMNI-Net or Diagnostic Center. Our confidence in the significance of the observations we report not only rests in the confirmation of two previous analyses but also on a concurrence of favorable circumstances in Rivne that foster and sustain the ongoing population-based CM surveillance process integrated with clinical services and public health programs.
Methods and Data Collection
In 1999, we designed and tested a birth medical report to concurrently fulfill Ministry of Health, provincial health care, and CM data collection needs. The scope and procedures of CM data collection and analysis are consistent with those upheld by the EUROCAT (European Surveillance of Congenital Anomalies) and ICBDSR (International Clearinghouse for Births Defects Surveillance and Research) consortia of which OMNI-Net became a full partner.
Following approval by Ukrainian health authorities, formal CM population-based data collection started in 2000 and is ongoing. Rivne neonatologists are mandated by health authorities to personally complete a medical birth document that is equivalent to an expanded birth certificate. This investigation is focused on the population-based frequencies of eight CM collectively referred to as core-CM (cCM). Occasionally, five of these eight cCM are referred to as a pentad-cCM composed of conjoined twins (CTW), teratomas (TER), neural tube defects (NTD), microcephalies (MIC), and microphthalmias (mOPH), or as a triad-cCM composed of omphaloceles (OM), gastroschises (GASTR), and urinary bladder exstrophies (BLEXTR), respectively. Two sentinel or index CM, cleft lip with or without concurrent cleft palate (CL/P) and Down syndrome are presented separately (Table S1d). The frequencies of other CM and malformation complexes mentioned are not population-based. Descriptions and patterns of Fetal Alcohol Spectrum Disorders (FASD) reflect collaborative investigations in partnership with investigators sponsored by the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) promoted by the National Health Institute on Alcohol Abuse and Alcoholism (NIAAA). Occasionally, observations stemming from mirror CM surveillance systems in two provinces that adjoin Rivne (Volyn and Khmelnytsky) are included. A companion Data Supplement includes a list of abbreviations, supplemental tables and figures indicated by a prefix (S), as well as individual summaries of clinical highlights, clues to which are inserted in the text between parentheses.
Definitions of cCM and other CM are those instituted by EUROCAT supplemented as needed by those found in authoritative reviews (Willis 1962; Warkany 1971; Warkany et al. 1981; Stevenson & Hall 2006). Regarding gastroschisis and other abdominal schises, criteria presented by Mastroiacovo et al. (2007) also apply. The computed NTD subcategories are encephaloceles, anencephaly, iniencephaly, cranio-rachis-schisis or anencephaly-spina bifida, and spina bifida. Spina bifida is further subcategorized as cervical, thoracic, and lumbo-sacral-coccygeal on the basis of the highest level and extent of the anomaly. These subcategories are combined into three groups, cephalad-NTD, spina bifida-NTD, and encephaloceles. The cephalad-NTD group includes anencephaly, iniencephaly and cranio-rachis-schisis. Microcephaly implies an occipital-frontal circumference of at least 3 standard deviations (SD) below the mean. When the number of observations permits, cCM are categorized as isolated and the rest as syndromic or not (Tables 1, S1c).
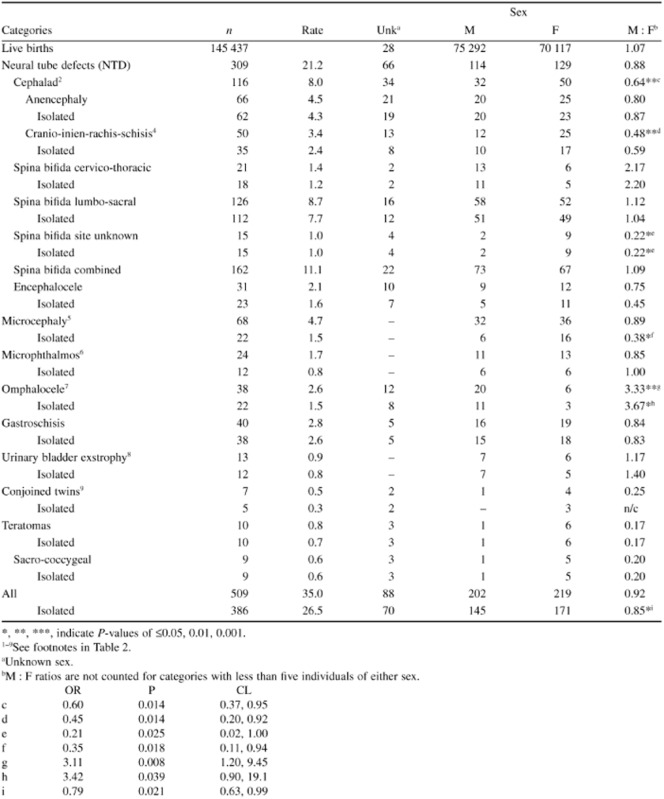
Table 1.
Population-based rates of unduplicated individuals and male-female (MF) proportions and ratios (M:F) of selected congenital malformations among live births in Rivne, Ukraine (2000–2009)1
 |
To convey the notion that conjoined twins, teratomas, NTD, OM, and BLEXTR arise prior to embryonal implantation, we may refer to these cCM as “blastopathies”. In the same spirit, those blastopathies associated with body wall defects can be referred to as “celosomias”. Regarding Down syndrome, and CL/P, these may be referred to as “sentinel” CM.
Data collection
Routine data collection is initiated by trained neonatologists who examine every liveborn and complete Part A of the birth document and Part B in the event that CM or dysmorphic signs are noted. Regarding stillbirths, obstetricians complete the birth document. Legal interruptions of pregnancies between 12th and 22nd weeks of gestation are registered separately. Part A of the birth document provides information about parents, conception, pregnancies, current gestation, birth measurements, and health status of every newborn. In Part B are recorded descriptions of anomalies in sufficient detail to fulfill reporting requirements by the Ministry of Health of Ukraine and parameters reported to EUROCAT. Training and formal interactions with neonatologists are at least twice yearly and with obstetricians quarterly in the context of reviews of prenatal fetal examinations. Reported CM or dysmorphic features are reviewed by OMNI-Net clinical geneticists and for the most part trigger tele-consultations and clinical referral of patients for further examinations. Parts A and B of the birth document are incorporated into individual clinical records. Clinical records integrate all other medical information concerning the patient, the parents, and relatives with disregard of the patient's age. Summaries of clinical records of patients with cCM are included in the Data Supplement. The monitoring of CM in Rivne is an active surveillance system. Twice yearly, children under state care in children's homes (former orphanages) are examined by OMNI-Net clinicians. Active search of CM also includes review of admissions to pediatric services, autopsies, and requests for social assistance programs related to childhood disabilities. Family histories are collected routinely from all individuals seeking clinical services from OMNI-Net clinicians. Currently, nearly 70% of pregnant women in Rivne seek prenatal clinical services at the Diagnostic Center generally between 18th–20th weeks of gestation. Virtually all assessed pregnant women agree to undergo fetal ultrasonographic examinations. Also, pregnant women are routinely screened concerning nutrition patterns and alcohol consumption and a substantial proportion agree to participate in clinical investigations sponsored by CIFASD alluded to earlier (Arenson et al. 2010; Mattson et al. 2010). Since 2008, pregnant women are urged and most agree to undergo whole body counts of Bq reflecting levels of incorporated 137Cs. The procedures adhered to are approved by the provincial health authorities and the Ethics Committee of the Lviv National Medical University.
Surveys of teratogenic risk factors
We include in this report initial results of ongoing surveys focused on three known teratogenic risk factors in Rivne: isonomy as an index of elevated rates of consanguinity among the rural isolated Polishchuk native population in Rivne-P, the patterns of alcohol consumption by pregnant women, and the incorporated levels of IR by pregnant women and ambulatory patients. Regarding consanguinity, a survey of isonomy rates of family names (surnames) assigned to all neonates was computed in every county in Rivne. Isonomy rates are computed as the aggregate frequency (percentage) of the five most common family names in each county in Rivne. Other methods are either described in this section or in footnotes of the corresponding figures and tables presenting results. Information regarding nutrition and alcohol consumption is routinely requested from pregnant women seeking medical services from the Rivne Diagnostic Center. In addition, all patients are routinely asked to voluntarily participate in an expanded survey of nutritional habits, sources of inhalation of wood smoke or dust, and consumption of alcohol as well as to voluntarily undergo whole body counts of their incorporated 137Cs levels. These recordings are obtained by use of a single officially-calibrated device operated by the Rivne Diagnostic Center staff. An additional survey determined incorporated 137Cs and 90Sr by potato plants grown in P.
Case-by-case review, categorization and computation of rates
After a review by at least two clinical geneticists, an individual with a CM is included in the analyses. Singletons and non-singletons, live born or stillborn (fetal death after the 22nd week of gestation) and instances of interruption of pregnancy are included in the analyses, both by OMNI-Net and EUROCAT. Rivne cCM population-based rates are computed in dual terms; as unduplicated individuals or u-rates or as overall rates or t-rates. Implicitly and as shown in Tables 1, 2, and S1 a,b, u-rates may be lower than corresponding t-rates in function of individuals with multiple cCM (Tables 5,10,11, S2). EUROCAT and most of the literature on the subject report t-rates. Comparisons of Rivne with EUROCAT t-rates are calculated on the basis of 10 000 births. Frequencies within Rivne are in u-rates and t-rates computed on the basis of 10 000 live births. To compute u-rates, an individual with multiple core-CM is represented in the first applicable cCM category in a priority or hierarchical sequence (NTD, MIC, mOPH, OM, GSTR, BLEXTR, CTW and TER). All individuals with NTD are represented only in this category regardless of any other associated CM. The group of individuals with MIC excludes those with NTD and the group of those with mOPH excludes individuals who have either NTD or microcephaly, and so on. Male-Female proportions (M-F) or ratios (M : F) are presented when there are at least five known individuals of either sex.
Table 2.
Population-based unduplicated rates per 10 000 live births of individuals with selected congenital malformations in Rivne, Ukraine1
| Categories | Polissia | Non-Polissia | Polissia | Non-Polissia | Polissia vs. non-Polissia | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2000–2004 | 2005–2009 | 2000–2004 | 2005–2009 | 2000–2009 | 2000–2009 | OR | P-value | CL | |
| Neural tube defects (NTD) | 29.1 | 23.5 | 18.4 | 14.8 | 26.1 | 16.4 | 1.59 | *** | 1.26, 2.02 |
| Cephalad2 | 9.5 | 10.1 | 7.8 | 4.8 | 9.8 | 6.2 | 1.59 | ** | 1.08, 2.37 |
| Anencephaly | 6.2 | 4.1 | 5.1 | 3.0 | 5.1 | 4.0 | – | n/s | – |
| Isolated | 5.6 | 3.9 | 4.8 | 3.0 | 4.7 | 3.8 | – | n/s | – |
| Non-NTD malformations3 | n/c | n/c | n/c | – | n/c | n/c | – | n/c | – |
| Cranio-inien-rachis-schisis4 | 3.3 | 5.9 | 2.7 | 1.8 | 4.7 | 2.2 | 2.15 | ** | 1.15, 4.16 |
| Isolated | 2.1 | 4.1 | 1.8 | 1.5 | 3.2 | 1.6 | 1.94 | * | 0.92, 4.27 |
| Other malformations | n/c | 1.8 | n/c | n/c | 1.5 | n/c | – | n/s | – |
| Spina bifida cervico-thoracic | 3.3 | 1.6 | – | n/c | 2.3 | n/c | 4.29 | ** | 1.40, 17.5 |
| Isolated | 2.4 | 1.6 | – | n/c | 1.9 | n/c | 3.53 | * | 1.11, 14.7 |
| Other malformations | n/c | – | – | – | n/c | – | – | n/c | – |
| Spina bifida lumbo-sacral | 10.1 | 10.3 | 7.2 | 7.0 | 10.2 | 7.1 | 1.44 | * | 0.99, 2.09 |
| Isolated | 8.9 | 9.3 | 6.0 | 6.5 | 9.1 | 6.3 | 1.45 | * | 0.98, 2.16 |
| Syndromes | n/c | – | – | – | n/c | – | – | n/c | – |
| Other malformations | n/c | n/c | n/c | n/c | 0.97 | 0.8 | – | n/s | – |
| Spina bifida site unknown | 3.3 | – | n/c | – | 1.5 | n/c | – | n/s | – |
| Isolated | 3.3 | – | n/c | – | 1.5 | n/c | – | n/s | – |
| Spina bifida combined | 16.6 | 11.9 | 8.4 | 8.0 | 14.1 | 8.2 | 1.71 | *** | 1.24, 2.40 |
| Encephalocele | 3.0 | 1.6 | 2.1 | 2.0 | 2.2 | 2.1 | – | n/s | – |
| Isolated | 2.1 | 1.3 | 1.5 | 1.5 | 1.7 | 1.5 | – | n/s | – |
| Syndromes | n/c | n/c | – | – | n/c | n/c | – | n/c | – |
| Other malformations | n/c | – | n/c | n/c | n/c | n/c | – | n/c | – |
| Microcephaly5 | 5.3 | 6.7 | 3.3 | 3.3 | 6.1 | 3.3 | 1.85 | ** | 1.10, 3.18 |
| Isolated | 2.7 | 1.3 | n/c | 1.3 | 1.9 | 1.1 | – | n/s | – |
| Syndromes | n/c | 2.8 | 2.1 | 1.8 | 2.1 | 1.9 | – | n/s | – |
| Other malformations | 1.5 | 2.6 | n/c | n/c | 2.1 | n/c | 7.57 | *** | 1.76, 68.2 |
| Microphthalmos6 | 1.8 | 3.1 | n/c | n/c | 2.5 | 0.8 | 3.03 | * | 1.15, 9.32 |
| Isolated | n/c | 1.6 | – | n/c | 1.1 | n/c | – | n/s | – |
| Syndromes | – | n/c | n/c | – | n/c | n/c | – | n/c | – |
| Other malformations | n/c | n/c | n/c | – | 1.0 | n/c | 7.07 | * | 0.91, 318 |
| Omphalocele7 | 1.8 | 1.8 | 2.4 | 4.3 | 1.8 | 3.4 | 0.52 | (*) | 0.25, 1.07 |
| Isolated | n/c | n/c | 1.8 | 2.8 | 0.7 | 2.3 | 0.30 | (**) | 0.09, 0.84 |
| Syndromes | n/c | n/c | n/c | – | n/c | n/c | – | n/c | – |
| Other malformations | n/c | n/c | n/c | 1.5 | 0.8 | 1.0 | – | n/s | – |
| Gastroschisis | n/c | 3.4 | 3.3 | 3.0 | 2.3 | 3.1 | – | n/s | – |
| Isolated | n/c | 3.4 | 3.0 | 3.0 | 2.2 | 3.0 | – | n/s | – |
| Other malformations | n/c | – | n/c | – | n/c | n/c | – | n/c | – |
| Urinary bladder exstrophy8 | n/c | n/c | n/c | n/c | 1.1 | 0.7 | – | n/s | – |
| Isolated | n/c | n/c | n/c | n/c | 1.1 | n/c | – | n/s | – |
| Other malformations | – | – | n/c | – | – | n/c | – | n/c | – |
| Conjoined twins9 | n/c | – | n/c | n/c | n/c | 0.7 | – | n/s | – |
| Isolated | n/c | – | n/c | n/c | n/c | n/c | – | n/c | – |
| Other malformations | – | – | n/c | n/c | – | n/c | – | n/c | – |
| Teratomas | 1.5 | n/c | n/c | n/c | 0.8 | n/c | – | n/s | – |
| Isolated | 1.5 | n/c | n/c | n/c | 0.8 | n/c | – | n/s | – |
| Sacro-coccygeal | 1.5 | n/c | n/c | n/c | 0.8 | n/c | – | n/s | – |
| Isolated | 1.5 | n/c | n/c | n/c | 0.8 | n/c | – | n/s | – |
| All | 42.4 | 39.8 | 29.8 | 28.4 | 41.0 | 29.0 | 1.42 | *** | 1.18, 1.70 |
| Isolated | 32.6 | 28.2 | 22.3 | 23.3 | 30.3 | 22.9 | 1.33 | ** | 1.08, 1.63 |
| Syndromes | 2.4 | 4.1 | 2.7 | 1.8 | 3.3 | 2.2 | – | n/s | – |
| Other malformations | 7.4 | 7.5 | 4.8 | 3.3 | 7.5 | 4.0 | 1.88 | ** | 1.18, 3.06 |
Actual counts of unduplicated individuals are found in Table S1b. All births in Rivne are 145 437, in Polissia, 72 379, and in non-Polissia, 73 058 (observations in non-Polissia are in italics). Individuals with congenital malformations (CM) are represented only in one category and include those liveborn or not and singletons or not; individuals with holoprosencephaly are excluded from the Tables 2, and S1a,b,c series; population rates of CM detected up to one year of age are calculated per 10 000 live births. One-tailed P-values are calculated with 95% confidence limits for categories with at least five individuals represented or alternatively are shown as not computed (n/c). P-values are denoted by
***, ≤0.001; **, ≤0.01; *, ≤0.05, or n/s for non-significant. P-values in parentheses indicate a negative association. Odds ratio (OR) and confidence limits (CL) are also shown.
Cephalad includes iniencephaly, anencephaly (or acrania) associated or not with rachis-schisis or spina bifida. Encephaloceles are computed separately.
Microphthalmia (c-1); cleft lip (c-2); omphalocele (c-3); esophageal atresia (d-1).
Includes iniencephaly and anencephaly-rachis-schisis.
Excluded are two singletons and one twin with holoprosencephaly (hol-6, 20; tw-1); also excluded is an individual incompletely described (z-2) diagnosed at 20 weeks of gestation with a holoprosencephaly spectrum and a single orbit, who is represented among conjoined twins.Included in this category as isolated microcephaly are two individuals who had microcephaly and microphthalmia not associated with extra-ocular anomalies (ri-8, 12).
Excluded is one individual represented among anencephalics (c-1); four individuals with microcephaly (ri-8, 12; t-8; s-2); and one individual (z-2), who is represented among conjoined twins and further described in footnote (5).
Excluded is one individual represented among anencephalics (c-3); seven individuals with cranio-rachis-schises (f-2, 4–8; g-4); six individuals with spina bifida (h-1, 3; l-5, m-2, 5, 6); two individuals with microcephaly (t-9, u-2); and two individuals with microphthalmia (v-1; w-1).
Excluded is one individual with spina bifida (h-3).
Excluded is one member of a set of conjoined twins (h-1) with spina bifida and represented among other individuals with spina bifida.
Table 5.
All individuals with microcephaly and associated anomalies in Rivne Province, Ukraine (2000–2009)a
| Categorya | Polissia | Non-Polissia | Rivne Province | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Rate* | Sex | All | Rate | Sex | All | Rate | M : F | |||
| M | F | M | F | ||||||||
| MIC (all individuals)b | 46 | 6.4 | 22 | 24 | 25 | 3.4 | 13 | 12 | 71 | 4.9 | 0.97 |
| MIC (no HOLOP, no mOPH)c | 26 | 3.6 | 10 | 16 | 10 | 1.4 | 4 | 6 | 36 | 2.5 | 0.64 |
| Isolated | 12 | 1.7 | 3 | 9 | 8 | 1.1 | 3 | 5 | 20 | 1.4 | 0.43 |
| MIC and HOLOP (no mOPH)c | 1 | n/c | 1 | 1 | n/c | 1 | 2 | n/c | n/c | ||
| Isolated | 1 | n/c | 1 | 1 | n/c | n/c | |||||
| MIC and mOPH (no HOLOP)c | 3 | n/c | 3 | 3 | n/c | n/c | |||||
| Isolated | 2 | n/c | 2 | 2 | n/c | n/c | |||||
| MIC and mOPH and HOLOPc | 1 | n/c | 1 | 1 | n/c | n/c | |||||
| Syndromic MIC | 15 | 2.1 | 10 | 5 | 14 | 1.9 | 8 | 6 | 29 | 2.0 | 1.64 |
| Patau Syndrome | 1 | n/c | 1 | 1 | n/c | n/c | |||||
| FASDd | 9 | 1.2 | 7 | 2 | 11 | 1.5 | 6 | 5 | 20 | 1.4 | 1.86 |
| mOPH (no MIC) (all individuals) | 22 | 3.0 | 10 | 12 | 8 | 1.1 | 4 | 4 | 30 | 2.1 | 0.88 |
| mOPH (no HOLOP)c | 16 | 2.2 | 7 | 9 | 5 | 0.7 | 2 | 3 | 21 | 1.4 | 0.75 |
| Isolated | 8 | 1.1 | 5 | 3 | 4 | n/c | 1 | 3 | 12 | 0.8 | 1.00 |
| mOPH and HOLOPc | 2 | n/c | 1 | 1 | 2 | n/c | n/c | ||||
| Isolated | 1 | n/c | 1 | 1 | n/c | n/c | |||||
| Syndromic mOPH | 6 | 0.8 | 3 | 3 | 1 | n/c | 1 | 7 | 0.5 | n/c | |
| mOPH and Patau Syndromee | 5 | 0.7 | 3 | 2 | 1 | n/c | 1 | 6 | 0.4 | n/c | |
Statistically significantly higher t-rates in Polissia are noted among all MIC individuals (P-value 0.008; OR 1.86; CL 1.12, 3.16); among MIC excluding HOLOP and mOPH (P-value 0.005; OR 2.63; CL 1.23, 6.10); among mOPH excluding MIC (P-value 0.008; OR 2.78; CL 1.19, 7.21); among mOPH excluding HOLOP (P-value 0.01; OR 3.23; CL 1.13, 11.3); and nearly significantly higher rates among syndromic mOPH (P-value 0.06; OR 6.06; CL 0.73, 278.6).
Individuals may be represented in various categories. Unduplicated individuals are presented in Table 2 and further information is given in the Data Supplement.
Includes 4 individuals with mOPH.
Excludes associated syndromic malformations.
Includes one individual (s-2) with concurrent MIC-FASD-mOPH.
Includes two individuals with mOPH-Patau-HOLOP combination (hol-22, 23).
F, female; FASD, fetal alcohol spectrum disorder; HOLOP, holoprosencephaly; M, male; M : F, male : female ratio; MIC, microcephaly; mOPH, microphthalmia; n/c, not computed.
Statistical comparisons are included in various tables particularly for their descriptive values. We rely on the one-tailed Fisher's exact test, Cochran-Mantel-Haenszel and Breslow-Day tests and for head circumference data analysis on methods as described by Wang and Wertelecki (2013). We chose a significance level of 0.05 and test an alternative hypothesis that the risk is higher in Rivne-P than in Rivne-nP. The P-values and 95% confidence intervals are computed using SAS 9.2 software (http://www.sas.com/). Generally, the CM rates observed in Rivne are compared with rates from elsewhere reported to EUROCAT, ICBDSR and rates based on analyses of MACDP data (Metropolitan Atlanta Congenital Defects Programs of the Centers for Disease Control and Prevention) or population-based studies. Regarding health effects of exposure to low levels of IR, the core reference source is the BEIR V report (1990).
Results
Among 145 437 live births in Rivne between 2000 and 2009 are included 2348 (1.61%) infants with anomalies noted before one year of age. This analysis concerns eight congenital malformations henceforth referred to as a group of cCM that includes conjoined twins, teratoma, NTD, microcephaly, mOPH, OM, gastroschisis, and exstrophy of the bladder. In the Tables 2 are shown population-based rates of unduplicated individuals (u-rates) of cCM and male-female proportions (M-F) as well as ratios (M : F) and the actual numbers of observed individuals are shown in Tables S1b and S2 and other companion tables.
The overall M : F ratio in Rivne is 1.07 and in Rivne-P and Rivne-nP is 1.08 and 1.07 respectively (Tables 1 and S2). The relative frequencies of mothers in Rivne-P and Rivne-nP who are under 20 years of age are 8.98% to 9.92%, respectively, and of mothers who are at least 35 years old are 8.87% to 6.75%, respectively. It is beyond the scope of this report to address other demographic characteristics in Rivne-P or Rivne-nP.
In Rivne, large proportions of pregnancies associated with cCM are detected prenatally and are medically interrupted. In Polissia, during the first and second 5-year study periods, 69% and 88% of NTD were detected prenatally, respectively. In nP the percent was 82–97%. Many of these NTD related pregnancies were medically interrupted; in Polissia, during the first and second 5-year periods, 47% and 55% respectively and in Rivne-nP the percent was 59–75%, respectively (Data Supplement). The above described temporal and Rivne-P-nP contrasts reflect, in our view, a gradual introduction of upgrades of technical resources combined with cognitive enhancements, particularly in the sphere of fetal ultrasonography. These trends spread from the capital city where OMNI-Net is located toward the peripheries such as the most distant northern counties in the Polissia region. The frequency of NTD-related pregnancy terminations across Europe compared with Polissia and Rivne-nP are summarized in Table 3 and in Table S5, which also include observations concerning OM and data from most partners of EUROCAT. The frequency of terminations of OM-related pregnancies is consistently lower than terminations of NTD-related pregnancies. The terminations of NTD-related pregnancies in Rivne-P are the lowest reported to EUROCAT. These comparisons sustain our view that higher rates of prenatal detection are not a basis for the pentad-cCM rates in Polissia to be among the highest in Europe.
Table 3.
Highest population ratesa in Europe (2005–2009)b of four congenital malformations (including percent of NTD-impacted terminated pregnancies)c compared to rates in Polissia and non-Polissia regions of Rivne Province, Ukraine (2000–2009)
| Neural tube defects | Microcephaly | Microphthalmia | Conjoined twins |
|---|---|---|---|
| 25.96 (51)c Polissia | 6.35 Polissia | 3.57 Polissia | 0.55 Rivne |
| 16.33 (68) non-Polissia | 5.35 Wales | 1.63 Wales | 0.49 North England |
| 14.47 (81) N. England | 5.03 South-West England | 1.51 Dublin | 0.35 Wales and Wessex |
| 13.60 (84) Wales | 4.52 Valencia | 1.22 N Netherlands and non-Polissia | 0.31 E. Midlands and South Yorkshire |
| 12.77 (87) Paris | 3.88 Basque C. (Spain) | 1.21 South-West England | 0.26 Wiekopolska |
Rate per 10 000 births of congenital malformations (not individuals) inclusive of live births, fetal deaths of 20 or more weeks of gestation and termination of pregnancies.
Rates reported by full member registries located in Europe who reported at least 30 000 births during the 2006–2008 and at least 30 instances of neural tube defects (NTD). These criteria were met by the 19 registries shown. Excluded were registries from Zagreb (Croatia), Odense (Denmark), Strasbourg (France), Mainz (Germany), Cork and Kerry (Ireland), SE Ireland, Malta, Barcelona (Spain), Vaud (Switzerland); South Portugal. Excluded are registries from Saxony-Anhalt (Germany) and Styria (Austria) whose data are under review. Ukraine is excluded and instead, rates from Rivne province in Ukraine subdivided as Polissia and non-Polissia regions are presented. The rates are calculated adhering to EUROCAT methods. Eurocat data are accessible on the web-site. Note: EUROCAT occasionally introduces data updates. The data shown above was accessed on 29 November 2013.
Percent of pregnancy terminations.
Concerning other temporal contrasts of CM frequencies in Rivne-P and Rivne-nP, while the overall frequencies of cCM, NTD, and spina bifida are statistically significantly higher in P during both 5-year study periods, the frequencies of cranio-inien-rachis-schisis, MIC, and mOPH are statistically significantly higher only during the second 5-year study period. However, it is also evident that the frequencies of all of these cCM are higher in P during the first and second study periods. This fact, in our view, is biologically significant although in some instances such contrasts do not reach statistical significance, which is at least in part due to a limited number of observations (Table S1a,b).
NTD
In Rivne, there are 309 individuals with NTD, of whom 31 (10%) have an encephalocele (Table 1 and S1b). The prevalence of encephaloceles is similar in Polissia and Rivne-nP. The overall M-F proportion in Rivne among non-syndromic encephaloceles is 7–12 (Table S9). To provide sufficient data to compute subcategories of encephaloceles, an analysis expanded to include encephaloceles observed in Rivne and two adjoining provinces where the t-rates are 2.13 and 1.54, respectively. Among 63 non-syndromic encephaloceles, 41 (65%) were occipital and the M-F proportion was 10–18 (Table S9).
In Rivne, and in contrast to encephaloceles, NTD subcategories are statistically significantly more frequent in Polissia. The overall M : F ratio in Rivne is 1.07 compared to 0.64 among those with cephalad-NTD and 1.09 among those with spina bifida, respectively. Another NTD preferential association is with OM noted in 4.5% of individuals or conversely in 25% of individuals with OM. An analysis of this association noted in Rivne and the two adjoining provinces demonstrates that it is the strongest among instances of cephalad-NTD (Table S7). The association was noted in 1.8%, 29%, 9%, 3.2% and 2.3% among 223, 17, 85, 62 and 280 instances of anencephaly, iniencephaly, cranio-rachis-schisis, “high” spina bifida (above the first lumbar vertebra), and lumbo-sacral spina bifida, respectively. In Rivne, a female prevalence was evident among the cephalad-NTD-OM and absent among those with spina bifida-OM associations where the M-F proportions were 2-9 and 3-2 respectively.
The 14 instances of NTD-OM preferential associations or dyads is indicative of a significantly higher risk for individuals to have this association (P < 0.001, odds ratio in Rivne-P is 93.12 and the 95% confidence limits are from 23.28 to 273.05; in Rivne-nP, the odds ratio is 268.81 and the 95% confidence limits are from 106.24 to 648.18). The Cochran-Mantel-Haenzel and Breslow-Day tests show that the strength of the NTD-OM association is similar in Polissia and Rivne-nP (P-value = 0.1157), and that the overall risks are still very significant (P-value <0.0001; OR = 174.95; 95% CL, 95.59 to 325.79). Another NTD preferential association is with twinning, which was noted in eight individuals or 2.6% of NTD instances (Table S4). A tendency to engender twinning events is also evident among relatives of NTD patients, in particular if maternal (Fig. 3).
Figure 3.

Twin individuals with neural tube defects (NTD) and twinning events among their relatives (2000–2009).
Microcephaly u-rates and t-rates are summarized in Tables 5 and S1b,2. The Rivne u-rate of 4.7 reflects the ascertainment of 68 unique individuals with microcephaly inclusive of instances of concurrent mOPH and exclusive of instances associated with holoprosencephaly or NTD. The u-rates in Polissia and Rivne-nP are 6.1 and 3.3, respectively, which represents a statistically significant contrast (Table 2 above). Among individuals with microcephaly, female prevalence is most evident in instances of isolated microcephaly where the M-F proportion is 6–16 (Table 1). In contrast, there is no female prevalence among those with syndromic microcephaly including instances of FASD or Patau or trisomy 13 syndromes. There are 30 individuals with mOPH who are not concurrently microcephalic, 12 of whom have no associated CM. Among these 30 individuals, 22 and eight are from Polissia and Rivne-nP, respectively, which represents a statistically significant contrast. Concerning sex prevalence, the observed M-F proportions are equivocal. In Table 4, syndromes and CM complexes often associated with microcephaly and/or mOPH are shown.
Table 4.
Individualsa with prevalent malformation syndromes associated with microcephaly in Rivne Province (2000–2009)
| Categorya | Polissia | Non-Polissia | Rivne Province | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratea | All | Sex | Rate | All | Sex | Rate | All | Sex | ||||
| M | F | M | F | M | F | |||||||
| FASDb | 5.1 | 37 | 19 | 18 | 5.7 | 42 | 25 | 17 | 5.4 | 79 | 44 | 35 |
| Included in alternative categories | 1.4 | 10 | 7 | 3 | 1.5 | 11 | 6 | 5 | 1.4 | 21 | 13 | 8 |
| among Microcephaly | 1.2 | 9 | 7 | 2 | 1.5 | 11 | 6 | 5 | 1.4 | 20 | 13 | 7 |
| PATAU SYNDROME/TRISOMY 13 | n/c | 9 | 5 | 4 | n/c | 11 | 8 | 2 | n/c | 20 | 13 | 6 |
| Included in alternative categories | n/c | 8 | 5 | 3 | n/c | 9 | 6 | 2 | n/c | 17 | 11 | 5 |
| Holoprosencephaly | n/c | 5 | 3 | 2 | n/c | 6 | 4 | 1 | n/c | 11 | 7 | 3 |
| Microphthalmia | n/c | 2 | 1 | 1 | n/c | 1 | 1 | n/c | 3 | 2 | 1 | |
| Omphalocele | n/c | 1 | 1 | n/c | 1 | 1 | n/c | 2 | 1 | 1 | ||
| Microcephaly | n/c | 1 | 1 | n/c | 1 | 1 | ||||||
| HOLOPROSENCEPHALYc | n/c | 13 | 8 | 5 | n/c | 23 | 12 | 7 | n/c | 36 | 20 | 12 |
| Associations | ||||||||||||
| and Microcephalyd | n/c | 2 | 2 | n/c | 1 | 1 | n/c | 3 | 3 | |||
| and Microphthalmiae | n/c | 3 | 2 | 1 | n/c | 2 | 1 | 1 | n/c | 5 | 3 | 2 |
| and Teratomaf | n/c | 1 | 1 | n/c | 1 | 1 | ||||||
In contrast to Table 2 and its derivatives where individuals are only represented once, in this table the rate is calculated as total impacted individuals who may be represented in several malformation categories (shown in capital letters). Individuals represented in sub-categories are mutually exclusive. Also note an alternative presentation of similar data in Table 5 and clinical summaries of all individuals contained in the Data Supplement. M indicates males and F indicates females.
FASD, Fetal Alcohol Spectrum Disorders.
Includes Patau syndrome (see above).
Includes one individual (hol-6) with holoprosencephaly-microcephaly-microphthalmos association.
Excludes above individual (hol-6).
Included one individual with cervico-faringeal teratoblastoma (neo-1).
n/c, not computed.
In Table 5, we present the instances and t-rates of microcephaly and/or mOPH associated with holoprosencephaly, FASD and Patau-trisomy 13 complexes.
Among 71 individuals with microcephaly, 28% represent instances of FASD and 1.4% represent Patau syndrome. From a perspective of FASD, 75% of individuals did not meet the strict definition of microcephaly adopted for this investigation (3 SD below norm). However, these individuals may have lesser degrees of microcephaly or reductions of head circumferences. Among 36 individuals with holoprosencephaly, 22% had concurrent microcephaly, mOPH or both and furthermore, nearly 50% of individuals with Patau syndrome had concurrent holoprosencephaly. These observations are among the reasons for the exclusion of holoprosencephaly from computations of microcephaly u-rates reported in Tables 2. In summary, instances of isolated microcephaly represent 32% and instances associated with non-syndromic CM represent another 25% (Table S1b and Data Supplement).
Regarding alcohol teratogenesis, a survey of alcohol consumption by pregnant women demonstrated, as summarized in Table 6, that alcohol consumption during pregnancy was least prevalent in Polissia and most prevalent in the eponymous capital cities of Rivne and Khmelnytsky. Furthermore, as shown in Table S3 the frequency of instances of FASD is higher in Rivne-nP than in Polissia.
Table 6.
Alcohol consumption by pregnant women (%)
| Area of Residence | Women | AE1 | OR | P | CL |
|---|---|---|---|---|---|
| Polissia | 852 | 13 (1.53) | – | – | – |
| Non-Polissia | 1417 | 67 (4.73) | 0.31 | <0.001 | 0.16, 0.58 |
| Rivne City | 566 | 36 (6.36) | 0.23 | <0.001 | 0.11, 0.45 |
| Khmelnytsky City | 1062 | 47 (4.43) | 0.33 | <0.001 | 0.17, 0.63 |
Data from Rivne (2009–2010) and Khmelnytsky (2010–2011).
“Alcohol Exposed” implies occasional consumption prior or during pregnancy of at least ≥5 standard drinks (sd), three times, or 3–4 sd, four times, or 1–2 sd, ≥10 times or alternatively almost daily consumption of small amounts or alternatively, a positive answer to at least two questions that follow: “in the past year”.
“Has a friend or family member ever told you about things you said or did while drinking that you could not remember?”.
“Have close friends or relatives worried or complained about your drinking?”.
“Have you had a drink first thing in the morning to steady your nerves or to get rid of a hangover?”.
“Have you felt you ought to cut down on your drinking?”.
“Have people annoyed you by criticizing your drinking?”.
“Have you felt bad or guilty about your drinking?” (adapted from Kfir M, Yevtushok L, Onishchenko S et al. in Ultrasound ObstetGynecol 2009;33:683–689; Bakhireva L, Wilsnack S, Kristjanson A et al. in J Stud Alcohol Drugs 2011;72(4):536–544).
Microcephaly may be caused by autosomal recessive and other genetic mutations as well as by teratogenic impacts such as from IR or alcohol, among other environmental insults. In anticipation of prospective investigations oriented to determine the pathogenesis and etiology of microcephaly and other cCM, there are several ongoing surveys in Rivne. Concerning genetic mutations, initial results of a survey of isonomy rates of family surnames assigned to all neonates in every county in Rivne are summarized in Figure S1 where the highest rates are evident in the most northern counties of Polissia.
Concerning IR, an analysis of whole body counts of incorporated 137Cs obtained from 6026 pregnant women shows that the highest levels are found among those who reside in the same three northernmost counties of Rivne-P with the highest isonomy rates. The actual IR levels incorporated by pregnant women, by site of residence, are illustrated in Table 7 and in Figure S2 of the Data Supplement. In terms of official IR protection standards, 48% of pregnant women residing in northern counties of Polissia incorporated 137Cs above the maximum permissible level. The analysis of 12 327 and 6706 recordings obtained from ambulatory pediatric and adult male patients demonstrates that 12% and 6%, respectively, incorporated 137Cs above official norms.
Table 7.
Whole body counts of incorporated ionizing radiation in Rivne diagnostic center's ambulatory outpatients
| Distant Polissiaa | Non-Distant Polissiab | Non- Polissiac | |
|---|---|---|---|
| Pregnant Womend | 1156 | 2534 | 2336 |
| Above Bq norme (%) | 557 (48.2) | 155 (6.1) | 3 (0.1) |
| Childrenf | 1338 | 3671 | 1697 |
| Above Bq norm (%) | 162 (12.1) | 50 (1.4) | 1 (0.1) |
| Adult Malesf | 2117 | 5885 | 4325 |
| Above Bq norm (%) | 136 (6.4) | 22 (0.4) | – |
Includes Zarichne, Dubrovytsia, and Rokytne counties.
Includes Volodymyrets, Sarny, Berezne, and Kostopil counties.
Includes remaining Rivne counties not mentioned in (a) or (b).
Pregnant women seeking prenatal ultrasound examinations at the Rivne Regional Diagnostic Center (2008–2011) who volunteered to undergo the procedure.
Official limits (norms) are 3700 and 14 800 Bq of 137Cs for subjects under 15 years of age and adults respectively.
2000–2011 data.
Taking into consideration the high consumption by the Polissia population of locally grown potatoes, the results of a survey of incorporated IR by these plants shown in Table 8 are of interest. An analysis of dry stems of potato plants shows 137Cs and 90Sr incorporated levels by these plants in approximately 2:1 proportions.
Table 8.
Radiometry of dried stems of potato plants from Rivne Polissia region
| Sample | Measurements | ||
|---|---|---|---|
| 90Sr, Bq/kg | 137Cs, Bq/kg | ||
| Initial | Repeat | ||
| A | 43.4 ± 17.2 | 46.8 ± 21.4 | 88.3 ± 36.4 |
| B | 49.9 ± 17.9 | 32.1 ± 24.1 | 63.6 ± 39.3 |
| C | 41.3 ± 19.9 | 46.4 ± 19.2 | 24.0 ± 22.0 |
| D | 82.3 ± 21.3 | 72.2 ± 20.0 | |
| E | 88.3 ± 23.1 | 84.4 ± 28.1 | 46.1 ± 34.6 |
| F | 95.6 ± 23.1 | 143.2 ± 29.6 | |
| G | 327.2 ± 86.6 | 87.3 ± 25.1 | 54.8 ± 31.4 |
Table 9 illustrates major sources of incorporation of nuclides by pregnant women in Polissia. Main sources of inhalation are from smoke and dust and sources of ingestion are from the use of water from shallow water-wells and consumption of locally produced nutrients. The calculated levels of 137Cs ingested daily are 268 Bq or above the declared daily upper limit of 210 Bq by the Ministry of Health (Decree 106, 1991).
Table 9.
| Water (%)b | Well | Spring | Bottled | Piped |
|---|---|---|---|---|
| 85.4 | 9.4 | 50.0 | 5.3 | |
| Fuel (%) | Wood | Gas | Central | Peat |
| Heating | 76.7 | 17.5 | 9.0 | 1.8 |
| Cooking | 52.3 | 48.9 | – | – |
| Food (%) | Own | Local | Imported | |
| Pork | 91.3 | 9.9 | 0 | |
| Chicken | 77.3 | 16.2 | 2.0 | |
| Milk | 71.8 | 15.1 | 0 | |
| Vegetables | 98.0 | 6.4 | 1.2 | |
| Apples | 91.6 | 17.2 | 3.2 | |
| Estimated daily137Cs intake | Bq | |||
| Polissia | 268.25 | |||
| Upper permissible limitc | 210.00 | |||
Extract from Dancause et al. (2010).
Mixed use, not additive percents.
Ministry of Health 1997 guidelines.
The current analysis of microcephaly defined as an occipito-frontal circumference (OFC) at least 3 SD below norms excludes lesser degrees of head size reductions. To assess this omission, we analyzed birth weights and at-birth OFC measurements obtained from all infants born in a Polissia county (Zarichne) and Rivne city located in Rivne-nP. The analysis compared 2476 male and 2305 female infants born in Zarichne county with 13 086 male and 12 155 female infants born in Rivne city. The physiologic slightly larger birth weights of male infants were evident among infants from the Rivne-P and Rivne-nP sites. As shown in Figure S3, sex-specific birth weights in Rivne-nP and Rivne-P were similar. On the other hand, the occipito-frontal head circumferences (OFC) of the same infants were smaller among those from Polissia (Fig. 2). Analyses limited to infants born after at least 38 weeks of gestation or limited to the same infants and free from anomalies detected at birth showed the same contrast. The average OFC values, from largest to smallest, were 34.57 (males from Rivne-nP), 34.31 (males from Polissia), 34.11 (females from Rivne-nP), and 33.84 cm (females from Polissia), respectively. The average OFC values of the same infants born after ≥38 weeks of gestation were 34.74, 34.45, 34.27, 33.96 cm, respectively. Statistically, the differences of OFC between infants of the same sex from Polissia and Rivne-nP are significant (P-value <0.0001). Clinically, the significance of these observations remains to be determined.
Figure 2.
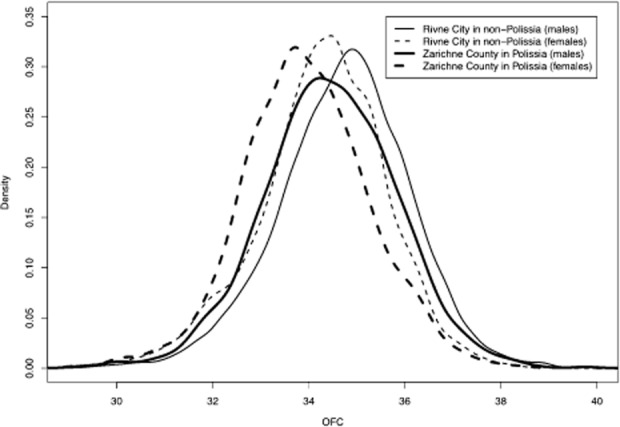
Occipito-frontal circumferences (OFC in cm) of infants born after at least 38 weeks of gestation. Measurements were obtained from 2398 males and 2240 females from Zarichne county in the Polissia region compared to those of 12 542 males and 11 649 females from Rivne city located in the non-Polissia region of the Rivne province. The OFC values of males and females are smaller in Zarichne county. The contrast is statistically significant (P-value <0.0001 using permutation test and other) (Wang and Wertelecki 2013).
In contrast to pentad-cCM, the triad-cCM (OM, GASTR and BLEXTR) are neither more frequent in Rivne-P nor more prevalent among females (Tables 2 and Table 10). Aside from 18 instances of OM associated with NTD (14 instances), MIC (two instances) or with mOPH (two instances), there are 38 other instances of OM (Table 10). The M-F proportion among those of known sex is 20-6. Among these 38 instances, 12 (32%) were detected before the 15th week of gestation. Such early detections often limit the depth of clinical descriptions necessary to fully categorize OM as representing particular patterns of CM such as syndromes or complexes such as the OEIS (omphalocele-exstrophy of the cloaca-imperforate anus-low lumbo-sacral spina bifida). Among these 38 individuals with OM, 16 (42%) had associated CM including two instances of Patau or trisomy 13-like syndrome (aa-2, bb-1) and one instance of Wiedemann Beckwith syndrome (aa-1). One individual had a concurrent skeletal dysplasia (dd-6). The remaining 12 individuals we dichotomized into those with cephalad or caudad associated CM, 10 had cephalad and two had caudad CM.
Table 10.
All individuals with omphaloceles or gastroschisis (2000–2009)
| Categorya | Polissia | Non-Polissia | Rivne Province | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | All | Sex | Rate | All | Sex | Rate | All | M : F | |||
| M | F | M | F | ||||||||
| OMPHALOCELE | 3.5 | 25 | 14 | 5 | 4.2 | 31 | 14 | 5 | 3.9 | 56 | 2.80 |
| Isolated | 0.7 | 5 | 4 | 2.3b | 17 | 7 | 3 | 1.5 | 22 | 3.67 | |
| Not isolated | 2.8 | 20 | 10 | 5 | 1.9 | 14 | 7 | 2 | 2.3 | 34 | 2.43 |
| Syndromicc | n/c | 3 | 3 | n/c | 2 | 1 | 1 | 0.3 | 5 | n/c | |
| Non-Syndromic | 2.3 | 17 | 7 | 5 | 1.6 | 12 | 6 | 1 | 2.0 | 29 | 2.17 |
| Neural tube defects | 1.4 | 10 | 2 | 4 | n/c | 4 | 2 | 1.0 | 14 | n/c | |
| Microcephaly | n/c | 1 | 1 | n/c | 1 | 1 | n/c | 2 | n/c | ||
| Other Anomalies | 0.8 | 6 | 4 | 1 | 1.0 | 7 | 3 | 1 | 0.9 | 13 | 3.50 |
| GASTROSCHISIS | 2.3 | 17 | 5 | 12 | 3.1 | 23 | 11 | 7 | 2.8 | 40 | 0.84 |
| Isolated | 2.2 | 16 | 5 | 11 | 3.0 | 22 | 10 | 7 | 2.6 | 38 | 0.83 |
Individuals represented in sub-categories are mutually exclusive.
A statistically significantly higher rate (P-value 0.009; OR 0.30; CL 0.09, 0.84).
Includes four individuals (v-1; aa-2; w-1; bb-1) with Patau syndrome; includes two individuals (v-1; w-1) with omphalocele-Patau syndrome-microphthalmos.
F, females; M, males; M : F, male : female ratio; n/c, not computed.
The OM-cephalad CM associations included a twin (dd-1) with ectopia cordis (suspected as an instance of a pentalogy of Cantrell complex), CL/P (cc-2); cleft lip-hypoplastic heart (dd-3); truncus arteriosus (cc-1); A-V septal defect (dd-4), a-v canal-upper limbs reductions (cc-5); stenotic pulmonary artery (dd-5); dextrocardia (dd-7), and diaphragmatic hernia (cc-4, dd-2); the caudad array of OM-CM associations included one individual with cloacal exstrophy (cc-6) and another with male genital anomalies and an accessory spleen (cc-3). We found this schematic cephalad-caudad dichotomization of OM-CM associations to be simpler and easier to implement and to be more acceptable to fetal ultrasonographers than alternative categorizations of celosomias calling for categorizations such as OEIS or other complexes referred to by other acronyms. The comparison of OM rates in Polissia vs. Rivne-nP is based upon u-rates which are 1.8 and 3.4, respectively, and are indicative of a statistically significantly higher frequency in Rivne-nP. In terms of isolated OM, u-rates in Polissia vs. Rivne-nP are 0.7 and 2.3, respectively, which demonstrates a higher prevalence in Rivne-nP. The overall M-F proportion among OM individuals is 20-6, a clear demonstration of a statistically significant male prevalence, which is likewise evident among subcategories of OM.
Regarding gastroschisis, among 40 individuals, 38 (95%) did not have associated CM, one had associated duodenal atresia (ff-1) and another (ff-2) had renal anomalies. The u-rates in Polissia and Rivne-nP are 2.3 and 3.1, respectively, and the M : F are 0.42 and 1.57, respectively. If confirmed by follow-up monitoring, the female prevalence in Polissia and male prevalence in Rivne-nP will be surprising. We also note that in 23 (58%) of individuals had birth weights under 2500 g. The association of gastroschisis with younger maternal ages is evident in Rivne as well as in the adjoining two provinces. In Rivne, 40% of mothers of gastroschisis infants were under the age of 20 years compared to an overall frequency of 9.45% (Table S8).
Concerning a group of 12 individuals with urinary bladder exstrophy, which excludes one individual (h-3) included in the NTD category and suspected to represent an OEIS complex – the noted CM include spina bifida, OM, anomalous male genitals and anal atresia. Another excluded individual (arj-2) has abdomino-caudal complex anomalies described later. Nearly half of the 12 individuals with urinary bladder exstrophy have a concurrent epispadias, while no instances of epispadias occurred in Rivne that were not associated with an exstrophy of the bladder. Four of the five individuals with epispadias were males. Among this group of 12 individuals, the M-F proportion was 3-5 among individuals from Polissia and 4-0 among those from Rivne-nP. None of the 12 individuals had other concurrent CM, except for one who had undescended testicles. Noteworthy is that all 12 individuals were liveborn and that their birth weights were above 3000 g with the exception of two instances whose birth weight was nearly 3000 g (Data Supplement). It may be of interest to note that in contrast to individuals with urinary bladder exstrophies, the birth weights of infants born near term with isolated microcephaly or recto-anal anomalies are frequently reduced. Among 22 individuals with isolated microcephaly and 20 individuals with isolated recto-anal anomalies delivered at ≥38 weeks of gestation, 12 (55%) and five (25%) had birth weights ≤3000 g, respectively, (Data Supplement).
The t-rates of CM in Rivne can be compared with the t-rates observed elsewhere in Europe and reported by EUROCAT. As summarized in Table 3 and reported in more detail in Table S5 in the Data Supplement, the pentad-cCM rates in Rivne are among the highest in Europe. On the other hand, the triad-cCM t-rates in Rivne fall within the range of those reported to EUROCAT.
Salient cCM inter-associations and associations with other CM are illustrated in Table 11. The most salient dyads are: cephalad NTD-OM, recto-anal-renal anomalies, renal-limb anomalies, CL/P-OM, spina bifida-OM, spina bifida-limb anomalies, microcephaly-mOPH; CL/P-renal anomalies; and CL/P-limb anomalies.
Table 11.
Non-syndromic associations of congenital malformationsa, Rivne Province, Ukraine (2000–2009)
| Category | CTW | CEPH | SB | MIC | mOPH | CL/P | OM | GSTR | ESOPH | DIAPH | RENALb | BLEXTR | RECTAN | LIMB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | 3 | 71 | 102 | 46 | 26 | 78 | 25 | 17 | 26 | 24 | 62 | 9 | 16 | 31 |
| nP | 5 | 45 | 60 | 25 | 9 | 79 | 31 | 23 | 17 | 26 | 63 | 5 | 29 | 49 |
| LB | 1 | 0 | 72 | 69 | 30 | 129 | 20 | 17 | 32 | 27 | 85 | 14 | 39 | 58 |
| S | 1 | 19 | 10 | 2 | 3 | 3 | 7 | 2 | 3 | 9 | 14 | 0 | 6 | 4 |
| ToP | 5 | 87 | 70 | 0 | 2 | 21 | 24 | 19 | 7 | 13 | 23 | 0 | 0 | 15 |
| SA | 1 | 10 | 10 | 0 | 0 | 4 | 5 | 2 | 1 | 1 | 3 | 0 | 0 | 3 |
| U | 2 | 34 | 22 | 0 | 0 | 2 | 18 | 5 | 3 | 6 | 11 | 0 | 3 | 9 |
| M | 1 | 32 | 73 | 35 | 16 | 100 | 28 | 16 | 22 | 26 | 69 | 8 | 22 | 37 |
| F | 5 | 50 | 67 | 36 | 19 | 55 | 10 | 19 | 18 | 18 | 45 | 6 | 20 | 34 |
| Twin | 0 | 5 | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 4 |
| CTW | 8 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CEPH | 0 | 116 | ||||||||||||
| SB | 1 | 0 | 162 | |||||||||||
| MIC | 0 | 0 | 0 | 71 | ||||||||||
| mOPH | 0 | 1 | 0 | 5 | 35 | |||||||||
| CL/P | 0 | 2 | 2 | 4 | 4 | 157 | ||||||||
| OM | 1 | 8 | 6 | 2 | 2 | 6 | 56 | |||||||
| GSTR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | ||||||
| ESOPH | 0 | 4 | 0 | 2 | 2 | 3 | 0 | 0 | 43 | |||||
| DIAPH | 0 | 3 | 1 | 1 | 2 | 3 | 3 | 0 | 2 | 50 | ||||
| RENALb | 0 | 2 | 3 | 4 | 2 | 5 | 3 | 1 | 4 | 3 | 125 | |||
| BLEXTR | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 14 | ||
| RECTAN | 0 | 0 | 2 | 1 | 1 | 1 | 2 | 0 | 5 | 2 | 8 | 2 | 45 | |
| LIMB | 0 | 1 | 5 | 4 | 2 | 5 | 2 | 0 | 1 | 1 | 7 | 1 | 4 | 80 |
The regional and perinatal observations are presented above the diagonal of grey cells. In the grey cells are given the total numbers of individuals with the particular malformation (including those with holoprosencephaly). The numbers below the diagonal of grey cells reflect the associations with other malformations. Individuals with multiple malformations may be represented in multiple cells.
Associations with other unlisted congenital malformations are not included in the table.
Included are individuals with renal agenesis, hypoplasia, cystic kidneys and excluded are hydronephroses and ureteral obstructions.
ANOR, anorectal anomalies; BLEXTR, urinary bladder exstrophy; CEPH, cephalic neural tube defects; CL/P, cleft lip/palate; CTW, conjoined twins; DIAPH, diaphragmatic defects; ESOPH, esophageal anomalies; F, female; GSTR, gastroschisis; LB, live birth; LIMB, limb reduction defects; M, male; MIC, microcephaly; mOPH, microphthalmia; nP, non-Polissia region; OM, omphalocele; P, Polissia region; RENAL, renal anomalies; S, stillbirth; SA, spontaneous abortion; SB, spina bifida; ToP, termination of pregnancy; U, unknown sex.
To complete a perspective of body wall schises or celosomias, we describe seven additional individuals with ectopia cordis, two of which are included among individuals with other cCM. These two individuals with ectopia cordis associated with cCM include one (q-3) who had an encephalocele and was included in the NTD category and another (dd-1) who had complex associated CM suggestive of a pentalogy of Cantrell and was included among other instances of OM. The remaining five individuals with ectopia cordis (ect-1–5) include individual ect-3 who had severe spinal deformities and reduction anomalies of the left arm and individual ect-4 who had a retroflexed spine and eviscerated organs adjoining the placenta, anomalies that probably represent an instance of a body stalk anomaly complex. Regarding four other individuals with thoraco-abdomino-schises not associated with ectopia cordis, the patterns of associated CM were similar to those associated with ectopia cordis. One of these four individuals was an anencephalic (g-2) with severe “s-like” spine deformity; a second individual (thab-1) was detected by ultrasonographic fetal examination during the 16th week of gestation that failed to visualize the body stalk and demonstrated a fetal spine adjacent to the uterine wall and severe reduction of a lower limb. A third individual (thab-2) had body stalk anomalies detected during the 12th week of gestation and a forth individual (thab-3) was detected by prenatal ultrasound during the 19th week of gestation and had evisceration without a membranous envelope and amniotic bands not connected to the fetal body. These four individuals with thoraco-abdomino-schisis associated with other CM and feto-placental anomalies illustrate the difficulties inherent in the categorization of early celosomias. On the basis of a total of 11 instances of thoraco-abdomino-schisis, associated or not with ectopia cordis, the t-rate in Rivne, at a minimum is 0.76 or the t-rate of ectopia cordis at a minimum is 0.48. The computation of M-F proportions was unfeasible due to limitations inherent in early gestational prenatal ultrasonographic examinations.
Among other caudal malformation complexes is included a sole individual (cc-6) with an exstrophy of the cloaca, a large male infant (3900 g) whose mother was not diabetic. One stillborn of ambiguous sex (arj-1) had body stalk anomalies, a caudal regression sequence, absent kidneys, adrenals, spleen, urinary bladder, rectum, and one foot. A liveborn female (arj-2) had urinary bladder exstrophy and absent left kidney, hemipelvis, lower limb, and recto-anal agenesis. She survived and demonstrated normal intellectual and social skills by finishing standard high school and being bi-lingual. Two individuals, both stillborns, had sirenomelia and inherently recto-anal agenesis. The first individual (sir-1) had a nephroblastoma, renal hypoplasia and the second individual (sir-2) was a di-amniotic, di-chorionic twin who had bilateral renal agenesis, absence of the left arm and ambiguous sexual development. His co-twin was a stillborn male whose legs were not visualized by ultrasound.
Regarding recto-anal anomalies, among 45 individuals, six had associated cCM, the first (h-3) had an OM-spina bifida-urinary bladder exstrophy and is included in the NTD group; a second individual (cc-6) had OM-exstrophy of the cloaca; a third individual (m-4) had a spina bifida-diaphragmatic hernia; a fourth(s-5) had Down syndrome-microcephaly; a fifth individual (clr-4) had a CL/P- ectopic kidney dyad; and a sixth individual (hol-29) had holoprosencephaly-mOPH association. Excluding the abovementioned six individuals and two other individuals with sirenomelia (implicitly associated with recto-anal anomalies), there are 37 instances of recto-anal anomalies in Rivne, 14 of which are from Polissia and 23 are from Rivne-nP, which translates in corresponding u-rates, at a minimum, of 1.93 and 3.15, respectively (Table S2). Among these 37 individuals, 20 had isolated recto-anal urinary bladder exstrophy except that 14 had concurrent fistulas. The u-rates of isolated recto-anal CM in Rivne, Polissia and Rivne-nP are, at a minimum, 1.38, 0.97 and 1.78 respectively (Data Supplement). It is also evident that the birth weight of infants born near term with non-syndromic recto-anal anomalies is often diminished – 25% of such infants had birth weights under 3000 g. Another contrast concerns M-F among infants with and without concurrent fistulas – among those with fistulas, males were prevalent 5-1 and among those without fistulas, the M-F was 6–8. Among 15 individuals with recto-anal anomalies associated with other CM, the M : F was 1.03 and the most salient associations were with esophago-gastro-intestinal anomalies (ari-24, 26, 27, 28, 30, 33, 34) in particular esophageal (ari-24, 27, 33, 34). Other individuals with recto-anal anomalies include one individual with body stalk anomalies (arj-1), and another individual with caudal dysplasia and lower limb reduction anomalies (arj-2).
Conjoined twins, teratomas
Among 145 437 births, there were 347 MF, 423 MM and 419 FF twin pairs (Table S4). The MM : FF pairs ratio is 1.01 and is virtually the same in Polissia and Rivne-nP as is the overall frequency of twin pairs. There were five instances of acardia among the 842 same-sex pairs (0.59%). Four of the five acardics were noted among 415 same-sex twin pairs in Polissia (0.96%). Eight conjoined twin pairs were born in Rivne during the 2000–2009 period, a ninth set was born during 2010 and five other pairs of conjoined twins were born in the two adjoining provinces of Rivne. The u-rate in Rivne is 0.55 and in the two adjoining provinces combined is 0.19 (Table S6). Family histories of conjoined twins show a considerable frequency of twinning events (Fig. S4).
An analysis of teratomas also included observations in the two provinces adjoining Rivne. The u-rate in Rivne is 0.76, which is similar to the 0.77 u-rate in the two adjoining provinces combined (Table S6). Among a total of 29 teratomas in the three provinces, 25 (86%) were sacro-coccygeal and the M-F proportions in Rivne and adjoining provinces were similar, 7–19 and 7–15 respectively. Among the 29 teratomas, two (7%) included tissues characteristic of teratoblastomas, one was a sacro-coccygeal (vter-11) and the other a cervico-pharyngeal teratoma (neo-1).
Concerning CM-neoplasia associations, in addition to a pharyngeal congenital teratoblastoma (neo-1) mentioned above, there were two other instances: an ependymoma (clr-5) associated with cataracts and a nephroblastoma (sir-1) associated with renal hypoplasia and sirenomelia.
Discussion
This investigation seeks to document population-based rates of congenital malformations (CM) computed with adherence to EUROCAT and international standards in the Rivne province of Ukraine, which was impacted by the IR following the 1986 Chornobyl disaster. The investigation concerns eight CM visually evident at birth and collectively referred to as core-CM (cCM) as well as surveys concerned with three prominent teratogenic risk factors in Rivne; consanguinity rates, alcohol, and ionizing radiation (IR). The object of this discussion is to emphasize contrasts of the frequencies and patterns of cCM in Rivne compared with those in the rest of Europe as well as to compare observations in Polissia with those in the rest of the province. The nature of each of the cCM noted is consonant with reports by recognized experts (Willis 1962; Warkany 1971; Stevenson & Hall 2006). Concerning the health effects of exposure to low levels of IR, this subject is expertly summarized by the Committee on Biological Effects of Ionizing radiation, which was established by the National Research Council (BEIR V Report 1990). The object of this discussion is focused on rates and patterns of cCM in Rivne in the context of related observations elsewhere.
Blastopathies
“Blastopathy” and “celosomia” are notions we find useful to convey an overview of the cCM observed in Rivne. Blastopathies are anomalies that arise prior to embryonal implantation and organogenesis and the term “celosomia” refers to blastopathies characterized by a failure of closure of the anterior body wall reflecting abnormalities of the embryonal folding process (Schinzel et al. 1979; Czeizel and Opitz 1981; Martinez-Frias et al. 1997; Opitz et al. 2002). The array of blastopathies includes monozygotic twinning, conjoined twinning, acardia, teratomas, NTD, OM and urinary bladder exstrophy, which are emphasized in this report as well as thoraco-abdomino-schisis-ectopia-cordis, body stalk defects, diaphragmatic anomalies, renal agenesis, esophageal-gastric-intestinal-colonic-recto-anal anomalies, cloacal dysgenesis-exstrophy, recto-anal-sacral and other caudal anomalies including sirenomelia and lower limb dysgenesis, among other complexes. The unifying notion implicit in blastopathies, in particular celosomias, is that these CM are the result of an altered process of embryonic folding (Duhamel 1963; Stevenson & Hall 2006). In brief, prior to the onset of embryonic folding process, during gastrulation, the embryonic disc develops in a cephalad-caudad and dorsal-ventral axes followed by the emergence of embryonic ecto-endo-meso-blast layers. The mesoblast becomes interposed between the expanding ecto and endoblast layers of the embryonic disc with the exception of two didermic sites, the oral and cloacal membranes. Dorsally, the ectoblast forms a medial notochord. Somites and nephrotomes develop laterally and extend peripherally to give rise to lateral laminae, which divide to form dorsally the amniotic and ventrally the celomic cavities. The embryonal folding process results from a more vigorous dorso-cephalic growth of the tri-laminar germ disc, which induces the transformation of a flat embryonal plate into a cylindrical ventrally bent embryo. The faster growth of the cephalad-dorso-cephalic area induces a cephalic folding from which the ventral zone will give rise to the heart, foregut and anterior diaphragm. Normally, the concurrent cephalad, lateral, and caudad embryonic folding converge to form an apex or omphalon preceded by a body stalk that evolves into an umbilical stalk to become the base of the umbilical cord. Altered growth and closure of the dorsal neural tube may impede normal embryonal ventral folding and result in celosomias, as is probably reflected in the preferential cephalad-NTD-omphalocele associations observed in Rivne. Symmetric anterior body wall growth failures give rise to a “middle celosomia” or omphaloceles (OM). Less symmetric folding failures may result in “upper” (cephalad) or “low” (caudad) celosomias. The “upper” celosomia group, for example, includes the pentalogy of Cantrell complex and the “low” group includes complexes like the OEIS defined earlier. Generally, celosomias are detected in early gestation and impacted infants are non-viable, which enhances a tendency for prenatal diagnosis detection to be promptly followed by terminations of pregnancy and descriptions of the anomalies noted to be limited. Often, descriptions allude to “suspected” instances of OEIS or other CM complexes without substantiation of such conclusions. One alternative is to rely on schematic descriptions such as outlined above, which prompt the actual description of anomalies noted during fetal ultrasound or post-mortem examinations. “Tail end” embryonal anomalies arise after the formation of the embryonal axis and closure of the posterior neuropore during the 4th week. The tail-bud during the 5–6th week, the Hensen's node and the caudal end of the cord are in continuity with the notochord and the post-anal dimple marks the site of the final closure of the posterior neuropore and site of post-anal sinuses. As the retrogression of the tail occurs during the 7–8th weeks, duplications and secondary posterior neuropores and other anomalies are relatively common in human embryos. These malformations of the hind end of the embryo show little constancy of patterns and include absence of one or both lower limbs and sirenomelia. Our observations in Rivne demonstrate an excess of “cephalad” cCM and no evidence of an excess of “caudad” or “tail end” malformations.
Rates
A perspective highlighting frequencies of cCM demonstrates that those of pentad-cCM in Rivne are among the highest reported to EUROCAT. Also, the rates in Polissia are statistically higher than in the rest of Rivne (Tables 3 and S5). After Polissia, the next highest rates of conjoined twins, NTD, microcephaly and mOPH noted in Europe are reported from Northern England, South West England and Wales. Two other independent investigations determined that in Finland the rates of conjoined twins and sacro-coccygeal teratomas also are among the highest in Europe although they are not higher than in Polissia (Mutchinick et al. 2011; Pauniaho et al. 2013). The mentioned regions in the UK and the central regions of Norway and Sweden are, like Polissia, particularly impacted by Chornobyl IR (Gillett et al. 2001). Although teratoma rates are not reported to EUROCAT, other investigations in Northern England demonstrated a prevalence of sacro-coccygeal teratomas of 0.37 compared to that in Rivne of 0.62 (Table S6). The frequency of triad-cCM in Polissia or Rivne is not distinct from elsewhere in Europe.
The frequency of NTD and NTD-OM preferential associations in Rivne and in Polissia in particular, is among the highest in Europe. On the other hand, an extensive review of OM in Europe by Calzolari et al. (1995, 1997) determined that the total OM prevalence is 2.52 or nearly the same as that in Rivne. The NTD-OM preferential association, as presented later, is more frequent in regions with high NTD rates, particularly in Polissia and the British Isles. Regarding individuals with “para-omphaloceles” or gastroschisis, this cCM represents a localized aplasia or dysplasia of the abdominal wall lateral and generally to the right of the umbilicus and is unrelated to the formation of the umbilicus or amniotic sac. In Rivne, 43% of instances of gastroschisis were liveborn and as noted in the Data Supplement another 48% of instances were associated with induced pregnancy terminations. A worldwide survey of 3322 instances of gastroschisis showed that 86% had no associated CM compared to 95% in Rivne. The 2.34 and 3.13 t-rates of gastroschisis in Polissia and Rivne-nP are within the range of 0.89–6.22 t-rates reported to EUROCAT from across Europe (Table S5). Another celosomia in the cCM triad is urinary bladder exstrophy, which is discussed later jointly with other caudad or “tail end” blastopathies.
Among “high” celosomias are included instances of thoraco-schisis, which often is associated with ectopia cordis, and both of these anomalies are included in the spectrum of the pentalogy of Cantrell complex. In Rivne, thoraco-abdomino-schisis-ectopia-cordis complexes are nearly as frequent as urinary bladder exstrophies. Recto-anal anomalies are the most frequent category of caudal or “low” celosomias, followed in frequency by urinary bladder exstrophies. Urinary bladder exstrophy rates in Rivne fall within the range of rates in Europe reported to EUROCAT. In contrast to individuals who are liveborn near term with isolated urinary bladder exstrophies and birth weights near the norm, those with isolated recto-anal anomalies who are born near term have reduced birth weights (Data Supplement).
Twinning and female prevalence
Many investigations demonstrated that children from multiple births, in particular monozygotic twins, are at a higher risk to have CM. A population-based cohort study by Li et al. (2003) demonstrated a relative risk ratio increase for CM of 1.9 for twins, 2.7 for triplets and 4.6 for quadruplets and higher. The rate of anencephaly among singletons and multiple births was 1.1 and 2.9, respectively, and equivalent to a statistically significant risk ratio of 2.64 in contrast to spina bifida without anencephaly, which had a similar frequency among singletons and twins. The proposition forwarded by Schinzel et al. (1979) and grounded in solid clinical observations holds that blastopathies reflect mechanisms in common with those of the monozygotic twinning process. Experts assert that twinning impacts approximately 1 in 40 (2.5%) individuals and therefore, in Rivne, an estimated 3636 twin infants were expected to have been born, in contrast to the 2378 (1.94%) observed (Phelan and Hall 2006). This contrast suggests that the frequency of twinning in Rivne is unlikely to be elevated. Experts also assert that population rates of dizygotic twinning are higher in proportion to the number of pregnancies reflecting advanced maternal age or reliance on IVF-ART (in vitro fertilization – assisted reproductive technology) procedures. IVF-ART procedures are rarely performed in Rivne and although maternal ages at delivery tend to be higher in Polissia than in Rivne-nP, we conclude that these factors are unlikely to significantly impact the observed twinning patterns in the province. Regarding conjoined twinning, the association with an altered monozygotic twinning process is self-evident, a process that may also be associated with the pathogenesis of sacro-coccygeal teratomas. The NTD-twinning association is also well established (Table S4) (Windham and Sever 1982; Garabedian and Fraser 1994; Kallen et al. 1994). The degrees of twinning associations with various subcategories of NTD and among relatives of conjoined twins, teratoma and NTD individuals are discussed below along with other aspects of the nosology of cCM.
In humans, the proportion of males at birth is generally slightly above 0.51 and is remarkably consistent among different populations over time. The sex proportion is slightly lower among twins and higher-order multiple births than among single births. Among monozygotic twins the M : F is lower than among dizygotic twins. Among monozygotic twins, the frequency of male pairs and even more so among conjoined twins is lower. A prevalent interpretation is that dichorionic, monoamniotic and conjoined twins reflect increasingly late embryonic duplications resulting in conjoined or twins pairs (Derom et al. 1988; James 1988; Phelan and Hall 2006). The question arises whether females are more prone to embryonic splitting since during early embryonic formation their development is slower, a point elaborated upon later.
Among the leading investigations of sex differences in the prevalence of CM are those by Lary and Paulozzi (2001) and Shaw et al. (2003). Both studies demonstrated, as observed in Rivne, a female prevalence among instances of NTD as well as among instances of microcephaly. Female prevalence among microcephalic individuals was also demonstrated by two independent investigations in Hungary (Abdel-Salam and Czeizel 2000; Szabo et al. 2010). It is provocative that several reviews of reports of microcephalia vera demonstrating female prevalence fail to stress this phenomenon (Warkany et al. 1981, chapter 1). The study by Shaw et al. (2003) included an analysis of individuals with mOPH among whom females were likewise prevalent in contrast to Kallen et al. (1996) who found no evidence of sex preference. Female prevalence among conjoined twins and teratomas, as noted in Rivne, is also well known (Table S2) (Warkany 1971; Phelan and Hall 2006). A repeatedly demonstrated phenomenon, also evident in Rivne, is a provocative shift away from the female prevalence among cephalad-NTD compared to spina bifida. An analysis of 226 fetuses with NTD demonstrated clear contrasts of M : F ratios among NTD reflecting their location. Among anencephalics (exclusive of mero-acrania), spina bifida above the lumbar spine and spina bifida below the thoracic spine the M : F ratio was <0.6, 0.4 and >1.4. Among individuals with mero-acrania (intact posterior skull and foramen magnum) the M : F ratio was 1.08 (Tapper and Lack 1983; Seller 1987, 1995). Another shift of M-F proportions is noted among encephaloceles when subdivided as occipital and non-occipital. In Rivne and two adjoining provinces there were 31 and 36 instances of encephaloceles among which 20 and 21, respectively, were non-syndromic occipital encephaloceles. The M-F proportions in Rivne and the two adjoining provinces were 9–12 and 12–12, respectively, compared to non-syndromic occipital encephaloceles which were 5–9 and 5–9, respectively. The total encephalocele population-based rates in Rivne and two adjoining provinces combined were 2.13 and 1.54, respectively; and the respective non-syndromic occipital encephaloceles were 1.38 and 0.90 (Table S9). Another shift away from a female prevalence evident among sacro-coccygeal teratomas is noted among individuals with teratomas in other locations (Tapper and Lack 1983; Stevenson & Hall 2006). An analysis of death certificates of children with teratomas by Fraumeni et al. (1973) determined that among 198 instances, 56 were malignant and that the M-F proportions reflected their location. The M-F proportions were 24–60, 5–17, 15–14 and 4–5 among instances of sacro-coccygeal, retroperitoneal, cranial and cervico-pharyngeal teratomas, respectively. These investigators also noted a high frequency of anomalies associated with sacro-coccygeal teratomas, in particular of the lower vertebrae and pelvic anomalies attributable to twinning or duplications of the hind gut. Among the 198 teratoma patients investigated by Fraumeni et al. (1973), five individuals were twins and one was a triplet.
Both in Rivne and Denmark, investigations did not produce evidence of an OM-twinning association (Bugge 2010). However, OM as a component of the OEIS complex is associated with twinning and likewise with body stalk anomalies (Bugge 2012). Furthermore, two investigations led by Mastroiacovo et al. (1992, 2007) and by Lee et al. (1999) summarized autopsy and clinical findings of the OEIS complex with an emphasis on its association with monozygotic twinning. The cited studies by Mastroiacovo noted that large body wall defects may be reported as body stalk anomalies or as a large gastroschisis. Perhaps this possibility may be reflected in observations by Hwang and Kousseff (2004) of an elevated frequency of twins among patients with OM and gastroschisis. Reid et al. (1986) also noted elevated twinning rates among patients with gastroschisis when associated with amyoplasia-related anomalies. Also, Moore and Nur (1986) noted an OM-twinning association and not with gastroschisis. These complexities probably reflect the heterogeneity of OM and inadequacies of the current classifications.
Of note is that among recto-anal anomalies, instances with and without fistulas have different M-F proportions, which in Rivne are 5–1 and 6–8, respectively. In Rivne and among 1846 cases of anal atresia surveyed by Cushieri (2001), 44% and 36% of instances were isolated anomalies, respectively (Table S2). Among recto-anal anomalies, 10% are above the level of the levator ani muscle and are prevalent among males. In Rivne, the M : F ratio among those with supralevator and infralevator anal atresias was 6.2 and 2.3, respectively.
Preferential associations of CM
Non-syndromic CM associations are illustrated in Table 11. The most frequent CM dyads are: cephalad-NTD-OM, renal-recto-anal; renal-limbs; spina bifida-OM; and CL/P-OM. An expanded analysis of NTD-OM dyads in Rivne and in two adjoining provinces showed 27 instances – two were encephaloceles (ov-2, 5). This group of 27 individuals included 17 (63%) instances of cephalad-NTD, among which eight (47%) had cranio-rachis-schisis, and the overall M-F proportion was 2-9 (Table S7). These preferential OM associations with cephalad-NTD and the prevalence among females are similar to observations by McKeown et al. (1953), Smithells et al. (1964), Windham and Sever (1982), and Doyle et al. (1990). An extensive analysis by Calzolari et al. (1995, 1997) showed that in the British Isles (UK and Ireland) among 70 OM-NTD dyads, 48 were associated with cephalad-NTD, 21 with spina bifida and six with encephaloceles. In Continental Europe, only 18 individuals had an OM-NTD dyad and among these, 12 had spina bifida and five had cephalad-NTD, a reversed proportion from that observed among instances in the British Isles. In this context, the number of NTD-OM dyads observed in Rivne and two adjoining provinces is probably greater than in the rest of Continental Europe combined. The studies by Calzolari et al. (1995, 1997) demonstrate that the high frequency of NTD-OM dyads in the British Isles reflects high frequency of NTD and are not correlated with the frequency of OM.
In Rivne there were 38 other instances of OM, not associated with NTD, 13 of which represent non-syndromic OM-CM associations. Schematically, most of these associated CM were cephalad with respect to the OM (Data Supplement). Regarding celosomias, these are increasingly detected by prenatal ultrasonography during early stages of pregnancy and often are incompatible with postnatal survival, thus terminations of pregnancies frequently follow. These circumstances foster relatively limited clinical descriptions. There also is a tendency to categorize early complex celosomias as suspected instances of complexes such as OEIS rather than description of actual visualized anomalies. An example of an alternative approach was used by Mastroiacovo et al. (1992) who studied a large number of “Gross Body Wall Defects” and categorized these as OM or gastroschisis inclusive of all degrees of limb reductions or the presence of sirenomelia. Among the 215 infants, 19% had NTD and 5.6% had a twin. Other investigators point out that an OM-twinning association is a landmark of OEIS (Martinez-Frias et al. 2000; Keppler-Noreuil et al. 2007). In our analysis, we tested a schematic categorization of OM-CM associations and found that the proportion of OM-cephalad CM and OM-caudad CM was 9-2. To what extent such topologic categorization of OM associations with other CM is nosologically informative remains to be seen. Of interest is that Russo et al. (1993) proposed that the Limb-Body-Wall complex consists of at least two distinguishable phenotypes. The first phenotype, which we refer to as cephalad-LBW includes encephalocele or anencephaly associated with facial cleft and a second phenotype, we refer to as caudad-LBW, which excludes cranio-facial defects and includes urogenital, recto-anal, lumbo-sacral meningocele, feto-placental attachments, short cord, and lower limb anomalies. Representative clinical observations in Rivne of the caudad-LBW complex are two infants (arj-1, arj-2) the second of which survived and is the high-school graduate described earlier.
In Rivne and elsewhere, gastroschisis is for the most part an isolated anomaly. However, Reid et al. (1986) demonstrated an association with twinning and amyoplasia. These investigators noted that in a series of 216 patients with amyoplasia, 5% had gastroschisis and another 6% had other defects, mainly bowel atresia and/or trunk wall musculature anomalies. In Rivne, the above associations have not been documented thus far. On the other hand, the well-known association of gastroschisis with younger maternal age at delivery is evident in Polissia, Rivne-nP as well as in the provinces adjoining Rivne (Table S8) (Curry et al. 2006). Also, gastroschisis is reported to be prevalent among males (Boyadjiev et al. 2004; Gambhir et al. 2007), although in Rivne, the M-F proportion is 16-19 and in Polissia and Rivne-nP is 5-12 and 11-7, respectively.
The array of cCM investigated, based on the number of observations, should be expanded to include instances of thoraco-abdomino-schises and ectopia cordis. The estimated rate of ectopia cordis in Rivne is, at a minimum 0.48 or several-folds higher than those reported by other studies (Carmi and Boughman 1992; Botto et al. 2011). Regarding caudal blastopathies, the perspective is complex. In addition to exstrophy of the urinary bladder, recto-anal anomalies and sacro-coccygeal teratomas, also noted are instances of combinations of exstrophy of the cloaca, sacral dysgenesis, sirenomelia, and lower limb dysgenesis. Furthermore, an analysis by Kallen et al. (1991) underscores that sirenomelia and cyclopia are prevalent among females and twins, which is conducive to considerations concerning holoprosencephaly, which was excluded from this analysis.
A salient characteristic, probably of most blastopathies, is that survivors, including individuals with the most severe CM complexes, can develop their full intellectual potential (Opitz et al. 2002). This fact is illustrated by a girl with severe caudal dysplasia (arj-2) and by an individual with sirenomelia studied by Pinette et al. (2005).
Teratogenic risks
This descriptive epidemiology study is not designed to investigate cause-effect relationships although it does provide some clues to orient prospective cause-effect investigations and prevention interventions. Three categories of teratogenic risk factors were addressed by surveys included in this analysis: gene mutations, alcohol, and IR. Regarding gene mutations, our concern is that Polishchuks represent a population isolate, a condition that would result in higher rates of consanguinity and homozygocity. This view is sustained by the survey of isonomy rates of county-specific family surnames of newborns. Isonomy rates are an indirect index of endogamy and consanguinity and the analysis summarized in Figure S1 shows that isonomy rates are highest in three northern counties of Polissia (Colantonio et al. 2003). The implications of this observation are described further in the context of nosology considerations.
Regarding alcohol teratogenesis, a survey of alcohol consumption by pregnant women and an analysis of Fetal Alcohol Spectrum Disorders frequencies demonstrated that neither is prevalent in Polissia. The results indicate that alcohol is unlikely to be a main teratogenic cause of the higher rates of microcephaly and mOPH noted in Polissia (Tables 5,S2 and S3).
Regarding IR, it is undeniable that since 1986, pregnant women and children in Polissia continue to be exposed to IR. Official estimates of levels of IR are based on measurements reflecting 137Cs, and reports rarely emphasize contrasts between Polissia and the rest of Rivne. Estimates of ingestion of 137Cs are, with few exceptions, not based on diets consumed in Polissia. With these considerations in mind, our past surveys demonstrated that in addition to ingestion, inhalation is an additional mode of incorporation of IR. In Polissia, smoke from frequent forest fires, incineration of biomass after harvests, and burning wood for cooking and heating are prevalent. Another prominent source of exposure to IR in Polissia is consumption of water from shallow wells, local fish, wild mushrooms and berries, and locally produced potatoes and dairy products (Dancause et al. 2010). We calculated the daily intake of 137Cs by pregnant women to be 268 Bq. Other investigators calculated a daily intake in Rivne at 571 Bq, the highest among 25 regions investigated in Ukraine, including Kyiv and Zhytomyr, which are most proximal to the Chornobyl site (Shiraishi et al. 2008). Such estimates are above the recommended 210 Bq daily upper limit for adults advocated by the Ukrainian Ministry of Health (Tables 9) (Decree 106, 1991). In Ukraine, the highest index of soil-to-food chain transfer of 137Cs is in Polissia (Likhtarev et al. 1996, 2000). Our surveys also show that in fact, nearly 50% of pregnant women residing in northern (distant) Polissia have incorporated levels of 137Cs above the upper limits considered as safe by the Ukrainian Ministry of Health (Table 7 and Fig. S2). Furthermore, these measurements of incorporated 137Cs do not account for additional incorporated nuclides such as 90Sr found in Polissia plants as demonstrated by our survey of potato plants, which represent a major dietary staple.
Skepticism and denials of teratogenic impacts of Chornobyl IR are centered on matters of dosimetry. The official view of many agencies is summarized in a 2006 Joint News Release by WHO/IAEA/UNDP (World Health Organization, International Atomic Energy Agency and the United Nations Development Programs) which asserts “… Because of the relatively low doses to residents of contaminated territories, no evidence or likelihood of … effect on the number of stillbirths, adverse pregnancy outcomes, delivery complications or overall health of children … A modest but steady increase in reported congenital malformations … appears related to better reporting, not radiation” (Hoffman and Fleming 2005; IAEA 2006). Until the present, such assertions remained untested by independent population-based investigations of well-defined populations living in relative proximity to the Chornobyl site and using established international methods. This report takes into account the manifest pervasive skepticism and presents the observations in Rivne in detail, which is further expanded in a Data Supplement.
Official assertions generally rest upon interpretation of investigations sponsored by ABCC (Atomic Bomb Casualty Commission) in Hiroshima and Nagasaki. Among the most influential ABCC sponsored investigations are the classic reports by Professor J. Neel and associates. Virtually all such investigations were focused on genetic rather than teratogenic impacts of the atomic blasts. The studies were initiated nearly 5 years after the blasts and concerned children unexposed to radiation whose parents survived the post-war circumstances. The investigation showed that the radiation impacts on parents were not statistically significantly associated with higher rates of congenital malformations in their offspring (Neel 1958, 1994; Neel and Schull 1991). We know of two sets of ABCC sponsored investigations focused on teratogenic impacts of the atomic bombs. The first investigation concerned 205 nearly 5-year-old children exposed in utero to the bomb blasts. Clinical examinations without a concurrent control group showed that 24 (12%) had anomalies including two instances of Down syndrome, six (3%) instances of microcephaly associated with mental subnormality, four instances of dislocations of the hip, three children were suspected of having congenital heart alterations; and nine others who had a variety of anomalies, some of which would currently not be categorized as CM (Plummer 1952). Another set of investigations was focused on mental retardation. The corresponding reports include information on microcephaly but do not focus on concurrent CM (Wood et al. 1967a, 1967b, 1967c; Otake and Schull 1984, 1998; Schull and Otake 1999). The study group consisted of 1613 children who were exposed to the atomic blasts during various states of gestation. The study group was subdivided according to the distance of the parent from the hypocenter of the blasts. The subjects were grouped as proximal to the blast (within 2000 m) and distal (beyond 3000 m). Children exposed in the 2001–2999 m category were excluded from the study “to create a distinct distance between the proximal and distal groups”. Significant effects were evident among those who survived infancy and were exposed at 8–15 and 16–25 weeks after ovulation, namely, reduction of cognitive function, severe mental retardation, and reduction of head size or frank microcephaly defined as head circumferences less than two SD from the mean. In 1987, it became possible to assign updated DS86 dose estimates to individual cases. Such an analysis estimated a decrease in IQ score at 25–29 IQ points per GY estimated uterine absorbed dose. The authors noted that doses as low as 10 cGY can impact migration of neocortical neurons. These investigations also established the widely accepted notion that the period of maximum sensitivity to radiation induced human cerebral anomalies is between 8 and 15 weeks of gestation. However, the investigators also point out that with respect to microcephaly, such a generalization did not hold.
Following the 1986 Chornobyl disaster, a series of clinical observations indicated a rise of the frequency of CM, in particular anencephaly. The impact of these reports was reviewed and eclipsed by an analysis of CM rates among populations residing in regions of Western Europe remote from Chornobyl. No evidence of a generalized detectable increase of the prevalence on CM within 2 years following the Chornobyl disaster was evident (Dolk and Nichols 1999). Regarding IR exposures from nuclear power plants, three investigations are of particular interest. Two independent and nearly concurrent investigations sponsored by the CDC (US Centers for Disease Control and Prevention) sought to determine teratogenic impacts of IR stemming from the Hanford Nuclear complex in Washington State. One investigation detected higher NTD rates in two counties proximal to the nuclear complex and the second investigation demonstrated higher rates of NTD associated with parental occupational exposures to low-level IR. The investigators considered the research results to be sound but rejected them as “falsely positive conclusions”. The results of the first study were rejected mainly because they could not be explained by reported patterns of IR emissions from the plant. The results of the second investigations were rejected because they were contradictory to conclusions drawn from the cited ABCC investigations (Sever et al. 1988a, 1988b). A third investigation concerned fathers employed at the Sellafield Nuclear Complex in the Cumbria region of Northern England. The results showed a positive association between total exposure to external IR before conception and a higher risk for stillbirths with congenital anomaly and highest for stillbirths with NTD (Parker et al. 1999). To our knowledge, this investigation has not been repeated. However, we note that the rates of NTD, microcephaly, mOPH, and conjoined twin rates in Northern England and Wales reported to EUROCAT are concurrently among the highest in the UK and Europe and approach the rates noted in Rivne (Table S5). It is relevant to note that the impact of the Chornobyl fallout on the UK was particularly significant on regions in Cumbria in Northern England, Wales, and Southwest England (Gillett et al. 2001). In the context of slightly reduced head circumferences among neonates born in Polissia compared to those born in Rivne city, two independent studies in Scandinavia, another region of Europe significantly impacted by Chornobyl IR, drew our attention. Both investigations, one in Norway and the other in Sweden, note that individuals most exposed in utero to Chornobyl IR show significant negative impacts on their cerebral functions (Almond et al. 2009; Heiervang et al. 2010). The observations in Scandinavia combined with those in Polissia indicate that further investigation of OFC measurements among Rivne neonates and children need to be expanded.
Pathogenesis
The nature of the cCM observed in Rivne is expertly reviewed and summarized by Warkany (1971) and Stevenson and Hall (2006). Likewise, a review concerning the impact of twinning is elegantly presented by Phelan and Hall (2006). A large study by Windham and Sever (1982) explored in detail the NTD-female and NTD-twinning preferential associations. The notion that blastopathies reflect an altered process of twinning was advanced by Schinzel et al. (1979), and this has been sustained by other investigators (Czeizel and Opitz 1981; Carmi and Boughman 1992; Martinez-Frias et al. 1997). This notion is rooted in embryologic observations summarized by Newman (1917, 1923). Newman and other students of embryogenesis established that in vertebrates, early cleavage cells appear to be totipotent and essentially are germinal in character and able to become a new apical point to establish new axes of polarity and symmetry. Newman highlighted experiments by Spemann and Falkenberg (1919) demonstrating that twins may be produced experimentally by various means that lower the embryonic developmental rate. Clinical observations combined with the notion of “arrested or delayed development” led to propositions of impacts of anomalous X chromosome inactivation patterns proposed by James (1988) and more recently reviewed by Curry et al. (2006, chapter 23).
In the context of failures of neural tube closure being more prevalent among females, Seller (1987, 1995) points out that the neural tube is formed before gonadal differentiation and that although both sexes start to cleave concurrently, males reach the blastocysts stage before females which may result in a greater propensity for NTD among females. There is evidence that in the mouse, males reach the blastocysts stage before females and that in bovine embryos, females develop slightly slower than males, observations that led to the notion of “epigenetic lag” or “developmental drag” which impact the process of neural folding and may be attributed to the inactivation of an X chromosome in females. Further investigations suggest that the female excess among human instances of anencephaly or cranial-NTD probably are not due to gonadal hormones or slower overall female developmental rate or an excess of male deaths, but instead, probably reflect the presence of two X chromosomes, a cause of what is referred to as an “inactive X sink”. This proposition points out that females methylate most of the DNA in the inactive X chromosome after every cell division (“inactive X sink”), which reduces methylation elsewhere (Juriloff and Harris 2012). Some investigators speculate that the “inactive X sink” relates to the female “development drag”. Currently, such issues are in the research domain of molecular embryology and planar cellular polarity (PCP) concerned with epithelial cellular signaling mechanisms controlling early developmental stages. Disturbances of the PCP process are conducive to neoplasia and prenatally, to alterations of the gastrulation process, twinning, somite and cilia development, duplications, anomalies of usual symmetry or asymmetry patterns, failures of NTD or body wall closures, and CM syndromes such as Meckel-Gruber and situs inversus, among others (Ueno and Greene 2003; Wallingford 2006; Wu et al. 2011; Vandenberg and Levin 2011; Yang 2012). Investigations show that impacts on PCP may alter patterning and polarity of neurons, perhaps a point relevant to the observed elevated rates of microcephaly in Polissia. In any case, the Polissia high rates of pentad-cCM are not associated with an increased frequency of monozygotic twinning, while higher rates of conjoined twins are associated with high rates of pentad-cCM. It appears that the causes of monozygotic twinning per se and alterations of the process giving rise to conjoined twins and blastopathies may reflect distinct risk factors.
Female prevalence among instances of sacro-coccygeal teratomas and conjoined twins is well established. There are occasional observations of individuals with an incorporated conjoined-twin or fetus-in-fetu and a concurrent teratoma. It is unlikely that such concurrent anomalies represent independent events particularly in instances where DNA markers were identical (Du Plessis et al. 1974; Spencer 2001; Gilbert-Barness et al. 2003; Higgins and Coley 2005). The notion of “fetus-fetus” as a destructive process was elaborated upon by Knox (1974) who proposed that it is a cause of NTD reflecting the destruction of one fetus with the surviving fetus having NTD anomalies. A linkage of anencephaly, spina bifida, twins and teratoma was proposed by Rogers (1976) who, in brief, notes that twins may develop epithelial defects that may be conducive to embryonic adhesions and destruction of the interamniotic partition resulting in a secondary mono-amniotic pregnancy. Thus monozygotic or dizygotic pregnancies are liable to undergo fetus-fetus destructive interactions. Under such circumstances, one embryo may be reabsorbed or “vanish” and the remaining twin become malformed or a teratoma. Such hypotheses are consistent with observations that NTD affected twins are usually discordant for the anomaly and generally sex-concordant. This assertion is sustained by observations in Rivne where among eight twin-NTD discordant pairs, six were concordant for sex (Table S4). Edmonds and Hawkins (1941) noted that the incidence of twinning whether measured by the percentage of families with twins or by the ratio of twin births to total births is similar and consistently high in families with childhood teratomas – a suggestion that the pathogenesis of teratomas and twinning includes shared elements. This observation is also consistent with observations in Rivne of high frequency of twinning events among relatives of NTD and conjoined twins individuals (Figs 3 and S4). These hypotheses were framed with awareness that the frequency of NTD and dizygotic twins among populations are highly variable and that the rates of both are correlated. Most provocatively, a significant and unexplained correlation in frequencies of NTD and dizygous twinning was detected by one of the earliest investigations of these anomalies implemented by the World Health Organization in 24 centers in 16 countries (Stevenson et al. 1966). Another large investigation by Windham and Sever (1982) demonstrated that: NTD were more frequent among twins than among singletons among whom the M : F ratios were 0.55 and 0.77, respectively; that among female twins, NTD were more frequent than among singleton females; that the lowest M : F ratio was 0.36 observed among anencephalic twins; and that a reversal toward male prevalence was evident among twins with spina bifida among whom the M : F ratio was 5.0 compared to 0.93 among singletons with spina bifida. The female sex bias does not apply to all sites of the NTD axis, which led Seller (1987) and later Hall et al. (1988) to underscore that among putative pathogenic mechanisms of cephalad-NTD (defects above the first lumbar vertebra and including encephaloceles) reflect defects of neurulation in contrast to lumbo-sacral spina bifidas, which are anomalies of canalization of the neural caudal cord. Garabedian and Fraser (1994) proposed a maternal factor as a cause of a higher frequency of NTD among maternal than paternal relatives of patients with “high” (above the first lumbar vertebra) NTD. According to these investigators, NTD among relatives may reflect a maternal factor since such instances are more common among maternal relatives. A similar pattern is evident in Rivne (Fig. 3) and was also noted by Marriman and Hamel (1992). Familial twinning is also evident among relatives of conjoined twins (Fig. S4).
An analysis of 16 instances of sirenomelia by Thottungal et al. (2010) led to their conclusion that caudal dysplasia and sirenomelia have a single pathogenesis. A review by Orioli et al. (2011) confirmed such a view and noted a sirenomelia-twinning association in 9% of patients. A review by Siebert et al. (2005) correlated caudal duplications with the vanishing twin phenomenon, conjoined twinning, caudal dysplasia, sirenomelia, and OEIS. A clinical illustration of this concept was reported by Fowler (1998) who studied an individual with a large OM, duplicated lower spine, and an intra-abdominal sack containing a skin covered “leg”. An analysis of monozygotic twins discordant for body stalk anomalies led Daskalakis et al. (1997) and Daskalakis and Nicolaides (2002) to postulate an early amnion rupture before obliteration of the celomic cavity. The rupture allows parts of the fetal body to pass through the membranes out of the amniotic cavity into the celomic cavity leading to structural defects and amniotic bands associated with body wall defects, encephalocele, CL/P and limb amputations as well as a short umbilical cord and a fetus almost attached to the placenta. Among alternative hypotheses, an abnormal embryonic folding and persistence of the celomic cavity may render the amniotic cavity prone for rupturing. Such twins, if incorporated, may have characteristics of conjoined twins, fetus-in-fetu, fetiform tumor or teratoma (Phelan and Hall 2006; Dhingra et al. 2008; Fuentes et al. 2010). A demised twin may result in the “vanishing twin” phenomenon or be incompletely resorbed by the host and disrupt the development of the surviving twin. Daskalakis and Nicolaides (2002) suggest that incorporated blighted conjoined twins may be a cause of cloacal exstrophy, which are strongly associated with OM and twinning (Martinez-Frias et al. 2001; Casale et al. 2004; Siebert et al. 2005). A female prevalence among patients with exstrophy of the cloaca was also observed by Caton et al. (2007). A concordant view was formulated by Feldkamp et al. (2011), who noted that cloacal exstrophy is etiologically heterogeneous, more frequent among monozygotic twins, and recurrent in families.
In summary, the observations in Rivne sustain the view that conjoined twins, sacro-coccygeal teratomas, cephalad-NTD, isolated microcephaly and their association with female prevalence and among twins probably are outcomes of shared mechanisms.
Etiology
The teratogenic risk factors manifesting in the reported rates and patterns of cCM remain unknown. Among three principal teratogenic risk factors and the higher rates of cCM in Polissia, our surveys indicate that alcohol is an unlikely candidate. Concerning genomic mutations associated with higher sensitivity to teratogens, in particular IR, their frequency in Polissia may be higher but have not been directly investigated (Ziolkowska et al. 2006). The impact of folate deficiencies on DNA repair and rates of NTD remain to be investigated in Polissia and Ukraine in general. Recent observations of radiation induced transgenerational inheritance of genomic instability have focused attention on the role of hypomethylation of DNA. Such discoveries, as summarized below, provide an increasingly complete framework linking modest IR exposures and other environmental factors with twinning and blastopathies as well as associations with preferential sex. Studies show that the NTS1 Slavic or NBN (c.657_651del5) mutation is more prevalent in regions proximal to Polissia. Carriers of this mutation associated with the Nijmegen chromosome breakage syndrome (NBS) are more sensitive to IR DNA damage and suffer the consequences of impeded double-stranded DNA repair manifested as a large array of CM and higher cancer risks. The frequency of this mutation in the eastern part of Wielkopolska province in Poland and in adjoining Ukraine is estimated at 1.3% (Ziolkowska et al. 2006; Chrazanowska et al. 2012). Also, among a plethora of reports and reviews, of direct interest are those of Dubrova et al. (1996) and Pils et al. (1999) who noted that exposure to low dose radiation resulted in minisatellite DNA mutations and could be followed by teratogenic effects in the subsequent generation; Morgan et al. (2002) proposed that radiation induces factors that stimulate production of reactive oxygen species that induce genomic and chromosomal instability over multiple cell cycles; Kovalchuk and Baulch (2008) noted that bystander IR effects can span several generations and influence the progeny of exposed parents associated with altered DNA methylation patterns; Filkowski et al. (2010) noted hypomethylation and genome instability in the germ line of exposed parents (paternal germ lines are more sensitive) associated with altered miRNA expression; the integration in Developmental Biology of epigenetic transgenerational inheritance with genetics is reviewed by Skinner (2011) in particular, regulatory DNA methylation such as X chromosome inactivation as well as impacts of environmental disruptors of erasure of DNA methylation during gonadal sex determination; Hales et al. (2011) reviewed the epigenetic marks, DNA methylation, histone modifications and noncoding RNAs critical role in cell memory during the development of gametes to blastocysts; excess risk for monozygotic twinning after IVF (in vitro fertilization) was noted by Ericson and Kallen (2001); increased risk of blastogenesis related malformations after ART (assisted reproductive technologies) procedures was demonstrated by Ben-Ami et al. (2011). These hypotheses and those alluded to earlier are generally consistent with the view that blastopathies result from embryonal developmental “delays”. This notion was proposed as early as 1651 by William Harvey in his “De Generatione Animalium” which followed his teacher's Fabricius ab Aquapentende (1537–1610) report on “De Formato Foetu”. During the second half of the eighteenth century, von Haller and C. F. Wolff applied the principle of arrested development to explain ectopia cordis and exomphalos along with experimental studies of “duplicity” and twinning, which strengthened the notion of arrested development as epigenetic in nature. One landmark is the 1921 voluminous monograph “Developmental rate and Structural Expression” by Stockard and given a wider perspective by Newman (1923). It was Ballentyne (1904, chapter XI, page 158) who described human blastophaties and asserted that embryologic theories dealt with pathogenesis and not etiology. The incorporation and expansion of the notion of arrested development in Medical Teratology and Pathology is reviewed by Willis (1962, chapter 4, page 132) and in Clinical and Experimental Teratology by Warkany (1971, chapter 2, page 17). A review of current molecular hypotheses of epigenetic mechanisms impacting neural fold development conducive to elevated rates and female excess of NTD as observed in Polissia, is presented, among others, by Juriloff and Harris (2012).
The persistent learned debate of in utero exposures to low-levels of IR impacts on human health became invigorated by the investigations of Alice Stewart (2000). Her studies led her to conclude that the ABCC sponsored investigations of the acute injuries of atomic bomb survivors “are biased in favor of exceptionally low levels of radiosensitivity”. Such a conclusion poses a challenge to official assertions concerning health impacts related to Chornobyl IR exposures. This view is underscored by an early classic investigation by MacMahon (1962) who noted that in utero exposure to diagnostic doses of X-ray increased subsequent mortality from neoplastic disease during childhood. A cluster of childhood leukemia noted in the vicinity of the Krummel nuclear plant near Hamburg, Germany, led the government to sponsor an investigation that in 1992 found a statistically significant threefold increase of leukemia risk for children below age 5 residing in the 5 km zone of 20 German nuclear facilities. In 1997, a second official study reported a 50% increased leukemia risk for this target group, which was only marginally significant (P = 0.060). In 1999, Korblein and Hoffmann (1999) re-analyzed the same data but only for the 15 sites of operating German nuclear power plants, omitting five small research facilities and decommissioned plants. They discovered a significant 76% increased risk at the P < 0.01 level. These controversial results prompted the German government to sponsor the “Epidemiologische Studie zu Kinderkrebs in der Umgebung von Kernkraftwerken” known by the acronym KiKK. The results of this case-control study, a 120% increase relative leukemia risk for children under five in the 5 km zone, were published by Kaatasch et al. in 2008 (Kaatasch et al. 2008). But they also state that “based on the available information about radiation emissions from German nuclear power plants, a direct relation to radiation seems implausible. Many factors may conceivably cause leukemia …”. Fairlie (2009) suggests that leukemia in children under five is induced in utero and that the effect could be teratogenic in nature rather than stochastic. In Polissia, until proven otherwise, it is prudent to view the persistent incorporation of nuclides by pregnant women, both as teratogenic and oncogenic.
In the field of radiobiology, dilemmas such as those mentioned above are relatively common and call for adherence to the “precautionary principle” endorsed by the majority of the scientific community. Under this principle, those who dictate or advocate policies in the absence of scientific evidence or consensus have the responsibility to demonstrate that their proposed, imposed, or advocated policies, including those by silence or unresponsiveness, are not harmful to the public or the environment. Arguably, official assertions that Chornobyl IR is not teratogenic contradict this precautionary principle. In any case, the repeated unsubstantiated official denials of teratogenic impacts or even the possibility of such impacts posed by Chornobyl had a chilling effect on initiatives to investigate their validity. In terms of the Fukushima disaster, it is self-evident that the circumstances, ecology and populations are distinct from those in Rivne. On the other hand, it is also evident that IR is a measurable teratogen that impacts in a similar manner human embryos anywhere. It is prudent to assume that whatever is learned concerning the teratogenic impacts in Polissia may be relevant in the context of Fukushima and elsewhere.
Prevention
In Rivne, specific prevention interventions are needed to reduce the high rates of NTD and other CM, to reduce the high levels of incorporated IR by pregnant women and others, and to reduce exposures to alcohol teratogenesis. The existing population-based CM monitoring program can facilitate concurrent investigations of synergistic impacts of risk factors and effectiveness of their interruption by prevention-interventions. Likewise, such initiatives can expand conclusions of prior investigations including those that have shown that maternal alcohol consumption impedes absorption of folates and has no impact on NTD rates (Halsted et al. 2002; Makelarski et al. 2013). Assessment of the proven reduction of NTD rates by folic acid supplementation programs, in the context of IR exposures in Polissia, are likewise of considerable interest (Berry et al. 2010).
Conclusions
The results of this descriptive epidemiological study provide a starting point for prospective investigations of cause-effect associations. The strengths and weaknesses of this investigation can be described with consideration of the well-known principles set by Sir Austin Bradford Hill (1965) as criteria defining causal relationships from observed associations, in this instance, the association in Polissia of high rates of pentad-cCM with Chornobyl IR. Among the strengths of this study are: (i) adherence to prevalent international methods; (ii) concurrent study of two distinct large populations of similar size, only one of which is chronically exposed to IR; (iii) concurrent statistically significantly elevated population-based rates of cCM; (iv) the array of elevated cCM in Polissia includes those known to result from experimental and human exposures to IR; and (v) elevated incorporated IR levels in pregnant women are detected solely among those living in Polissia. Concerning plausibility, three teratogenic risks are of concern in Rivne: alcohol, genomic mutations, and IR. As noted earlier, alcohol teratogenesis is not prevalent in Polissia and genomic mutations are unlikely to cause the blastopathies observed. The concurrence of elevated rates of cCM with elevated IR levels in Polissia lends coherence to a hypothetical cause-effect association. Advances in molecular biology and embryology suggest that the cCM-IR association may reflect altered methylation processes associated with sex, greater sensitivity of paternal germ lines to some teratogens, and potential causes of genomic instability. Among the weaknesses of this investigation are: (i) lack of CM data prior to the onset of this investigation; (ii) lack of data of actual levels of parental incorporated IR of infants with cCM compared to matched controls; (iii) limited data concerning prenatal loss of conceptions; and (iv) limited data concerning cCM, microcephaly and reduced head size beyond 12 months of age.
In summary, the present descriptive investigation documents higher population-based rates of some CM in a population isolate impacted by Chornobyl IR. In our view, prospective cause-effect investigations by national and international scientists are warranted.
Acknowledgments
The authors thank Drs Ralf M. Garruto (Biomedical Anthropology program, Binghamton University, SUNY, Binghamton, NY) and Yuri S. Korzhynskyy (Department of Pediatrics, Lviv School of Medicine) for their helpful discussion. These studies were supported by the OMNI-Net for Children International Charitable Fund.
Supporting Information
Fig. S1 County Isonomy Rates of Neonates (Aggregate Frequency of Five Most Common Family Names).
Fig. S2 Whole Body Counts of incorporated ionizing radiation (Bq 137Cs) among 6026 pregnant women residing in Rivne Province (2008–2011). The (*) indicates the official upper norm for children under the age of 15 years (3700 Bq). (Decree #106 of Cabinet of Ministers of Ukrainian Soviet Socialist Republic of July 23, 1991. – See references).
Fig. S3 Birth weight of liveborns of all gestational ages (2000–2009) in Polissia and non-Polissia regions of Rivne province of Ukraine.
Fig. S4 Twinning Events among Relatives of Conjoined Twins (2000–2010).
Table S1a Temporal Contrasts between Polissia (POL) and non-Polissia (nPOL)(1) of Population-Based Rates of Core Congenital Malformations.
Table S1b Temporal Contrasts within Polissia and non-Polissia(1) of the Number of Observed Individuals with Core Congenital Malformations.
Table S1c Unduplicated Individuals with Core Congenital Malformations after Exclusion of Those with Clinically Recognizable Malformation Syndromes, Genomic Mutations, Cytogenetic Anomalies and Recognizable Teratogenic Effects(1).
Table S1d Overview of Unduplicated Individuals in Polissia and non-Polissia with Non-Syndromic Congenital Malformations(1) or Sentinel Anomalies (2000–2009).
Table S2 Total Number of Malformations and Rates per se (not Unique Individuals) and Male-Female (MF) Proportions and Ratios (M : F) in Polissia and non-Polissia(a).
Table S3 All Individuals with Fetal Alcohol Spectrum Disorder (FASD). Non-Population-Based Observations.
Table S4 All Non-Singletons Including Those with Congenital Malformations (2000–2009)(1).
Table S5 Population Rates of Neural and Other Malformations (not Individuals) in the Polissia and non-Polissia Regions of Ukraine and other regions of Europe.
Table S6 Teratomas – Rivne, Volyn (2000–2009) and Khmelnytsky (Kh) Provinces (2002–2009)(a).
Table S7 Non-Syndromic NTD Associated with Omphaloceles (OM), Body Wall and Other Anomalies in Rivne, Khmelnytsky and Volyn Provinces (2000–2009)(a).
Table S8 Gastroschisis and Maternal Age at Delivery: Rivne and Volyn Provinces (2000–2009) and Khmelnytsky Province (2002–2009).
Table S9 Encephaloceles and Male-Female (M-F) Proportions.
References
- Abdel-Salam G, Czeizel A. A case-control etiologic study of microcephaly. Epidemiology. 2000;11:571–575. doi: 10.1097/00001648-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Almond D, Edlund L, Palme M. Chernobyl's subclinical legacy: prenatal exposure to radioactive fallout and school outcomes in Sweden. Quart J Econ. 2009;124:1729–1772. [Google Scholar]
- Arenson A, Bakhireva L, Chambers C. Implementation of a shared data repository and common data dictionary for fetal alcohol spectrum disorders research. Alcohol. 2010;44:643–647. doi: 10.1016/j.alcohol.2009.08.007. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballentyne JW. Manual of antenatal pathology and hygiene. The embryo. Edinburgh: W. Green & Sons; 1904. . 697 p. [Google Scholar]
- BEIR V Report. Biologic effects of ionizing radiations. Health effects of exposure to low levels of ionizing radiation. Committee on Biological Effects of Ionizing Radiations. Board on Radiation Effects Research Commission on Life Sciences. National Research Council. Washington, DC: National Academy Press; 1990. [Google Scholar]
- Ben-Ami I, Edel Y, Vaknin Z, Herman A, Maymon R. Do assisted conception twins have an increased risk for anencephaly? Hum Reprod. 2011;26:3466–3471. doi: 10.1093/humrep/der326. [DOI] [PubMed] [Google Scholar]
- Berry RJ, Bailey L, Mulinare J, Bower C. Fortification of flour with folic acid. Food Nutr Bull. 2010;27:522–530. doi: 10.1177/15648265100311S103. [DOI] [PubMed] [Google Scholar]
- Botto LD, Feldkamp ML, Amar E. Acardia: epidemiologic findings and literature review from the International Clearinghouse of Birth Defects Surveillance and Research. Am J Med Genet C Semin Med Genet. 2011;157C:262–273. doi: 10.1002/ajmg.c.30318. et al. [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Dodson JL, Radford CL. Clinical and molecular characterization of the bladder exstrophy-epispadias complex: analysis of 232 families. BJU Int. 2004;94:1337–1343. doi: 10.1111/j.1464-410X.2004.05170.x. et al. [DOI] [PubMed] [Google Scholar]
- Bugge M. Twins with omphalocele in Denmark (1970–1989) Am J Med Genet A. 2010;152A:2048–2052. doi: 10.1002/ajmg.a.33494. [DOI] [PubMed] [Google Scholar]
- Bugge M. Body stalk anomaly in Denmark during 20 years (1970–1989) Am J Med Genet A. 2012;158A:1702–1708. doi: 10.1002/ajmg.a.35394. [DOI] [PubMed] [Google Scholar]
- Calzolari E, Bianchi F, Dolk H, Milan M. Omphalocele and gastroschisis in Europe: a survey of 3 million births 1980–1990. EUROCAT Working Group. Am J Med Genet. 1995;58:187–194. doi: 10.1002/ajmg.1320580218. [DOI] [PubMed] [Google Scholar]
- Calzolari E, Bianchi F, Dolk H, Stone D, Milan M. Are omphalocele and neural tube defects related congenital anomlaies?: data from 21 registries in Europe (EUROCAT). EUROCAT Working Group. Am J Med Genet. 1997;72:79–84. [PubMed] [Google Scholar]
- Carmi R, Boughman J. Pentalogy of Cantrell and associated midline anomalies: a possible ventral midline developmental field. Am J Med Genet. 1992;42:90–95. doi: 10.1002/ajmg.1320420118. [DOI] [PubMed] [Google Scholar]
- Casale P, Grady R, Waldhausen J, Joyner B, Wright J, Mitchell M. Cloacal exstrophy variants. Can blighted conjoined twinning play a role? J Urol. 2004;172:1103–1107. doi: 10.1097/01.ju.0000142108.62457.81. [DOI] [PubMed] [Google Scholar]
- Caton A, Bloom A, Druschel C, Kirby R. Epidemiology of bladder and cloacal exstrophies in New York state, 1983–1999. Birth Defects Res A Clin Mol Teratol. 2007;79:781–787. doi: 10.1002/bdra.20402. [DOI] [PubMed] [Google Scholar]
- Chrazanowska K, Gregork H, Dembowska-Baginska B, Kalina M, Digweed M. Nijmegen breakage syndrome (NBS) Orphanet J Rare Dis. 2012;7:1–19. doi: 10.1186/1750-1172-7-13. [online]. Available at: http://www.ojrd.com/content/7/1/13 [September 17, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantonio SE, Lasker GW, Kaplan BA, Fuster V. Use of surname models in human population biology: a review of recent developments. Hum Biol. 2003;75:785–807. doi: 10.1353/hub.2004.0004. [DOI] [PubMed] [Google Scholar]
- Curry C, Boyd E, Stevenson RE. Ventral wall of the trunk. In: Stevenson R, Hall JG, editors. Human malformations and related anomalies. 2nd edn. New York: Oxford University Press Inc; 2006. pp. 1377–1407. . In:, editors., Chapter 23. [Google Scholar]
- Cushieri A EUROCAT Working Group. Descriptive epidemiology of isolated anal anomalies: a survey of 4.6 million births in Europe. Am J Med Genet. 2001;103:207–215. doi: 10.1002/ajmg.1532.abs. [DOI] [PubMed] [Google Scholar]
- Czeizel A, Opitz J. Schisis-association. Am J Med Genet. 1981;10:25–35. doi: 10.1002/ajmg.1320100105. [DOI] [PubMed] [Google Scholar]
- Dancause K, Yevtushok L, Lapchenko S, Shevchenko G, Wertelecki W, Garruto R. Chronic radiation exposure in the Rivne-Polissia Region of Ukraine: implications for births defects. Am J Hum Biol. 2010;22:657–674. doi: 10.1002/ajhb.21063. [DOI] [PubMed] [Google Scholar]
- Daskalakis G, Nicolaides K. Monozygotic twins discordant for body stalk anomaly. Ultrasound Obstet Gynecol. 2002;20:79–81. doi: 10.1046/j.1469-0705.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- Daskalakis G, Sebire N, Jurkovic D, Snijders R, Nicolaides K. Body stalk anomaly at 10–14 weeks of gestation. Ultrasound Obstet Gynecol. 1997;10:416–418. doi: 10.1046/j.1469-0705.1997.10060416.x. [DOI] [PubMed] [Google Scholar]
- Decree 106. 1991. Regarding Realization of Decrees of Supreme Soviet of Ukrainian Soviet Socialist Republic about Realization of Laws of Ukrainian Soviet Socialist Republic concerning the Legal Status of Territories Polluted by the Chornobyl Catastrophe Radiation and regarding the Status and Social Protection of People Suffered from the Chornobyl Catastrophe. Decree Number 106 of Cabinet of Ministers of Ukrainian Soviet Socialist Republic of July 23, 1991 [online]. Available at: http://zakon.rada.gov.ua:80/cgi-bin/laws/main.cgi?nreg=106%E0-91-%EF&p=1236860994819643 [September 17, 2013] (in Ukrainian)
- Derom C, Vlietinck R, Derom R, Van den Berghe H, Thiery M. Population-based study on sex proportion in monoamniotic twins. N Engl J Med. 1988;319(2):119–120. doi: 10.1056/NEJM198807143190215. [DOI] [PubMed] [Google Scholar]
- Dhingra K, Mandal S, Roy S, Khurana N, Sarin Y. A Sacrococcygeal teratoma or conjoint parasitic twin: a diagnostic dilemma. Pathology. 2008;40:532–534. doi: 10.1080/00313020802197988. [DOI] [PubMed] [Google Scholar]
- Dolk H, Nichols R EUROCAT Working Group. Evaluation of the impact of Chernobyl on the prevalence of congenital anomalies in 16 regions of Europe. Int J Epidemiol. 1999;28:941–948. doi: 10.1093/ije/28.5.941. [DOI] [PubMed] [Google Scholar]
- Doyle P, Beral V, Botting B, Wale C. Congenital malformations in twins in England and Wales. J Epidemiol Community Health. 1990;45:43–48. doi: 10.1136/jech.45.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis J, Winship W, Kirstein J. Fetus in fetu and teratoma. S Afr Med J. 1974;48:2119–2122. [PubMed] [Google Scholar]
- Dubrova Y, Nesterov V, Kruochinsky N. Human minisatellite mutation rate after the Chernobyl accident. Nature. 1996;380:683–686. doi: 10.1038/380683a0. et al. [DOI] [PubMed] [Google Scholar]
- Duhamel B. Embryology of exomphalos and allied malformations. Arch Dis Child. 1963;38:142–147. doi: 10.1136/adc.38.198.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds H, Hawkins J. The relationship of twins, teratomas, and ovarian dermoids. Cancer Res. 1941;1:896–899. [Google Scholar]
- Ericson A, Kallen B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001;16:504–509. doi: 10.1093/humrep/16.3.504. [DOI] [PubMed] [Google Scholar]
- Fairlie I. Commentary: childhood cancer near nuclear power stations. Environ Health. 2009;8:43. doi: 10.1186/1476-069X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkamp M, Botto L, Amar E. Cloacal exstrophy: an epidemiologic study from the international clearinghouse for birth defects surveillance and research. Am J Med Genet C Semin Med Genet. 2011;157:333–343. doi: 10.1002/ajmg.c.30317. et al. [DOI] [PubMed] [Google Scholar]
- Filkowski J, Ilnytskyy Y, Tamminga J. Hypomethylation and genome instability in the germline of exposed parents and their progeny is associated with altered miRNA expression. Carcinogenesis. 2010;31:1110–1115. doi: 10.1093/carcin/bgp300. et al. [DOI] [PubMed] [Google Scholar]
- Fowler C. Intraabdominal leg: unique variant of split notochord syndrome. J Pediatr Surg. 1998;33:522–524. doi: 10.1016/s0022-3468(98)90104-x. [DOI] [PubMed] [Google Scholar]
- Fraumeni J, Li F, Dalager N. Teratomas in children: epidemiologic features. J Natl Cancer Inst. 1973;51:1425–1430. doi: 10.1093/jnci/51.5.1425. [DOI] [PubMed] [Google Scholar]
- Fuentes S, Moreno C, Tejedor R, Cabozali D, Benavent M, Gomez A. Highly differentiated sacrococcygeal teratoma. Internet J Pediatr Neonatol. 2010;12(1) [online]. ). Available at: http://ispub.com/IJPN/12/1/3204 [September 17, 2013] [Google Scholar]
- Gambhir L, Holler T, Muller M. Epidemiological survey of 214 families with exstrophy-epispadias complex (BEEC) J Urol. 2007;179:1539–1543. doi: 10.1016/j.juro.2007.11.092. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian B, Fraser C. A familial association between twinning and upper-neural tube defects. Am J Hum Genet. 1994;55:1050–1053. [PMC free article] [PubMed] [Google Scholar]
- Garruto R, Little M, James G, Brown D. Natural experimental models: the global search for biomedical paradigms among traditional, modernizing, and modern populations. Proc Natl Acad Sci USA. 1999;96:10 536–10 543. doi: 10.1073/pnas.96.18.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Barness E, Opitz J, Debich-Spicer D, Mueller T, Arnold S, Quintero R. Fetus-in-fetu form of monozygotic twinning with retroperitoneal teratoma. Am J Med Genet. 2003;120A:406–412. doi: 10.1002/ajmg.a.10153. [DOI] [PubMed] [Google Scholar]
- Gillett A, Crout N, Absalom J. Temporal and spatial predition of the radiocaesium transfer to food products. Radiat Environ Biophys. 2001;40:227–235. doi: 10.1007/s004110100107. et al. [online]. Available at: http://nora.nerc.ac.uk/17750/ [September 17, 2013] [DOI] [PubMed] [Google Scholar]
- Hales B, Grenier L, Lolancette C, Robaire B. Epigenetic programming: from gametes to blastocyst. Birth Defects Res A Clin Mol Teratol. 2011;91:642–665. doi: 10.1002/bdra.20781. [DOI] [PubMed] [Google Scholar]
- Hall J, Friedman J, Kenna A, Popkin J, Jawanda M, Arnold W. Clinical, genetic, and epidemiological factors in neural tube defects. Am J Hum Genet. 1988;43:827–837. [PMC free article] [PubMed] [Google Scholar]
- Halsted C, Villanueva J, Devlin A, Chandler C. Metabolic interactions of alcohol and folate. J Nutr. 2002;132:2367S–2372S. doi: 10.1093/jn/132.8.2367S. [DOI] [PubMed] [Google Scholar]
- Heiervang K, Mednick S, Sundet K, Rund B. Effect of low dose ionizing radiation exposure in utero on cognitive function in adolescence. Scand J Psychol. 2010;51:210–215. doi: 10.1111/j.1467-9450.2010.00814.x. [DOI] [PubMed] [Google Scholar]
- Higgins K, Coley B. Fetus in fetu and fetaform teratoma in 2 neonates. An embryologic spectrum? J Ultrasound Med. 2005;25:259–273. doi: 10.7863/jum.2006.25.2.259. [DOI] [PubMed] [Google Scholar]
- Hill A. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M, Fleming M. 2005. Chernobyl: the true scale of the accident: 20 years later, UN report provides definitive answers and ways to repair lives (press release) [online]. Available at: http://www.who.int/mediacentre/news/releases/2005/pr38/en/index1.html. [September 17, 2013]
- Hwang P, Kousseff B. Omphalocele and gastroschisis: an 18 year review study. Genet Med. 2004;6:232–236. doi: 10.1097/01.gim.0000133919.68912.a3. [DOI] [PubMed] [Google Scholar]
- IAEA. 2006. Report Chornobyl's legacy: health, environmental and socio-economic impacts and recommendations to the governments of Belarus, the Russian Federation and Ukraine [online]. Technical Report, International Atomic Energy Agency. Available at: http://www.iaea.org/Publications/Booklets/Chernobyl/chernobyl.pdf [September 17, 2013]
- James W. Anomalous X chromosome inactivation: the link between female zygotes, monozygotic twinning, and neural tube defects? J Med Genet. 1988;25:213–216. doi: 10.1136/jmg.25.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriloff D, Harris J. Hypothesis: the female excess in cranial neural tube defects reflects and epigenetic drag of the inactivating X chromsome on the molecular mechanisms of neural fold elevation. Birth Defects Res A Clin Mol Teratol. 2012;94:849–855. doi: 10.1002/bdra.23036. [DOI] [PubMed] [Google Scholar]
- Kaatasch P, Spix C, Jung I, Blettner M. Childhood leukemia in the vicinity of Nuclear Power Plants in Germany. Dtsch Arztebl Int. 2008;105:725–732. doi: 10.3238/arztebl.2008.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen B, Castilla EE, Lancaster PA. The Cyclops and the mermaid: and epidemiological study of two types of rare malformations. J Med Genet. 1991;29:30–35. doi: 10.1136/jmg.29.1.30. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen B, Cocchi G, Knudsen LB. International study of sex ratio and twinning of neural tube defects. Teratology. 1994;50:322–331. doi: 10.1002/tera.1420500503. et al. [DOI] [PubMed] [Google Scholar]
- Kallen B, Robert E, Harris J. The descriptive epidemiology of anophthalmia and microphthalmia. Int J Epidemiol. 1996;25:1009–1016. doi: 10.1093/ije/25.5.1009. [DOI] [PubMed] [Google Scholar]
- Keppler-Noreuil K, Gorton S, Foo F, Yankowitz J, Keegan C. Prenatal ascertainment of OEIS Complex/Cloacal Exstrophy – 15 new cases and Literature review. Am J Med Genet A. 2007;143A:2122–2128. doi: 10.1002/ajmg.a.31897. [DOI] [PubMed] [Google Scholar]
- Knox E. Twins and neural tube defects. Br J Prev Soc Med. 1974;28:73–80. doi: 10.1136/jech.28.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korblein A, Hoffmann W. Childhood cancer in the vicinity of German nuclear power plants. Med Glob Surviv. 1999;6:18–23. [Google Scholar]
- Kovalchuk O, Baulch J. Epigenetic changes and nontargeted radiation effects – is there a link? Environ Mol Mutagen. 2008;49:16–25. doi: 10.1002/em.20361. [DOI] [PubMed] [Google Scholar]
- Lary J, Paulozzi L. Sex differences in the prevalence of human birth defects: a population-based study. Teratology. 2001;64:237–251. doi: 10.1002/tera.1070. [DOI] [PubMed] [Google Scholar]
- Lee D, Cottrell J, Sanders R, Meyers C, Wulfsberg E, Sun C-CJ. OEIS complex (omphalocele-exstrophy-imperforate anus-spinal defects) in monozygotic twins. Am J Med Genet. 1999;84:29–33. [PubMed] [Google Scholar]
- Li S, Ford N, Meister K, Bodurtha J. Increased risk of birth defects among children from multiple births. Births Defects Res A Clin Mol Teratol. 2003;67:879–885. doi: 10.1002/bdra.10093. [DOI] [PubMed] [Google Scholar]
- Likhtarev I, Kovgan L, Vavilov S. Internal exposure from the ingestion of foods contaminated by 137Cs after the Chernobyl accident. Report 2. Ingestion doses of the rural population of Ukraine up to 12 y after the accident (1986–1997) Health Phys. 2000;79:341–357. doi: 10.1097/00004032-200010000-00002. et al. [DOI] [PubMed] [Google Scholar]
- Likhtarev IA, Kovgan LN, Vavilov SE. Internal exposure from the ingestion of foods contaminated by 137Cs after the Chernoby l accident. Report 1. General model: ingestion doses and countermeasure effectiveness for the adults of Rovno Oblast of Ukraine. Health Phys. 1996;70:297–317. doi: 10.1097/00004032-199603000-00001. et al. [DOI] [PubMed] [Google Scholar]
- MacMahon B. Prenatal x-ray exposure and childhood cancer. J Natl Cancer Inst. 1962;May(28):1173–1191. [PubMed] [Google Scholar]
- Makelarski J, Romitti P, Sun L. Periconceptional maternal alcohol consumption and neural tube defects. Birth defects research (Part A) Birth Defects Res A Clin Mol Teratol. 2013;97:152–160. doi: 10.1002/bdra.23122. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriman E, Hamel B. Sex ratios of affected and transmitting members of multiple case families with neural tube defects. J Med Genet. 1992;29:695–698. doi: 10.1136/jmg.29.10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Frias M, Frias J, Bermejo E, Rodriguez-Pinilla E, Urioste M. Epidemiologic analysis of the schisis association in the Spanish Registry of Congenital Malformations. Am J Med Genet. 1997;70:16–23. [PubMed] [Google Scholar]
- Martinez-Frias M, Bermejo E, Rodriguez-Pinilla E. Body stalk defects, body wall defects, amniotic bands with and without body wall defects, and gastroschisis: comparative epidemiology. Am J Med Genet. 2000;92:13–18. doi: 10.1002/(sici)1096-8628(20000501)92:1<13::aid-ajmg3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Frias M, Bermejo E, Rodriguez-Pinilla E, Frias J. Exstrophy of the cloaca and exstrophy of the bladder: two different expressions of a primary developmental field defect. Am J Med Genet. 2001;99:261–269. doi: 10.1002/ajmg.1210. [DOI] [PubMed] [Google Scholar]
- Mastroiacovo P, Kallen B, Knudsen L. Absence of limbs and gross body wall defects: an epidemiological study of related rare malformation conditions. Teratology. 1992;46:455–464. doi: 10.1002/tera.1420460510. et al. [DOI] [PubMed] [Google Scholar]
- Mastroiacovo P, Lisi A, Castilla E. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660–671. doi: 10.1002/ajmg.a.31607. et al. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44:635–641. doi: 10.1016/j.alcohol.2009.08.005. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown T, MacMahon B, Record R. An investigation of 69 cases of exomphalos. Am J Hum Genet. 1953;5:168–175. [PMC free article] [PubMed] [Google Scholar]
- Moore T, Nur K. An international survey of gastroschisis and omphalocele (490 cases) Pediatr Surg Int. 1986;1:105–109. [Google Scholar]
- Morgan W, Hartmann A, Limoli C, Nagar S, Ponnaiya B. Bystander effects in radiation-induced genomic instability. Mutat Res. 2002;504:91–100. doi: 10.1016/s0027-5107(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Mutchinick O, Luna-Munoz L, Amar E. Conjoined twins: a worldwide collaborative epidemiological study of the International Clearinghouse for Birth Defects Surveillance and Research. Am J Med Genet C Semin Med Genet. 2011;157C:274–287. doi: 10.1002/ajmg.c.30321. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. A study of major congenital defects in Japanese infants. Am J Hum Genet. 1958;10:398–445. [PMC free article] [PubMed] [Google Scholar]
- Neel JV. Physician to the gene pool: genetic lessons and other stories. New York: John Wiley and Sons Inc; 1994. . 457 p. [Google Scholar]
- Neel JV, Schull WJ. The children of atomic bomb survivors – a genetic study. Washington, DC: National Academy Press; 1991. . 511 p. [PubMed] [Google Scholar]
- Newman HH. The biology of twins. Chicago, IL: University of Chicago Press; 1917. [Google Scholar]
- Newman HH. The physiology of twinning. Chicago, IL: University of Chicago Press; 1923. [Google Scholar]
- Opitz J, Zanni G, Reynolds J, Jr, Gilbert-Barness E. Defects of blastogenesis. Am J Med Genet. 2002;115:269–286. doi: 10.1002/ajmg.10983. [DOI] [PubMed] [Google Scholar]
- Orioli I, Amar E, Arteaga-Vazquez J. Sirenomelia: an epidemiologic study in a large dataset from the International Clearinghouse of Birth Defects Surveillance and Research, and literature review. Am J Med Genet C Semin Med Genet. 2011;157:358–373. doi: 10.1002/ajmg.c.30324. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake M, Schull W. In utero exposure to A-bomb radiation and mental retardation; a reassessment. Br J Radiol. 1984;57:409–414. doi: 10.1259/0007-1285-57-677-409. [DOI] [PubMed] [Google Scholar]
- Otake M, Schull W. Radiation-related brain damage and growth retardation among the prenatally exposed atomic bomb survivors. Int J Radiat Biol. 1998;74:159–171. doi: 10.1080/095530098141555. [DOI] [PubMed] [Google Scholar]
- Parker L, Pearce M, Dickinson H, Aitkin M, Craft A. Stillbirths among offspring of male radiation workers at Sellafield nuclear reprocessing plant. Lancet. 1999;354:1407–1414. doi: 10.1016/S0140-6736(99)04138-0. [DOI] [PubMed] [Google Scholar]
- Pauniaho S, Heikinheimo O, Vettenranta K. High prevalence of sacrococcygeal teratoma in Finland – a nationwide population-based study. Acta Paediatr. 2013;102:e251–e256. doi: 10.1111/apa.12211. et al. [DOI] [PubMed] [Google Scholar]
- Phelan MC, Hall JG. Twins. In: Stevenson R, Hall JG, editors. Human malformations and related anomalies. 2nd edn. New York: Oxford University Press Inc; 2006. pp. 1377–1407. . In:, editors., Chapter 34. [Google Scholar]
- Pils S, Muller W, Streffer C. Lethal and teratogenic effects in two successive generations of the HLG mouse strain after radiation exposure of zygotes – association with genomic instability? Mutat Res. 1999;429:85–92. doi: 10.1016/s0027-5107(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Pinette M, Hand M, Hunt R, Blackstone J, Wax J, Cartin A. Surviving sirenomelia. J Ultrasound Med. 2005;24:1555–1559. doi: 10.7863/jum.2005.24.11.1555. [DOI] [PubMed] [Google Scholar]
- Plummer G. Anomalies occurring in children exposed in utero to the atomic bomb in Hiroshima. Pediatrics. 1952;10:687–693. . Available at: http://pediatrics.aappublications.org/content/10/6/687 [September 17, 2013] [PubMed] [Google Scholar]
- Reid CO, Hall JG, Anderson C. Association of amyoplasia with gastroschisis, bowel atresia, and defects of the muscular layer of the trunk. Am J Med Genet. 1986;24:701–710. doi: 10.1002/ajmg.1320240415. et al. [DOI] [PubMed] [Google Scholar]
- Rogers SC. Anencephalus, spina bifida, twins and teratoma. Br J Prev Soc Med. 1976;30:20–28. doi: 10.1136/jech.30.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, D'Armiento M, Angrisani P, Vecchione R. Limb body wall complex: a critical review and a nosological proposal. Am J Med Genet. 1993;47:892–900. doi: 10.1002/ajmg.1320470617. [DOI] [PubMed] [Google Scholar]
- Schinzel A, Smith D, Miller J. Monozygotic twinning and structural defects. J Pediatr. 1979;95:921–930. doi: 10.1016/s0022-3476(79)80278-4. [DOI] [PubMed] [Google Scholar]
- Schull W, Otake M. Cognitive function and prenatal exposure to ionizing radiation. Teratology. 1999;59:222–226. doi: 10.1002/(SICI)1096-9926(199904)59:4<222::AID-TERA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Seller M. Neural tube defects and sex ratios. Am J Med Genet. 1987;26:699–707. doi: 10.1002/ajmg.1320260325. [DOI] [PubMed] [Google Scholar]
- Seller M. Sex, neural tube defects, and multisite closure of the human neural tube. Am J Med Genet. 1995;58:332–336. doi: 10.1002/ajmg.1320580406. [DOI] [PubMed] [Google Scholar]
- Sever L, Hessol N, Gilbert E, McIntyre J. The prevalence at birth of congenital malformations in communities near the Hanford site. Am J Epidemiol. 1988a;127:243–254. doi: 10.1093/oxfordjournals.aje.a114800. [DOI] [PubMed] [Google Scholar]
- Sever L, Gilbert E, Hessol N, McIntyre J. A case-control study of congenital malformations and occupational exposure to low-level ionizing radiation. Am J Epidmiol. 1988b;127:226–242. doi: 10.1093/oxfordjournals.aje.a114799. [DOI] [PubMed] [Google Scholar]
- Shaw G, Carmichael S, Kaidarova Z, Harris J. Differential risks to males and females for congenital malformations among 2.5 million California births, 1989–1997. Birth Defects Res A Clin Mol Teratol. 2003;67:953–958. doi: 10.1002/bdra.10129. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Ko S, Ban-nai T. Dietary intakes of radioactive cesium for Ukrainians. J Radioanal Nucl Chem. 2008;275:411–415. et al. [Google Scholar]
- Siebert J, Rutledge J, Kapur R. Association of cloacal anomalies, caudal duplication, and twinning. Pediatr Dev Pathol. 2005;8:339–354. doi: 10.1007/s10024-005-1157-6. [DOI] [PubMed] [Google Scholar]
- Skinner M. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res C Embryo Today. 2011;93:51–55. doi: 10.1002/bdrc.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithells R, Chinn E, Franklin D. Anencephaly in Liverpool. Dev Med Child Neurol. 1964;6:231–240. doi: 10.1111/j.1469-8749.1964.tb10782.x. [DOI] [PubMed] [Google Scholar]
- Spemann H, Falkenberg H. Uberasymmetrische Entwicklung und Situs inversus viscerum bei Zwillingen und Doppelbildungen. Arch Entwick Org. 1919;45:371–422. [Google Scholar]
- Spencer R. Parasitic conjoined twins: external, internal (fetuses in fetu and teratomas), and detached (acardiacs) Clin Anat. 2001;14:428–444. doi: 10.1002/ca.1079. [DOI] [PubMed] [Google Scholar]
- Stevenson A, Johnston H, Stewart M, Golding D. Congenital malformations. A report of a study of series of consecutive births in 24 centres. Bull World Health Organ. 1966;34(Suppl):9–127. [PMC free article] [PubMed] [Google Scholar]
- Stevenson RE, Hall JC, editors. Human malformations and related anomalies. 2nd edn. New York: Oxford University Press Inc; 2006. , editors. 1495 p. [Google Scholar]
- Stewart A. The Role of Epidemiology in the detection of harmful effects of radiation. Environ Health Perspect. 2000;108:93–96. doi: 10.1289/ehp.0010893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo N, Pap C, Kobor J, Svekus A, Turi S, Sztriha L. Primary microcephaly in Hungary: epidemiology and clinical features. Acta Paediatr. 2010;99:690–693. doi: 10.1111/j.1651-2227.2009.01666.x. [DOI] [PubMed] [Google Scholar]
- Tapper D, Lack E. Teratomas in infancy and childhood. Ann Surg. 1983;198:398–409. doi: 10.1097/00000658-198309000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thottungal A, Charles A, Dickinson J, Bower C. Caudal dysgenesis and sirenomelia-single centre experience suggests common pathogenic basis. Am J Med Genet A. 2010;152A:2578–2587. doi: 10.1002/ajmg.a.33599. [DOI] [PubMed] [Google Scholar]
- Ueno N, Greene N. Planar cell polarity genes and neural tube closure. Birth Defects Res C Embryo Today. 2003;69(4):318–324. doi: 10.1002/bdrc.10029. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Polarity proteins are required for left-right axis orientation and twin-twin instruction. Genesis. 2012;50(3):219–234. doi: 10.1002/dvg.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J. Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet. 2006;15(Review No. 2):R227–R234. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- Wang B, Wertelecki W. Density estimation for data with rounding errors. Comput Stat Data Anal. 2013;65:4–12. [online]. Available at: http://www.sciencedirect.com/science/article/pii/S0167947312000989 [September 17, 2013] [Google Scholar]
- Warkany J. Congenital malformations: notes and comments. Chicago, IL: Year Book Medical Publishers Inc; 1971. . 1495 p. [Google Scholar]
- Warkany J, Lemire RJ, Cohen MM. Mental retardation and congenital malformations of the central nervous system. Chicago, IL: Year Book Medical Publishers Inc; 1981. . 459 p. [Google Scholar]
- Wertelecki W. Birth defects surveillance in Ukraine: a process. J Appl Genet. 2006;47:143–149. doi: 10.1007/BF03194614. [DOI] [PubMed] [Google Scholar]
- Wertelecki W. Malformations in a Chornobyl-impacted region. Pediatrics. 2010;125:836–843. doi: 10.1542/peds.2009-2219. [DOI] [PubMed] [Google Scholar]
- Willis RA. The borderland of embryology and pathoglogy. 2nd edn. Washington, DC: Batterworth Inc; 1962. . 641 p. [Google Scholar]
- Windham G, Sever L. Neural tube defects among twin births. Am J Hum Genet. 1982;34:988–998. [PMC free article] [PubMed] [Google Scholar]
- Wood J, Johnson K, Omori Y, Kawamoto S, Keehn R. Mental retardation in children exposed in utero to the atomic bombs in Hiroshima and Nagasaki. Am J Public Health Nations Health. 1967a;57:1381–1390. doi: 10.2105/ajph.57.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J, Keehn R, Kwamoto S, Johnson K. The growth and development of children exposed in utero to the atomic bombs in Hiroshima and Nagasaki. Am J Public Health Nations Health. 1967b;57:1374–1380. doi: 10.2105/ajph.57.8.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J, Johnson K, Omori Y. In utero exposure to the Hiroshima Atomic bomb: an evaluation of head size and mental retardation: twenty years later. Pediatrics. 1967c;39:385–392. [PubMed] [Google Scholar]
- Wu G, Huang X, Hua Y, Mu D. Roles of planar cell polarity pathways in the development of neural [correction of neutral] tube defects. J Biomed Sci. 2011;18:66. doi: 10.1186/1423-0127-18-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, editor. New York: Academic Press; 2012. Planar cell polarity during development. , editor. 360 p. [Google Scholar]
- Yuskiv N, Andelin C, Polishuk S. High rates of neural tube defects in Ukraine. Birth Defects Res A Clin Mol Teratol. 2004;70:400–402. doi: 10.1002/bdra.20020. et al. [DOI] [PubMed] [Google Scholar]
- Zamostian P, Moysich KB, Mahoney MC. Influence of various factors on individual radiation exposure from the Chernobyl disaster. Environ Health. 2002;1:4. doi: 10.1186/1476-069X-1-4. et al. Available at: http://www.ehjournal.net/content/1/1/4 [September 17, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska I, Mosor M, Nowak J. Regional distribution of heterozygous 657del5 mutation carriers of the NBS1 gene in Wielkopolska province (Poland) J Appl Genet. 2006;47:269–272. doi: 10.1007/BF03194635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 County Isonomy Rates of Neonates (Aggregate Frequency of Five Most Common Family Names).
Fig. S2 Whole Body Counts of incorporated ionizing radiation (Bq 137Cs) among 6026 pregnant women residing in Rivne Province (2008–2011). The (*) indicates the official upper norm for children under the age of 15 years (3700 Bq). (Decree #106 of Cabinet of Ministers of Ukrainian Soviet Socialist Republic of July 23, 1991. – See references).
Fig. S3 Birth weight of liveborns of all gestational ages (2000–2009) in Polissia and non-Polissia regions of Rivne province of Ukraine.
Fig. S4 Twinning Events among Relatives of Conjoined Twins (2000–2010).
Table S1a Temporal Contrasts between Polissia (POL) and non-Polissia (nPOL)(1) of Population-Based Rates of Core Congenital Malformations.
Table S1b Temporal Contrasts within Polissia and non-Polissia(1) of the Number of Observed Individuals with Core Congenital Malformations.
Table S1c Unduplicated Individuals with Core Congenital Malformations after Exclusion of Those with Clinically Recognizable Malformation Syndromes, Genomic Mutations, Cytogenetic Anomalies and Recognizable Teratogenic Effects(1).
Table S1d Overview of Unduplicated Individuals in Polissia and non-Polissia with Non-Syndromic Congenital Malformations(1) or Sentinel Anomalies (2000–2009).
Table S2 Total Number of Malformations and Rates per se (not Unique Individuals) and Male-Female (MF) Proportions and Ratios (M : F) in Polissia and non-Polissia(a).
Table S3 All Individuals with Fetal Alcohol Spectrum Disorder (FASD). Non-Population-Based Observations.
Table S4 All Non-Singletons Including Those with Congenital Malformations (2000–2009)(1).
Table S5 Population Rates of Neural and Other Malformations (not Individuals) in the Polissia and non-Polissia Regions of Ukraine and other regions of Europe.
Table S6 Teratomas – Rivne, Volyn (2000–2009) and Khmelnytsky (Kh) Provinces (2002–2009)(a).
Table S7 Non-Syndromic NTD Associated with Omphaloceles (OM), Body Wall and Other Anomalies in Rivne, Khmelnytsky and Volyn Provinces (2000–2009)(a).
Table S8 Gastroschisis and Maternal Age at Delivery: Rivne and Volyn Provinces (2000–2009) and Khmelnytsky Province (2002–2009).
Table S9 Encephaloceles and Male-Female (M-F) Proportions.


